In March 2020, "Coronavirus Disease 2019" (COVID- 19) outbreak due to SARS-CoV-2 was declared a pandemic by the World Health Organization. Clinical manifestations of COVID-19 are variable, ranging from complete absence of symptoms, to severe pneumonia, multiorgan failure, and death. Main risk factors for poor outcome of COVID-19 are advanced age and comorbidities, conditions that often recur in patients with monoclonal gammopathies. In this setting, several papers have reported more frequent and severe COVID-19, as well as higher fatality rate, in patients with multiple myeloma (MM), particularly in those older than 60 years, with high risk, active/progressive disease and/or renal failure, respect to general population.1-3 By contrast, very few data are available about patients with monoclonal gammopathies of undetermined significance (MGUS).4 In our retrospective study, we investigated the incidence of SARS-CoV-2 infection and COVID-19 outcomes in MGUS patients. Overall, we found that subjects with MGUS neither have an increased risk of contracting SARS-CoV-2, nor show poorer COVID-19 outcomes compared to controls.
Patients with MGUS are frequently asymptomatic and diagnosed by chance; therefore, differently from MM, one would not expect an increased incidence of SARSCoV- 2 infection or a worse outcome of COVID-19 respect to the normal population in these subjects. Notwithstanding, according to well recognized risk factors, they may have different risks of developing MM, as well as clinical findings, including older age, presence of medical comorbidities, and impaired humoral/cellular immunity, which could still play a role when assessing their risk during the COVID-19 pandemic.5,6 Notably, in epidemiological studies, people with MGUS were shown to have an increased risk of developing both venous and arterial thrombosis, bacterial and viral infections, as well as an excess mortality risk due to bacterial infections as compared to age and sex-matched healthy controls.7,8 On this basis, the presence of MGUS could possibly increase susceptibility to SARS-CoV-2 infection and severity of COVID-19, and might theoretically account for the increased mortality due to COVID-19 observed in the elderly population.9 However, a retrospective chart review aiming to study the vulnerability of 228 patients with MGUS (3 of whom resulted infected by SARS-CoV2, with 1 death) and their clinical outcomes during the COVID-19 pandemic, concluded that there were neither significant differences in the mean age or survival of the MGUS patients not infected by SARS-COV-2 who died before versus after the pandemic onset, nor an increase in venous thrombotic events.10 Furthermore, in a small case series of seven MGUS patients experiencing COVID-19, 71% were hospitalized, but none of the patients required mechanical ventilation or ICU (intensive care unit) management.4 Patients had an age range between 59 and 92 years and all had underlying high-risk comorbidities. One patient with acute kidney injury recovered after hemodialysis. The only death was a male patient with advanced age, nursing home residency, multiple comorbidities and elevated D-dimer. This small case series would suggest that MGUS does not pose additional risks for poor outcome in COVID-19 patients.
The aim of our observational, retrospective, single center study was to formally investigate the incidence of SARS-CoV-2 infection, as well as the characteristics and the clinical outcome of COVID-19 in a larger cohort of MGUS patients. The study was conducted within the context of the clinicaltrials gov. Identifier: NCT04352556.
Between March 1, 2020 and April 30, 2021, we collected, among 1.454 MGUS patients followed at our center, clinical data from 91 patients with SARS-CoV2 infection, diagnosed by RT-PCR on nasopharyngeal swabs. Data were mainly extracted from “GIAVA-COVID-19”, a regional platform where authorized medical health workers can view the results of the nasopharyngeal swabs for SARS-CoV-2 performed, along with other information. In MGUS patients a review of medical records was also carried out. Clinical data collected regarded age, cardiovascular, pulmonary, diabetic and neoplastic comorbidities, presence of symptoms (in detail: fever or chills, cough, shortness of breath or difficulty breathing, fatigue, muscle or body aches, headache, loss of taste or smell, sore throat, congestion or runny nose, nausea or vomiting, diarrhea), hospitalization, hospitalization in ICU, and outcome (alive/dead). Patients with monoclonal gammopathies of clinical (renal, dermatological or neurological) significance (MGCS) were excluded.
The characteristics of COVID-19 in MGUS patients were compared with those of 182 age- and sex-matched normal controls infected by SARS-CoV-2. Furthermore, the incidence of SARS-CoV-2 infection in MGUS patients was compared to that of the entire Apulian population (Apulia is a region of Southern Italy with about 4 million inhabitants). Wilcoxon test, chi-square tests and multivariate logistic regression were applied, as appropriate, by using STATA software MP17.
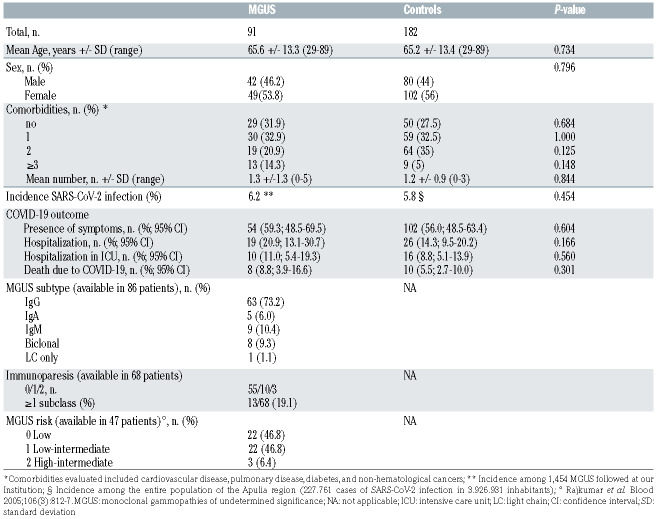
Table 1 summarizes the main characteristics of SARSCoV- 2 infected MGUS patients and controls. The two groups were comparable for age, sex, and presence of comorbidities. Among the MGUS group, 68 patients showed a non-IgM subtype and nine an IgM subtype: this information was missing in five patients. Nine patients had a double M-component. Immunoparesis (at least one uninvolved immunoglobulin below reference levels) was present in 19% of 68 evaluable patients. Regarding MGUS risk-stratification according to Mayo Clinic model, most (94%) of 47 patients with complete available dataset scored as low or low/intermediate risk. Sixty-two patients showed the presence of at least one co-existing, potentially clinically relevant comorbidity (cardiovascular disease 40.6%, diabetes 11%, nonhematological cancer 8.8%, pulmonary disease 6.6%).
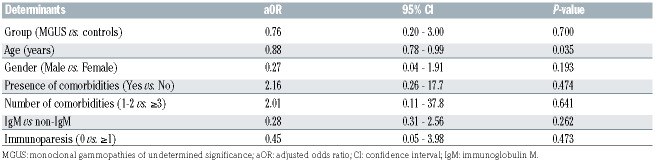
As shown in Table 1, rates of COVID-19-related symptoms, hospitalization, hospitalization in ICU and deaths due to COVID-19 were slightly higher in the MGUS group than in the control group, but these differences were not statistically significant. In MGUS patients, sex, immunoparesis, presence/number of comorbidities and IgM versus non-IgM isotype did not significantly influence the risk of death, while a statistically significant association was observed with older age; importantly, the risk of death was not correlated with the presence of MGUS (Table 2). Lastly, incidence of SARS-CoV2 infection in MGUS patients (91/1.454, 6.2%) was not statistically different from that observed in the entire population of the Apulia region (227.761/3.926.931, 5.8%) during the same period (Table 1).
Thus, in our study, patients with MGUS, contrarily to what is seen in MM, did not show an increased incidence of SARS-CoV-2 infection compared to the general population. Furthermore, although rates of symptoms, hospitalization, hospitalization in ICU and deaths were slightly higher than in controls, MGUS did not appear to represent a risk for a poorer COVID-19 outcome. The only factor associated with an increased risk of death was older age; however this was likely not related to the presence of MGUS, but rather to the well known predictive power of this clinical parameter for a worse outcome in the general population infected by SARS-CoV-2.9
Although, to the best of our knowledge, we conducted the largest study of SARS-CoV-2 infection in patients with MGUS, several limitations are present in our analysis. First, as with any observational retrospective study, there could be unintentional patient selection bias: MGUS may be misclassified and, conversely, individuals may be unaware of its presence. Second, not all MGUS patients had a complete dataset and some laboratory findings were lacking. Finally, long-term outcomes of MGUS and COVID-19 infection should also be explored. Therefore, further data are needed to achieve greater generalizability of our findings.
Other questions, particularly the possibility of a suboptimal response in people with MGUS to anti-SARSCoV- 2 vaccine (as observed in MM), possibly due to age and MGUS-related immune dysfunction, will be probably soon addressed by ongoing studies. In this setting, preliminary data show that MGUS patients receiving anti-SARS-CoV-2 vaccines could have a better antibody response than those with MM.11 Anyway, in patients with MGUS, as in those with MM, full vaccination against SARS-CoV-2 should be strongly recommended to reduce the possibility of infection rate and severity of the illness.12-14 Last, but not least, the psychosocial impact of the pandemic on MGUS patients and their management in the long term follow-up also would warrant to be further and specifically investigated.15
Table 1.
Characteristics of patients with monoclonal gammopathies of undetermined significance versus controls and COVID-19 outcome.
Table 2.
Risk for death in COVID-19 monoclonal gammopathies of undetermined significance patients, adjusted for different clinical parameters.
References
- 1.Chari A, Samur MK, Martinez-Lopez J, et al. Clinical features associated with COVID-19 outcome in multiple myeloma: first results from the International Myeloma Society data set. Blood. 2020;136(26):3033-3040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Martínez-López J, Mateos MV, Encinas C, et al. Multiple myeloma and SARS-CoV-2 infection: clinical characteristics and prognostic factors of inpatient mortality. Blood Cancer J. 2020;10(10):103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Engelhardt M, Shoumariyeh K, Rösner A, et al. Clinical characteristics and outcome of multiple myeloma patients with concomitant COVID-19 at Comprehensive Cancer Centers in Germany. Haematologica. 2020;105(12):2872-2878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gonzalez-Lugo JD, Bachier-Rodriguez L, Goldfinger M, et al. A case series of monoclonal gammopathy of undetermined significance and COVID-19. Br J Haematol. 2020;190(3):e130-e133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kyle RA, Larson DR, Therneau TM, et al. Long-term follow-up of monoclonal gammopathy of undetermined significance. N Engl J Med. 2018;378(3):241-249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.van de Donk NW, Palumbo A, Johnsen HE, et al. The clinical relevance and management of monoclonal gammopathy of undetermined significance and related disorders: recommendations from the European Myeloma Network. Haematologica. 2014;99(6):984-996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kristinsson SY, Björkholm M, Andersson TM, et al. Patterns of survival and causes of death following a diagnosis of monoclonal gammopathy of undetermined significance: a population-based study. Haematologica. 2009;94(12):1714-1720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kristinsson SY, Tang M, Pfeiffer RM, et al. Monoclonal gammopathy of undetermined significance and risk of infections: a populationbased study. Haematologica. 2012;97(6):854-858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Onder G., Rezza G., Brusaferro S. Case-fatality rate and characteristics of patients dying in relation to COVID-19 in Italy. JAMA. 2020;323(18):1775-1776. [DOI] [PubMed] [Google Scholar]
- 10.Lee H, Tay J, Street L, Duggan P, Jiménez-Zepeda VH. Monoclonal gammopathy of undetermined significance clinic during the coronavirus disease-19 pandemic: caring for the vulnerable in an academic medical center. Rev Invest Clin. 2021;73(4):259-264. [DOI] [PubMed] [Google Scholar]
- 11.Terpos E, Gavriatopoulou M, Ntanasis-Stathopoulos I, et al. The neutralizing antibody response post COVID-19 vaccination in patients with myeloma is highly dependent on the type of antimyeloma treatment. Blood Cancer J. 2021;11(8):138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. https://cms.cws.net/content/beta.myelomasociety.org/files/PM%20COVID%20vaccination%20in%20MM%20guidelines%20The%20Final.pdf . [Google Scholar]
- 13.Ludwig H, Meckl A, Engelhardt M. Compliance with vaccination recommendations among patients with multiple myeloma: a real world experience. Hemasphere. 2021;5(7):e597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gavriatopoulou M, Ntanasis-Stathopoulos I, Korompoki E, Terpos E, Dimopoulos MA. SARS-CoV-2 vaccines in patients with multiple myeloma. Hemasphere. 2021;5(3):e547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Quinn SJ, Anderson LA, Lohfeld L, McShane CM. The psychosocial impact of the COVID-19 pandemic on patients with monoclonal gammopathy of undermined significance, smouldering and active myeloma: findings from an international survey. Br J Haematol. 2021;194(2):294-297. [DOI] [PMC free article] [PubMed] [Google Scholar]