Abstract
We determined the value of a new serological assay detecting Toxoplasma-specific immunoglobulin M (IgM) and IgA antibodies at birth for use in mass neonatal screening. The incidence of congenital infection in newborns was compared with data from an epidemiological investigation on the seroprevalence of Toxoplasma in the studied population. Peripheral blood was collected on Guthrie cards during the first 3 days of life and tested for anti-Toxoplasma IgA and IgM using a noncommercial immunocapture enzyme-linked immunosorbent assay (ELISA). When the screening assay was positive, serum samples from the child and the mother were collected for use in Western blotting comparative immunological profile analysis and traditional serological tests for determination of specific IgG, IgM, and IgA antibodies. From December 1998 to April 2000, 17,653 filter paper samples from live-born neonates were successively screened. Congenital T. gondii infection was finally confirmed in 19 newborns. In traditional assays, 13 of 19 infants were IgM and IgA positive using filter paper eluates at birth, 1 child was positive only for IgM, 1 patient was positive for IgM and borderline for IgA, 1 had an equivocal level of IgA, and 3 cases were confirmed only by the Western blot assay. The prevalence of Toxoplasma-specific IgA and/or IgM in filter paper samples at birth was 1 per 929 live-born neonates (1.08/1,000) or about 1 per 523 children (1.9/1,000) born to nonimmune women with a potential risk of primary T. gondii infection during pregnancy, compared to the actual seropositivity rate of 43.7%. The diagnostic sensitivity of the combined IgA-IgM ELISA using neonatal filter paper specimens was not more than 95%, the positive predictive value of the test was 82.6%, and the diagnostic specificity was calculated to be 99.9%. The combined IgA-IgM ELISA is a valuable method for the diagnosis of congenital toxoplasmosis at birth and fulfills criteria for neonatal screening programs. The method showed a good diagnostic sensitivity in neonates untreated prenatally who were born in an area of high seroprevalence of T. gondii infection.
The clinical pattern of congenital Toxoplasma infection varies from an asymptomatic presentation to a clinical disease of the fetus or newborn with a risk of late development of new complications until adolescence and early adult life (3, 13, 25). Psychomotor retardation and secondary eye lesions, leading to severe vision impairment, may pose significant health problems in young people during the developmental period, and this requires consideration of the application of large-scale prophylactic measures.
The strategic approach for preventing congenital toxoplasmosis is strictly related to the incidence of primary infection during pregnancy in a given area, resulting from the absence of specific antibodies in women in the childbearing age group. The potential risk of maternal infection throughout pregnancy can be estimated by the increase in the prevalence of seropositivity in the female population of reproductive age over a 9-month period (18). Serological surveillance of nonimmune pregnant women at risk is widely practiced in France and Austria. Since a systematic nationwide screening during pregnancy followed by prenatal diagnosis has not been accepted by the National Health Services in Poland and since the seropositivity curve during the childbearing years is stable in Polish women (20, 21), we decided to develop a pilot study of neonatal screening for congenital toxoplasmosis. In the pilot screening program, an analysis of Toxoplasma-specific immunoglobulin M (IgM) antibody eluted from filter papers collected at birth showed that 1 per 2,117 live-born children (0.47/1,000) was infected (19). Since December 1998, the program was extended by the combined detection of IgA and IgM antibodies using a single assay procedure. The aim of the present study was to estimate the benefit of the combined IgA-IgM assay adapted for mass neonatal screening to provide more data on the prevalence of congenital Toxoplasma gondii infection on live-born babies in Poland.
MATERIALS AND METHODS
Study population.
Neonates born in the obstetric clinics of the University Hospital of Gynaecology and Obstetrics in Poznań and in maternity wards of the nine main district hospitals from the Grand Poland Province (about 12,000 births per year) were systematically screened for congenital T. gondii infection. Peripheral blood absorbed onto separate Guthrie cards (catalog no. 10321395; Schleicher and Schuell, Dassel, Germany) was collected on the first to third days of life and kept at 4°C before being delivered to the laboratory by ordinary mail twice a week.
Between December 1998 and April 2000, 17,653 filter paper samples from successively born infants were tested. This number is equivalent to approximately 90% of live-born neonates and 88% of the total number of births from the study area during this time period.
The study was approved by the Karol Marcinkowski University of Medical Sciences Ethical Council (Poznań, Poland).
Combined neonatal screening assay.
Toxoplasma-specific IgA and IgM antibodies were analyzed in filter paper eluates by a combined immunocapture enzyme-linked immunosorbent assay (ELISA). The technique was adapted for joint detection of IgA and IgM using a single determination.
Polystyrene microwells (Kartell, Noviglio, Italy) were coated with 5.3 μg of rabbit anti-human IgM (catalog no. A0425; DAKO, Glostrup, Denmark) per ml in 50 mM carbonate-bicarbonate buffer (pH 9.6) and incubated overnight at 4°C. After being washed with phosphate-buffered saline (PBS)–Tween 20 (pH 7.4), the microwells were coated a second time with a 4.4-μg/ml concentration of rabbit anti-human IgA antibody directed against α chains (catalog no. A0262; DAKO) and then incubated for another 4 h at 37°C. The 3.2-mm-diameter filter paper spots saturated with dried blood specimens were punched into precoated microtiter plates and eluted with 100 μl of 1% bovine serum albumin–PBS–Tween 20 per well on a Kline agitator (Jouan, Saint-Herblain, France) for 90 min at room temperature. After removal of the discs, bound T. gondii-specific IgM and/or IgA antibodies were reacted with T. gondii Tx12 antigen from in vitro culture. Bound Tx12 was visualized with monoclonal anti-SAG1 antibody (S13); the next step with rabbit anti-mouse immunoglobulin conjugate was unchanged from that of the procedure for the IgM screening ELISA (14).
The positive control came from a patient with high anti-Toxoplasma IgA and IgM levels. The cutoff value was calculated as the mean optical density (OD) plus three standard deviations for 276 filter paper spots from newborns known to be Toxoplasma negative by the reference direct agglutination assay (Toxo-Screen DA; bioMérieux, Marcy-l'Etoile, France) and the noncommercial IgM ELISA screening test (19). For 95 serum samples from patients who were IgM and/or IgA positive in commercial assays (PLATELIA TOXO IgM or IgA; Sanofi Diagnostics Pasteur, Marnes la Coquette, France), the OD values ranged from 0.302 to 2.244 (mean ± standard deviation, 0.726 ± 0.488). One hundred twenty-eight samples from patients suffering from other parasitic diseases (echinococcosis, 27 cases) or infectious diseases (borreliosis, 4 cases; cytomegalovirus infection, 74 cases; and Chlamydia pneumoniae infection, 23 cases) and IgM negative by the PLATELIA TOXO assay (Sanofi Diagnostics Pasteur) were evaluated to determine the test specificity.
Confirmation of congenital toxoplasmosis.
When the screening result was positive, the filter paper sample was retested twice and then additionally analyzed for Toxoplasma-specific IgM, IgA, and IgG antibodies using commercial assays (PLATELIA TOXO IgM, IgA, and IgG; Sanofi Diagnostics Pasteur). Filter paper spots were placed overnight at 4°C in a dilution of 1:100, and the eluates were then analyzed according to the manufacturer's procedure.
Toxoplasma infection was confirmed by testing serum samples from children with suspected infection and their mothers by conventional serology (ELISA; Sanofi Diagnostics Pasteur) and the IgG-IgM Western blot comparative immunological profile analysis (LDBIO Diagnostics, Lyon, France) after the second week of life. Congenital infection was defined by the presence of specific IgM and/or IgA antibodies in the infant's peripheral blood and by the detection of actively synthesized IgG antibody of an antigenic specificity different from that of the mother as shown by immunoblotting. The follow-up sera were tested 4 weeks after the primary confirmatory analysis and then every 2 months during the first year of life and every 4 months after treatment was stopped, together with pediatric, ophthalmic, and imaging examinations. Serum samples from the infants were tested for IgM and IgA at a dilution of 1:20.
Analysis of Toxoplasma seropositivity.
Maternal IgG is transferred to the fetus, and the seroprevalence of Toxoplasma-specific IgG antibodies in children born in 1998 to 2000 was therefore regarded as equivalent to the seroprevalence in the pregnant women. In total, 2,684 filter papers for newborns were analyzed: 905 samples from consecutive births in October 1998, 864 specimens from children born in August 1999, and the 915 samples collected in March 2000. Anti-Toxoplasma antibodies were measured by the direct agglutination assay (bioMérieux), which is positive from 4 IU/ml. Briefly, three filter paper discs of 3.2 mm in diameter were eluted overnight at 4°C in PBS and transferred to a new round-bottomed microtiter plate. Fifty microliters of Toxoplasma antigen suspension in borate albumin-buffered saline (pH 8.95) was applied to each well, and the reaction was read after 5 h of incubation at room temperature.
RESULTS
During 17 months of the study period, 17,653 Guthrie cards from live-born neonates were successively tested, and congenital T. gondii infection diagnosed by serological screening alone was confirmed in 19 newborns (11 males and 8 females; all single births). The cutoff OD value was calculated to be 0.205, and the OD values of the 19 IgA- and/or IgM-positive filter paper spots ranged from 0.322 to 0.830, with a mean of 0.554. Only 16 of the 19 infants (84.2%) were IgM and/or IgA positive or borderline at birth in the commercial PLATELIA IgM and/or IgA assay (Table 1).
TABLE 1.
Complementary detection of Toxoplasma-specific IgA, IgM, and IgG antibodies from filter paper eluates at birth in 19 newborns identified by the combined IgA-IgM ELISA
| Patient no. | Age (days) | Resulta with:
|
||||
|---|---|---|---|---|---|---|
| Noncommercial IgA-IgM ELISA
|
PLATELIA TOXO IgA (indexc) | PLATELIA TOXO IgM (ODd) | PLATELIA TOXO IgG (IU/mle) | |||
| ODb | OD % | |||||
| 1 | 2 | 0.830 | 96.8 | 33.9 (+) | 2.274 (+) | 1,140 |
| 2 | 1 | 0.705 | 81.5 | 5.7 (+) | 1.878 (+) | 197 |
| 3 | 1 | 0.526 | 59.4 | 33.9 (+) | 1.667 (+) | 1,840 |
| 4f | 1 | 0.460 | 48.2 | Neg | Neg | 480 |
| 5 | 1 | 0.727 | 86.0 | 65.4 (+) | 1.953 (+) | 410 |
| 6 | 1 | 0.335 | 38.5 | 28.5 (+) | 0.632 (+) | 1,220 |
| 7f | 1 | 0.322 | 38.7 | Neg | Neg | 219 |
| 8 | 7 | 0.574 | 58.8 | 30.3 (+) | 4.386 (+) | 1,980 |
| 9 | 1 | 0.458 | 51.1 | 34.8 (+) | 0.509 (+) | 266 |
| 10 | 3 | 0.594 | 45.3 | 3.0 (+) | 1.857 (+) | 1,440 |
| 11 | 1 | 0.327 | 40.5 | 4.2 (+) | 1.039 (+) | 318 |
| 12 | 1 | 0.772 | 67.2 | 0.4 (±) | 2.604 (+) | 570 |
| 13 | 1 | 0.615 | 61.6 | 0.6 (±) | Neg | 306 |
| 14 | 3 | 0.666 | 63.6 | Neg | 1.087 (+) | 139 |
| 15 | 1 | 0.545 | 62.2 | 1.7 (±) | 1.601 (+) | 416 |
| 16 | 1 | 0.496 | 49.3 | 16.4 (+) | 2.015 (+) | 590 |
| 17f | 1 | 0.622 | 66.1 | Neg | Neg | 221 |
| 18 | 1 | 0.386 | 44.3 | 64.0 (+) | 1.502 (+) | 400 |
| 19 | 1 | 0.561 | 64.0 | 5.1 (+) | 1.771 (+) | 370 |
All assays were performed on filter paper spots at a dilution of 1:100. +, positive; ±, borderline; Neg, negative.
Positive value, >0.205.
Positive value, >2.0.
The cutoff was variable.
Positive value, ≥6 IU/ml.
Infection was confirmed by IgG-IgM Western blotting.
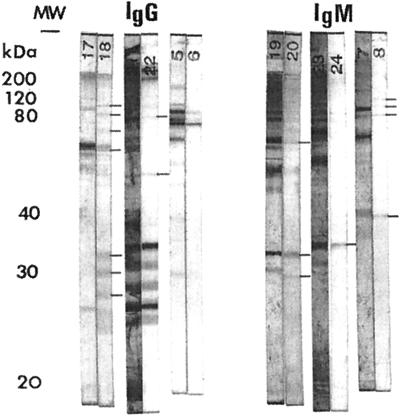
Serum samples from children suspected of having congenital toxoplasmosis and from their mothers were available from blood collected 12 to 47 days after birth (mean, 24 days) for a confirmatory analysis. Twelve follow-up sera from infants were positive for IgA and IgM (63.2%), three serum samples were positive for IgM (15.8%), and one serum was only IgA positive. Three infants who were IgM and IgA negative after the second week of life when retested were finally confirmed by the detection of neoantibodies in the Western blot assay (Fig. 1). The two of them with the longest observation times had a persistently positive IgG beyond 1 year of age.
FIG. 1.
Comparative Western blot immunological profile analysis for the three mothers and their children undiagnosed by traditional serology. Three pairs of Western blot strips from the mothers and newborns are compared for anti-Toxoplasma IgG and IgM antibodies. Paired serum samples were collected at 24, 25, and 12 days after birth, respectively: lines 17, 21, and 5, IgG in maternal sera; lines 18, 22, and 6, IgG in neonatal sera; lines 19, 23, and 7, IgM in maternal sera; lines 20, 24, and 8, IgM in neonatal sera. The bars indicate antibodies actively synthesized by the neonates. MW, molecular mass standards.
Fifteen of 19 mothers of the infected infants were IgM and IgA positive (78.9%) and 4 women were only IgM positive in the postpartum period. In nine of them (47.4%), a significant rise in the titer of specific IgG was observed between two examinations at 4-week intervals.
During the study period, we identified one premature infant (patient 20) who was IgA and IgM negative at birth and whose mother had seroconverted and was treated during pregnancy. The child began to synthesize IgM of an antigenic specificity different from that of her mother at 28 days of life as shown by the Western blotting. Two other infected newborns, whose blood samples were not collected for the screening assay, were also found. One was asymptomatic, but testing was requested by the mother and a high titer of specific IgG was found at 12 months; the other child died at 7 weeks of age from multiorgan failure presumably due to the Toxoplasma infection and was found to have neosynthesized IgG and IgM.
Four false-positive cases with low levels of IgM or IgA in the filter paper assay were found. Two of these infants were delivered by caesarean section, and the false-positive results were probably due to contamination with maternal blood by IgM or IgA (one case of each), as the mothers had Toxoplasma-specific IgM and IgA antibodies, respectively. The two remaining children had naturally occurring IgM antibodies, and they were born to IgG-negative mothers (23). No cross-reactivity with samples from other infectious diseases was observed, particularly in any patients IgM positive for cytomegalovirus.
The positive predictive value of the assay was 82.6%.
The prevalence of Toxoplasma-specific IgA and/or IgM in filter paper specimens at birth was 1 per 929 live-born infants (1.08/1,000). The diagnostic sensitivity of the combined IgA-IgM ELISA was not more than 95%, the false-positive rate of the assay was 2.3 per 10,000 determinations (4 of 17,653), and the diagnostic specificity was calculated to be 99.9%. Including one infected infant with a delayed synthesis of IgM at 4 weeks of life, the prevalence of congenital toxoplasmosis in the Poznań region was at least 1 per 883 live births (1.13/1,000).
Twelve of the 20 infected infants were asymptomatic during the neonatal period or demonstrated only nonspecific signs of intrauterine infection (Table 2). Clinical toxoplasmosis (mild or moderate) was observed in six newborns (30%), and two children had documented coexisting congenital cytomegalovirus infections. One infant developed a unilateral macular scar, and five patients had isolated asymmetry of the ventricular system or periventricular calcifications. During the mean follow-up period of 17 months (range, 10 to 25 months) and intensive antiparasitic chemotherapy, no clinical relapses occurred.
TABLE 2.
Clinical findings detected during the neonatal period in 20 newborns with congenital T. gondii infectiona
| Initial abnormalities before treatment | No. of affected newborns/total (% affected) |
|---|---|
| Unilateral retinochoroiditis | 1/20 (5) |
| Macular scar | 1 |
| Active retinal lesions | 0 |
| Intracranial calcifications | 5/20 (25)b |
| Periventricular region alone | 4 |
| Parenchymal and periventricular locations | 1 |
| Dilatation of ventricular system | 3/20 (15) |
| Hydrocephalus | 0 |
| Enlarged lateral ventricles on ultrasonogram | 3 |
| Muscular hypertonia | 2/20 (10) |
| Sensorineural hearing loss | 2/20 (10)b |
| Nonspecific signs | 11/20 (55) |
| Intrauterine hypotrophy or fetal distress | 4 |
| Prematurity | 7 |
| Anemia | 8 |
| Hyperbilirubinemia | 4 |
| Thrombocytopenia | 1 |
| Petechial skin rash | 1 |
| Hepatosplenomegaly | 1 |
| Respiratory disorders or interstitial pneumonia | 3 |
The 19 patients were identified solely by the combined IgA-IgM ELISA. Patient 20, who was born to a mother who seroconverted and was treated during pregnancy, was confirmed as having T. gondii infection by the Western blot assay at 4 weeks.
Two infants with coexisting congenital cytomegalovirus infection.
The results of the epidemiological investigation on Toxoplasma seropositivity in newborns and in pregnant women at delivery are presented in Table 3. Altogether, 2,684 filter paper samples from live-born neonates were examined for Toxoplasma-specific IgG antibody at birth, or 2,656 gravidae with adjustment for the 28 multiple pregnancies (1.05%). The proportions of samples collected at maternity departments from inhabitants of urban (n = 1,423) and rural (n = 1,261) areas were comparable (1:1.1). The mean seropositivity rate of 43.7% (range, 41.5 to 45.6%) was the same for the newborns and pregnant women, and it declined slightly during the study period (P = 0.06 [χ2 test]). Twin pregnancies were positive in 46.4% of the cases (13 of 28).
TABLE 3.
Prevalence of Toxoplasma-specific antibodies among newborns and pregnant women at delivery in 1998 to 2000
| Mo and yr | Births
|
Seropositive newborns
|
No. of infected infants | Women living in:
|
Seropositive gravidae
|
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Singletons (n) | Twins (pairs) | n | % | Urban areas (>500,000 inhabitants)
|
Towns
|
Rural areas
|
n | % | ||||||||
| Enrolled (n) | Seropositive
|
Enrolled (n) | Seropositive
|
Enrolled (n) | Seropositive
|
|||||||||||
| n | % | n | % | n | % | |||||||||||
| October 1998 | 881 | 12 | 399 | 44.1 | 1 | 71 | 26a | 36.6 | 393 | 158 | 40.2 | 429 | 207b | 48.3 | 391 | 43.8 |
| August 1999 | 848 | 8 | 358 | 41.4 | 1 | 167 | 55a | 32.9 | 321 | 127 | 39.6 | 368 | 173a | 47.0 | 355 | 41.5 |
| March 2000 | 899 | 8 | 418 | 45.7 | 3 | 111 | 39a | 35.1 | 349 | 146 | 41.8 | 447 | 230c | 51.5 | 415 | 45.8 |
| Total | 2,628 | 28 | 1,174 | 43.7 | 5 | 349 | 120a | 34.4 | 1,063 | 431 | 40.5 | 1,244 | 610a | 49.0 | 1,161 | 43.7 |
P < 0.00001.
P = 0.0001.
P = 0.00001.
The Toxoplasma seroprevalence of 49.0% in women from rural areas was significantly higher than the seropositivity rate of 34.4% in urban areas (P < 0.00001 [χ2 test]). Similarly, the Poznań inhabitants were less frequently seropositive than people living in other localities (P < 0.00001 [χ2 test]).
The prevalence of Toxoplasma-specific IgA and/or IgM in filter paper eluates at birth was about 1 per 523 deliveries of nonimmune mothers with a potential risk of primary T. gondii infection during pregnancy (1.9/1,000), and the total prevalence of congenital toxoplasmosis was 1 per 497 susceptible women (2/1,000).
DISCUSSION
The 19 children with congenital toxoplasmosis reported in this study, identified solely by the regional screening program, gave the highest incidence found in present-day Europe when compared with other centers conducting a similar preventive program for newborns (10, 14, 15, 22). The lack of clinical symptoms at birth in the majority of infected infants makes the diagnosis of congenital toxoplasmosis in newborns difficult if antenatal or neonatal screening is not conducted.
In the past, neonatal screening programs have used tests for Toxoplasma-specific IgM antibodies, but the sensitivity would be increased by concomitant analysis for IgA antibodies (22). The regional New England program detects Toxoplasma-specific IgM in filter paper eluates at birth. Fifty of 635,000 neonates were diagnosed as being congenitally infected (1 of 10,000), although they appeared healthy on routine pediatric examination after birth (10). In the Danish neonatal screening trial, combining an analysis of neonatal IgM with detection of maternal IgG seroconversion during pregnancy, the frequency of congenital toxoplasmosis was three times lower (1 of 3,000), with a proportion of symptomatic cases of only 15% (14). Recently, Malm et al. identified three infected IgM-positive babies of about 41,000 examined at birth (15).
Neonatal screening has not previously been proposed for use in areas with a high seroprevalence of Toxoplasma in women of procreative age. In Poland, the percentage of pregnancies at risk is significantly lower than in many other countries, where the seropositivity rate does not reach 40% (8, 11, 12, 14). The prevalence of specific antibodies in the Polish population has declined significantly since the early 1990s, when 58.9% of pregnant women were infected with Toxoplasma before pregnancy (20).
The diagnostic accuracy of Toxoplasma IgM and IgA assays in infants is a source of continuing discussion (1, 4, 5, 17). A study by Lebech et al. (14) documented that neonatal screening based on detection of specific IgM alone is able to diagnose more than 75% of infected infants who have not received antenatal therapy. Naessens et al. (16) have found IgM antibody in neonatal blood in 85% of congenitally infected children born to untreated mothers but in only 25% of infants who were treated prenatally. Similarly, the sensitivities of diagnosing IgA in untreated and treated infants were 80 and 57%, respectively, although the impact of maternal-fetal treatment on the suppression of the newborn's immunological response has not been proven.
Couvreur (3) reported that specific IgM is not visualized by the reference immunosorbent agglutination assay in 30% of infants at birth but the discordance rises to 82% if the mothers received pyrimethamine-sulfadiazine therapy during pregnancy. A higher reliability of diagnosing specific IgM or IgA in an infant's peripheral blood was shown by Wallon et al. (24), with an increase in the overall diagnostic sensitivity when the immunoglobulins were jointly assessed.
Compared with the previous IgM study, the significantly higher number of congenital infections diagnosed by this new serological screening was due to higher sensitivity of the combined IgA-IgM assay used. One immature infant identified by this method had only a weakly detectable level of specific IgA eluted from filter paper at birth. Some studies have reported that IgA is not useful in improving the sensitivity of a neonatal diagnosis (9); however, Lebech et al. (14) showed that testing newborns for Toxoplasma-specific IgA may identify an additional 5 to 10% of infected infants.
The test used in the New England Newborn Screening Program (10) showed a high false-positivity rate of 50% (50 of 100 IgM-positive infants were confirmed to be noninfected) compared to our 4 false-positive results of 17,653 examined samples.
The overall incidence of congenital toxoplasmosis of 1 to 2 per each 2,000 live births, or 250 to 500 infected infants per year, in this population seems to sufficiently justify the need for some nationwide preventive measures in Poland. So far, national screenings for the less frequently occurring phenylketonuria and congenital hypothyroidism are carried out only when followed by an elimination diet or substitution therapy of a doubtful efficiency (2, 7). Prenatal testing for pregnancies at risk of trisomy 18 or 21 (Edwards' or Down's syndrome) or central nervous system malformations is widely implemented by specialized centers in Poland (6).
The best strategy for the prevention and control of congenital T. gondii infections is still unknown. We are encouraged by the combined IgA-IgM ELISA, which showed a good diagnostic sensitivity for a high-seroprevalence population of newborns and successfully fulfills the criteria for a diagnostic test to be used in a neonatal screening program.
ACKNOWLEDGMENTS
We express our deep gratitude to Zbigniew S. Pawłowski, the late Chief of the Clinic of Parasitic and Tropical Diseases at the University of Medical Sciences in Poznań, Poland, for his professional help and to Krzysztof Drews, Daniela Skórzybut, and Dorota Wendland for making working facilities available at the Laboratory of Immunodiagnosis, University Hospital of Gynaecology and Obstetrics, Poznań, Poland. Lis Wassmann and Bodil Nielsen skillfully produced the T. gondii Tx12 antigen from in vitro culture.
This study received financial support from the Second Biomedicine and Health Research Program of the Commission of the European Union, Brussels, Belgium (contract BMH2-CT95-1688), and the Polish Research Committee, Warsaw, Poland (grant KBN 4PO5E 10017).
REFERENCES
- 1.Bessières H M, Roques C, Berrebi A, Barre V, Cazaux M, Séquéla J P. IgA antibody response during acquired and congenital toxoplasmosis. J Clin Pathol. 1992;45:605–608. doi: 10.1136/jcp.45.7.605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cabalska B M, Nowaczewska I, Sendecka E, Zorska K. Longitudinal study on early diagnosis and treatment of phenylketonuria in Poland. Eur J Pediatr. 1996;155(Suppl. 1):S53–S55. doi: 10.1007/pl00014250. [DOI] [PubMed] [Google Scholar]
- 3.Couvreur J. Toxoplasmose congénitale. Prise en charge et devenir. Méd Mal Infect. 1993;23(Spécial):176–182. [Google Scholar]
- 4.Decoster A, Caron A, Darcy F, Capron A. IgA antibodies against P30 as markers of congenital and acute toxoplasmosis. Lancet. 1988;ii:1104–1107. doi: 10.1016/s0140-6736(88)90523-5. [DOI] [PubMed] [Google Scholar]
- 5.Decoster A, Slizewicz B, Simon J, Bazin C, Darcy F, Vittu G, Boulanger C, Champeau Y, Demory J L, Duhamel M, Capron A. Platelia-Toxo IgA, a new kit for early diagnosis of congenital toxoplasmosis by detection of anti-P30 immunoglobulin A antibodies. J Clin Microbiol. 1991;29:2291–2295. doi: 10.1128/jcm.29.10.2291-2295.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Drews K, Pawelczyk L. Hormonal diagnostics in gynecology and screening examinations of genetic malformations in the fetus. Warsaw: Abbott Laboratories Poland; 2000. . (In Polish.) [Google Scholar]
- 7.Erenz B, Jerzykowska M, Wieja E, Smorawińska A, Korman E. Results of neonatal screening for congenital hypothyroidism in the Wielkopolska region. In: Szczapa J, Twarowska I, editors. Postȩpy w neonatologii. Problemy endokrynologiczne okresu noworodkowego. Wielkopolska Fundacja Neonatologii, Poznań, Poland. (In Polish.) 1998. pp. 68–72. [Google Scholar]
- 8.Evengård B, Lilja G, Capraru T, Malm G, Kussofsky E, Öman H, Forsgren M. A retrospective study of seroconversion against Toxoplasma gondii during 3,000 pregnancies in Stockholm. Scand J Infect Dis. 1999;31:127–129. doi: 10.1080/003655499750006146. [DOI] [PubMed] [Google Scholar]
- 9.Faure A K, Fricker-Hidalgo H, Pelloux H, Bost-Bru C, Goullier-Fleuret A, Ambroise-Thomas P. Lack of value of specific IgA detection in the postnatal diagnosis of congenital toxoplasmosis. J Clin Lab Anal. 1999;13:27–30. doi: 10.1002/(SICI)1098-2825(1999)13:1<27::AID-JCLA5>3.0.CO;2-Y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Guerina N G, Hsu H W, Meissner H C, Maguire J H, Lynfield R, Stechenberg B, Abroms I, Pasternack M S, Hoff R, Eaton R B G F, Grady the New England Regional Toxoplasma Working Group. Neonatal serologic screening and early treatment for congenital Toxoplasma gondii infection. N Engl J Med. 1994;330:1859–1863. doi: 10.1056/NEJM199406303302604. [DOI] [PubMed] [Google Scholar]
- 11.Jenum P A, Stray-Pedersen B, Melby K K, Kapperud G, Whitelaw A, Eskild A, Eng J. Incidence of Toxoplasma gondii infection in 35,940 pregnant women in Norway and pregnancy outcome for infected women. J Clin Microbiol. 1998;36:2900–2906. doi: 10.1128/jcm.36.10.2900-2906.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Joynson D H M. Epidemiology of toxoplasmosis in the U.K. Scand J Infect Dis. 1992;84:65–69. [PubMed] [Google Scholar]
- 13.Koppe J G, Loewer-Sieger D H, de Roever-Bonnet H. Results of 20-year follow-up of congenital toxoplasmosis. Lancet. 1986;i:254–255. doi: 10.1016/s0140-6736(86)90785-3. [DOI] [PubMed] [Google Scholar]
- 14.Lebech M, Andersen O, Christensen N C, Hertel J, Nielsen H E, Peitersen B, Rechnitzer C, Larsen S O, Nørgaard-Pedersen B, Petersen E the Danish Congenital Toxoplasmosis Study Group. Feasibility of neonatal screening for Toxoplasma infection in the absence of prenatal treatment. Lancet. 1999;353:1834–1837. doi: 10.1016/s0140-6736(98)11281-3. [DOI] [PubMed] [Google Scholar]
- 15.Malm G, Teär Fahnehjelm K, Wiklund S, Engman M L, Ivarsson S A, Petersson K, Evengård B. Three children with congenital toxoplasmosis: early report from a Swedish prospective screening study. Acta Paediatr. 1999;88:667–670. doi: 10.1080/08035259950169369. [DOI] [PubMed] [Google Scholar]
- 16.Naessens A, Jenum P A, Pollak A, Decoster A, Lappalainen M, Villena I, Lebech M, Stray-Pedersen B, Hayde M, Pinon J M, Petersen E, Foulon W. Diagnosis of congenital toxoplasmosis in the neonatal period: a multicenter evaluation. J Pediatr. 1999;135:714–719. doi: 10.1016/s0022-3476(99)70090-9. [DOI] [PubMed] [Google Scholar]
- 17.Naot Y, Desmonts G, Remington J. IgM enzyme-linked immunosorbent assay test for the diagnosis of congenital Toxoplasma infection. J Pediatr. 1981;98:32–36. doi: 10.1016/s0022-3476(81)80528-8. [DOI] [PubMed] [Google Scholar]
- 18.Papoz L, Simondon F, Saurin W, Sarmini H. A simple model relevant to toxoplasmosis applied to epidemiologic results in France. Am J Epidemiol. 1986;123:154–161. doi: 10.1093/oxfordjournals.aje.a114210. [DOI] [PubMed] [Google Scholar]
- 19.Paul M, Petersen E, Pawłowski Z S, Szczapa J. Neonatal screening for congenital toxoplasmosis in the Poznań region of Poland by analysis of Toxoplasma gondii-specific IgM antibodies eluted from filter paper blood spots. Pediatr Infect Dis J. 2000;19:30–36. doi: 10.1097/00006454-200001000-00007. [DOI] [PubMed] [Google Scholar]
- 20.Pawłowski Z S. Control of congenital Toxoplasma infection in Poland. Wiad Parazytol. 1993;39:331–338. . (In Polish.) [PubMed] [Google Scholar]
- 21.Pawłowski Z S. Clinical epidemiology of congenital toxoplasmosis in the Poznań region. Klin Perin Gin Suppl. 1995;11:5–11. . (In Polish.) [Google Scholar]
- 22.Petersen E, Eaton R. Control of congenital infection with Toxoplasma gondii by neonatal screening based on detection of specific immunoglobulin M antibodies eluted from phenylketonuria filter paper blood-spot samples. Acta Paediatr Suppl. 1999;432:36–39. doi: 10.1111/j.1651-2227.1999.tb01154.x. [DOI] [PubMed] [Google Scholar]
- 23.Sharma S D, Mullenax J, Araujo F G, Erlich H A, Remington J S. Western blot analysis of the antigens of Toxoplasma gondii recognized by human IgM and IgG antibodies. J Immunol. 1983;131:977–983. [PubMed] [Google Scholar]
- 24.Wallon M, Dunn D, Slimani D, Girault V, Gay-Andrieu F, Peyron F. Diagnosis of congenital toxoplasmosis at birth: what is the value of testing for IgM and IgA? Eur J Pediatr. 1999;158:645–649. doi: 10.1007/s004310051168. [DOI] [PubMed] [Google Scholar]
- 25.Wilson C B, Remington J S, Stagno S, Reynolds D W. Development of adverse sequelae in children born with subclinical Toxoplasma infection. Pediatrics. 1980;66:767–774. [PubMed] [Google Scholar]