Abstract
Objective
Intraarticular (IA) administration of platelet-rich plasma (PRP) has been proposed as a new strategy to halt osteoarthritis (OA) progression. In patients with severe OA, its potential is limited because it is unable to reach the subchondral bone, so a new strategy is needed, and intraosseous (IO) infiltration has been suggested. The purpose is to assess the impact of IA together with IO infiltration of plasma rich in growth factors (PRGF) in serum hyaluronic acid (HA) and type II collagen cleavage neoepitope (C2C) levels.
Design
A total of 32 rabbits were included in the study and randomly divided into 2 groups: control and treatment. A 4-mm chondral defect was created in the medial femoral condyle and IA followed by IO infiltration were performed. Serum C2C and HA levels were measured using enzyme-linked immunosorbent assay (ELISA) tests before infiltration and 28, 56, and 84 days post-infiltration.
Results
Significant lower C2C serum levels were obtained in treatment group (IA + IO infiltration of PRGF) at 84 days post-infiltration than in control group (IA infiltration of PRGF + IO infiltration of saline solution), while no significant differences between groups were reported at any other study times. Regarding HA, at 56 days post-infiltration, greater significant levels were seen in the treatment group. However, at 84 days post-infiltration, no significant differences were obtained, although lower levels were reported in the treatment group.
Conclusions
Despite inconclusive, the results suggest that the combination of IA and IO infiltration with PRGF may enhance cartilage and subchondral bone regeneration, but further studies are needed.
Keywords: platelet-rich plasma, intraosseous infiltration, biomarkers, cartilage, osteoarthritis
Introduction
Osteoarthritis (OA) is a chronic disease commonly associated with damage and loss of articular cartilage, together with progressive destruction of intraarticular (IA) structures, which leads to pain, loss of function, and poor quality of life. 1 Even though traditionally OA was considered a cartilage-driven disease, during the last decade, some studies have suggested a direct implication of the subchondral bone (SB) and synovial membrane (SM) in the degradation process of articular cartilage (AC).2-4 All the joint tissues are crucial for maintaining the homeostasis, and a disruption in the anabolic-catabolic balance results in cartilage degeneration, osteophyte formation, and inflammation of SM. 5 Additionally, in severe cases of OA, the SB undergoes changes, including structural defects such as progressive replacement of the subchondral marrow with fibroneurovascular mesenchymal tissue, demineralization of bone, microcracks, and bone marrow lesions (BMLs).4,6
Early diagnosis of the disease is crucial for its management, and with this purpose, several biomarkers have been identified and validated. In addition, these biomarkers allow clinicians to detect people at high risk of developing OA, monitor disease progression, and assess the response to treatments. 7 Type II collagen cleavage neoepitope (C2C) is a collagenous biomarker, and it is a neoepitope created by the cleavage of type II collagen by collagenases. Type II collagen is the major form of collagen in the AC, and it has been proven that its cleavage products, including C2C, are upregulated in early stages of OA, while in chronic stages they are downregulated.8,9 Hyaluronic acid (HA) is the most important component of the synovial fluid (SF) and provides smoothness to the joint and resistance of cartilage to compression. Its serum and SF levels are correlated with radiographic progression of the disease and with the clinical severity of OA.10,11 HA has proven to be useful not only in OA diagnosis but also in identifying disease’s severity and prognosis. The serum concentration of HA is thought to reflect the extent of synovitis, which accelerates the progression of the disease by producing proteases and cytokines. 12 Serum HA concentration is elevated in patients with osteoarthritis, and higher concentrations are correlated to radiological and clinical worsening of the pathology. 13 On the contrary, HA levels decrease in the SF of patients with osteoarthritis. 14
Conservative treatments, including nonsteroidal anti-inflammatory drugs, analgesics, and IA infiltrations of HA or steroids, are focused on relieving the symptoms, but none of these treatments can reverse the damage or halt the progression of OA, making arthroplasty the only solution for patients with osteoarthritis. 15 In recent years, IA infiltrations with regenerative therapies such as mesenchymal stem cells (MSCs) and platelet-rich plasma (PRP) have emerged as an alternative treatment with promising results. 16 Despite the positive outcomes reported in several studies, this therapy still has some limitations, such as the administration mode and the differences between PRP obtention protocols. 5 The most used form of administration is IA injection, which is effective in patients with mild OA, but, in patients with severe OA, the therapeutic potential is limited because the PRP is unable to reach the deeper layers of the AC and the SB.6,17,18 Recent studies have shown the importance of the SB in the pathogenesis of OA, and the existing communication between the cartilage and the SB. 19 Following these basis, intraosseous (IO) infiltration with PRP has been proposed.
We hypothesize that the combination of IA and IO infiltration of PRP will improve cartilage healing and regenerative properties observed when PRP is only IA infiltrated in patients with osteoarthritis, especially in those in which the SB is affected. Based on this hypothesis, the purpose of the study is to assess the impact of IO infiltration of PRP together with IA infiltration of PRP in rabbits with acute chondral lesion by measuring serum OA biomarkers’ (C2C and HA) levels.
Methods
This study was approved by the Ethics Committee of Animal Welfare (CEEA) of the university CEU Cardenal Herrera of Valencia (Spain) according to the Spanish Policy for Animal Protection (RD118/2021), which complies with European Union Directive 2010/63/UE, with the following approval code: 2019/VSC/PEA/0153.
The sample size was calculated by a power analysis considering results (mean = 10.83 and standard deviation = 2.041) published by Wang et al. 20 in a study in which only IA PRP was administered. An alpha level of 0.02 and a power of 80% were established.
Animals
A total of 32 healthy 6 months old, female New Zealand rabbits, with an average weight of 4.63 kg, were used to carry out a prospective randomized and blinded experimental study. Animals were housed in individual big cages, with room enough to move freely, and with water and food ad libitum. Rabbits were daily monitored for signs of pain, infection, and weight loss. An acclimatization period of 15 days was established before starting the experiment to allow animal adaptation.
Before surgery, a complete physical examination, hematology, and serum biochemical analyses were also performed, and all results were within normal reference range values, therefore no animals were excluded from the study.
After surgery, an anti-inflammatory drug (meloxicam 0.3 mg/kg SC q24h; Metacam®, Boehringer Ingelheim, Spain) together with an antibiotic (enrofloxacin 10 mg/kg SC q24h; Ganadexil®, Invesa, Spain) was given to each animal during a 7-day period. In addition, the rabbit Grimace scale was daily performed and if the score was equal or higher than 4, rescue analgesia was provided (buprenorphine 0.1 mg/kg SC q8h: Bupaq, Richter Pharma AG, Austria).
At the end of the study, all animals were sacrificed in compliance with Spanish Policy for Animal Protection (RD118/2021). After sedation with dexmedetomidine (0.05 mg/kg IM; Dexdomitor®, Esteve, Spain) and ketamine (10 mg/kg IM; Imalgene®, Merial, Spain), an IV injection of pentobarbital sodium (150 mg/kg) in the marginal ear vain was given.
Study Groups
Animals were randomly divided into 2 different groups following a simple random sampling method by using the random number function in Microsoft Excel. Sixteen animals were included in each treatment group:
Control group (CT): Animals in CT group received a single IA injection of Plasma Rich in Growth Factors® (PRGF) and a single IO injection of saline solution.
Treatment group (TRT): Animals in TRT group received a single IA injection together with a single IO injection of PRGF.
Plasma Rich in Growth Factors Preparation
PRGF-Endoret® technology was used to obtain an autologous preparation of PRP. A total of 15 mL of blood was collected from the auricular artery of each rabbit under sterile conditions in vacutainer sodium citrate 3.8% tubes (BD Vacutainer® 9NC, New Jersey, USA). Animals were sedated with intramuscular dexmedetomidine (0.05 mg/kg; Dexdomitor®, Esteve, Spain), ketamine (10 mg/kg; Imalgene®, Merial, Spain), and morphine (1 mg/kg; B-Braun, Germany).
The tubes were centrifuged at 460g for 8 minutes (PRGF® System III, Biotechnology Institute®, Álava, Spain) to separate the different blood phases. A sterile pipette was used to collect the PRGF fraction and transfer it into a sterile tube.
Immediately before the IA and IO infiltration, 10% calcium chloride was added to PRGF (50 μL/mL of PRGF) to activate platelets for growth factors release.
Chondral Defect Model and Treatments
Rabbits were intramuscular premedicated with dexmedetomidine (0,05 mg/kg; Dexdomitor®, Esteve, Spain), ketamine (10 mg/kg; Imalgene®, Merial, Spain) and morphine (1 mg/kg; B-Braun, Germany). The medial area of both forelimbs was clipped and prepared for aseptic surgery. General anesthesia was mask induced and maintained with sevoflurane (Sevoflo®, Esteve, Spain).
A cutaneous skin incision of 10 mm was made in the margin of the medial femoral condyle, while a complete flexion of the knee was maintained. After the incision of the fascia and joint capsule, the medial femoral condyle was exposed. Keeping the joint in complete flexion, the loading area was identified and a defect of 4 mm in diameter and 5 mm in depth was created with a drill bit with the help of a drilling guide. A layer suture was performed using 3/0 polyglyconate (Novosyn® Quick, B-Braun, Germany), simple stitches were used in the joint capsule and fascia, while cross stitches were used for the subcutaneous tissue and the skin. Then, the same procedure was carried out in the contralateral knee.
Consecutively, IA infiltration of both knees with PRP was conducted with a 22-G needle. With the knee in flexion, the needle was inserted lateral to the patellar tendon and an infiltration with 0.25 mL of PRP was performed in all animals. Finally, the IO infiltration was performed. An 18-G spinal needle was inserted with smooth rotation movements perpendicular to the femur, in the lateral supracondylar area, and the infiltration with 0.5 mL of saline solution or PRP (depending on the treatment group) was conducted.
Biomarkers’ Study
A total of 3.5 mL of blood was collected from the auricular artery of each rabbit in vacutainer serum tubes with clot accelerator and gel serum separator (BD Vacutainer® SST™ II Advance, BD, New Jersey, USA). Blood samples were collected just before the surgery and 28, 56, and 84 days after the infiltration.
Blood samples were centrifuged at 3,000g for 5 minutes and the obtained serum was frozen at −80°C in eppendorf tubes. Enzyme-linked immunosorbent assay (ELISA) tests for C2C (IBEX® 60-1001-001) and HA (TECO® TE1017-2) were used to determine serum concentration of these biomarkers.
These commercially available kits consist of a multi-well microtiter plate, and these wells are coated with the antigen of interest. The wells are filled with dilutions of the rabbits’ serum and if antibodies against the antigen are present, they will bind to the antigen fixed to the wells. The wells are then washed out to remove all unbound antibodies and then a secondary antibody covalently conjugated to an enzyme, bind to the primary antibodies. The wells are washed out again and a substrate which reacts with the enzymes is added producing a color change. Finally, the microtiter plate is placed into a plate reader to measure the coloring reaction.
Statistical Analysis
The data were processed using the SPSS 20.0 program for Windows (SPSS®Inc., Chicago, USA).
A descriptive study of the mean, standard deviation, and confidence intervals was made for each variable. A value of P < 0.05 was considered significant. Analysis of variance (ANOVA) tests were used to compare normally distributed variables, while nonparametric Kruskal-Wallis tests were used to compare the variables that did not follow a normal distribution. Normality of data was tested in every quantitative variable with Shapiro-Wilk test and variance homogeneity with Levene test.
Results
Serum Hyaluronic Acid Levels
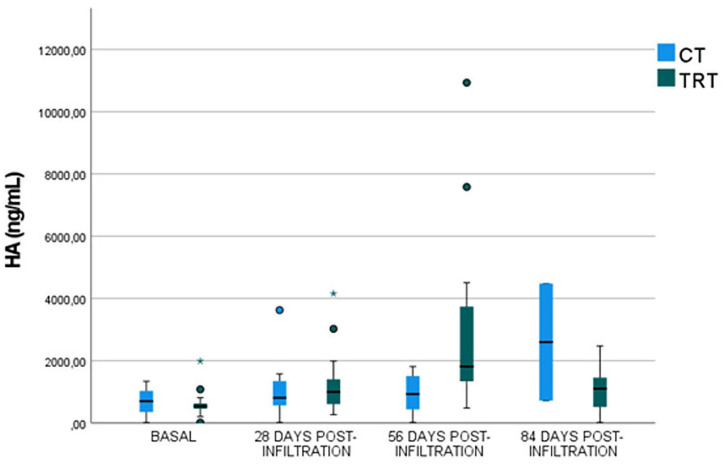
No significant differences (P-value= 0.3254) between groups were demonstrated at baseline and both groups exhibited the lowest serum HA concentration (CT: 683.86 ± 413.68 ng/mL; TRT: 631.62 ± 480.83 ng/mL) ( Fig. 1 ).
Figure 1.
Serum HA levels before infiltration and 28, 56, and 84 days post-infiltration in both control and treatment groups. HA = hyaluronic acid; CT = control group; TRT = treatment group.
No significant differences were observed in serum HA levels between groups (P-value = 0.5082) 28 days post-infiltration. However, rabbits in CT group showed lower HA concentrations (1,058.54 ± 933.49 ng/mL) than rabbits included in TRT group (1,336.20 ± 1,114.80 ng/mL) ( Fig. 1 ).
Significantly greater (P-value = 0.02072) serum HA levels were obtained in animals included in TRT group, which received an IA infiltration of PRGF together with an IO infiltration of PRGF (3,119.60 ± 3,040.71 ng/mL), than in CT rabbits (974.47 ± 623.86 ng/mL), 56 days post-infiltration ( Fig. 1 ).
At 84 days post-infiltration, no significant differences were observed in serum HA concentration (P-value = 0.0721), although lower values were reported in TRT group (1,099.86 ± 762.32 ng/mL) than in CT group (1,554.59 ± 1,722.73 ng/mL) ( Fig. 1 ).
Serum Type II Collagen Neoepitope Levels
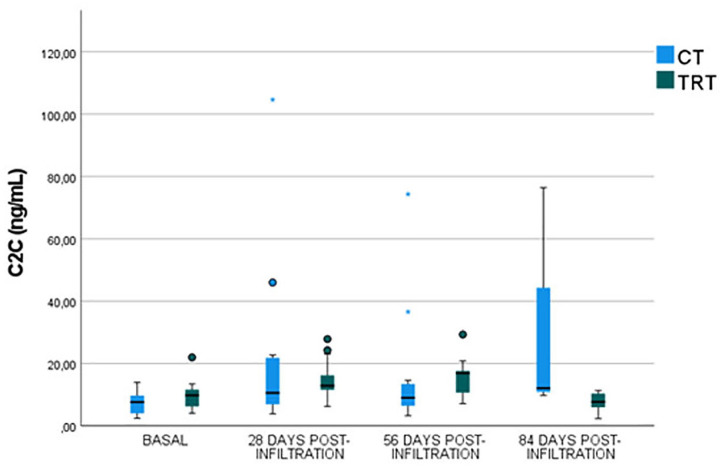
At baseline, no significant differences (P-value = 0.4872) between groups were reported (CT group: 7.520 ± 3.91 ng/mL; TRT group: 9.64 ± 4.83 ng/mL) ( Fig. 2 ).
Figure 2.
Serum C2C levels before infiltration and 28, 56, and 84 days post-infiltration in both control and treatment groups. C2C = type II collagen cleavage neoepitope; CT = control group; TRT = treatment group.
No significant differences were obtained between groups 28 days post-infiltration (P-value = 0.5139); however, lower serum C2C concentration was reported in TRT group (14.59 ± 6.64 ng/mL) than in CT group (21.961 ± 28.52 ng/mL) ( Fig. 2 ).
Furthermore, no significant differences were observed between groups (P-value = 0.8625) 56 days post-infiltration, but lower levels were reported in TRT group (15.13 ± 6.27 ng/mL) than in CT group (16.17 ± 20.27 ng/mL) ( Fig. 2 ).
Significant lower C2C serum values were obtained 84 days post-infiltration (P-value = 0.0376) in TRT group (7.26 ± 3.29 ng/mL) than in CT group (24.15 ± 29.24 ng/mL) ( Fig. 2 ).
Discussion
In our study, lower C2C and HA serum levels were obtained in rabbits 28 days after IA combined with IO PRGF infiltration, although no significant differences were reported. At 56 days follow-up, lower C2C serum levels were reported in TRT group, but contrarily, at the same study time, significant greater HA serum levels were reported in this group. On the contrary, significant lower C2C serum levels were obtained in rabbits 84 days after IA and IO infiltration of PRGF, as well as lower HA serum levels. In addition, no complications or adverse events were described.
The use of PRP to treat OA and other musculoskeletal disorders has gained interest in clinical practice during the last decade. The most used form of PRP administration is IA infiltration, and even though promising outcomes have been observed in patients with mild OA, this technique only targets the AC and the SM without reaching the SB. 21 IA infiltrations combined with IO infiltrations to additionally target SB can provide a more comprehensive treatment. 22 Moreover, it has been proposed that infiltrating PRP directly into the SB could act on the MSCs of this tissue, and these cells would be maintained within the PRP matrix and modulate the repair process of the SB. 23
Although the role of SB in OA etiology, development, and treatment still being discussed, for now, a crosstalk between cartilage and SB has been demonstrated in animal models, showing that vessels and channels from the SB reach the cartilage. 24 These vessels are more abundant in patients with OA and they are important to allow the transit of molecules involved in joint homeostasis and cartilage nutrition.18,19,25-27 Furthermore, it has been revealed that SB remodeling is an important process in OA pathogenesis. 28
Some IO injection techniques have been used in the past to treat other bony pathologies, such as BMLs with an injectable form of calcium phosphate. This technique has shown good results in pain reduction with small risk of complications.29-32 IO biologics have also been used to treat osteonecrosis by injecting PRP or MSCs into the area of necrosis, reporting positive radiological and clinical results.33,34 The literature regarding IO infiltration with PRP to treat OA is scarce, but in the last years, some small pilot or observational studies with favorable results have been published.5,21,35-38 The novel technique of IA combined with IO injections of PRP to treat patients suffering from OA was introduced by Sánchez et al. 5 in 2014 and since then, some pilot studies have shown this treatment to be an effective method in relieving pain and improving joint functionality.5,21,35-38 All 4 published studies in this field showed improvements relative to baseline in patient-reported outcome measures (PROMs), and the 3 studies presenting visual analogue scale (VAS) measures reported a reduction in pain compared to baseline, as did the 3 studies reporting Western Ontario and McMaster Universities scores (WOMAC). Also, Knee Injury and Osteoarthritis Outcome Score (KOOS) rose compared with baseline scores in all studies.5,21,35-38 In addition to these scales, Sánchez et al. 35 assessed the inflammatory response by quantifying MSCs in SF and concluded that MSC levels were lower in patients after IO infiltration. On the contrary, in a study conducted by Lychagin et al., 38 serum cartilage oligomeric matrix protein (COMP) levels were initially high in the patients included in the experimental group compared to the same biomarker in healthy control patients, and continued to rise 1 and 3 months following the infiltration, but posterior analysis showed stable COMP levels.
In our study, C2C and HA biomarker levels were assessed in rabbits with acute cartilage full depth defect 28, 56, and 84 days after IA infiltration of PRGF together with IO infiltration of saline solution or PRGF (CT and TRT group, respectively). The utility of C2C concentration, both in serum and SF, to monitor disease progression and response to treatment has been reported in various animal models, including dogs,39-41 sheep, 42 horses, 43 mice, 44 guinea pigs, 45 and rabbits.46,47 In all studies, a correlation between C2C levels and OA severity was observed, and its serum or SF concentration was higher after joint damage. In our study, serum C2C levels were lower in rabbits treated with IA and IO infiltration of PRGF at 28, 56, and 84 days follow-up. No statistically significant differences were reported between groups at 28 and 56 days follow-up, while significant differences were obtained at 84 days follow-up. These results may suggest that IA PRGF combined with IO PRGF infiltration enhance cartilage healing better than IA infiltration of PRGF alone, and these differences are more remarkable 84 days after treatment.
Serum HA concentration is raised in patients with osteoarthritis, and higher concentrations are correlated with radiological and clinical worsening of the pathology in humans, 13 dogs,40,48,49 and rabbits. 50 In our study, at 28 days follow-up, lower serum HA levels were reported in the TRT group, but contrarily, significant greater serum HA levels were observed in rabbits that underwent IA and IO infiltration of PRGF at 56 days, followed by a sudden fall of these levels at 84 days follow-up. Although no significant differences between groups were obtained 84 days after infiltration, lower serum levels were reported in TRT group. On the contrary, serum HA levels in CT group remained low and stable during all the study times, showing a little increase at 84 days follow-up. The high increase, followed by the abrupt fall in HA levels in rabbits treated with IA and IO PRGF, might be suggestive of an accelerated chondrogenesis process compared with rabbits only treated with IA PRGF, but further investigation is required.
The results obtained in the present study cannot be fully compared with the ones obtained in pilot or observational studies, in which better clinical outcomes were reported in patients with osteoarthritis after IO infiltration of PRP.5,21,35-38
The present study is the first experimental animal study in this field; in addition, it is one of the first in which objective data have been assessed. The main limitation is the short follow-up period and the lack of studies to compare our results with. Moreover, only 2 OA biomarkers have been analyzed. Despite the positive results that IO infiltration of PRP have shown in previous pilot studies, further experimental trials are needed to evaluate histological and biomechanical changes after treatment that could better explain the results obtained in our study. In addition, further clinical trials with greater sample size and longer follow-up period are required, so long-term IO PRP effects can be assessed. This study leaves a door open for future research in which other biological therapies, such as MSCs, could be IO infiltrated.
Conclusions
PRP is a promising, minimally invasive therapeutic tool for bone and cartilage pathologies, including OA; however, the route of administration seems to be important in its clinical efficacy. Some studies have reported hopeful results when PRP is IA administered in patients with mild OA; nevertheless, its efficacy is limited in patients with severe OA. It seems that the combination of IA application with IO infiltration targets AC, the SM as well as the SB, all the key tissues in the development of OA, which makes this administration route an auspicious tool in the pathology management.
Our results are inconclusive, but better outcomes in rabbits treated with IA together with IO PRGF are suggested, although further studies are needed in this field to correlate these results with histological chondral changes, and biomechanical and clinical outcomes. Even if no clinical complications were reported, studies with longer follow-up periods are needed to evaluate long-term IO PRGF infiltration impact.
Supplemental Material
Supplemental material, sj-pdf-1-car-10.1177_19476035211057246 for Treating Full Depth Cartilage Defects with Intraosseous Infiltration of Plasma Rich in Growth Factors: An Experimental Study in Rabbits by Marta Torres-Torrillas, Elena Damiá, José J. Cerón, José M. Carrillo, Pau Peláez, Laura Miguel, Ayla Del Romero, Mónica Rubio and Joaquín J. Sopena in CARTILAGE
Footnotes
Supplementary material for this article is available on the Cartilage website at http://cart.sagepub.com/supplemental.
Acknowledgments and Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This research was funded by García Cugat Foundation CEU-UCH Chair of Medicine and Regenerative Surgery.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Ethical Approval: This study was approved by the Ethics Committee of Animal Welfare (CEEA) of the university CEU Cardenal Herrera of Valencia (approval code: 2019/VSC/PEA/0153) and was conducted in accordance with the ARRIVE (Animal Research: Reporting In Vivo Experiments) guidelines.
ORCID iD: Elena Damiá  https://orcid.org/0000-0003-2156-9207
https://orcid.org/0000-0003-2156-9207
References
- 1. Kloppenburg M, Berenbaum F. Osteoarthritis year in review 2019: epidemiology and therapy. Osteoarthritis Cartilage. 2020;28(3):242–8. [DOI] [PubMed] [Google Scholar]
- 2. Barr AJ, Campbell TM, Hopkinson D, Kingsbury SR, Bowes MA, Conaghan PG. A systematic review of the relationship between subchondral bone features, pain and structural pathology in peripheral joint osteoarthritis. Arthritis Res Ther. 2015;17:228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Scanzello CR, Goldring SR. The role of synovitis in osteoarthritis pathogenesis. Bone. 2012;51(2_suppl):249–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Campbell TM, Churchman SM, Gomez A, McGonagle D, Conaghan PG, Ponchel F, et al. Mesenchymal stem cell alterations in bone marrow lesions in patients with hip osteoarthritis. Arthritis Rheumatol. 2016;68(7):1648–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Sanchez M, Fiz N, Guadilla J, Padilla S, Anitua E, Sánchez P, et al. Intraosseous infiltration of platelet-rich plasma for severe knee osteoarthritis. Arthrosc Tech. 2014;3(6):e713–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Suri S, Walsh DA. Osteochondral alterations in osteoarthritis. Bone. 2012;51(2_suppl):204–11. [DOI] [PubMed] [Google Scholar]
- 7. van Spil WE, Szilagyi IA. Osteoarthritis year in review 2019: biomarkers (biochemical markers). Osteoarthritis Cartilage. 2020;28(3):296–315. [DOI] [PubMed] [Google Scholar]
- 8. Conrozier T, Poole AR, Ferrand F, Mathieu P, Vincent F, Piperno M, et al. Serum concentrations of type II collagen biomarkers (C2C, C1, 2C and CPII) suggest different pathophysiologies in patients with hip osteoarthritis. Clin Exp Rheumatol. 2008;26(3):430–5. [PubMed] [Google Scholar]
- 9. Garnero P, Ayral X, Rousseau JC, Christgau S, Sandell LJ, Dougados M, et al. Uncoupling of type II collagen synthesis and degradation predicts progression of joint damage in patients with knee osteoarthritis. Arthritis Rheum. 2002;46(10):2613–24. [DOI] [PubMed] [Google Scholar]
- 10. Garvican ER, Vaughan-Thomas A, Clegg PD, Innes JF. Biomarkers of cartilage turnover. part 2: non-collagenous markers. Vet J. 2010;185(1):43–9. [DOI] [PubMed] [Google Scholar]
- 11. Filková M, Senolt L, Braun M, Hulejová H, Pavelková A, Sléglová O, et al. Serum hyaluronic acid as a potential marker with a predictive value for further radiographic progression of hand osteoarthritis. Osteoarthritis Cartilage. 2009;17(12):1615–9. [DOI] [PubMed] [Google Scholar]
- 12. Jiao Q, Wei L, Chen C, Li P, Wang X, Li Y, et al. Cartilage oligomeric matrix protein and hyaluronic acid are sensitive serum biomarkers for early cartilage lesions in the knee joint. Biomarkers. 2016;21(2_suppl):146–51. [DOI] [PubMed] [Google Scholar]
- 13. Kraus VB, Hargrove DE, Hunter DJ, Renner JB, Jordan JM. Establishment of reference intervals for osteoarthritis-related soluble biomarkers: the FNIH/OARSI OA biomarkers consortium. Ann Rheum Dis. 2017;76(1):179–85. [DOI] [PubMed] [Google Scholar]
- 14. Kumavat R, Kumar V, Malhotra R, Pandit H, Jones E, Ponchel F, et al. Biomarkers of joint damage in osteoarthritis: current status and future directions. Mediators Inflamm. 2021;2021:5574582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ambra LF, de Girolamo L, Mosier B, Gomoll AH. Review: interventions for cartilage disease: current state-of-the-art and emerging technologies. Arthritis Rheumatol. 2017;69(7):1363–73. [DOI] [PubMed] [Google Scholar]
- 16. Kennedy MI, Whitney K, Evans T, LaPrade RF. Platelet-rich plasma and cartilage repair. Curr Rev Musculoskelet Med. 2018;11(4):573–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kwan Tat S, Lajeunesse D, Pelletier JP, Martel-Pelletier J. Targeting subchondral bone for treating osteoarthritis: what is the evidence? Best Pract Res Clin Rheumatol. 2010;24(1):51–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Pan J, Wang B, Li W, Zhou X, Scherr T, Yang Y, et al. Elevated cross-talk between subchondral bone and cartilage in osteoarthritic joints. Bone. 2012;51(2_suppl):212–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Goldring SR, Goldring MB. Changes in the osteochondral unit during osteoarthritis: structure, function and cartilage-bone crosstalk. Nat Rev Rheumatol. 2016;12(11):632–44. [DOI] [PubMed] [Google Scholar]
- 20. Wang Z, Zhai C, Fei H, Hu J, Cui W, Wang Z, et al. Intraarticular injection autologous platelet-rich plasma and bone marrow concentrate in a goat osteoarthritis model. J Orthop Res. 2018;36(8):2140–6. [DOI] [PubMed] [Google Scholar]
- 21. Sanchez M, Delfado D, Pompei O, Pérez JC, Sánchez P, Garate A, et al. Treating severe knee osteoarthritis with combination of intra-osseous and intra-articular infiltrations of platelet-rich plasma: an observational study. Cartilage. 2019;10(2_suppl):245–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Delgado D, Garate A, Vincent H, Bilbao AM, Patel R, Fiz N, et al. Current concepts in intraosseous platelet-rich plasma injections for knee osteoarthritis. J Clin Orthop Traum. 2019;10(1):36–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Yuan XL, Meng HY, Wang YC, Peng J, Guo QY, Wang AY, et al. Bone-cartilage interface crosstalk in osteoarthritis: potential pathways and future therapeutic strategies. Osteoarthritis Cartilage. 2014;22(8):1077–89. [DOI] [PubMed] [Google Scholar]
- 24. Sundaram K, Vargas-Hernandez JS, Sanchez TR, Moreu NM, Mont MA, Higuera CA, et al. Are subchondral intraosseous injections effective and safe for the treatment of knee osteoarthritis? A systematic review. J Knee Surg. 2019;32:1046–57. [DOI] [PubMed] [Google Scholar]
- 25. Lories RJ, Luyten FP. The bone-cartilage unit in osteoarthritis. Nat Rev Rheumatol. 2011;7(1):43–9. [DOI] [PubMed] [Google Scholar]
- 26. Gerter R, Kruegel J, Miosge N. New insights into cartilage repair—the role of migratory progenitor cells in osteoarthritis. Matrix Biol. 2012;31(3):206–13. [DOI] [PubMed] [Google Scholar]
- 27. Aaron RK, Racine JR, Voisinet A, Evangelista P, Dyke JP. Subchondral bone circulation in osteoarthritis of the human knee. Osteoarthritis Cartilage. 2018;26(7):940–4. [DOI] [PubMed] [Google Scholar]
- 28. Mayerhoefer ME, Breitenseher MJ, Kramer J, Aigner N, Norden C, Hofmann S. STIR vs. T1-weighted fat-suppressed gadolinium-enhanced MRI of bone marrow edema of the knee: computer-assisted quantitative comparison and influence of injected contrast media volume and acquisition parameters. J Magn Reson Imaging. 2005;22(6):788–93. [DOI] [PubMed] [Google Scholar]
- 29. Chua K, Kang JYB, Ng FDJ, Pang HN, Lie DTT, Silva A, et al. Subchondroplasty for bone marrow lesions in the arthritic knee results in pain relief and improvement in function. J Knee Surg. 2021;34(6):665–71. [DOI] [PubMed] [Google Scholar]
- 30. Bessa F, Rasio J, Newhouse A, Nwachukwu BU, Nho S. Surgical treatment of subchondral bone cysts of the acetabulum with calcium phosphate bone substitute material in patients without advanced arthritic hips. Arthrosc Tech. 2020;9(9):e1375–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Bonadio MB, Giglio PN, Helito CP, Pécora JR, Camanho GL, Demange MK. Subchondroplasty for treating bone marrow lesions in the knee—initial experience. Rev Bras Ortop. 2017;52(3):325–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Cohen SB, Sharkey PF. Subchondroplasty for treating bone marrow lesions. J Knee Surg. 2016;29(7):555–63. [DOI] [PubMed] [Google Scholar]
- 33. Hernigou P, Trousselier M, Roubineau F, Bouthors C, Chevallier N, Rouard H, et al. Stem cell therapy for the treatment of hip osteonecrosis: a 30-year review of progress. Clin Orthop Surg. 2016;8(1):1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Guadilla J, Fiz N, Andia I, Sánchez M. Arthroscopic management and platelet-rich plasma therapy for avascular necrosis of the hip. Knee Surg Sports Traumatol Arthrosc. 2012;20(2_suppl):393–8. [DOI] [PubMed] [Google Scholar]
- 35. Sánchez M, Delgado D, Sanchez P, Muiños-López E, Paiva B, Granero-Moltó F, et al. Combination of intra-articular and intraosseous injections of platelet rich plasma for severe knee osteoarthritis: a pilot study. Biomed Res Int. 2016;2016:4868613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Fiz N, Perez JC, Guadilla J, Garate A, Sánchez P, Padilla S, et al. Intraosseous infiltration of platelet-rich plasma for severe hip osteoarthritis. Arthrosc Tech. 2017;6(3):E821–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Su K, Bai Y, Wang J, Zhang H, Liu H, Ma S. Comparison of hyaluronic acid and PRP intra-articular injection with combined intra-articular and intraosseous PRP injections to treat patients with knee osteoarthritis. Clin Rheumatol. 2018;37(5):1341–50. [DOI] [PubMed] [Google Scholar]
- 38. Lychagin A, Lipina M, Garkavi A, Islaieh O, Timashev P, Ashmore K, et al. Intraosseous injections of platelet rich plasma for knee bone marrow lesions treatment: one year follow-up. Int Orthop. 2021;45(2_suppl):355–63. [DOI] [PubMed] [Google Scholar]
- 39. Chu Q, Lopez M, Hayashi K, Ionescu M, Billinghurst RC, Johnson KA, et al. Elevation of a collagenase generated type II collagen neoepitope and proteoglycan epitopes in synovial fluid following induction of joint instability in the dog. Osteoarthritis Cartilage. 2002;10(8):662–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Vilar JM, Rubio M, Spinella G, Cuervo B, Sopena J, Cugat R, et al. Serum collagen type II cleavage epitope and serum hyaluronic acid as biomarkers for treatment monitoring of dogs with hip osteoarthritis. PLoS ONE. 2016;11(2_suppl):e0149472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Goranov NV. Serum markers of lipid peroxidation, antioxidant enzymatic defense, and collagen degradation in an experimental (Pond-Nuki) canine model of osteoarthritis. Vet Clin Pathol. 2007;36(2_suppl):192–5. [DOI] [PubMed] [Google Scholar]
- 42. Lu Y, Markel MD, Swain C, Kaplan LD. Development of partial thickness articular cartilage injury in an ovine model. J Orthop Res. 2006;24(10):1974–82. [DOI] [PubMed] [Google Scholar]
- 43. Coppelman EB, David FH, Toth F, Ernst NS, Trumble TN. The association between collagen and bone biomarkers and radiographic osteoarthritis in the distal tarsal joints of horses. Equine Vet J. 2020;52(3):391–8. [DOI] [PubMed] [Google Scholar]
- 44. Ameye LG, Deberg M, Oliveira M, Labasse A, Aeschlimann JM, Henrotin Y. The chemical biomarkers C2C, Coll2-1, and Coll2-1NO(2) provide complementary information on type II collagen catabolism in healthy and osteoarthritic mice. Arthritis Rheum. 2007;56(10):3336–46. [DOI] [PubMed] [Google Scholar]
- 45. Huebner JL, Kraus VB. Assessment of the utility of biomarkers of osteoarthritis in the guinea. Osteoarthritis Cartilage. 2006;14(9):923–30. [DOI] [PubMed] [Google Scholar]
- 46. Lindhorst E, Wachsmuth L, Kimmig N, Raiss R, Aigner T, Atley L, et al. Increase in degraded collagen type II in synovial fluid early in the rabbit meniscectomy model of osteoarthritis. Osteoarthritis Cartilage. 2005;13(2_suppl):139–45. [DOI] [PubMed] [Google Scholar]
- 47. Nemirovskiy OV, Dufield DR, Sunyer T, Aggarwal P, Welsch DJ, Mathews WR. Discovery and development of a type II collagen neoepitope (TIINE) biomarker for matrix metalloproteinase activity: from in vitro to in vivo. Anal Biochem. 2007;361(1):93–101. [DOI] [PubMed] [Google Scholar]
- 48. de Bakker E, Stroobants V, VanDael F, Van Ryssen B, Meyer E. Canine synovial fluid biomarkers for early detection and monitoring of osteoarthritis. Vet Rec. 2017;180(13):328–9. [DOI] [PubMed] [Google Scholar]
- 49. Cuervo B, Rubio M, Sopena J, Dominguez JM, Vilar J, Morales M, et al. Hip osteoarthritis in dogs: a randomized study using mesenchymal stem cells from adipose tissue and plasma rich in growth factors. Int J Mol Sci. 2014;15(8):13437–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Ohnishi A, Osaki T, Matahira Y, Tsuka T, Imagawa T, Okamoto Y, et al. Evaluation of the chondroprotective effects of glucosamine and fish collagen peptide on a rabbit ACLT model using serum biomarkers. J Vet Med Sci. 2013;75(4):421–9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-pdf-1-car-10.1177_19476035211057246 for Treating Full Depth Cartilage Defects with Intraosseous Infiltration of Plasma Rich in Growth Factors: An Experimental Study in Rabbits by Marta Torres-Torrillas, Elena Damiá, José J. Cerón, José M. Carrillo, Pau Peláez, Laura Miguel, Ayla Del Romero, Mónica Rubio and Joaquín J. Sopena in CARTILAGE