Abstract
Objective
Curcumin monoglucuronide (TBP1901) is highly water soluble and can convert to free form curcumin, which has pharmacological effects, on intravenous administration. This study aimed to investigate the effectiveness of TBP1901 intra-articular injections in an osteoarthritis (OA) rat model.
Methods
Sixty-four male Wistar rats (12 weeks old) who underwent destabilized medial meniscus (DMM) surgery were randomly separated into the TBP1901 injection or saline solution (control) injection group. They were sacrificed at 1, 2, 6, or 10 weeks postoperatively (weeks 1, 2, 6, and 10; n = 8 for each group). TBP1901 (30 mg/mL) or saline solution of 50 μL was injected into the knee joints twice a week during weeks 1 and 2 to investigate the effects in the acute phase of posttraumatic (PT) OA or once a week during weeks 6 and 10 to investigate it in the chronic phase of PTOA. Histology, immunohistochemistry, and micro-computed tomography were performed to evaluate the changes in OA.
Results
TBP1901 injections significantly reduced synovial inflammation at weeks 1 and 2, and tumor necrosis factor-α expression in the articular cartilage at week 6. The TBP1901 injections also significantly suppressed articular cartilage damage, subchondral bone (SB) plate thickening, SB plate perforation, and osteophyte formation at week 10.
Conclusions
TBP1901 intra-articular injections suppressed synovial inflammation in the acute phase of PTOA in DMM rats. In the chronic phase, TBP1901 suppresses articular cartilage damage and regulates SB plate changes.
Keywords: TBP1901, curcumin monoglucuronide, osteoarthritis, intra-articular injection, articular cartilage
Introduction
Osteoarthritis (OA) is a progressive joint disease characterized by articular cartilage loss, subchondral bone (SB) alterations, and osteophyte formation. OA progression affects an individual’s activities of daily living. Inflammation plays a central role in OA pathogenesis. It contributes to chondrocyte apoptosis, extracellular matrix disruption, and cartilage degeneration through pro-inflammatory cytokines such as interleukin (IL)-1β, IL-6, and tumor necrosis factor-α (TNF-α).1-4
Curcumin, a polyphenol compound present in turmeric (Curcuma longa, long used as a spice and herbal medicine), has many positive health benefits, including anti-inflammatory, 5 antioxidative, 6 anticancer, 7 and antidepressant 8 effects. Oral curcumin ingestion for OA was shown to reduce pain and improve function in clinical studies.9-11 Animal studies have shown that curcumin inhibits OA progression by decreasing inflammation by blocking the Toll-like receptor/nuclear factor-κB (TLR4/NF-κB) signaling pathway and inhibiting chondrocyte apoptosis through enhanced autophagy.12-16 Curcumin has few side effects; therefore, its use is expected to be a potential adjunct to pharmaceutical therapies. On the other hand, orally ingested curcumin has low bioavailability because of limited absorption with low hydrophilicity and conjugation in the intestinal tract.17-20 Only a few ingested curcumin molecules are absorbed and present in the conjugated form (mainly curcumin monoglucuronide [CMG]) in the blood.21,22 To improve its low absorption, bio-optimization by liposomal delivery systems, chemical modification of curcumin, nanoparticulation of curcumin, and use of polysorbate as an emulsifier have been performed.23-26 However, the low absorbability and hydrophilicity remain, although their efficacy is greater than that of pure curcumin. To overcome the disadvantages of oral curcumin ingestion, intra-articular administration, an ideal drug delivery method commonly used in OA therapies, has been used to deliver a concentrated therapeutic dose of curcumin into the knee joint and has been shown to suppress articular cartilage degeneration and synovial inflammation in an OA rat model.24,27 We hypothesized that the intra-articular administration of CMG could overcome the issue of low hydrophilicity due to its high water solubility, could be absorbed into the deep layer of the cartilage and maximize its pharmacological effects. CMG exhibits 10-fold lower antioxidative activity than unconjugated, free form curcumin. 28 Therefore, CMG must be metabolized to free form curcumin in vivo to execute its antioxidative activity. 29 β-Glucuronidase is an important hydrolase for the deconjugation of CMG to free form curcumin. It is released from neutrophils, injured cells at sites of inflammation, and cells in bone.30-33 In addition, β-glucuronidase secretion is enhanced in the articular cartilage, synovium, and synovial fluid with inflammation and OA.34-37 Therefore, administered CMG would be deconjugated to free form curcumin in OA joint. To confirm our hypothesis, we utilized the synthesized CMG (TBP1901), which has already been investigated and demonstrated the efficacy of intravenous administration in tumor-bearing mice with colorectal cancer. 29 TBP1901, which has high water solubility, may be deconjugated to free form curcumin in OA joint and suitable for intra-articular administration. Although the efficacy of intra-articular administration of TBP1901 is unknown, the pharmaceutical value of curcumin will be enhanced if it is shown to have an anti-osteoarthritic effect in intra-articular administration.
To verify the efficacy of TBP1901, we chose rats with destabilized medial meniscus (DMM) as an OA animal model.38,39 Although posttraumatic (PT) OA models include anterior cruciate ligament transection, meniscus resection, combination with ligament transection and meniscectomy, and DMM model, 40 the DMM model was chosen to be the slower progress model and closer to human OA progression. 38 PTOA has an acute phase and a chronic phase for each period from trauma. 41 Although curcumin is effective for treating chronic inflammation, 42 it was recently reported as effective for treating acute inflammation. 43 Therefore, to comprehensively confirm the efficacy of TBP1901 in DMM rats, we observed its effects from the acute phase to the chronic phase of PTOA.
This study aimed to investigate the effectiveness of intra-articular administration of TBP1901 on inflammation and OA progression in a rat model with DMM.
Methods
Animal Preparation and Surgical Procedures
This study was conducted in accordance with the ARRIVE guidelines. 44 Sixty-four male Wistar rats (12 weeks old), purchased from SHIMIZU Laboratory Supplies Co. Ltd. (Kyoto, Japan), were placed in a plastic cage with paper bedding on a 12-hour light/dark cycle at a constant temperature. The rats could move freely in cages and had free access to food and water. The rats were randomly separated into TBP1901 and saline solution (control) intra-articular injection groups (n = 32 for each group). All rats underwent DMM surgery for the OA model.38,39 Briefly, DMM surgery was performed with an anteromedial capsule incision and transection of the medial meniscotibial ligament in the right knee under anesthesia with 1.0 mL/kg pentobarbital sodium (Somnopentyl; Kyoritsu Seiyaku Corp., Tokyo, Japan). The DMM rat model rapidly progresses to moderate OA with severe inflammation by 4 weeks postoperatively and to severe OA by 8 weeks postoperatively, by which time inflammation is milder.39,45,46 Therefore, the rats were sacrificed at 1 and 2 weeks postoperatively to confirm the effect of TBP1901 as the acute phase of PTOA and at 6 and 10 weeks postoperatively to confirm the effect as the chronic phase of PTOA (n = 8 for each).
The synthesis of TBP1901 was performed as described previously. 29 TBP1901 and saline intra-articular injections of 50 μL were administered to the right knee joints through the patellar tendon. The concentration of TBP1901 was chosen to be 30 mg/mL (1.5 mg/50 μL) based on a previous study, 29 which showed no abnormalities in mice administered with 125 mg/kg of TBP1901, and a preliminary study (Supplementary Fig. S1). The preliminary study showed no significant effects on TBP1901 injections at 10 mg/mL for 6 weeks in DMM rats. The rats sacrificed at 6 and 10 weeks postoperatively were injected once a week from 1 week postoperatively. All rats were injected an hour before sacrifice to confirm intra-articular curcumin by fluorescence observation. The rats sacrificed at 1 and 2 weeks postoperatively were injected twice a week since the observation periods were short. The rats sacrificed at 1 week were injected on days 3 and 7, and the rats sacrificed at 2 weeks were injected on days 3, 7, 10, and 14. One hour after the final intra-articular injection in each observation period, the rats were sacrificed by a lethal dose of pentobarbital sodium, and their right knee joints were harvested for micro-computed tomography (micro-CT), histochemical, and immunohistochemical analyses. Body weight was measured weekly for all rats.
Micro-CT Analysis
After fixation of the knees with 4% paraformaldehyde overnight, the tibiofemoral (TF) joints were scanned using a micro-CT system (SMX-100CT, Shimadzu, Kyoto, Japan) at 43 kV and 43 μA with a scan time of approximately 10 minutes. After scanning, the 3-dimensional (3D) reconstruction and assessment of the TF joint were performed using ImageJ (National Institutes of Health, Bethesda, MD, USA) and Amira (version 5.5, EFI Visualization Science Group, Burlington, MA, USA) software. The SB plate thickness, number of SB plate perforations (holes), diameter of SB plate perforations, bone volume (BV), total volume (TV), and osteophyte volume in the medial tibia SB (above epiphyseal plate) were calculated according to protocols described in previous studies.39,47 SB plate thickness was measured at the thickest region around the center of the medial articular surface. The diameter of the SB plate perforation was defined as the largest diameter of the largest perforation in each sample. To assess the bone density of the SB, bone volume was divided by the total volume (BV/TV).
Histological Analysis
After micro-CT, the knee joint samples were decalcified in 10% ethylenediaminetetraacetic acid (EDTA) for 3 to 4 weeks and cut along the mid-sagittal plane at the halfway point. The DMM model causes rapid OA progression in the TF joint and also affects the patellofemoral (PF) joint. 48 Therefore, the histological evaluation was performed on the TF and PF joints. After decalcification, the samples were paraffin-embedded and cut into 6-μm sections at 50-μm intervals. Fluorescence histochemistry was performed using sections with an epifluorescence microscope (MVX10, Olympus Corporation, Tokyo, Japan) to confirm the presence of curcumin in the articular cartilage and synovium. Curcumin is a fluorescent substance with an excitation wavelength of 300 to 550 nm (maximum excitation wavelength: 467 nm) and an emission wavelength of 548 to 600 nm (maximum emission wavelength: 571 nm). 49 Fluorescence detection is used for curcumin quantification. 50 Based on these reports, curcumin fluorescence was observed with the mirror unit of U-MYFPHQ/XL (Olympus Corporation, Tokyo, Japan). In addition, other paraffin sections were stained with hematoxylin and eosin (H&E) to evaluate inflammation of the synovium in knee joints and safranin O/fast green to evaluate the severity of cartilage degeneration. Inflammation was assessed using an inflammation scoring system described in a previous study. 51 Three membrane features (synovial lining cell layer, stroma cell density, and inflammatory infiltrate) were assessed in the whole knee joint as 0 (none), 1 (slight), 2 (moderate), or 3 (strong). The inflammation score was determined as the summed score for all parameters. The values of the parameters were summarized and interpreted as follows: 0-1, no synovitis; 2-4, low-grade synovitis; and 5-9, high-grade synovitis. Cartilage degeneration was assessed using the Osteoarthritis Research Society International (OARSI) score 52 and the modified Mankin (MM) score. 53 The OARSI score consists of 6 grades and 4 stages on a scale from 0 (intact) to 24 (severe damage). The MM score consists of 3 features (pericellular matrix staining, spatial arrangement of chondrocytes, and interterritorial matrix staining) on a scale from 0 (intact) to 8 (severe). Cartilage degeneration was evaluated in the medial TF and PF joints. The maximum score was used for all scoring systems and samples.
Immunohistochemical Analysis
Immunohistochemistry of type I collagen, type II collagen, IL-1β, IL-6, and TNF-α was performed to determine the principal collagen expression and inflammation in the articular cartilage. Antigen retrieval was performed for 20 minutes by heating with HistoVT One (Nacalai Tesque, Inc., Kyoto, Japan). Blocking was performed using 0.3% H2O2 for 15 minutes and 5% goat serum for 20 minutes at room temperature. The sections were then incubated at 4 °C overnight with primary antibodies against type I collagen (AB755P, diluted 1:1000; Merck KGaA, Darmstadt, Germany), type II collagen (diluted 1:100; Kyowa Pharma Chemical Co., Ltd., Toyama, Japan), IL-1β (ab9722, diluted 1:100; Abcam Co., Cambridge, UK), IL-6 (ab9324, diluted 1:250; Abcam Co., Cambridge, UK), and TNF-α (ab6671, diluted 1:100; Abcam Co., Cambridge, UK). Detection was performed using the streptavidin-biotin-peroxidase complex technique with an Elite ABC kit (Vector Laboratories, Burlingame, CA, USA). Immunoreactivity was visualized by incubation with a diaminobenzidine solution (Vector Laboratories, Burlingame, CA, USA) followed by counterstaining with hematoxylin. The primary antibody was omitted from negative control slides. The expression of type I and type II collagen in the medial tibia and patella cartilage was analyzed by measuring the minimum and mean pixel intensity values using TIFF images (magnification ×40) taken by microscopy and ImageJ, respectively, on a scale of 0 (minimum; dark) to 255 (maximum; no staining), according to a previous study. 54 The expression of IL-1β, IL-6, and TNF-α in the medial tibia and patella cartilage was analyzed by measuring the percentage of positive chondrocytes to detect the severity of cartilage inflammation within the middle region of the medial tibia with an anteroposterior width of 0.5 mm or the central region of the patella with a superoinferior width of 0.5 mm.
Statistical Analyses
The median and interquartile range (IQR) of the inflammation score, OARSI score, and MM score (nonparametric data) were calculated for each group. The Wilcoxon test was used to compare differences between the 2 groups. In addition, the mean and 95% confidence intervals (CIs) of body weight, immunohistochemical, and micro-CT analysis data (parametric data) were calculated, and Welch’s t test was used to compare differences between the groups. The normality of the data was assessed using normal quantile–quantile plots and the Shapiro-Wilk test. Statistical significance was set at P < 0.05. Statistical analyses were performed using JMP Pro 14 software (SAS Institute Inc., Cary, NC, USA).
Results
Body weight is shown in Supplementary Figure S2. Body weight in the TBP1901 group was significantly lower than that in the saline control group at week 1 (mean [95% CIs]: 276.59 g [272.65–280.54]; TBP1901, 282.31 g [278.40–286.23]; control, P = 0.040), but the difference was small. There were no significant differences between the groups at weeks 2, 6, and 10. No other adverse events were observed.
Histology
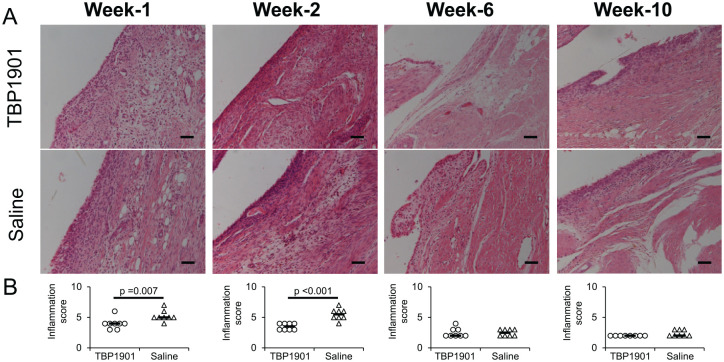
The fluorescence expression of curcumin was confirmed on the tibial articular cartilage and was found on the synovium in the TBP1901 group but not in the saline control group ( Fig. 1 ). Severe synovial inflammation was observed at weeks 1 and 2 as an enlargement of the synovial lining cell layer, increased cellularity, and situated lymphocytes or plasma cells, especially in the saline control ( Fig. 2 ). TBP1901 Intra-articular injections significantly reduced the synovial inflammation at week 1 (P = 0.007) and week 2 (P < 0.001). However, no significant effects were observed after 6 weeks with mild inflammation.
Figure 1.
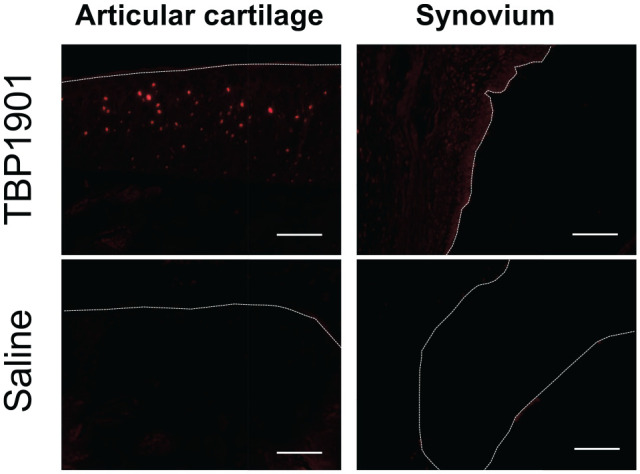
Fluorescence microscopy images of the articular cartilage and synovium of knee joints. Representative histological images. Curcumin fluorescence (red) was confirmed in the articular cartilage and slightly in the synovium of rats injected with TBP1901 intra-articularly. The dotted line indicates the surface line of the articular cartilage and synovium. Magnification: ×125. Scale bar: 100 μm.
Figure 2.
Histological images stained with hematoxylin and eosin and synovial inflammation scores in knee joints. (A) Representative histological images of the knee synovium are shown. Severe synovial inflammation was observed at weeks 1 and 2 as enlargement of the synovial lining cell layer, increased cellularity, and situated lymphocytes or plasma cells, especially in the saline control group. Magnification: ×200. Scale bar: 100 μm. (B) TBP1901 intra-articular injections significantly reduced the inflammation scores at weeks 1 and 2 compared with the saline control group. Values are the medians in the TBP1901 and the saline control group at several time points (n = 8). P values were calculated using the Wilcoxon test.
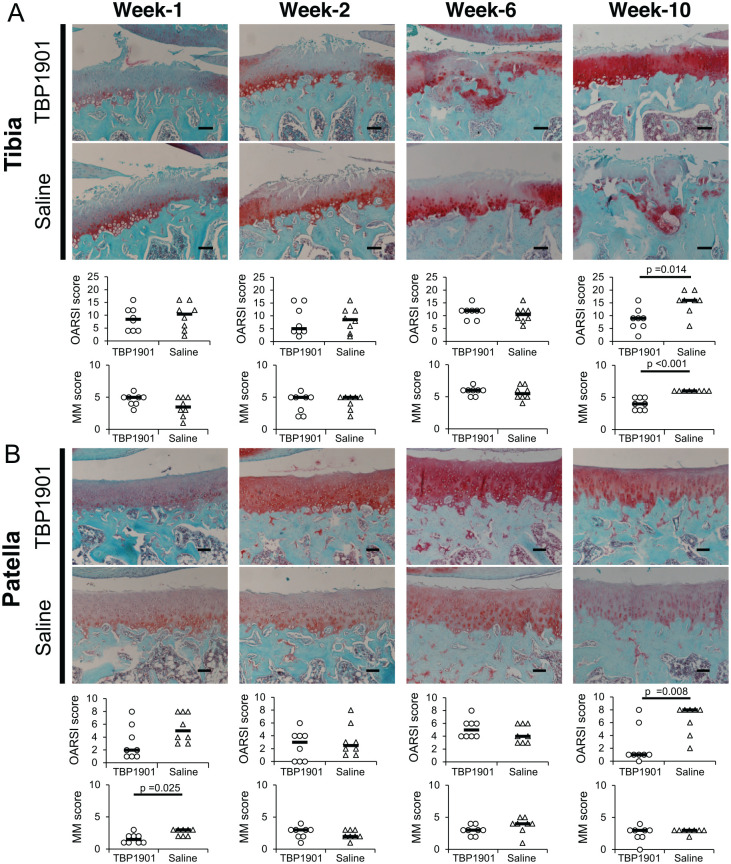
Articular cartilage damage of the medial tibia was found as fissures and matrix staining depletion at week 1 in both groups. Cartilage damage did not change much until week 6 in both groups. In week 10, the cartilage damage in most of the TBP1901 did not progress, but it progressed and eroded in most of the saline control ( Fig. 3A ). The OARSI and MM scores in the TBP1901 group were significantly lower than those in the saline control group at week 10 (P = 0.014 and P < 0.001, respectively). In the patella, the cartilage in the saline control group showed fibrillation and matrix staining depletion at all observation periods ( Fig. 3B ). However, the cartilage damage in the TBP1901 group at weeks 1 and 10 was lower than that in the saline control group. At week 1, the MM score was significantly lower than that in the saline control group (P = 0.025), and at week 10, the OARSI score was significantly lower than that in the saline control group (P = 0.008).
Figure 3.
Histological images of safranin O/fast green–stained specimens and osteoarthritis (OA) scores. (A) Representative histological images of the articular cartilage and the Osteoarthritis Research Society International (OARSI) score and the modified Mankin (MM) score on the tibial plateau are shown. At week 10, the cartilage structure in the TBP1901 was relatively preserved. Furthermore, the cartilage matrix staining and the chondrocyte cellularity in the TBP1901 group were less reduced than those in the saline control group. There were no significant intergroup changes in OA scores until week 6. However, TBP1901 intra-articular injections significantly reduced the OARSI score and the MM score at week 10. (B) Representative histological images of the articular cartilage, the OARSI score, and the MM score on the patella are shown. At week 1, the matrix staining in the TBP1901 group was less reduced than that in the saline control group. TBP1901 intra-articular injections significantly reduced the MM score in the patella at 1 week. Furthermore, at week 10, TBP1901 intra-articular injections reduced the fibrillation of the cartilage surface, and the OARSI score was significantly lower than that in the saline control. Values are the medians in the TBP1901 and saline control groups at several time points (n = 8 each). P values were calculated using the Wilcoxon test. Magnification: ×100. Scale bar: 100 μm.
Immunohistochemistry
Tibiofemoral Joint
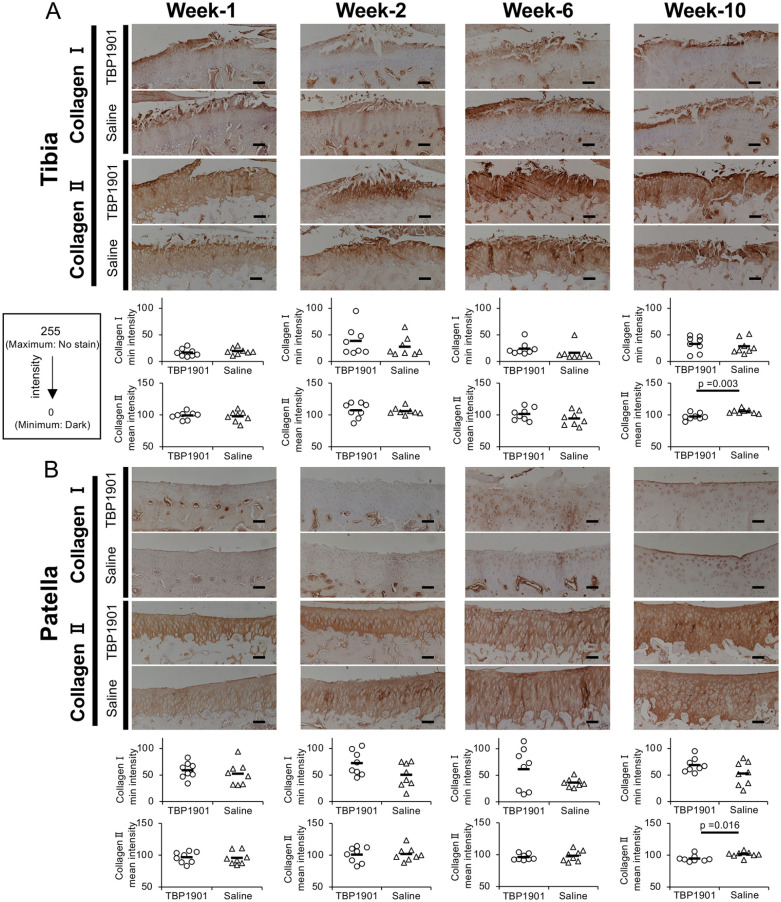
The expression of type I collagen was confirmed on the surface of the articular cartilage in the medial tibia during all observation periods. There were no significant differences between the groups ( Fig. 4A ). The expression of type II collagen in both groups at weeks 1 and 2 was strong in the surface layer of the cartilage, while it was weakly expressed in the other layers. There were no significant differences in mean intensity at weeks 1, 2, and 6 between the groups. However, at week 10, TBP1901 intra-articular injections significantly increased type II collagen expression (P = 0.003).
Figure 4.
TBP1901 intra-articular injections increased type II collagen expression at 10 weeks. (A) Immunohistochemical images with type I and II collagen and quantitative analyses in the tibia of the tibiofemoral (TF) joint. TBP1901 intra-articular injections significantly increased the type II collagen expression at week 10. (B) Immunohistochemical images with type I and II collagen and quantitative analyses in the patella of the patellofemoral joint. TBP1901 intra-articular injections significantly increased the type II collagen expression at week-10. The expressions of type I and type II collagen were analyzed by measuring the minimum and mean intensity values using ImageJ, respectively, on a scale from 0 (dark) to 255 (no staining). Values are the means in the TBP1901 and saline control groups at several time points (n = 8 each). P values were calculated using Welch’s t test. Magnification: ×100. Scale bar: 100 μm.
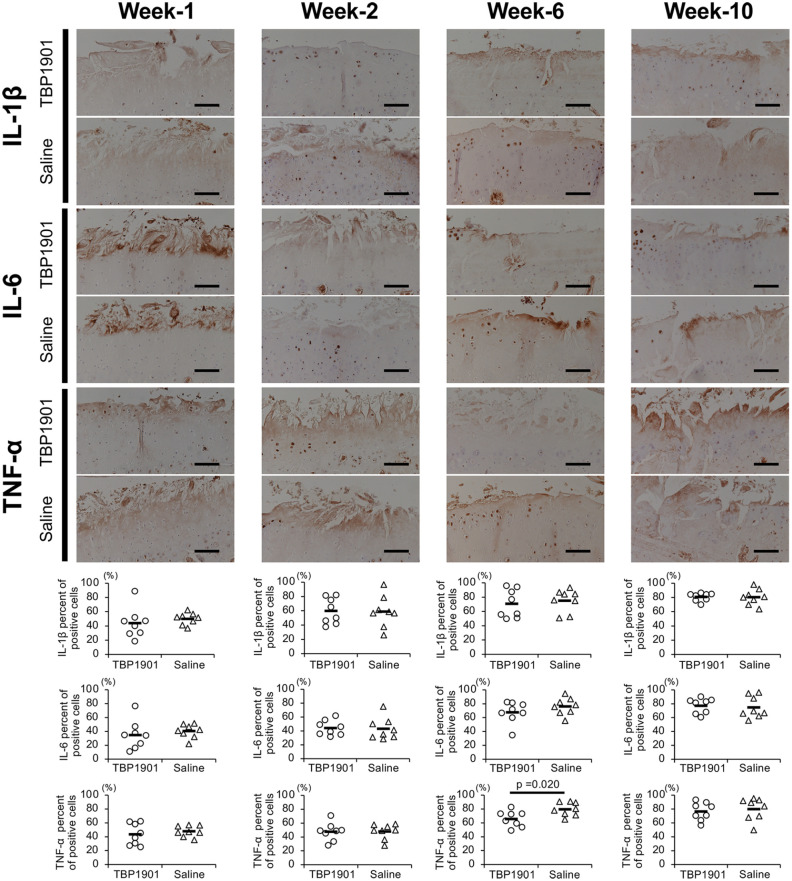
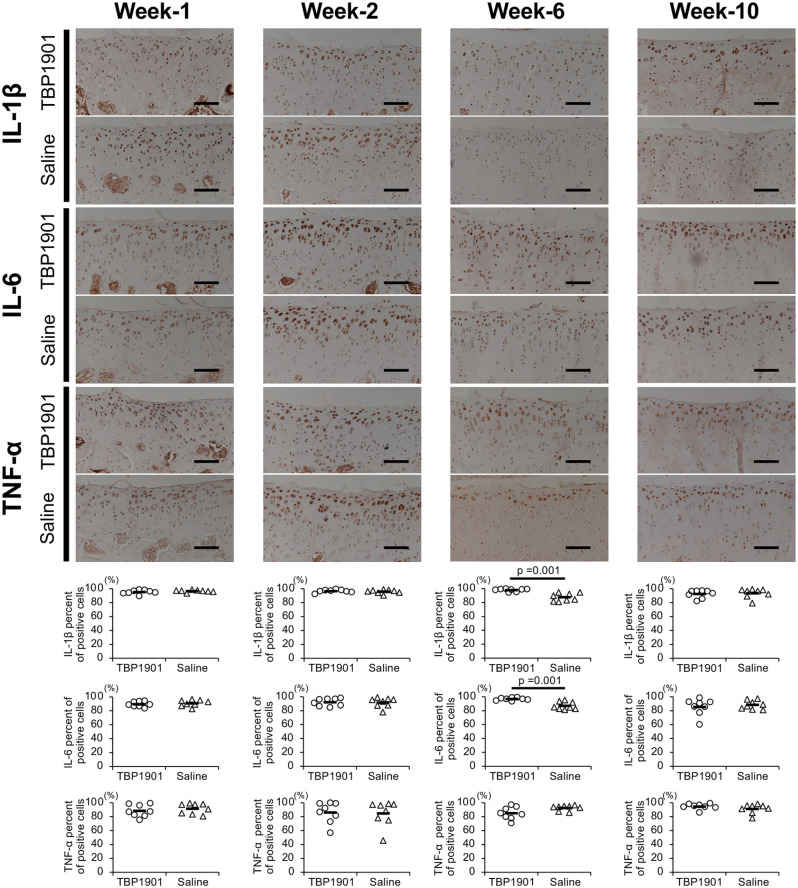
The expression of pro-inflammatory cytokines IL-1β, IL-6, and TNF-α was confirmed in the articular cartilage ( Fig. 5 ). The percentage of their positive chondrocytes increased from week 1 to 10 in both groups. There were no significant differences between groups at weeks 1, 2, and 10, but TBP1901 intra-articular injections significantly reduced the TNF-α expression at week 6 (P = 0.020). In addition, TBP1901 intra-articular injections showed a tendency to reduce IL-6 expression at week 6.
Figure 5.
TBP1901 intra-articular injections suppressed the expression of pro-inflammatory cytokines in the articular cartilage of the tibiofemoral joint. Representative immunohistochemical images of interleukin (IL)-1β, IL-6, and tumor necrosis factor-α (TNF)-α in the articular cartilage of the tibia are shown in the upper row. The percentages of positive cells are shown in the lower row. The percentage of TNF-α positive cells in the TBP1901 group was significantly reduced at week 6 compared with that in the saline control group. Values are the means of the TBP1901 and saline control groups at several time points (n = 8 each). P values were calculated using Welch’s t test. Magnification: ×200. Scale bar: 100 μm.
Patellofemoral Joint
The expression of type I collagen was almost undetectable in the patellar articular cartilage at weeks 1 and 2. At week 6, it was confirmed in the peri-chondrocytes and the middle layer of the cartilage at week 6 in the TBP1901 group. At week 10, it was confirmed on the cartilage surface ( Fig. 4B ). There were no significant differences between the groups at any time point. Type II collagen in the patella was stably expressed throughout the cartilage, unlike that in the tibia. There were no differences between the groups at weeks 1, 2, and 6. However, at week 10, TBP1901 intra-articular injections significantly increased type II collagen expression (P = 0.016).
The pro-inflammatory cytokines IL-1β, IL-6, and TNF-α were more highly expressed in the articular cartilage of the patella than in the tibia ( Fig. 6 ). The percentage of positive chondrocytes decreased slightly from week 1 to 10. There were no significant differences between the groups at weeks 1, 2, and 10, but TBP1901 intra-articular injections significantly increased IL-6 and IL-1β expression at week 6 (P = 0.001 each). However, TNF-α expression in the TBP1901 group showed a tendency to decrease at week 6.
Figure 6.
TBP1901 intra-articular injections did not suppress the expression of pro-inflammatory cytokines in the articular cartilage of the patellofemoral joint. Representative immunohistochemical images of interleukin (IL)-1β, IL-6, and tumor necrosis factor-α (TNF)-α in the articular cartilage of the patella are shown in the upper row. The percentages of positive cells are shown in the lower row. The percentages of IL-1β and IL-6 positive cells were significantly higher at week 6 in the TBP1901 group than in the saline control group. Values are the means of the TBP1901 and saline control groups at several time points (n = 8 each). P values were calculated using Welch’s t test. Magnification: ×200. Scale bar: 100 μm.
Micro-CT Analysis
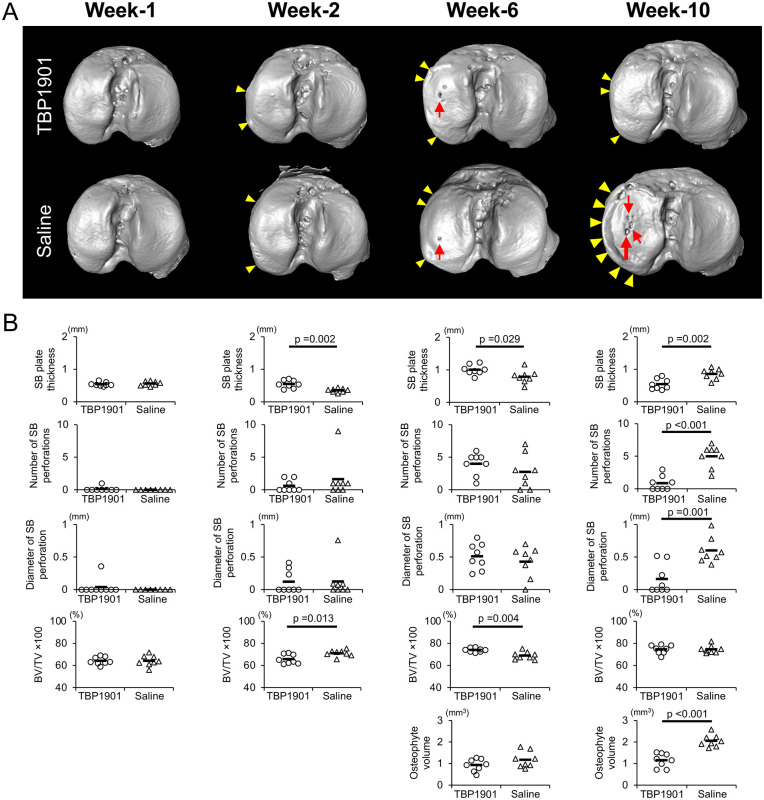
DMM surgery with saline or TBP1901 injections induced SB changes and osteophyte formation ( Fig. 7A and B). There were significant differences in the SB plate thickness at week 2 (P = 0.002), week 6 (P = 0.029), and week 10 (P = 0.002) between the groups ( Fig. 7B ). In particular, TBP1901 intra-articular injections reduced the thickness at week 10 compared with the saline control. SB plate perforations were observed in both groups, especially after 6 weeks ( Fig. 7A ). There were no differences in the number and diameter of SB plate perforations at week 6 between the groups. However, at week 10, TBP1901 intra-articular injections significantly reduced them compared to the control group (P < 0.001 and P = 0.001, respectively; Fig. 7B ).
Figure 7.
TBP1901 intra-articular injections regulated subchondral bone (SB) plate formation and prevented excessive bone formation. (A) Representative 3-dimensional micro-computed tomography images of the SB in the tibial plateau. SB plate perforations (holes; red arrows) were found in the medial tibial plateau, especially at weeks 6 and 10. Osteophytes (yellow arrowheads) were confirmed on the medial margin at weeks 2, 6, and 10. (B) TBP1901 intra-articular injections significantly suppressed the SB plate thinning in week 2, reduced SB plate thickness, the number of SB plate perforations, the diameters of the SB plate perforations, and the osteophyte volume at week 10. Values are the means of all measurements at several time points (n = 8 each). P values were calculated using Welch’s t test for all measurements.
The BV/TV tended to increase from week 1 to week 10 in both groups ( Fig. 7B ). However, TBP1901 injections delayed the timing of the increase and decrease in BV/TV. In the TBP1901 group, the BV/TV was significantly decreased at week 2 (P = 0.013) and significantly increased at week 6 (P = 0.004) compared with the control group. There was no significant difference at week 10 between the groups.
The osteophytes had formed from week 2 (quantitative data not shown). In the TBP1901 group, the osteophyte volume tended to be lower than that in the control group at week 6. In week 10, that in the TBP1901 group was significantly lower than that in the control group (P < 0.001).
Discussion
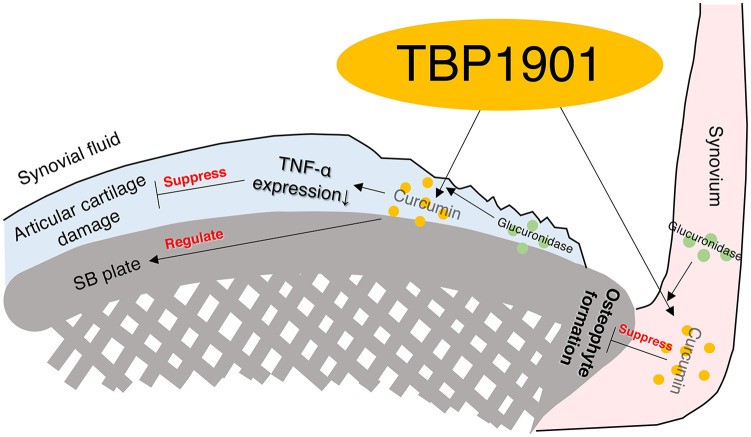
In this study, we performed an intra-articular injection of TBP1901 for 1, 2, 6, or 10 weeks in DMM rats to investigate its effects on inflammation and OA progression. First, the fluorescence of curcumin was confirmed in the articular cartilage and synovium of the TBP1901 group. This indicates that TBP1901 is incorporated into the cartilage and synovium and might induce biological effects. In addition, we found that TBP1901 intra-articular injections suppressed synovial inflammation in the acute phase and degeneration of cartilage and SB in the chronic phase ( Fig. 8 ).
Figure 8.
Graphical abstract of the effects of the intra-articular injection of TBP1901 on the suppression of osteoarthritis (OA) development in an OA rat model (destabilized medial meniscus). Curcumin was absorbed in the articular cartilage and synovium by the TBP1901 injection. Curcumin suppressed inflammation, osteophyte formation, articular cartilage, and subchondral bone (SB) pathology.
Suppression of Inflammation and Articular Cartilage Degeneration by TBP1901 Intra-Articular Injections
This study showed the suppression of synovial inflammation in the acute phase of PTOA by TBP1901 intra-articular injections in an OA rat model. The mammalian target of the rapamycin (mTOR) pathway plays an important role in regulating cell growth, proliferation, differentiation, and apoptosis. It has been reported that curcumin inhibits synovial inflammation and hyperplasia via the mTOR pathway in rats with collagen-induced arthritis. 55 The present study showed the suppression of synovial inflammation in a rat model of PTOA. In contrast, TBP1901 did not suppress cartilage damage during the acute phase. In a previous report, oral administration of curcumin or curcumin-loaded poly lactic-co-glycolic acid nanoparticles for 14 days showed a preventative effect on articular cartilage in a monoiodoacetate (MIA) model of osteoarthritis in rats. 56 MIA inhibits glyceraldehyde-3-phosphate dehydrogenase of the Krebs cycle, leading to the death of chondrocytes, leading to unique pathophysiology that does not correlate with PTOA. 57 In the present study, the progression of cartilage damage by DMM surgery may have been too rapid for TBP1901 to be sufficiently effective in the acute phase.
In the chronic phase, articular cartilage degeneration is suppressed by TBP1901. Curcumin suppresses inflammatory cytokines, such as TNF-α, IL-1β, and IL-6. It inhibits the production of pro-inflammatory mediators and matrix-degrading enzymes, metalloproteinase (MMP)-3 and MMP-13.58,59 In the present study, TNF-α expression in the cartilage was also reduced by TBP1901 injections. In addition, type II collagen deposition was higher in the TBP1901 group at week-10. It has been reported that curcumin promotes chondrogenic differentiation, proliferation, and migration of mesenchymal stem cells (MSCs).60,61 These effects may improve articular cartilage conditions. Furthermore, SB and articular cartilage interact with each other and work as a unit. 62 The protection of the SB damage by TBP1901 injections may have suppressed cartilage degeneration.
SB Improvement by TBP1901 Intra-Articular Injections
There are few reports on the effects of curcumin on SB. The present study evaluated the effect of TBP1901 on SB in detail using μ-CT. Some reports have shown that curcumin attenuates bone resorption by suppressing osteoclast proliferation 63 and osteoblast apoptosis. 64 The SB plate thins as bone remodeling increases in early OA and thickens as the remodeling balance changes as OA progresses.65,66 The attenuation of bone resorption may have suppressed the SB plate thinning in the acute phase and the SB plate thickening and perforating in the chronic phase in the present study. A previous study demonstrated histologically that the oral administration of curcumin reduced SB plate thickness in the chronic phase, 12 similar to our results.
In addition, TBP1901 injection suppressed osteophyte formation. Osteophytes are formed by synovial cells in a process similar to endochondral ossification and limit range of motion. 67 Transforming growth factor-β (TGF-β) and macrophages play an important role in osteophyte formation.68,69 In a previous report, the administration of oral curcumin suppressed TGF-β activation in the synovium. 70 TBP1901 injections may have suppressed the osteophyte formation by the inactivation of TGFβ on synovium. The remarkable effects on SB in our study may have been due to the presence of rich β-glucuronidase, which hydrolyzes TBP1901 to free form curcumin in the bone and synovium.29,32
Limitations and Conclusion
The present study has some limitations. First, it is still unclear when and how much TBP1901 should be administered, although it was administered at a dose of 30 mg/mL by intra-articular injection from postoperative day 3 or 7 in this study. Additional investigations are necessary to determine the optimal postoperative timing and dose of TBP1901. Second, the working mechanism of TBP1901 in SB and osteophytes is unclear. Additional comprehensive studies focusing on TGF-β and macrophages are required. Third, although TBP1901 suppressed articular cartilage in a rat OA model, its effects on human OA are unclear. Fourth, it has not been determined whether TBP1901 is more effective than traditional curcumin or other curcumin-related products. We plan to conduct comparative studies to demonstrate the efficacy of TBP1901 in the future.
In conclusion, this study showed that intra-articular injections of TBP1901 reduced synovial inflammation at weeks 1 and 2 in the acute phase of PTOA. In the chronic phase of PTOA, TBP1901 reduced TNF-α expression at week 6 and suppressed articular cartilage degeneration, SB plate thickening, SB plate perforation, and osteophyte formation at week 10.
Supplemental Material
Supplemental material, sj-pdf-1-car-10.1177_19476035211043202 for Intra-Articular Injections of Curcumin Monoglucuronide TBP1901 Suppresses Articular Cartilage Damage and Regulates Subchondral Bone Alteration in an Osteoarthritis Rat Model by Akihiro Nakahata, Akira Ito, Ryo Nakahara, Atsuhiro Kishimoto, Atsushi Imaizumi, Tadashi Hashimoto, Shogo Mukai, Yasuaki Nakagawa and Hiroshi Kuroki in CARTILAGE
Supplemental material, sj-pdf-2-car-10.1177_19476035211043202 for Intra-Articular Injections of Curcumin Monoglucuronide TBP1901 Suppresses Articular Cartilage Damage and Regulates Subchondral Bone Alteration in an Osteoarthritis Rat Model by Akihiro Nakahata, Akira Ito, Ryo Nakahara, Atsuhiro Kishimoto, Atsushi Imaizumi, Tadashi Hashimoto, Shogo Mukai, Yasuaki Nakagawa and Hiroshi Kuroki in CARTILAGE
Supplemental material, sj-pdf-3-car-10.1177_19476035211043202 for Intra-Articular Injections of Curcumin Monoglucuronide TBP1901 Suppresses Articular Cartilage Damage and Regulates Subchondral Bone Alteration in an Osteoarthritis Rat Model by Akihiro Nakahata, Akira Ito, Ryo Nakahara, Atsuhiro Kishimoto, Atsushi Imaizumi, Tadashi Hashimoto, Shogo Mukai, Yasuaki Nakagawa and Hiroshi Kuroki in CARTILAGE
This article is distributed under the terms of the Creative Commons Attribution 4.0 License (https://creativecommons.org/licenses/by/4.0/) which permits any use, reproduction and distribution of the work without further permission provided the original work is attributed as specified on the SAGE and Open Access pages (https://us.sagepub.com/en-us/nam/open-access-at-sage).
sj-pptx-1-car-10.1177_19476035211043202 for Intra-Articular Injections of Curcumin Monoglucuronide TBP1901 Suppresses Articular Cartilage Damage and Regulates Subchondral Bone Alteration in an Osteoarthritis Rat Model by Akihiro Nakahata, Akira Ito, Ryo Nakahara, Atsuhiro Kishimoto, Atsushi Imaizumi, Tadashi Hashimoto, Shogo Mukai, Yasuaki Nakagawa and Hiroshi Kuroki in CARTILAGE
Footnotes
Supplementary material for this article is available on the Cartilage website at http://cart.sagepub.com/supplemental.
Acknowledgments and Funding: The author(s) disclosed receipt of the following financial support for research, authorhship and/or publication of this article: This study was supported in part by JSPS KAKENHI Grant Numbers JP18H03129 and JP18K19739, and a sponsored research grant from Therabiopharma Inc. to HK. We would like to thank Editage (www.editage.com) for English language editing.
Declaration of Conflicting Interests: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: This study was supported in part by a sponsored research grant from Therabiopharma Inc. to HK. AIm and TH have equity in Therabiopharma Inc. and serve on the board of directors of Therabiopharma Inc. AK is an employee of Therabiopharma Inc.
Ethical Approval: This study was approved by the animal research committee of Kyoto University (approval number: Med Kyo 19016).
ORCID iDs: Akihiro Nakahata  https://orcid.org/0000-0001-7462-9774
https://orcid.org/0000-0001-7462-9774
Akira Ito  https://orcid.org/0000-0002-9645-9777
https://orcid.org/0000-0002-9645-9777
Yasuaki Nakagawa  https://orcid.org/0000-0002-5664-963X
https://orcid.org/0000-0002-5664-963X
References
- 1. Shen J, Abu-Amer Y, O’Keefe RJ, McAlinden A. Inflammation and epigenetic regulation in osteoarthritis. Connect Tissue Res. 2017;58(1):49-63. doi: 10.1080/03008207.2016.1208655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Lieberthal J, Sambamurthy N, Scanzello CR. Inflammation in joint injury and post-traumatic osteoarthritis. Osteoarthritis Cartilage. 2015;23(11):1825-34. doi: 10.1016/j.joca.2015.08.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Kapoor M, Martel-Pelletier J, Lajeunesse D, Pelletier JP, Fahmi H. Role of proinflammatory cytokines in the pathophysiology of osteoarthritis. Nat Rev Rheumatol. 2011;7(1):33-42. doi: 10.1038/nrrheum.2010.196 [DOI] [PubMed] [Google Scholar]
- 4. Jia PT, Zhang XL, Zuo HN, Lu X, Li L. Articular cartilage degradation is prevented by tanshinone IIA through inhibiting apoptosis and the expression of inflammatory cytokines. Mol Med Rep. 2017;16(5):6285-9. doi: 10.3892/mmr.2017.7340 [DOI] [PubMed] [Google Scholar]
- 5. Srimal RC, Dhawan BN. Pharmacology of diferuloyl methane (curcumin), a non-steroidal anti-inflammatory agent. J Pharm Pharmacol. 1973;25(6):447-52. doi: 10.1111/j.2042-7158.1973.tb09131.x [DOI] [PubMed] [Google Scholar]
- 6. Sugiyama Y, Kawakishi S, Osawa T. Involvement of the β-diketone moiety in the antioxidative mechanism of tetrahydrocurcumin. Biochem Pharmacol. 1996;52(4):519-25. doi: 10.1016/0006-2952(96)00302-4 [DOI] [PubMed] [Google Scholar]
- 7. Aggarwal BB, Kumar A, Bharti AC. Anticancer potential of curcumin: preclinical and clinical studies. Anticancer Res. 2003;23(1A):363-98. [PubMed] [Google Scholar]
- 8. Kulkarni SK, Bhutani MK, Bishnoi M. Antidepressant activity of curcumin: involvement of serotonin and dopamine system. Psychopharmacology (Berl). 2008;201(3):435-42. doi: 10.1007/s00213-008-1300-y [DOI] [PubMed] [Google Scholar]
- 9. Panahi Y, Rahimnia AR, Sharafi M, Alishiri G, Saburi A, Sahebkar A. Curcuminoid treatment for knee osteoarthritis: a randomized double-blind placebo-controlled trial. Phyther Res. 2014;28(11):1625-31. doi: 10.1002/ptr.5174 [DOI] [PubMed] [Google Scholar]
- 10. Madhu K, Chanda K, Saji MJ. Safety and efficacy of Curcuma longa extract in the treatment of painful knee osteoarthritis: a randomized placebo-controlled trial. Inflammopharmacology. 2013;21(2_suppl):129-36. doi: 10.1007/s10787-012-0163-3 [DOI] [PubMed] [Google Scholar]
- 11. Srivastava S, Saksena AK, Khattri S, Kumar S, Dagur RS. Curcuma longa extract reduces inflammatory and oxidative stress biomarkers in osteoarthritis of knee: a four-month, double-blind, randomized, placebo-controlled trial. Inflammopharmacology. 2016;24(6):377-88. doi: 10.1007/s10787-016-0289-9 [DOI] [PubMed] [Google Scholar]
- 12. Zhang Z, Leong DJ, Xu L, He Z, Wang A, Navati M, et al. Curcumin slows osteoarthritis progression and relieves osteoarthritis-associated pain symptoms in a post-traumatic osteoarthritis mouse model. Arthritis Res Ther. 2016;18(1):128. doi: 10.1186/s13075-016-1025-y [DOI] [PMC free article] [PubMed] [Google Scholar] [Research Misconduct Found]
- 13. Zhang G, Cao J, Yang E, Liang B, Ding J, Liang J, et al. Curcumin improves age-related and surgically induced osteoarthritis by promoting autophagy in mice. Biosci Rep. 2018;38(4):BSR20171691. doi: 10.1042/BSR20171691 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 14. TenBroek EM, Yunker L, Nies MF, Bendele AM. Randomized controlled studies on the efficacy of antiarthritic agents in inhibiting cartilage degeneration and pain associated with progression of osteoarthritis in the rat. Arthritis Res Ther. 2016;18:24. doi: 10.1186/s13075-016-0921-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Feng K, Ge Y, Chen Z, Li X, Liu Z, Li X, et al. Curcumin inhibits the PERK-eIF2α-CHOP pathway through promoting SIRT1 expression in oxidative stress-induced rat chondrocytes and ameliorates osteoarthritis progression in a rat model. Oxid Med Cell Longev. 2019;2019:8574386. doi: 10.1155/2019/8574386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Zhang Y, Zeng Y. Curcumin reduces inflammation in knee osteoarthritis rats through blocking TLR4/MyD88/NF-κB signal pathway. Drug Dev Res. 2019;80(3):353-9. doi: 10.1002/ddr.21509 [DOI] [PubMed] [Google Scholar]
- 17. Anand P, Kunnumakkara AB, Newman RA, Aggarwal BB. Bioavailability of curcumin: problems and promises. Mol Pharm. 2007;4(6):807-18. doi: 10.1021/mp700113r [DOI] [PubMed] [Google Scholar]
- 18. Yang KY, Lin LC, Tseng TY, Wang SC, Tsai TH. Oral bioavailability of curcumin in rat and the herbal analysis from Curcuma longa by LC-MS/MS. J Chromatogr B Anal Technol Biomed Life Sci. 2007;853(1-2):183-9. doi: 10.1016/j.jchromb.2007.03.010 [DOI] [PubMed] [Google Scholar]
- 19. Shoji M, Nakagawa K, Watanabe A, Tsuduki T, Yamada T, Kuwahara S, et al. Comparison of the effects of curcumin and curcumin glucuronide in human hepatocellular carcinoma HepG2 cells. Food Chem. 2014;151:126-32. doi: 10.1016/j.foodchem.2013.11.021 [DOI] [PubMed] [Google Scholar]
- 20. Pal A, Sung B, Bhanu Prasad BA, Schuber PT, Jr, Prasad S, Aggarwal BB, et al. Curcumin glucuronides: Assessing the proliferative activity against human cell lines. Bioorg Med Chem. 2014;22(1):435-9. doi: 10.1016/j.bmc.2013.11.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Pan MH, Huang TM, Lin JK. Biotransformation of curcumin through reduction and glucuronidation in mice. Drug Metab Dispos. 1999;27(4):486-94. [PubMed] [Google Scholar]
- 22. Vareed SK, Kakarala M, Ruffin MT, Crowell JA, Normolle DP, Djuric Z, et al. Pharmacokinetics of curcumin conjugate metabolites in healthy human subjects. Cancer Epidemiol Biomarkers Prev. 2008;17(6):1411-7. doi: 10.1158/1055-9965.EPI-07-2693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Sahebkar A, Henrotin Y. Analgesic efficacy and safety of curcuminoids in clinical practice: a systematic review and meta-analysis of randomized controlled trials. Pain Med. 2016;17(6):1192-202. doi: 10.1093/pm/pnv024 [DOI] [PubMed] [Google Scholar]
- 24. Zhou Y, Ming J, Deng M, Li Y, Li B, Li J, et al. Chemically modified curcumin (CMC2.24) alleviates osteoarthritis progression by restoring cartilage homeostasis and inhibiting chondrocyte apoptosis via the NF-κB/HIF-2α axis. J Mol Med (Berl). 2020;98(10):1479-91. doi: 10.1007/s00109-020-01972-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Henrotin Y, Malaise M, Wittoek R, de Vlam K, Brasseur JP, Luyten FP, et al. Bio-optimized curcuma longa extract is efficient on knee osteoarthritis pain: a doule-blind multicenter randomized placebo controlled study. Arthritis Res Ther. 2019;27:179. doi: 10.1016/j.joca.2019.02.562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Nakagawa Y, Mukai S, Yamada S, Matsuoka M, Tarumi E, Hashimoto T, et al. Short-term effects of highly-bioavailable curcumin for treating knee osteoarthritis: a randomized, double-blind, placebo-controlled prospective study. J Orthop Sci. 2014;19(6):933-9. doi: 10.1007/s00776-014-0633-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Yan D, He B, Guo J, Li S, Wang J. Involvement of TLR4 in the protective effect of intra-articular administration of curcumin on rat experimental osteoarthritis. Acta Cir Bras. 2019;34(6):e201900604. doi: 10.1590/s0102-865020190060000004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Choudhury AK, Raja S, Mahapatra S, Nagabhushanam K, Majeed M. Synthesis and evaluation of the anti-oxidant capacity of curcumin glucuronides, the major curcumin metabolites. Antioxidants. 2015;4(4):750-67. doi: 10.3390/antiox4040750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Ozawa H, Imaizumi A, Sumi Y, Hasihimoto T, Kanai M, Makino Y, et al. Curcumin β-D-glucuronide plays an important role to keep high levels of free-form curcumin in the blood. Biol Pharm Bull. 2017;40(9):1515-24. doi: 10.1248/bpb.b17-00339 [DOI] [PubMed] [Google Scholar]
- 30. Marshall T, Shult P, Busse WW. Release of lysosomal enzyme beta-glucuronidase from isolated human eosinophils. J Allergy Clin Immunol. 1988;82(4):550-5. doi: 10.1016/0091-6749(88)90964-5 [DOI] [PubMed] [Google Scholar]
- 31. Shimoi K, Nakayama T. Glucuronidase deconjugation in inflammation. Methods Enzymol. 2005;400(1994):263-72. doi: 10.1016/S0076-6879(05)00015-7 [DOI] [PubMed] [Google Scholar]
- 32. Kunihiro AG, Brickey JA, Frye JB, Luis PB, Schneider C, Funk JL. Curcumin, but not curcumin-glucuronide, inhibits Smad signaling in TGFβ-dependent bone metastatic breast cancer cells and is enriched in bone compared to other tissues. J Nutr Biochem. 2019;63:150-6. doi: 10.1016/j.jnutbio.2018.09.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Kunihiro AG, Luis PB, Brickey JA, Frye JB, Chow HHS, Schneider C, et al. Beta-glucuronidase catalyzes deconjugation and activation of curcumin-glucuronide in bone. J Nat Prod. 2019;82(3):500-9. doi: 10.1021/acs.jnatprod.8b00873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Chang H, Israel H. Analysis of inflammatory mediators in temporomandibular joint synovial fluid lavage samples of symptomatic patients and asymptomatic controls. J Oral Maxillofac Surg. 2005;63(6):761-5. doi: 10.1016/j.joms.2005.02.009 [DOI] [PubMed] [Google Scholar]
- 35. Evequoz V, Schnyder J, Trechsel U. Influence of macrophage products on the release of plasminogen activator, collagenase, β-glucuronidase and prostaglandin E2 by articular chondrocytes. Biochem J. 1984;219(2_suppl):667-77. doi: 10.1042/bj2190667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Henderson B. Alterations in the activity of lysosomal hydrolases in the synovial lining cell population of the knee joint of the rabbit during the development of chronic arthritis. Pathol Res Pract. 1981;172(4):363-71. [PubMed] [Google Scholar]
- 37. Fawthrop F, Hornby J, Swan A, Hutton C, Doherty M, Dieppe P. A comparison of normal and pathological synovial fluid. Br J Rheumatol. 1985;24(1):61-9. doi: 10.1093/rheumatology/24.1.61 [DOI] [PubMed] [Google Scholar]
- 38. Glasson SS, Blanchet TJ, Morris EA. The surgical destabilization of the medial meniscus (DMM) model of osteoarthritis in the 129/SvEv mouse. 2007;15(9):1061-9. doi: 10.1016/j.Joca.2007.03.006 [DOI] [PubMed] [Google Scholar]
- 39. Iijima H, Ito A, Nagai M, Tajino J, Yamaguchi S, Kiyan W, et al. Physiological exercise loading suppresses post-traumatic osteoarthritis progression via an increase in bone morphogenetic proteins expression in an experimental rat knee model. Osteoarthritis Cartilage. 2017;25(6):964-75. doi: 10.1016/j.joca.2016.12.008 [DOI] [PubMed] [Google Scholar]
- 40. Narez GE, Fischenich KM, Donahue TLH. Experimental animal models of post-traumatic osteoarthritis of the knee. Orthop Rev (Pavia). 2020;12(2_suppl):95-103. doi: 10.4081/or.2020.8448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Punzi L, Galozzi P, Luisetto R, Favero M, Ramonda R, Oliviero F, et al. Post-traumatic arthritis: overview on pathogenic mechanisms and role of inflammation. RMD Open. 2016;2(2_suppl):e000279. doi: 10.1136/rmdopen-2016-000279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. He Y, Yue Y, Zheng X, Zhang K, Chen S, Du Z. Curcumin, inflammation, and chronic diseases: how are they linked? Molecules. 2015;20(5):9183-213. doi: 10.3390/molecules20059183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Chen B, Li H, Ou G, Ren L, Yang X, Zeng M. Curcumin attenuates MSU crystal-induced inflammation by inhibiting the degradation of IκBα and blocking mitochondrial damage. Arthritis Res Ther. 2019;21(1):193. doi: 10.1186/s13075-019-1974-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Kilkenny C, Browne WJ, Cuthill IC, Emerson M, Altman DG. Improving bioscience research reporting: The arrive guidelines for reporting animal research. PLoS Biol. 2010;8(6):e100412. doi: 10.1371/journal.pbio.1000412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Iijima H, Aoyama T, Ito A, Tajino J, Nagai M, Zhang X, et al. Destabilization of the medial meniscus leads to subchondral bone defects and site-specific cartilage degeneration in an experimental rat model. Osteoarthritis Cartilage. 2014;22(7):1036-43. doi: 10.1016/j.joca.2014.05.009 [DOI] [PubMed] [Google Scholar]
- 46. Salamanna F, Giavaresi G, Parrilli A, Martini L, Aldini NN, Abatangelo G, et al. Effects of intra-articular hyaluronic acid associated to Chitlac (arty-duo®) in a rat knee osteoarthritis model. J Orthop Res. 2019;37(4):867-76. doi: 10.1002/jor.24259 [DOI] [PubMed] [Google Scholar]
- 47. Iijima H, Aoyama T, Ito A, Tajino J, Yamaguchi S, Nagai M. i. Exercise intervention increases expression of bone morphogenetic proteins and prevents the progression of cartilage-subchondral bone lesions in a post-traumatic rat knee model. Osteoarthritis Cartilage. 2016;24(6):1092-102. doi: 10.1016/j.joca.2016.01.006 [DOI] [PubMed] [Google Scholar]
- 48. Fang H, Huang L, Welch I, Norley C, Holdsworth DW, Beier F, et al. Early changes of articular cartilage and subchondral bone in the DMM mouse model of osteoarthritis. Sci Rep. 2018;8(1):2855. doi: 10.1038/s41598-018-21184-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Ali Z, Saleem M, Atta BM, Khan SS, Hammad G, et al. Determination of curcuminoid content in turmeric using fluorescence spectroscopy. Spectrochim Acta A Mol Biomol Spectrosc. 2019;213:192-8. doi: 10.1016/j.saa.2019.01.028 [DOI] [PubMed] [Google Scholar]
- 50. Jasim F, Ali F. A novel and rapid method for the spectrofluorometric determination of curcumin in curcumin spices and flavors. Microchem J. 1992;46(2_suppl):209-14. doi: 10.1016/0026-265X(92)90040-A [DOI] [Google Scholar]
- 51. Krenn V, Morawietz L, Burmester GR, Kinne RW, Mueller-Ladner U, Muller B, et al. Synovitis score: Discrimination between chronic low-grade and high-grade synovitis. Histopathology. 2006;49(4):358-64. doi: 10.1111/j.1365-2559.2006.02508.x [DOI] [PubMed] [Google Scholar]
- 52. Pritzker KPH, Gay S, Jimenez SA, Ostergaard K, Pelletier JP, et al. Osteoarthritis cartilage histopathology : grading and staging. Osteoarthritis Cartilage. 2006;14:13-29. doi: 10.1016/j.joca.2005.07.014 [DOI] [PubMed] [Google Scholar]
- 53. Bomsta BD, Bridgewater LC, Seegmiller RE. Premature osteoarthritis in the disproportionate micromelia (Dmm) mouse. Osteoarthritis Cartilage. 2006;14(5):477-85. doi: 10.1016/j.joca.2005.11.011 [DOI] [PubMed] [Google Scholar]
- 54. Iijima H, Aoyama T, Ito A, Tajino J, Nagai M, Zhang X, et al. Immature articular cartilage and subchondral bone covered by menisci are potentially susceptive to mechanical load. BMC Musculoskelet Disord. 2014;15(1):101. doi: 10.1186/1471-2474-15-101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Dai Q, Zhou D, Xu L, Song X. Curcumin alleviates rheumatoid arthritis-induced inflammation and synovial hyperplasia by targeting mTOR pathway in rats. Drug Des Devel Ther. 2018;12:4095-105. doi: 10.2147/DDDT.S175763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Niazvand F, Khorsandi L, Abbaspour M, Orazizadeh M, Varaa N, Maghzi M, et al. Curcumin-loaded poly lactic-co-glycolic acid nanoparticles effects on mono-iodoacetate -induced osteoarthritis in rats. Vet Res Forum. 2017;8(2_suppl):155-61. [PMC free article] [PubMed] [Google Scholar]
- 57. Kuyinu EL, Narayanan G, Nair LS, Laurencin CT. Animal models of osteoarthritis: classification, update, and measurement of outcomes. J Orthop Surg Res. 2016;11(1):19. doi: 10.1186/s13018-016-0346-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Liacini A, Sylvester J, Li WQ, Huang W, Dehnade F, Ahmad M, et al. Induction of matrix metalloproteinase-13 gene expression by TNF-α is mediated by MAP kinases, AP-1, and NF-κB transcription factors in articular chondrocytes. Exp Cell Res. 2003;288(1):208-17. doi: 10.1016/S0014-4827(03)00180-0 [DOI] [PubMed] [Google Scholar]
- 59. Mathy-Hartert M, Jacquemond-Collet I, Priem F, Sanchez C, Lambert C, Henrotin Y. Curcumin inhibits pro-inflammatory mediators and metalloproteinase-3 production by chondrocytes. Inflamm Res. 2009;58(12):899-908. doi: 10.1007/s00011-009-0063-1 [DOI] [PubMed] [Google Scholar]
- 60. Buhrmann C, Mobasheri A, Matis U, Shakibaei M. Curcumin mediated suppression of nuclear factor-κB promotes chondrogenic differentiation of mesenchymal stem cells in a high-density co-culture microenvironment. Arthritis Res Ther. 2010;12(4):R127. doi: 10.1186/ar3065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Zhang R, Zhang Q, Zou Z, Li Z, Jin M, An J, et al. Curcumin supplementation enhances bone marrow mesenchymal stem cells to promote the anabolism of articular chondrocytes and cartilage repair. Cell Transplant. 2021;30:096368972199377. doi: 10.1177/0963689721993776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Mahjoub M, Berenbaum F, Houard X. Why subchondral bone in osteoarthritis? the importance of the cartilage bone interface in osteoarthritis. Osteoporos Int. 2012;23(Suppl 8):S841-S846. doi: 10.1007/s00198-012-2161-0 [DOI] [PubMed] [Google Scholar]
- 63. Oh S, Kyung TW, Choi HS. Curcumin inhibits osteoclastogenesis by decreasing receptor activator of nuclear factor-κB ligand (RANKL) in bone marrow stromal cells. Mol Cells. 2008;26(5):486-9. [PubMed] [Google Scholar]
- 64. Chen Z, Xue J, Shen T, Ba G, Yu D, Fu Q. Curcumin alleviates glucocorticoid-induced osteoporosis by protecting osteoblasts from apoptosis in vivo and in vitro. Clin Exp Pharmacol Physiol. 2016;43(2_suppl):268-76. doi: 10.1111/1440-1681.12513 [DOI] [PubMed] [Google Scholar]
- 65. Aho OM, Finnilä M, Thevenot J, Saarakkala S, Lehenkari P. Subchondral bone histology and grading in osteoarthritis. PLoS One. 2017;12(3):e0173726. doi: 10.1371/journal.pone.0173726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Burr DB, Gallant MA. Bone remodelling in osteoarthritis. Nat Rev Rheumatol. 2012;8(11):665-73. doi: 10.1038/nrrheum.2012.130 [DOI] [PubMed] [Google Scholar]
- 67. van der Kraan PM, van den Berg WB. Osteophytes: relevance and biology. Osteoarthritis Cartilage. 2007;15(3):237-44. doi: 10.1016/j.joca.2006.11.006 [DOI] [PubMed] [Google Scholar]
- 68. Van Lent PLEM, Blom AB, Van Der Kraan P, Holthuysen AEM, Vitters E, van Rooijen N, et al. Crucial role of synovial lining macrophages in the promotion of transforming growth factor β-mediated osteophyte formation. Arthritis Rheum. 2004;50(1):103-11. doi: 10.1002/art.11422 [DOI] [PubMed] [Google Scholar]
- 69. Blom AB, van Lent PLEM, Holthuysen AEM, van der Kraan PM, Roth J, van Rooijen N, den Berg WB. Synovial lining macrophages mediate osteophyte formation during experimental osteoarthritis. Osteoarthritis Cartilage. 2004;12(8):627-35. doi: 10.1016/j.joca.2004.03.003 [DOI] [PubMed] [Google Scholar]
- 70. Wang Q, Ye C, Sun S, Li R, Shi X, Wang S, et al. Curcumin attenuates collagen-induced rat arthritis via anti-inflammatory and apoptotic effects. Int Immunopharmacol. 2019;72:292-300. doi: 10.1016/j.intimp.2019.04.027 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-pdf-1-car-10.1177_19476035211043202 for Intra-Articular Injections of Curcumin Monoglucuronide TBP1901 Suppresses Articular Cartilage Damage and Regulates Subchondral Bone Alteration in an Osteoarthritis Rat Model by Akihiro Nakahata, Akira Ito, Ryo Nakahara, Atsuhiro Kishimoto, Atsushi Imaizumi, Tadashi Hashimoto, Shogo Mukai, Yasuaki Nakagawa and Hiroshi Kuroki in CARTILAGE
Supplemental material, sj-pdf-2-car-10.1177_19476035211043202 for Intra-Articular Injections of Curcumin Monoglucuronide TBP1901 Suppresses Articular Cartilage Damage and Regulates Subchondral Bone Alteration in an Osteoarthritis Rat Model by Akihiro Nakahata, Akira Ito, Ryo Nakahara, Atsuhiro Kishimoto, Atsushi Imaizumi, Tadashi Hashimoto, Shogo Mukai, Yasuaki Nakagawa and Hiroshi Kuroki in CARTILAGE
Supplemental material, sj-pdf-3-car-10.1177_19476035211043202 for Intra-Articular Injections of Curcumin Monoglucuronide TBP1901 Suppresses Articular Cartilage Damage and Regulates Subchondral Bone Alteration in an Osteoarthritis Rat Model by Akihiro Nakahata, Akira Ito, Ryo Nakahara, Atsuhiro Kishimoto, Atsushi Imaizumi, Tadashi Hashimoto, Shogo Mukai, Yasuaki Nakagawa and Hiroshi Kuroki in CARTILAGE
This article is distributed under the terms of the Creative Commons Attribution 4.0 License (https://creativecommons.org/licenses/by/4.0/) which permits any use, reproduction and distribution of the work without further permission provided the original work is attributed as specified on the SAGE and Open Access pages (https://us.sagepub.com/en-us/nam/open-access-at-sage).
sj-pptx-1-car-10.1177_19476035211043202 for Intra-Articular Injections of Curcumin Monoglucuronide TBP1901 Suppresses Articular Cartilage Damage and Regulates Subchondral Bone Alteration in an Osteoarthritis Rat Model by Akihiro Nakahata, Akira Ito, Ryo Nakahara, Atsuhiro Kishimoto, Atsushi Imaizumi, Tadashi Hashimoto, Shogo Mukai, Yasuaki Nakagawa and Hiroshi Kuroki in CARTILAGE