Abstract
Introduction
Autism spectrum disorder (ASD) is a complicated diffuse developmental disorder that commonly involves gastrointestinal distress and dysbacteriosis. Emerging lines of evidence have shown faecal microbiota transplantation (FMT) to be a potential therapeutic strategy for improving the clinical outcomes of patients with ASD by re-establishing their intestinal microflora. We are undertaking the first-ever multicentre, double-blind, randomised controlled trial of FMT for the treatment of children with both ASD and gastrointestinal symptoms and will assess the feasibility and efficacy outcomes of this strategy.
Methods
In total, 318 children with both ASD and gastrointestinal symptoms will be enrolled (from 15 hospitals in China) to receive either FMT intervention (n=212) or a placebo (control, n=106). Children aged 3–6 years will take two capsules two times a day, and those older than 6 years will take three capsules two times a day. Each patient will receive four treatment courses, with each 12-day course being repeated every month. Outcomes will be evaluated at baseline, throughout the period of intervention, and at subsequent follow-ups for 2 months. The primary trial objective is to investigate the remodelling effect of FMT on the intestinal microflora in patients with ASD. The secondary objective focuses on the clinical efficacy and safety of FMT, including its improvement of the clinical response and metabonomics.
Ethics and dissemination
Ethical approval was obtained from the hospital Ethics Committee of each Faecal Transfer for ASD China Multicenter Trial Working Group. The ongoing FMT clinical trial is intended to support the approval of the new technology and its administration. The results of this trial will provide high-quality evidence to inform the future clinical application of this new therapy.
Trial registration number
ChiCTR2100043906; Pre-results.
Keywords: microbiology, child & adolescent psychiatry, gastroenterology
Strengths and limitations of this study.
This multicentre, double-blind, randomised controlled trial will be the first-ever study to provide the strongest evidence of the feasibility and efficacy of faecal microbiota transplantation (FMT) for treating children with autism spectrum disorder (ASD).
The trial will verify the correlation between ASD core symptoms and the microbiota and metabonomics, which may further explain the role of the brain–gut axis in this disorder.
The intervention period is 6 months and will involve the equivalent of approximately 24–36 g of human stool in total, making it one of the longest studies with the largest usage of FMT.
Although selective digestive tract decontamination through combination antibiotics and FMT may have further effects on the gut microbiota, antibiotics will not be used before FMT in this study, given that they may have a long-term impact on the children.
Introduction
Autism spectrum disorder (ASD), a complicated diffuse developmental disorder of the central nervous system, responds poorly to treatment.1 2 Most children with ASD have gastrointestinal (GI) problems, such as constipation, diarrhoea and indigestion.3 4 Autism and GI symptoms may be related to intestinal microbes via the brain–gut axis.5 The gut microbiota of children with ASD is significantly different from that of healthy children, with the Firmicutes and Proteobacteria being increased and the Bacteroidetes reduced in individuals with the disorder.6 Particularly, the main species of the Firmicutes that is increased is Clostridium histolyticum, which releases neurotoxins through the vagus nerve into the central nervous system, thereby inhibiting the release of neurotransmitters and resulting in ASD behaviour.7 Treatment with Clostridium-targeting oral vancomycin ameliorates GI symptoms in some children with ASD and to improve their communication and social skills.8 9 However, the difficulty in eliminating the bacterial spores completely can lead to recurrence after treatment discontinuation.10 Additionally, the metabolites of some gut microbiota have also been thought to be possible causes of ASD;11 for example, the excessive production of short-chain fatty acids, p-methylphenol, and ammonia.12 13 Propionic acid, a type of short-chain fatty acid produced mainly by Clostridium and Bacteroides, was shown to limit behavioural interest and impair social behaviour and cognitive functions in rats, suggesting its association with ASD.14
Therefore, modulation of the gut microbiota and microbiome may play a therapeutic role in ASD, as was demonstrated through the improvement of symptoms following dietary and probiotic interventions.15 16 However, the effects of food, probiotics and prebiotics are limited when faced with the huge amount of microorganisms in the gut. Faecal microbiota transplantation (FMT) is a treatment method that involves the transplantation of a whole healthy gut microbiota into the gut of patients to reconstruct their intestinal microflora and cure associated diseases.17 When faeces collected from a patient with ASD were transplanted into sterile mice, the offspring of the mice developed symptoms of ASD.18 In a long-term open-ended study on the safety, tolerability and efficacy of this strategy for ASD treatment,19 16 of 18 children with ASD who had received FMT for 8 weeks showed significant improvement of their autism and GI symptoms, as determined using the Gastrointestinal Symptom Rating Scale (GSRS), Childhood Autism Rating Scale (CARS), Parent Global Impressions—III questionnaire and Vineland Adaptive Behavior Scale.20 The effects lasted at least 8 weeks after the treatment, and the CARS score improved by 50% after 2 years.19 In an early exploratory study conducted by our centre, the efficacy of FMT in the treatment of ASD was approximately 64.6%,21 with core symptoms such as social communication disorder and GI symptoms being apparently improved and changes in the composition of the intestinal microflora evident. Because standard FMT procedures were lacking and the sample size was limited in previous studies, the efficacy and safety of this treatment method have not been verified with strong evidence, which can only be gathered through well-designed randomised controlled trials (RCTs). Therefore, we have initiated a phase II study targeted at a paediatric population, with a well-calculated sample size and randomised controls. The systematic preparation of the faecal microbiota and the procedure used in this trial for its transfer will provide a reference for standard FMT, which is essential for its further development and clinical application.
Significance
The treatment of patients with ASD through FMT may interfere with and improve the GI and core symptoms of the disorder. Our centre is the first in China to initiate a multicentre, double-blind, randomised, placebo-controlled study on FMT in children with ASD. To the best of our knowledge, no multicentre RCT or a trial with a large sample size has been conducted on FMT in ASD. Moreover, there are no reports of studies exploring the pathophysiological mechanisms of ASD from the point of view of the microbiota and their metabolites, GI symptoms, and autism symptoms in children.
Objectives
The objectives of this study are to evaluate the efficacy and safety of oral administrations of faecal microbiota-filled capsules (cFM) in children with both ASD and GI symptoms. This trial will test the hypothesis that four courses of cFMs from standardised faecal donors will result in improved therapeutic effects.
Primary objective: to evaluate the effect of repeated FMT on the remodelling of the gut microecology in children with GI symptoms.
Secondary objectives:
To study the efficacy of FMT in treating core symptoms and emotional symptoms in children with ASD.
To study the efficacy of FMT in treating GI symptoms in children with ASD.
To study the efficacy of FMT in treating other comorbidities in children with ASD, such as allergy, dermatitis, asthma and the metabolic syndrome.
To study the safety of FMT in children with ASD.
The exploratory objective is to study the dynamic changes in the intestinal microflora and host metabolism following FMT treatment to identify the mechanisms and microbes related to ASD. A further aim is to study the impacts of FMT on the intestinal permeability, immune response and nervous system in ASD.
Methods and analysis
Study design
The Fecal Transfer for ASD China Multicenter Trial (FTACMT) study will be a multicentre, double-blind, randomised, placebo-controlled trial. The study will recruit 318 children with both ASD and GI symptoms from 15 hospitals in China. Patient enrolment began in May 2021.
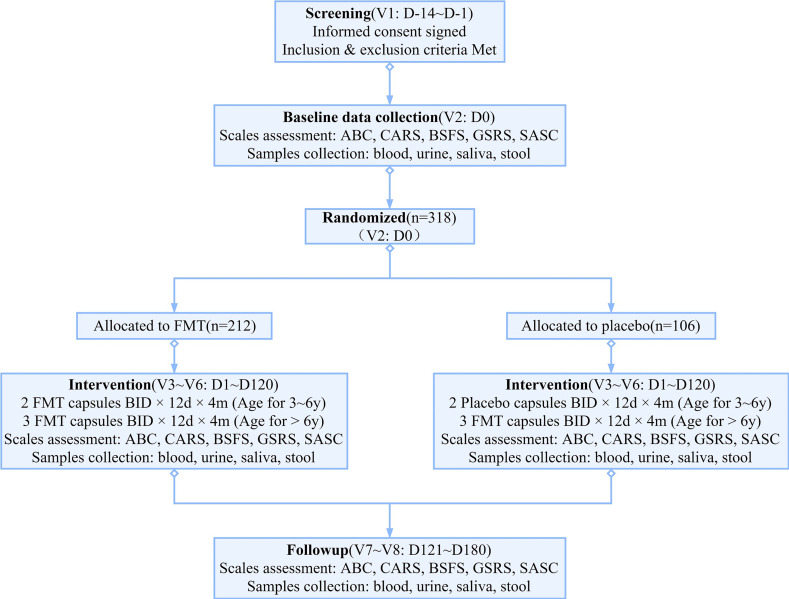
In accordance with the protocol, the study participants will be seen on-site for all scheduled visits (figure 1). The study will use a parallel-arm approach composed of an FMT intervention group (n=212) and a placebo group (n=106) at a 2:1 ratio. The FMT group will receive cFMs prepared through a standard procedure, whereas the control group will receive placebo capsules containing microcrystalline cellulose (MCC). The placebo capsules are similar to the cFMs in appearance, smell and weight. Previous reports and our own research have indicated that MCC is not harmful to children with ASD.16 Four cFMs have an equivalent of approximately 1.0 g of human stool. The number of live bacteria is more than 5.0×109/g, composing more than 70% of each capsule. The study duration will be 6 months per patient, composed of 4 months of treatment and 2 months of follow-up.
Figure 1.
Protocol flow chart of the study. ABC, Autism Behavior Checklist; BSFC, Bristol Stool Form Scale; CARS, Childhood Autism Rating Scale; FMT, faecal microbiota transplantation; GSRS, Gastrointestinal Symptom Rating Scale; SASC, Social Anxiety Scale for Children.
Inclusion/exclusion criteria
The inclusion criteria for study participation are as follows:
Age 3–13 years, male or female.
Meeting the ASD diagnostic criteria of the Diagnostic and Statistical Manual of Mental Disorders-5 of the American Psychiatric Association.
A recurrence of diarrhoea, constipation, abdominal distension, and/or food allergy/intolerance for at least 6 months and these same symptoms occurring within the last 3 months.
An informed consent form signed by the study participant or their guardian and agreement of cooperation with the researcher (who will collect clinical data and blood, saliva, urine and faecal samples) throughout the whole process of the clinical trial.
An ability to swallow two or three cFMs unaided, depending on the age group.
The exclusion criteria are as follows:
Diagnosis of Rett syndrome.
Previous history of brain injury, cerebral palsy, encephalitis or other organic brain diseases.
Diagnosis of other mental diseases.
Diagnosis of organic GI diseases, such as congenital megacolon, intestinal obstruction, intussusception and inflammatory bowel disease.
Severe obesity or malnutrition.
Use of probiotics, antibiotics and other drugs affecting the intestinal microflora in a planned way within the last 3 months or in the following 6 months.
Recipient of FMT treatment in the past 12 months.
An inability to swallow two or three cFMs, depending on the age group.
Sample size estimation
The proposed sample size for the present study is 318. According to a previous study conducted at our centre, the mean Shannon index for the alpha diversity of intestinal microflora in children with ASD is approximately 2.923, with an SD of 0.674. After receiving cFMs, the mean Shannon index increased by 0.298, with an SD of 0.705. The superiority of the means of the two groups will be tested using Power Analysis and Sample Size software, with an α value of 0.025 (one-sided), power-of-test value of 0.9, difference value of 0.3, SD of 0.7 and dividing value of 0. When calculated at 2:1, the effective sample size should not be less than 260. Given the high natural attrition rate of children with ASD, the dropout rate of study participants is assumed to be 20%. Thus, we finally set the sample size to 318, including 212 for the FMT group and 106 for the control group.
Randomisation
All patients who meet the eligibility criteria will be enrolled. Patients will be allocated in a 2:1 ratio to the FMT intervention and control groups, respectively, via computer-generated block randomisation (block size=6). Competitive enrolment programmes will be adopted. The study will have only one unblinded pharmacist, who will prepare and account for the bags of cFMs and placebo capsules according to set guidelines. After all patients have completed the study and the data have been locked, the pharmacist will unblind the materials.
Intervention
The FMT group will receive oral administrations of cFMs two times a day. The 3–6 years old children will ingest two capsules each time, whereas the children aged over 6 years will swallow three capsules. In total, four treatment courses will be conducted, with each course lasting for 12 days and repeated every month. The control group will receive MCC-containing placebo capsules that will be similar to the cFMs in appearance, smell and weight. The placebo doses and courses will be the same as those of the cFMs (figure 1).
Follow-up
Screening will be conducted at the first visit (V1), following which baseline data will be collected (V2). The intervention will be carried out according to the plan for 4 months, during which monthly visits will be conducted (V3–V6). Follow-up will be conducted for 2 months, during which the participants will be visited once a month (V7 and V8). The last visit (V8) will be conducted at the end of the sixth month, when the trial ends. In accordance with the requirements of the study protocol, the participants should not change their original eating habits but must also not ingest edible fermented and preserved foods or probiotic products during the trial period (table 1).
Table 1.
Visit form of the study
| Examinations | Screening | Baseline | Intervention | Follow-up | ||||
| Time window |
V1
D-14~D-1 |
V2
D0 |
V3
D1 ~D30 |
V4
D31 ~D60 |
V5
D61 ~D90 |
V6
D91 ~D120 |
V7
D121 ~D150 |
V8
D151 ~D180 |
| Informed consent | ★ | |||||||
| Inclusion and exclusion criteria | ★ | |||||||
| Clinical assessment | ||||||||
| ABC | ★ | ★ | ★ | ★ | ★ | ★ | ★ | |
| CARS | ★ | ★ | ★ | ★ | ★ | ★ | ★ | |
| BSFS | ★ | ★ | ★ | ★ | ★ | ★ | ★ | |
| GSRS | ★ | ★ | ★ | ★ | ★ | ★ | ★ | |
| SASC | ★ | ★ | ★ | ★ | ★ | ★ | ★ | |
| Other symptoms | ★ | ★ | ★ | ★ | ★ | ★ | ★ | |
| Biological activity | ||||||||
| Stool samples | ★ | ★ | ★ | ★ | ★ | ★ | ||
| Urine samples | ★ | ★ | ★ | ★ | ★ | ★ | ||
| Blood samples | ★ | ★ | ★ | ★ | ★ | ★ | ||
| Saliva samples | ★ | ★ | ★ | ★ | ★ | ★ | ||
| Safety assessment | ||||||||
| Adverse events | ★ | ★ | ★ | ★ | ★ | ★ | ||
| Vital signs | ★ | ★ | ★ | ★ | ★ | ★ | ★ | |
| Physical examination | ★ | ★ | ★ | ★ | ★ | ★ | ★ | |
| Concomitant medications | ★ | ★ | ★ | ★ | ★ | ★ | ★ | |
| Haematology | ★ | ★ | ★ | ★ | ★ | ★ | ★ | |
| Biochemistry | ★ | ★ | ★ | ★ | ★ | ★ | ★ | |
| Urinalysis | ★ | ★ | ★ | ★ | ★ | ★ | ★ | |
| Infectious markers | ★ | ★ | ★ | ★ | ★ | ★ | ★ | |
| Records | ||||||||
| Capsules distribution | ★ | ★ | ★ | ★ | ||||
ABC, Autism Behavior Checklist; BSFC, Bristol Stool Form Scale; CARS, Childhood Autism Rating Scale; GSRS, Gastrointestinal Symptom Rating Scale; SASC, Social Anxiety Scale for Children.
Fecal microbiota donor screening criteria
General information and laboratory tests:
Age 18–30 years; men or women; body mass index of 18.5–22.9 kg/m2.
Haematological tests: normal routine blood test results, liver and kidney function, and electrolyte and C reactive protein levels; negative for infectious hepatitis, HIV, syphilis, Epstein-Barr virus, cytomegalovirus, nematodes, amoebas and other pathogens.
Faecal test: normal routine stool test results; negative occult-blood test results; negative for Clostridium difficile, Campylobacter, Salmonella, Shigella, toxigenic Escherichia coli, parasitic ova, vesicles, and spores, norovirus, and rotavirus; and negative for pathogens with multidrug resistance genes (including extended-spectrum beta-lactamase-producing bacteria, carbapenem-resistant Enterobacteriaceae among others).
Medical history:
Previous history: no GI symptoms in the last 2 weeks; no use of antibiotics, acid inhibitors, immune suppressors, or chemotherapeutic drugs for nearly 3 months; no chronic pain symptoms; no history of digestive surgery; no history of infections or exposure to infectious diseases; no history of allergic disease, autoimmune disease, metabolic disease, cardiovascular disease, and nervous system or mental disease; no history of malignancy; and no injections of growth hormones, insulin, or clotting factors.
Personal history: having a regular schedule, healthy diet and domestic peace; no promiscuity; no smoking, drinking or drug addictions; no vaccinations or drug tests in the past 6 months; no tattoos or skin damage in the past 6 months; no contact with tropical areas in the past 6 months.
Family history: no family history of GI diseases, malignant tumours or infectious diseases.
Others: not pregnant or menstruating.
Capsule quality control standards
The preparation of the cFMs meets the Chinese expert consensus on the standardised methodology and clinical application of FMT.22 The donated stools are processed into lyophilised powder using a lyophiliser and then filled into capsules. For each donor sample, capsules will be randomly sampled after their formulation to test for the presence of pathogenic organisms, infectious sources and multidrug-resistant bacteria, which should all be negative. The number of live bacteria in each capsule should not be less than 2.5×108/g, and the 16S rDNA bacterial sequencing results should be consistent in the same batch samples. Capsules will be refrigerated and transported at –20℃.
Patient and public involvement
The study design was informed by two follow-up studies on the perceptions that patients and parents had about FMT. One of the follow-up studies has been published,23 and the other is under preparation.
Statistical program
Outcome measures
The primary outcome measure will be the increase in the Shannon index for the alpha diversity of intestinal microflora in children with ASD, from the baseline (V1) to the end (V8) of the study.
The secondary outcome measures constitute specific biological aims from the baseline (V1) to the end (V8) of the study.
Comparison of autism symptoms between the treated and control patients (assessed using the Autism Behavior Checklist (ABC) and CARS).
Comparison of GI symptoms between the treated and control patients (assessed using the Bristol Stool Form Scale (BSFS) and GSRS).
Comparison of emotional symptoms between the treated and control patients (assessed using the Social Anxiety Scale for Children (SASC)).
Determination of the safety and tolerability of FMT in children with ASD through routine blood, urine and stool tests; liver and kidney function tests; serum antibody detection; stool pathogenic bacteria detection; and the presence of abdominal pain, distension, constipation, diarrhoea, allergies, asthma and other symptoms.
Determination of changes in other comorbidities (eg, allergy, asthma, dermatitis and the metabolic syndrome) and in other symptoms.
Analysis of faecal, urine, saliva and blood samples to evaluate and compare the metabolic profiles between the two groups.
Analysis of changes in intestinal permeability indexes (including plasma lipopolysaccharide (LPS) and LPS-binding protein).
Analysis of neuroinflammatory biomarkers (including serum levels of interleukin (IL)−1b, IL-6, IL-8, IL-12, IL-13, IL-17, interferon-gamma and tumour necrosis factor-alpha).
Adverse events and safety
According to the International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use, adverse events (AEs) refer to all adverse medical events that occur after the study participant receives the investigational drug, including signs, symptoms and laboratory abnormalities, all of which should be recorded. However, such events do not necessarily have a causal relationship with the experimental drugs. Severe AEs (SAEs) refer to any of the following AEs: death, life-threatening symptoms resulting in hospitalisation or prolonging hospitalisation, congenital abnormalities/malformation, and permanent/major disabilities.
Many studies have suggested that FMT is a safe therapy, and few associated SAEs have been reported. The incidence of AEs after FMT remains at approximately 1%, with the most common event being abdominal discomfort. Other common AEs associated with FMT are diarrhoea, transient allergy, transient fever, nausea and vomiting, most of which are mild to moderate adverse reactions and self-limited. Thus, we will conduct the following to account for AEs:
An adverse reaction reporting system will be established.
Strict inclusion and exclusion criteria will be implemented, and the risk of complications before FMT will be assessed.
Mild symptoms, such as dizziness, nausea and abdominal discomfort, can be observed clinically.
Moderate symptoms will be treated according to the usual clinical practice guidelines; for example, oral antidiarrhoeal agents for diarrhoea, oral non-steroidal antipyretics for fever, spasmolysis for abdominal pain and metoclopramide for nausea and vomiting.
In the event of severe symptoms, FMT will be suspended or terminated and the patient must receive appropriate treatments, such as oral antidiarrheal agents, fluid replacement therapy and the maintenance of homeostasis when diarrhoea occurs more than five times. Moreover, for patients whose body temperature exceeds 39°C, tests should be performed to exclude intestinal infection.
When intestinal infection is suspected, FMT treatment must be immediately terminated, testing for pathogens in the faeces of both the donor and recipient should be performed, blood culture tests should be conducted, and anti-infective and fluid replacement therapies should be administered.
In the event of symptoms that could be considered SAEs occur, the investigators are responsible for reporting those to the appropriate ethics committee.
Data recording and monitoring
Case report forms will be prepared for data recording. A certified contract research organisation (CRO) is responsible for monitoring the study. The CRO is in contact with the principal investigators and will visit each centre to discuss and collect data. The CRO has organised meetings before the start of the study to discuss the protocol and obligations of the investigators and will monitor every centre from the beginning to the end. All observations and findings in the clinical trial will be verified to ensure the reliability of the data and that the conclusions of the trial are derived from the original data. There are corresponding data management measures in the clinical trial and data processing stage. All records will be made available, including original medical documents, protocol copies, patient identification codes, source data, informed consent forms, case report forms and other documents related to the study. Medical records and medical record forms are regarded as original records and generally cannot be altered. Should any corrections be needed, the original record will remain unaltered. Corrections should only be an additional statement with reasons given and must be signed and dated by the physician participating in the clinical trial. No study document can be destroyed. All original documents and copies of the study will be stored for inspection.
Statistical analysis
Data will be statistically analysed by professional statisticians. All statistical tests will be conducted as a two-sided test, with a p value of less than 0.05 considered to be statistically significant. For clinical outcome measures, we will compare changes in the ABC, CARS, BSFS, GSRS and SASC total scores and those in the ABC and CARS subscores, from baseline to each time point, in the treatment and control groups. A logistic regression model will be used to compare clinical endpoints in each group. The mean values for different biomarkers will be assessed. Mean differences between the treatment groups are to be calculated using the t-test or Wilcoxon-Mann-Whitney test. One-way repeated-measures analysis of variance will be used to assess the average change over time for the same variables mentioned above. Different prognostic factors will be tested between the two treatment groups. Microbiome analysis will be performed using specific software and different models, each with different microbiomes or biomarkers as dependent variables. Records of any AEs will be kept until the end of the study, and a safety analysis will be performed accordingly for each study participant receiving at least one course of cFM. The Cox proportional risk model will be used to adjust for any possible irregularities.
Ethics and dissemination
All researchers involved in this study will follow the Regulations of Ethical Reviews of Biomedical Research Involving Human Subjects (2016), the Declaration of Helsinki, as amended by the 64th WMA General Assembly (2013), and the International Ethical Guide for Human Biomedical Research (CIOMS, 2002). Under the guidance of the Good Practice for Quality Management of Drug Clinical Trials, the study shall be conducted according to the requirements of the protocol approved by the ethics committee to ensure the scientific nature of the study and protect the health and rights of the study participants.
The trial has been registered with the Chinese Clinical Trial Registry (ChiCTR; https://www.chictr.org.cn) under the reference number ChiCTR2100043906. After being provided written and oral information, all study participants and guardians will sign an approved informed consent form that explains the rationale, procedure, duration and possible risks and benefits of the study. Participants or their guardians will be informed that participation in the study is voluntary and that refusal or withdrawal from the study at any time will not result in any penalty or loss of benefit. Additionally, the phone number of the FTACMT leader will be provided in the informed consent form to facilitate a point of contact for participants or guardians needing to obtain independent information. Data privacy and confidentiality will be addressed according to the constitution of the People’s Republic of China. The lead ethics committee will be responsible for addressing all ethical issues during the study. The CRO and FTACMT will be responsible for addressing security and validity issues. The handling of all clinical data on the patients will be supervised by the funding committee of the Clinical Research Plan of SHDC. This study involves the collection of clinical data and blood, faeces, saliva and other samples from the participants. It does not involve trading, export, exit and other activities as well as highly pathogenic microorganisms. The study will be conducted in accordance with the relevant provisions of the Interim Measures for the Management of Human Genetic Resources. The results will be published on the ChiCTR website and in international peer-reviewed journals.
Discussion
Studies have shown that the gut microbiota has a potential therapeutic effect on ASD.16 18 19 Moreover, prebiotics, probiotics and FMT can improve GI and autism symptoms.16 18 19 Unlike probiotics, which typically contain only a few bacterial strains, FMT involves the intake of more than 1000 strains of the gut microbiota, covering a full spectrum of microbial species.24 It is also more effective than prebiotics and probiotics in regulating the nervous system, endocrine system and behavioural performance.5 25 However, most studies on FMT in ASD involved animal experiments.14 18 26 Considering that most of the patients are children, the impairment from ASD should be controlled and safety is of utmost importance. In earlier studies, the oral intake of human faecal suspensions was considered disgusting and highly unpleasant by patients and might even cause other problems in those taking acid inhibitors.27 28 Thus, the best formulation for children would be oral colon-release capsules, which are made of acid-resistant hydroxypropyl cellulose.23
In recent studies, FMT was demonstrated to be safe and achieved promising clinical results,19 23 with almost half of the children demonstrating continuously improved behaviour for 2 years after the treatment.19 In another study on the long-term efficacy and complications of FMT treatment, 64% of the 3932 participants showed improvement of their ASD symptoms, with 59% of them retaining the positive effects for 4 years, and the treatment efficacy was found to increase with a higher number of admitted courses.21
However, in previous studies, placebo controls were lacking and the sample sizes were limited. The present study will be the first-ever multicentre, double-blind RCT of FMT for the treatment children with both ASD and GI symptoms. Donors of the faecal microbiota are managed through standardised procedures and are anonymous and non-household.22 Every child will receive a total of four courses of cFMs produced from homogeneous donors. To ensure the safety of FMT and clarify its effect, the administration of antibiotics will be forbidden. Although vancomycin could improve the communication and social skills of patients with regressive-onset autism, antibiotics may have side effects on children.9 Moreover, the spores of certain bacilli are difficult to destroy, and the abuse of antibiotics may result in pathogens acquiring resistance to the drugs.10 Such risks need to be minimised. Additionally, gut microbiota dysbiosis involves large amounts and different types of bacteria, viruses and other microorganisms.29 If one type of bacteria is reduced, other microorganisms will increase. Based on the clinical results, we hypothesise that the metabonomics of the microbiota play an equally important role in treatment. Although many blood, salivary, urinary and faecal metabolites (eg, 5-hydroxytryptamine, exorphin, sulfate, propionic acid, and S-adenosylmethionine) have been reported as being associated with ASD, no representative biomarker of the disorder has been identified to date.12 13 30 31 Therefore, amino acid metabolism, sulphur metabolism, fatty acid metabolism, redox balance, nutrients, allergy and autoimmunity will all be tested to search for biomarkers of ASD and to clarify the involvement of the brain–gut axis.
In conclusion, we have initiated an innovative and high-quality study to clarify the feasibility and safety of FMT for the treatment of children with both ASD and GI symptoms. Since 2012, our intestinal microecology treatment centre has treated more than 4000 patients with various diseases.23 We have accumulated extensive experience and established a Chinese consensus on the selection and establishment of FMT delivery routes for use in clinical practice as well as an expert consensus on the establishment of a standardised methodology for the clinical application for FMT.22 32 Furthermore, this study will provide preliminary evidence on potential biomarkers of ASD. We believe that this study is gathering increasing interest from the international scientific community.
Supplementary Material
Acknowledgments
The authors acknowledge Faecal Transfer for ASD China Multicenter Trial (FTACMT) Working Group. The authors also acknowledge the contribution of the members of the CRO, Shanghai Xinshen Medical Consulting Center (Prof. Zhong Ming, Prof. Huang Chunyan, etc), Finch Therapeutics (Prof. Martina Schinkeand, Christopher Weidenmaier, etc) and all hospital ethics committees (Prof. Xu Huixiong, Dr. Fu Jin, etc).
Footnotes
YC and ZX contributed equally.
Collaborators: FTACMT Working Group: Department of Colorectal Disease, the Tenth People’s Hospital, Tongji University, Shanghai, China: Qin Huanlong, Li Ning, Chen Qiyi, Ye Chen, Zhang Xueying, Cui Jiaqu, Yang Rong, Lu Jubao, Yan Yinmei, Ma Chunlian, Lv Xiaoqiong, Yan Peihua, Huang Linsheng, Zhang Shaoyi. Shanghai Mental Health Center, Shanghai Jiao Tong University School of Medicine, Shanghai, China: Du Yasong, Zhao Xiaoxin. Shanghai Children’s Hospital, Shanghai Jiao Tong University, Shanghai, China: Wang Yu, Ma Chenhuan. Shanghai General Hospital, Shanghai Jiao Tong University, Shanghai, China: Chen Shidong, Miao Yun. Qingdao Women and Children’s Hospital, Qingdao University, Shandong, China: Kuang Guifang, Ling Wenqi. Qilu Children’s Hospital, Shandong University, Shandong, China: Zhao Dongmei, Li Ning. Henan Children’s Hospital, Zhengzhou University, Henan, China: Fang Shuanfeng, Li Ke. Hebei Mental Center, Hebei, China: Zhang Xujing, Geng Bojing, Zhang Xin. Shenzhen Children’s Hospital, Guangdong, China: Yang Binrang. The Affiliated Hospital of Qingdao University, Shandong, China: Wang Yanxia, Feng Xueying. Hainan Women and Children’s Medical Center, Hainan, China: Li Ling, Li Huilin, Liu Liyan, Wei Wei, Wu Weijia. Zhoushan Second People’s Hospital, Zhejiang, China: Yuan Song. Department of Children Psychology, Zhuhai Maternal and Child Health Care Hospital, Guangdong, China: Zhou Xiang, Li Dexin. Shanghai YangZhi Rehabilitation Hospital, Tongji University School of Medicine, Shanghai, China: Zhang Beihua, Jiang Qianru. Dalian Seventh People’s Hospital, Liaoning, China: Jiang Lin. Wuhu No.1 people's hospital, Anhui, China: Ji Hong, Gong Weida, Zhao Tiantian.
Contributors: YC, ZXue, and CJ drafted of the manuscript and accounted for all aspects of the work, contributing equally to the study. QH, LN, CQ substantially contributed to conception and design of the study. DY, LJ and ZXiao contributed to design of the study and planned outcome measures. LX coped with samples and collected data. MC produced FMT capsules. YR, WYu, CS, KG, ZD, FS, ZXuj, YB, WYa, LL, YS, ZXian, ZB, JL, and JH recruited and managed subjects. All members of FTACMT group contributed manuscript revision, read and approved the submitted version.
Funding: This project was supported by Clinical Research Plan of SHDC (No.SHDC2020CR1030B and No.SHDC2020CR4026) and Shanghai Action Plan on Science, Technology and Innovation (21Y11908300).
Competing interests: None declared.
Patient and public involvement: Patients and/or the public were involved in the design, or conduct, or reporting, or dissemination plans of this research. Refer to the Methods section for further details.
Provenance and peer review: Not commissioned; externally peer reviewed.
Contributor Information
FTACMT Working Group:
Qin Huanlong, Li Ning, Chen Qiyi, Ye Chen, Zhang Xueying, Cui Jiaqu, Yang Rong, Lu Jubao, Yan Yinmei, Ma Chunlian, Lv Xiaoqiong, Yan Peihua, Huang Linsheng, Zhang Shaoyi, Du Yasong, Zhao Xiaoxin, Wang Yu, Ma Chenhuan, Chen Shidong, Miao Yun, Kuang Guifang, Ling Wenqi, Zhao Dongmei, Li Ning, Fang Shuanfeng, Li Ke, Zhang Xujing, Geng Bojing, Zhang Xin, Yang Binrang, Wang Yanxia, Feng Xueying, Li Lin, Li Huilin, Liu Liyan, Wei Wei, Wu Weijia, Yuan Song, Zhou Xiang, Li Dexin, Zhang Beihua, Jiang Qianru, and Jiang Lin
Ethics statements
Patient consent for publication
Consent obtained from parent(s)/guardian(s).
References
- 1. Yuen RKC, Thiruvahindrapuram B, Merico D, et al. Whole-Genome sequencing of quartet families with autism spectrum disorder. Nat Med 2015;21:185–91. 10.1038/nm.3792 [DOI] [PubMed] [Google Scholar]
- 2. Kim S, Kim H, Yim YS, et al. Maternal gut bacteria promote neurodevelopmental abnormalities in mouse offspring. Nature 2017;549:528–32. 10.1038/nature23910 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Buie T, Campbell DB, Fuchs GJ, et al. Evaluation, diagnosis, and treatment of gastrointestinal disorders in individuals with ASDs: a consensus report. Pediatrics 2010;125 Suppl 1:S1–18. 10.1542/peds.2009-1878C [DOI] [PubMed] [Google Scholar]
- 4. Brondino N, Fusar-Poli L, Rocchetti M, et al. Complementary and alternative therapies for autism spectrum disorder. Evid Based Complement Alternat Med 2015;2015:1–31. 10.1155/2015/258589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Carabotti M, Scirocco A, Maselli MA, et al. The gut-brain axis: interactions between enteric microbiota, central and enteric nervous systems. Ann Gastroenterol 2015;28:203–9. [PMC free article] [PubMed] [Google Scholar]
- 6. Parracho HM, Bingham MO, Gibson GR, et al. Differences between the gut microflora of children with autistic spectrum disorders and that of healthy children. J Med Microbiol 2005;54:987–91. 10.1099/jmm.0.46101-0 [DOI] [PubMed] [Google Scholar]
- 7. Finegold SM, Summanen PH, Downes J, et al. Detection of Clostridium perfringens toxin genes in the gut microbiota of autistic children. Anaerobe 2017;45:133–7. 10.1016/j.anaerobe.2017.02.008 [DOI] [PubMed] [Google Scholar]
- 8. Finegold SM, Molitoris D, Song Y, et al. Gastrointestinal microflora studies in late-onset autism. Clin Infect Dis 2002;35:S6–16. 10.1086/341914 [DOI] [PubMed] [Google Scholar]
- 9. Sandler RH, Finegold SM, Bolte ER, et al. Short-Term benefit from oral vancomycin treatment of regressive-onset autism. J Child Neurol 2000;15:429–35. 10.1177/088307380001500701 [DOI] [PubMed] [Google Scholar]
- 10. Bolte ER. Data from: autism and Clostridium tetani. Med. Hypotheses 1998;51:133–44. [DOI] [PubMed] [Google Scholar]
- 11. Yang Y, Tian J, Yang B. Targeting gut microbiome: a novel and potential therapy for autism. Life Sci 2018;194:111–9. 10.1016/j.lfs.2017.12.027 [DOI] [PubMed] [Google Scholar]
- 12. Altieri L, Neri C, Sacco R, et al. Urinary p-cresol is elevated in small children with severe autism spectrum disorder. Biomarkers 2011;16:252–60. 10.3109/1354750X.2010.548010 [DOI] [PubMed] [Google Scholar]
- 13. MacFabe DF. Enteric short-chain fatty acids: microbial messengers of metabolism, mitochondria, and mind: implications in autism spectrum disorders. Microb Ecol Health Dis 2015;26:28177. 10.3402/mehd.v26.28177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Shultz SR, Aziz NAB, Yang L, et al. Intracerebroventricular injection of propionic acid, an enteric metabolite implicated in autism, induces social abnormalities that do not differ between seizure-prone (fast) and seizure-resistant (slow) rats. Behav Brain Res 2015;278:542–8. 10.1016/j.bbr.2014.10.050 [DOI] [PubMed] [Google Scholar]
- 15. Adams J, Audhya T, Geis E, et al. Comprehensive nutritional and dietary intervention for autism spectrum Disorder—A randomized, controlled 12-month trial. Nutrients 2018;10:369. 10.3390/nu10030369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Liu Y-W, Liong MT, Chung Y-CE, et al. Effects of Lactobacillus plantarum PS128 on Children with Autism Spectrum Disorder in Taiwan: A Randomized, Double-Blind, Placebo-Controlled Trial. Nutrients 2019;11:820. 10.3390/nu11040820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Bibbò S, Ianiro G, Gasbarrini A, et al. Fecal microbiota transplantation: past, present and future perspectives. Minerva Gastroenterol Dietol 2017;63:420–30. 10.23736/S1121-421X.17.02374-1 [DOI] [PubMed] [Google Scholar]
- 18. Sharon G, Cruz NJ, Kang D-W, et al. Human gut microbiota from autism spectrum disorder promote behavioral symptoms in mice. Cell 2019;177:e1617:1600–18. 10.1016/j.cell.2019.05.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kang D-W, Adams JB, Coleman DM, et al. Long-Term benefit of microbiota transfer therapy on autism symptoms and gut microbiota. Sci Rep 2019;9:5821. 10.1038/s41598-019-42183-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kang D-W, Adams JB, Gregory AC, et al. Microbiota transfer therapy alters gut ecosystem and improves gastrointestinal and autism symptoms: an open-label study. Microbiome 2017;5:10. 10.1186/s40168-016-0225-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Qiyi C, Bo Y, Hongliang T. Data from: a five-year follow-up analysis of efficacy and complications of 3 932 cases of fecal microbiota transplantation treatment. Chinese Journal of Digestion 2020;40:768–77. [Google Scholar]
- 22. Parenteral . Enteral Nutrition Branch of Chinese Medical A, Enhanced Recovery After Surgery Branch of China International Health Care P, Exchange A, China Microecological Treatment Innovation A, Microecology Committee of Shanghai Preventive Medicine A. Data from: [Chinese experts consensus on standardized methodology and clinical application of fecal microbiota transplantation]. Zhonghua Wei Chang Wai Ke Za Zhi 2020;23:5–13. [DOI] [PubMed] [Google Scholar]
- 23. Li N, Tian HL, Chen QY, et al. [Efficacy analysis of fecal microbiota transplantation in the treatment of 2010 patients with intestinal disorders]. Zhonghua Wei Chang Wai Ke Za Zhi 2019;22:861–8. 10.3760/cma.j.issn.1671-0274.2019.09.011 [DOI] [PubMed] [Google Scholar]
- 24. Cani PD. Human gut microbiome: hopes, threats and promises. Gut 2018;67:1716–25. 10.1136/gutjnl-2018-316723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Wang Y, Kasper LH. The role of microbiome in central nervous system disorders. Brain Behav Immun 2014;38:1–12. 10.1016/j.bbi.2013.12.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Buffington SA, Di Prisco GV, Auchtung TA, et al. Microbial reconstitution reverses maternal diet-induced social and synaptic deficits in offspring. Cell 2016;165:1762–75. 10.1016/j.cell.2016.06.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Zhang F, Luo W, Shi Y, et al. Should we standardize the 1,700-year-old fecal microbiota transplantation? Am J Gastroenterol 2012;107:1755. author reply p 1755-1756. 10.1038/ajg.2012.251 [DOI] [PubMed] [Google Scholar]
- 28. Minalyan A, Gabrielyan L, Scott D, et al. The gastric and intestinal microbiome: role of proton pump inhibitors. Curr Gastroenterol Rep 2017;19:42. 10.1007/s11894-017-0577-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Minot S, Bryson A, Chehoud C, et al. Rapid evolution of the human gut virome. Proc Natl Acad Sci U S A 2013;110:12450–5. 10.1073/pnas.1300833110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Amidon GL, Lee HJ. Absorption of peptide and peptidomimetic drugs. Annu Rev Pharmacol Toxicol 1994;34:321–41. 10.1146/annurev.pa.34.040194.001541 [DOI] [PubMed] [Google Scholar]
- 31. Geier DA, Kern JK, Garver CR, et al. A prospective study of transsulfuration biomarkers in autistic disorders. Neurochem Res 2009;34:386–93. 10.1007/s11064-008-9782-x [DOI] [PubMed] [Google Scholar]
- 32. Parenteral Enteral Nutrition Branch of Chinese Medical A, Enhanced Recovery After Surgery Branch of China International Health Care P, Exchange A, China Microecological Treatment Innovation A, Microecology Committee of Shanghai Preventive Medicine A. Data from: [Chinese experts consensus on clinical practice of the selection and Parenteralestablishment of fecal microbiota transplantation delivery routes]. Zhonghua Wei Chang Wai Ke Za Zhi 2020;23:14–20. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.