Abstract
Objectives
Mesenchymal stem/stromal cells (MSCs) are a well-established cell source for cartilage engineering, but challenges remain as differentiation often results in chondrocyte hypertrophy. Chondrogenic potential also varies with MSC source and donor age. We assessed the chondrogenic potential of first-trimester and term placental MSCs and compared their response to commonly used bone marrow MSCs (BM-MSCs).
Design
MSCs were isolated from first-trimester and term placentae. BM-MSCs were commercially obtained. Chondrogenesis was induced by micromass culture in commercial chondrogenic media for 7, 14, or 21 days. Pellets were assessed for glycosaminoglycan (GAG) content, and types I, II, and X collagen. Gene expression was profiled using Qiagen RT2 human MSC arrays.
Results
At day 0, first-trimester and term MSCs expression levels of many chondrogenic genes to BM-MSC after 21 days of culture. Only first trimester MSCs showed significant changes in chondrogenic gene expression during induction compared to day 0 undifferentiated MSCs (greater BMP4, KAT2B, and reduced GDF6 expression). Additionally, first-trimester MSCs showed significantly greater expression of ABCB1 (at days 14 and 21) and BMP4 (at days 7, 14, 21) compared with term MSCs. Both first-trimester and term pellets showed increased GAG content over time and term MSCs had significantly GAG greater compared with BM-MSCs at days 7 and 14. Type II collagen was present in all pellets but unlike BM-MSCs, type I collagen was not observed in first-trimester or term MSC pellets.
Conclusions
These data highlight differences in BM-MSC and placental MSC chondrogenesis and demonstrate that placental MSCs may be an alternative cell source.
Keywords: term placenta, microarray, chondrogenesis, PCR array, glycosaminoglycans
Introduction
Articular cartilage is an avascular, aneural, connective tissue that lines the ends of bones within synovial joints such as the knee and hip, providing a smooth weightbearing surface to protect the underlying bone. Clinically, articular cartilage becomes problematic when it is damaged or diseased due to its extremely poor capacity to heal or repair. Mesenchymal stem/stromal cells (MSCs), known for their capacity to differentiate into fat, cartilage, or bone, are seen as an attractive cell type for cartilage regeneration. However, poor chondrocyte induction, dedifferentiation, and hypertrophy remain significant challenges in this area. 1
Protocols that induce in vitro differentiation of MSCs mimic the process of embryonic chondrogenesis and typically involve culture in exogenous growth factors such as transforming growth factor β (TGF-β1/β3), dexamethasone, and insulin for several weeks.2,3 This process induces the upregulation of chondrogenic transcription factor Sox9 followed by type II collagen and aggrecan.4,5 However, in current models there is also an upregulation of markers of osteogenesis (collagen type I alpha 1 chain, COLIA; RUNX family transcription factor 2, RUNX2) and hypertrophy/endochondral ossification (collagen type X alpha 1, COL10A1; alkaline phosphatase, ALP), which can lead to apoptosis and calcification.6,7 In particular, while bone marrow MSCs (BM-MSCs), can be differentiated into articular chondrocytes in 3-dimensional (3D) micromass cultures or artificial “scaffolds,” the resulting cartilage is transient and ultimately undergoes chondrocyte hypertrophy and mineralization similar to that observed during endochondral ossification.1,8 It is perhaps not surprising that BM-MSCs are particularly prone to becoming “bone-like” via this process, and this highlights the manner by which MSC differentiation can be highly influenced by cell source (e.g., adipose, bone marrow, placenta) and donor age.9-12
The placenta is a unique source of MSCs for regenerative medicine. The accessibility of this tissue makes it very suitable as a clinical source of MSCs. Furthermore, its fetal origin means that placental MSCs expand faster and are more plastic than their counterparts derived from adult tissues. 13 This makes placenta-derived MSCs a promising cell source for improving articular cartilage generation. MSCs derived from chorionic villi can generate cartilage-like tissue in vitro using atelocollagen gels with chondrogenic induction media. 14 Multiple studies have reported improved lineage specificity when culturing placental derived cells on decelluarized matrix15,16 and also demonstrated maintenance of a chondrocyte phenotype when cultured in collagen sponges or functionalized gel scaffolds containing integrin binding sequences.17,18 Importantly, MSCs derived from the decidua and chorionic membranes were converted to chondrocytes by transfection with chondrogenic transcription factors and produced cartilaginous tissue with no obvious histological evidence of endochondral ossification when implanted in nude mice for 7 weeks. 19 Despite these promising findings, few studies have directly compared the chondrogenic potential of placenta-derived MSCs with commonly used MSC sources in the field (bone marrow– or adipose-derived MSCs); they have also not investigated the underlying mechanisms that contribute to the successful generation of cartilage from placental MSC populations.
Placental MSCs obtained from earlier in gestation are translationally advantageous as they have faster growth kinetics and greater multipotency and immune-modulatory properties than those derived from term placentae or adult tissues, including the ability to influence macrophage M1/M2 polarization. 13 While not ethically suitable for clinical use, the plasticity of first trimester MSCs combined with their early embryonic origins suggests that these cells have great potential to improve our molecular understanding of how cells respond to chondrogenic cues. However, no studies to date have examined the role of first-trimester MSCs in cartilage generation. In order to understand how first-trimester, term, and bone marrow–derived MSCs may respond differently to chondrogenic cues, here we have evaluated differences in gene expression, protein expression, and morphology in 3D chondrogenic micromass cultures from each of these cell sources.
Methods
MSC Isolation and Culture
First-trimester placentae were collected from Auckland Medical Aid Centre following elective surgical termination of pregnancy from 6 to 12 weeks of gestation. For ethical reasons, first-trimester tissues are donated anonymously to the research team, but clinic staff screen all donors to ensure that samples with gross chromosomal or anatomical abnormalities are excluded. Term placentae from normal healthy deliveries were collected from Auckland City Hospital. The collection and use of both first-trimester and term placentae for this project was approved by the Northern Regional Health and Disability Ethics Committee (reference number NTX/12/06/057/AM06).
MSCs were isolated in a manner adapted from that previously described. 20 For first-trimester placentae, tissue was washed in phosphate-buffered saline (PBS) until free of blood. Membranes were dissected away from the villous tissue, which was then dissected into small explants of approximately 200 mg weight. For term placentae, the superficial decidual layer (approximately 2-mm thick) was dissected away before explants of approximately 1 cm3 (250 mg wet weight) were dissected from at least 6 regions of the placenta, generating a total of 12 to 20 explants. Explants were agitated with forceps and washed extensively with PBS until no detectable blood was present and separated villi were evident. Once villous explants had been prepared the same methodology was used to isolate MSCs from both first-trimester and term placentae. To do this, the outer trophoblast layers of the placentae were removed by digesting the explants in 10 mL of PBS containing 0.25% trypsin-EDTA (trypsin–ethylenediamine tetraacetic acid) (Invitrogen, Auckland) and 5 mg/mL DNase (Sigma, USA) per gram of placental tissue in a 37 °C water bath for 10 minutes. Villous explants were then washed approximately 10 times with PBS until no visible debris was present in the supernatant. A further 10 mL of PBS containing 0.25% trypsin-EDTA and 5 mg/mL DNase was added per gram of tissue, and tubes were incubated on a rocker for 7 minutes at 4 °C and then incubated stationary at 4 °C overnight.
The following morning, explants were washed as above, until the supernatant appeared free of visible debris. Denuded explants were then cultured in 12-well plates (BD Falcon, Australia) in MSC culture medium (advanced Dulbecco’s modified Eagle medium: Nutrient Mixture F12 (Advanced DMEM-F12, Life Technologies Ltd., New Zealand) containing 1% penicillin-streptomycin (Invitrogen, New Zealand), 1% Glutamax (Life Technologies), and 2% MSC-qualified fetal bovine serum (MSC-FBS) (Life Technologies) at 37 °C in a humidified 5% CO2 environment. Media was replaced completely every 2 days. MSCs were visible as clusters of plastic-adherent cells within 1 week of culture. Once cell clusters reached confluence (after approximately 2-3 weeks), they were passaged with TrypLE and cultured in a 25 cm2 flask (BD Falcon). Placental MSCs were used up to passage 7.
StemPro bone marrow–derived MSC (BM-MSC) at passage 4 were purchased from Life Technologies. BM-MSC were initially cultured on CELLStart substrate (Life Technologies) according to the manufacturer’s instructions, and then expanded on plastic and maintained in StemPro CTS MSC serum-free media (Life Technologies,). Media was changed every 2 to 3 days, and cells were cultured up to passage 8.
Multicolor Flow Cytometry
In order to ensure that isolated placental cells had a MSC phenotype, all cultures used in this study were profiled by multicolor flow cytometry for the expression of six cell surface proteins (3 positive markers; CD73, CD90, CD105 and 3 markers of contaminating cells; CD45, CD31, CD34) as defined by the International Society for Cellular Therapy as minimal criteria for characterizing MSC. 21 Placental MSCs (passage 4-6) were dissociated with TrypLE and centrifuged at 250 × g for 7 minutes. Fluorophore-conjugated antibodies ( Table 1 ) were added to 50 µL of a 2 × 106 cells/mL suspension in MSC media and incubated on ice for 30 minutes. Cells were washed with 1 mL of FACS buffer (PBS containing 1% HT-FBS) and centrifuged at 220 × g for 5 minutes at 4 °C. The wash was repeated and cells were resuspended in FACS buffer for analysis. Two microliters of DAPI were added to each FACS tube to distinguish between live and dead cells. Single stain compensation controls for were run simultaneously using the same protocol to establish fluorescence baselines for each of the fluorophores used. For CD73, CD90, and CD105, these compensation controls were performed on MSCs as described above. For CD45, CD34, or CD31, 50 µL of Compbeads Plus Anti-Mouse IgG beads (BD Biosciences, New Zealand) were used. A CD31 APCCy-7 fluorescence minus one (FMO) control was run to ensure to established gates. Unstained controls were run simultaneously with stained samples to determine the auto-fluorescence intensity of the placental MSCs. Cells were analyzed on a BD FACS Aria II Flow Cytometer (BD Biosciences). Data were analyzed using FlowJo v.10.1 software (BD Biosciences). Doublets and dead cells were gated out to ensure only single live cells were used for analysis.
Table 1.
Fluorophore-Conjugated Antibodies Used in the Multicolor Flow Cytometry Panel.
| Antibody | Manufacturer | Catalogue Number | Working Concentration (µg/mL) |
|---|---|---|---|
| CD73-PE | BD Biosciences | 344004 | 10 |
| CD90-Alexa Fluor700 | Bio Legend | 328120 | 10 |
| CD105-APC | BD Biosciences | 323208 | 2.5 |
| CD45-BUV395 | BD Biosciences | 563792 | 1.25 |
| CD34-BV510 | BD Biosciences | 343528 | 10 |
| CD31- APC/Cy7 | BD Biosciences | 303120 | 1.25 |
Adipogenic Differentiation
Adipogenic staining was performed as described previously. 22 First-trimester placental MSC (n = 3, passage 3-4), or adipose-derived MSC (n = 3, passage 2-4) obtained by plastic adherence of the stromal vascular fraction derived from lipoaspirate were plated at 5 × 103 cells/well in 8 wells of a Nunclon 96-well flat bottom plate (Life Technologies) in 200 μL of adipose stem cell (ASC) media (DMEM/F12 media [Life Technologies] supplemented with 10% FBS, 1% GlutaMax, and 1% penicillin/streptomycin) and cultured at 37 °C with 5% CO2. On day 4, 100 μL of media in 4 wells was replaced with adipogenic differentiation media (ASC media with 1 µM dexamethasome, 10 µM insulin, 0.5 mM 3-isobutyl-1-methylxanthine [IBMX] and 200 µM indomethacin [Sigma Aldrich, Auckland]) and standard ASC media was added to 4 negative control wells. Fifty percent of the media was removed and replenished with an equal volume of fresh media every 3 days until day 14. Cells were then fixed with ice-cold methanol for 5 minutes, washed once with 1 × Tris buffered saline (TBS), and blocked with 0.25% casein supplemented with 10% human serum for 10 minutes to reduce nonspecific staining. After a second wash, cells were incubated with a 1:200 dilution of rabbit anti-human FABP4 antibody (Cat #10004944, Cayman Chemicals, MI, USA) in dilution buffer (10% human serum in TBS) for 1 hour. Two wells (one exposed to adipogenic differentiation media and one control media well) were incubated with dilution buffer to serve as secondary antibody only controls. Cells were washed 3 times for 5 minutes then incubated with a 1:200 dilution AlexaFluor-488 conjugated goat anti-rabbit IgG secondary antibody (Cat#A11008, Molecular Probes, New Zealand) and 1:2000 diluted DAPI (Molecular Probes) in dilution buffer for 1 hour in the dark. Excess antibody was removed with two 15-minute TBS washes. Fluorescent images were captured using the ImageXpress Micro XLS high content screening system (Molecular Devices, USA). Nine images were taken per well at 10× magnification and quantitative data were generated using the MetaXpress v5.3.0.1 (Molecular Devices) software.
Osteogenic Differentiation
For osteogenic differentiation, first-trimester MSCs (n = 5, passage 4-6) were seeded in triplicate at 1000 cells/well in 96-well plates, and cultured for 28 days using a StemPro Osteogenic Differentiation Kit (ThermoFisher Scientific, New Zealand) according to the manufacturer’s instructions. Duplicate control wells for each placental replicate were cultured in MSC basal culture medium. Following culture, cells were fixed in 4% paraformaldehyde in PBS for 30 minutes at 37 °C, washed with distilled water, and then stained with 2% alizarin red S (pH 4.2) (Sigma Aldrich) for 3 minutes. Wells were washed 3 times with distilled water and visualized on a Nikon Eclipse Ti inverted microscope (Nikon, Japan).
Chondrogenic Differentiation Assays
In order to differentiate MSC down the chondrogenic pathway, a StemPro Chondrogenic Differentiation kit (Life Technologies, Auckland) was used according to the manufacturer’s instructions. Briefly, cells were removed from culture flasks with TrypLE express, and resuspended in chondrogenic differentiation media (Life Technologies) at a concentration of 250,000 cells/mL. Each chondrogenic pellet was generated by placing 1 mL of this cell solution in a 15-mL tube (BD Falcon), followed by centrifugation at 400 × g for 5 minutes. Tubes were transferred directly to a humidified 37 °C ambient oxygen incubator containing 5% CO2. After 24 hours, pellets were floated by complete media replacement. Chondrogenic pellets were cultured for 7, 14, or 21 days, with media replaced thrice weekly for the duration of the culture period.
Real-Time PCR Arrays
Qiagen RT2 Profiler Arrays (Bio-Strategy, New Zealand) were used to compare the expression of 84 genes between first-trimester MSCs, term MSCs, and BM-MSCs from 0 to 28 days of chondrogenic culture. A Machery-Nagel Nucleospin RNA XS kit (MediRay, New Zealand) was used according to the manufacturer’s instructions to extract RNA from first-trimester MSCs (n = 4 donors, passage 6), term MSCs (n = 3 donors, passage 6), and BM-MSCs (n = 3 different cultures of commercially acquired cells, passage 8) immediately after removal from culture flasks, as well as pellets generated from each of these MSC preparations that had undergone chondrogenic differentiation for 7, 14, or 21 days. To ensure adequate RNA extraction, 2 chondrogenic pellets from each time point were pooled, and pellets were crushed with a pipette tip during the first lysis buffer step. RNA quality and quantity was determined on an Agilent Bioanalyzer using an Agilent RNA 6000 Pico Kit (Bio-Strategy, NZ). cDNA was transcribed and amplified using a Qiagen RT2 PreAmp cDNA synthesis kit (Bio-Strategy, New Zealand). Quantitative real-time polymerase chain reaction (PCR) of amplified cDNA was undertaken using Qiagen RT2 Profiler Human Mesenchymal Stem Cell Arrays (Bio-Strategy, New Zealand). All assays were conducted according to the manufacturer’s instructions unless otherwise stated. Gene expression was normalized to the geometric mean of 4 housekeeping genes (glyceraldehyde-3-phosphate dehydrogenase, GAPDH; beta-2 microglobulin, B2M; hypoxanthine phosphoribosyltransferase 1, HPRT1; ribosomal protein large P0, RPLPO).
Pellet Histology
Pellets (days 7, 14, 21) from the 3 cell sources were fixed in 4% paraformaldehyde at 37 °C for 1 hour and washed 3 times for 5 minutes in PBS. Pellets underwent standard tissue processing, paraffin embedding, and were sectioned at 10-µm thickness and stained using hematoxylin and eosin, Alcian blue, or toluidine blue. 8
Pellet Immunohistochemistry
Sections (10 µm) from day 21 paraffin-embedded pellets were dewaxed, incubated in testicular hyaluronidase (2 mg/mL) and pronase (0.1% w/v) for 1 hour at 37 °C, then rinsed in distilled water prior to labeling with either mouse monoclonal type I collagen (1:500 clone AB6308, Abcam, UK), type IIa collagen (DHSB hybridoma product II-II6B3, deposited by Linsenmayer, T.F.), or type X collagen (1:200; clone X53, eBioscience, USA) for 2 hours at room temperature, then detected using the Novolink Polymer Detection System Novacastra (Leica Biosystems Inc, USA), according to the manufacturer instructions. Sections were counterstained with hematoxylin, dehydrated, and mounted in DPX.
Glycosaminoglycan Content
Following culture, supernatants were removed, and pellets were digested for the extraction of cartilage extracellular matrix (ECM) (glycosaminoglycans [GAG]) by lysis in 1 mL of papain buffer (150 mM NaCl, 55 mM sodium citrate, 5 mM EDTA, 5 mM cysteine hydrochloride, and 0.6 units/mL papain in PBS) for 12 hours at 60 °C, with tubes vortexed every 2 hours. DNA was extracted from digested pellets by isopropanol precipitation and quantified using a SYBR Green (Life Technologies, New Zealand) assay as previously described. 23 Calf thymus DNA (Sigma-Aldrich) was used to generate a standard curve. The 1,9-dimethylmethylene blue (DMMB) dye binding assay was used to measure total sulfated glycosaminoglycan content as previously described. 24 Bovine chondroitin sulfate (Sigma-Aldrich) was used to generate a standard curve. GAG content within each pellet was normalized to DNA content in the same sample using the SYBR Green assay.
Statistical Analysis
A 1-way analysis of variance (ANOVA) followed by Holm-Sidak’s posttest was used to compare differences in gene expression between cell populations and timepoints using Prism GraphPad software (version 8.0). GAG/DNA differences were assessed using a 1-way ANOVA and Tukey’s post hoc analysis to compare between cell types and culture duration (independent factors). P values <0.05 were considered statistically significant.
Results
Phenotypic Characterization of Isolated Placental MSCs
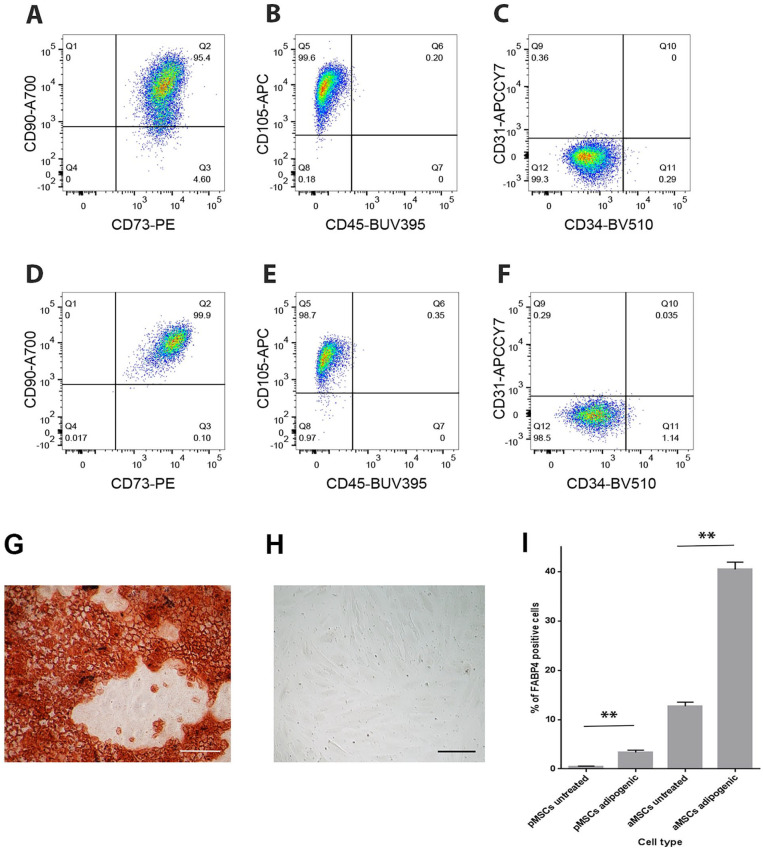
All placental first-trimester (n = 4) and term (n = 3) MSC isolates exhibited a typical MSC phenotype, being predominantly positive for the MSC markers CD73, CD90, and CD105, and predominantly negative for CD31, CD34, and CD45 ( Fig. 1 ). Placental MSC isolates from both first-trimester and term placentae were evident as a single homogenously stained population on flow cytometry dot plots ( Fig. 1A-F ).
Figure 1.
Representative flow cytometry dot plots from first trimester (A, B, C) and term (D, E, F) placental mesenchymal stem/stromal cells (MSCs) demonstrating the fluorescence intensity of CD90-A700/CD73/PE (A, D), CD105-APC/CD45-BUV395 (B, E), or CD31-APCCY7/CD34-BV510 (C, F) antibodies run as a multicolor flow cytometry panel. Alizarin red S staining of first-trimester MSCs following osteogenic induction (G) or after maintenance in an undifferentiated state in control conditions (H). (I) Mean (±SEM) percentage (%) FAPB4-positive cells in first trimester MSCs and adipose-derived MSCs in control or pro-adipogenic culture.
While chondrogenic differentiation was the focus of this work, we also confirmed the multipotency of isolated MSCs by inducing osteogenic and adipogenic differentiation. Exposure of isolated first-trimester MSCs to osteogenic differentiation medium for 28 days resulted in strong alizarin red S staining in all cultures (n = 5 isolates assayed in triplicate) demonstrating calcium sulfate deposition indicative of osteogenic differentiation ( Fig. 1G ). No staining was demonstrated in isolated MSCs cultured in control media ( Fig. 1H ).
First-trimester placental MSCs exposed to adipogenic media significantly upregulated the proportion of FABP4-positive adipocytes present in the culture in comparison with untreated controls (3.34% vs. 0.49%, n = 3 cell isolates assayed in triplicate, P < 0.001, Fig. 1I ). BM-MSCs exhibited similar levels of adipogenesis to both first-trimester and term placental MSCs (data not shown). The total level of adipogenic differentiation in placental MSC cultures was lower compared with the levels of adipogenic differentiation observed in cultures of adipose-derived MSCs ( Fig. 1I ). However, the fold increase in the proportion of adipocytes in cultures exposed to adipogenic media compared with untreated cultures was greater in placental MSCs (6.8-fold) than for adipose-derived MSCs (3.2-fold). Taken together, these data demonstrate that placental MSCs can undergo adipogenic differentiation, but to a lesser degree than adipose-derived MSC populations.
First-Trimester and Term Placenta-Derived MSCs Have Different Gene Expression Profiles in Chondrogenic Culture
In order to determine the molecular mechanisms by which placental MSCs are more chondrogenic, the gene expression profile of pellets generated from first-trimester, term, or BM-MSCs was examined after 0 (flask cultures), 7, 14, or 21 days of chondrogenic culture using Qiagen Mesenchymal Stem Cell RT2 Profiler PCR Arrays. RNA used in the RT2 Profiler PCR Arrays had an mean RIN value of 8.94 (±0.20, n = 11).
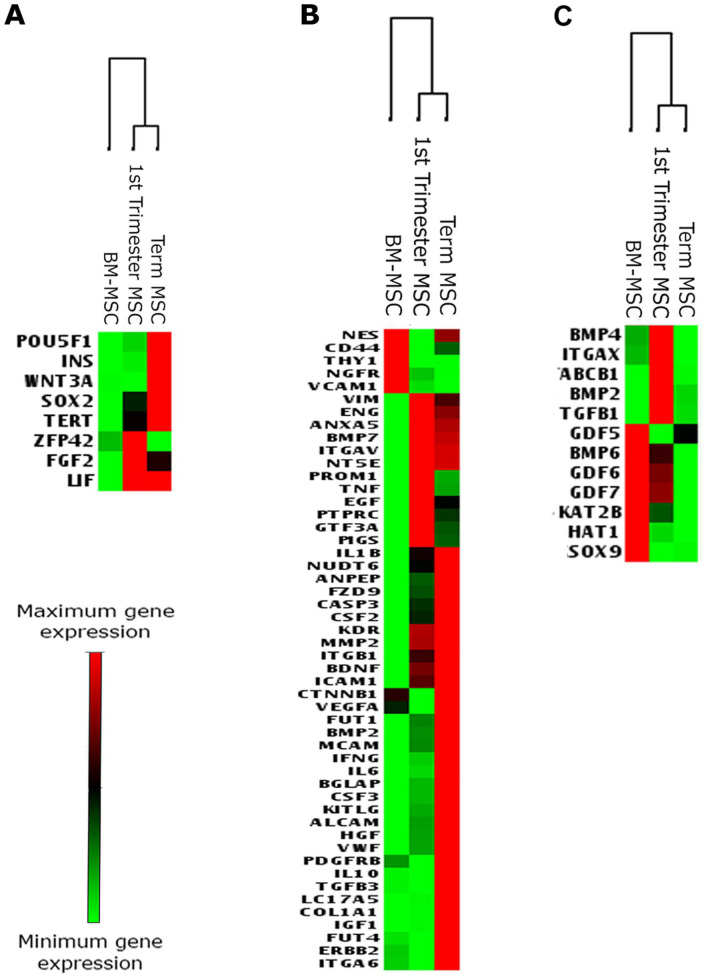
First, we assessed basal differences in gene expression in undifferentiated MSCs used as starting material for our chondrogenic assays (day 0 flask cultures). Clustering analysis of pluripotency and MSC-associated gene expression revealed that globally first-trimester and term MSCs have a different gene expression profile from BM-MSCs ( Fig. 2A and B ). However, when first-trimester MSCs were compared with BM-MSCs, only one gene (phosphatidylinositol glycan anchor biosynthesis class S, PIGS) was significantly upregulated, and no genes were significantly downregulated (Supplementary Table 1). Similarly, when term MSCs were compared with BM-MSCs, four genes (endoglin, ENG; fibroblast growth factor 2, FGF2; fucosyltransferase 1, FUT1; KIT ligand, KITLG) were significantly upregulated, and no genes were significantly downregulated (Supplementary Table 1). There were no statistically significant differences in gene expression between first trimester and term MSCs in any of the genes examined (Supplementary Table 1).
Figure 2.
Heat maps generated from Qiagen RT2 Profiler Array showing relative expression of genes associated with (A) pluripotency, (B) mesenchymal stem/stromal cells (MSCs) at day 0 of culture, and (C) genes associated with early chondrogenesis in pellets after 21 days of chondrogenic culture. Heat maps were generated using data from 4 individual first-trimester donors, 3 individual term donors, and from 3 different cultures of commercially acquired bone marrow (BM) MSCs.
The Effect of Gestational Age on Placental MSC Chondrogenic Gene Expression
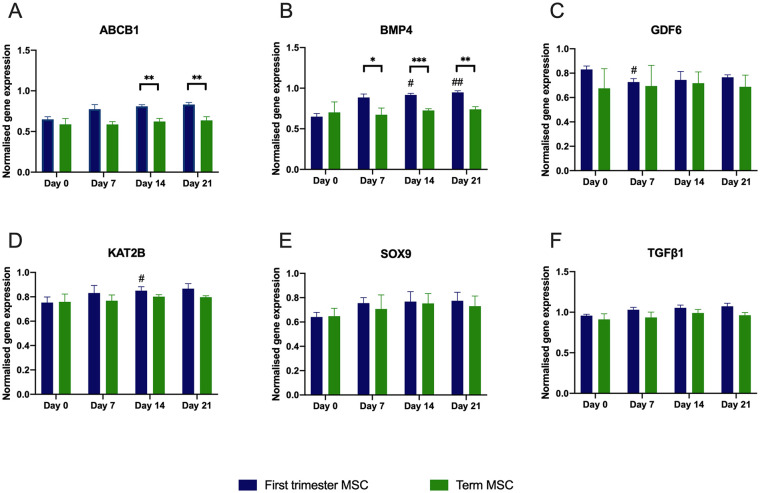
In order to understand how gestational age may affect the chondrogenic potential of placental MSCs, we first examined the expression of chondrogenesis-associated genes during chondrogenic induction within each placental MSC type ( Figs. 2C and 3 ). Only first-trimester MSCs showed significant changes in gene expression compared with day 0. Bone morphogenetic protein 4 (BMP4) (P < 0.05 at day 14 and P < 0.01 at day 21) and lysine acetyltransferase 2B (KAT2B; P < 0.05 at day 14) significantly increased, whereas growth differentiation factor 6 (GDF6) expression decreased at day 7 compared with day 0 (P < 0.05), as shown in Figure 3 . No significant changes in gene expression were observed in SRY-box transcription factor 9 (SOX9) or transforming growth factor beta 1 (TGFB1). As chondrogenesis progressed, term MSC pellets showed significantly greater expression than first-trimester MSC pellets for ABCB1 on days 14 and 21 (P < 0.01 for both, Fig. 3A ). In contrast, first-trimester MSC pellets had increased expression of BMP4 on day 7 (P < 0.05), day 14 (P < 0.001), and day 21 (P < 0.01) compared with term MSC pellets ( Fig. 3B ).
Figure 3.
Expression of ABCB1, BMP4, BMP2, KAT2B, GDF5, and SOX9 genes in cells in first-trimester or term MSCs pellets cultured over 21 days in chondrogenic conditions. Data are obtained from real-time polymerase chain reaction (PCR) arrays (Qiagen RT2 Profiler Array). # represents a significant increase in chondrogenic gene expression with time in first-trimester MSCs. ## indicates a significant difference within each cell type. Asterisks indicate significant differences between first-trimester and term MSCs with *P < 0.05, **P < 0.01, and ***P < 0.001.
Placental and Bone Marrow MSCs Exhibit Similar Gene Expression during Chondrogenesis
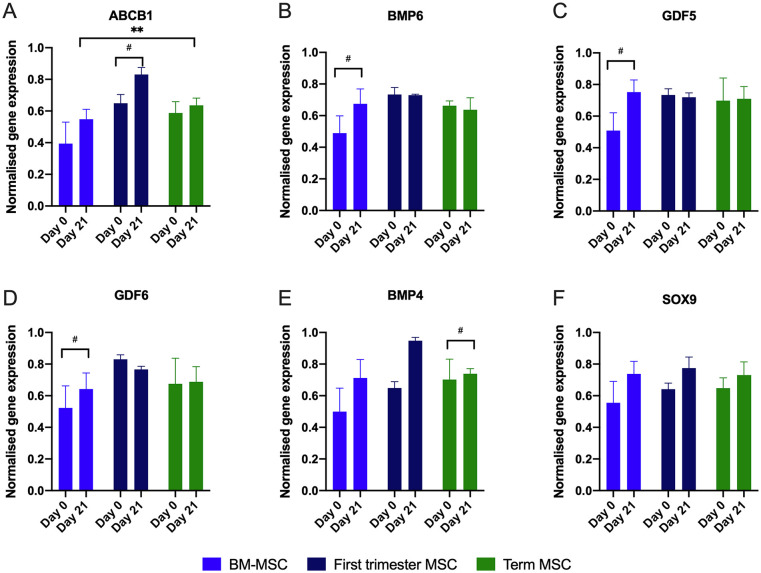
Chondrogenic gene expression in placental-derived MSC pellets was not significantly different to that of BM-MSCs after 21 days, with the exception of the ABCB1 gene, which was expressed at significantly greater levels at day 21 in first-trimester MSCs compared with BM-MSCs and term MSCs ( Fig. 4 ). When expression of chondrogenic genes was compared in all 3 MSC sources at day 0 and day 21 of culture, several genes (ABCB1, BMP4, GDF5, GDF6, GDF7) in undifferentiated first-trimester and term MSCs were equivalent to that in BM-MSC chondrogenic pellets after 21 days of chondrogenic induction. This high level of basal gene expression was further upregulated for first-trimester MSCs ( Fig. 4B , C , and E ). Expression levels of SOX9, a transcription factor essential for chondrogenesis, which is also a key factor for maintaining chondrocyte phenotype in adult articular cartilage were very similar across the 3 cell types after 21 days and no significant increase in expression compared with undifferentiated cells in all MSC groups over the 21-day culture period, although the mean expression was greater at day 21 compared with day 1 ( Fig. 4D ).
Figure 4.
Gene expression obtained from real-time polymerase chain reaction (PCR) arrays (Qiagen RT2 Profiler Array) in bone marrow mesenchymal stem/stromal cells (BM-MSCs), first-trimester MSCs, or term MSCs at day 0, or following 21 days of chondrogenic pellet culture. For several genes (A, B, C, E), basal levels of chondrogenic gene expression in first-trimester MSCs were similar to day 21 BM-MSCs pellets. # indicates a significant difference comparing changes within each cell type. *P < 0.05 and **P < 0.01 indicate significant differences in gene expression between the 3 difference sources of MSCs.
Differentiated Placenta-Derived MSCs Express Chondrogenic Markers
Histological Characterization
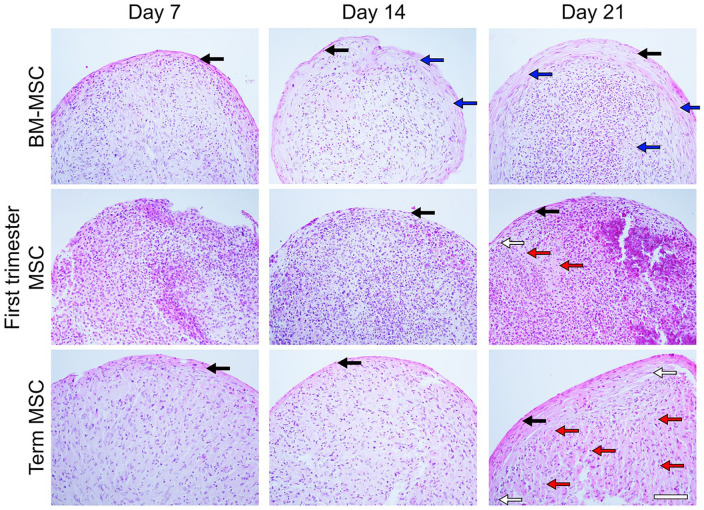
In order to understand how placental MSCs and BM-MSCs may respond differently to chondrogenic cues, we induced chondrogenesis in 3D micromass cultures, and evaluated chondrogenesis across a 21-day time course. First, pellet morphology was qualitatively assessed histologically by hematoxylin and eosin staining ( Fig. 5 ). All pellet cultures formed a layer of fibroblast-like cells around the periphery of pellets, marked by spindle-shaped cell soma with flat, oval nuclei, with minimal deposition of ECM. This perichondrium-like structure was between 1 and 3 cell layers thick in day-7 cultures and thickened with culture length. In term MSC and BM-MSC cultures the increase in cell layers was accompanied by ECM deposition. In BM-MSC cultures, a loose fibrous ECM was observed which aligned with cell nuclei, characteristic of the formation of fibrocartilage, while term MSC cultures showed more densely staining ECM. In first-trimester MSC cultures, the structure remained highly cellular, with no significant ECM deposition. Cells with large ovoid nuclei running parallel to the perichondrium-like structure were observed; and these cells were more frequent in both placental MSC cultures than in BM-MSC cultures.
Figure 5.
Pellet histology. Representative images of hematoxylin and eosin–stained sections of bone marrow mesenchymal stem/stromal cell (BM-MSC), first-trimester MSC, and term MSC pellet cultures (n = 2-5 per cell source). Fibroblast-like cells (black arrow; spindle cell soma with flat, oval nuclei) formed a structure around the periphery of pellets. Superficially, chondroblast-like cells (white arrow) are parallel to the apical structure. Fibrocartilage-like structures (blue arrow) and hyaline cartilage-like structures with lacunae forming around chondrocytes (red arrow). Scale bar represents 200 µm.
By day 21, the fibrocartilage-like structures observed in BM-MSC micromass cultures were also present toward the pellet center ( Fig. 5 ). Here, some cells appeared flattened and aligned with ECM fibers; however cells with large round cell soma and rounded nuclei, characteristic of chondrocytes, were also observed in areas of high ECM deposition. In contrast, no deposition of fibrous ECM was observed in either of the placental MSC pellet cultures. Instead, in these cultures ECM deposition more closely resembled hyaline cartilage; here the ECM has an amorphous, translucent appearance with the formation of lacunae-like structures around chondrocytes ( Fig. 5 ). Term placental MSC pellets showed uniform formation of hyaline cartilage. In contrast, first trimester cultures are more cellular at all time points compared with both term MSC and BM-MSC cultures, with highly heterogenous cell populations. The pockets of hyaline cartilage observed here vary in the level of cellularity between regions, and some regions of the pellet show no ECM deposition ( Figs. 5 and 6A ) with cell populations that show large round nuclei with low nuclei to cytoplasm ratios and are not characteristic of either chondroblasts or fibroblasts.
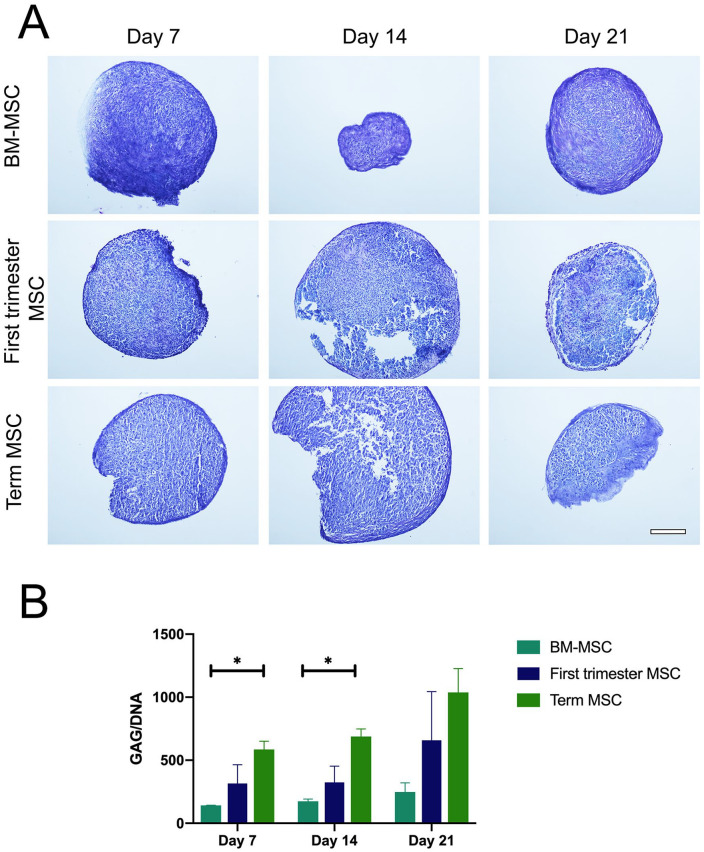
Figure 6.
Glycosaminoglycan (GAG) distribution and quantification. (A) Representative images of toluidine blue stained sections of bone marrow mesenchymal stem/stromal cell (BM-MSC), first-trimester MSC, and term MSC pellets on days 7, 14, and 21 of culture, demonstrating the deposition of GAG with most intense staining present in BM-MSC derived pellets. Scale bar represents 200 µm. (B) Mean (± SEM) GAG (µg) content normalized to DNA (ng) in BM-MSC, first-trimester MSC, and term MSC pellets on days 7, 14, and 21 of culture. Bars represent the mean of 2 pellets from each of 2 to 4 donors per cell type. *P < 0.05 between BM-MSC and term MSC on days 7 and 14.
Glycosaminoglycan Content
Toluidine blue staining of cell pellets demonstrated that cartilage formation was morphologically evident from all 3 cell sources (first trimester n = 4, term n = 4, and BM-MSC n = 2; Fig. 6A ). Toluidine blue staining of BM-MSC pellets appeared much darker and pellets in section appeared smaller and more compact compared with both first-trimester and term MSC pellets; although wet weight samples were not recorded to verify this. Pellets were also stained with Alcian blue (Supplementary Figure 1), and showed a similar staining pattern as Figure 6A . Quantitative assessment of GAG demonstrated that all cell types increased GAG production over the 21-day culture period. Term MSC showed a significant greater GAG content normalized to DNA content compared with BM-MSC pellets at days 7 and 14 of culture (P < 0.05; Fig. 6B ). Although this trend is evident at day 21, there were no significant differences in GAG content between cell types.
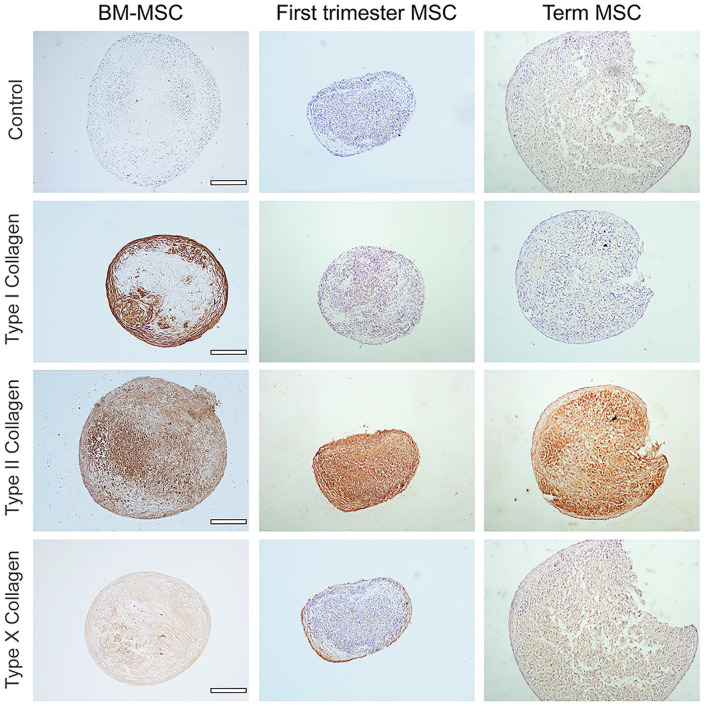
Collagen Deposition
The expression of collagens associated with hyaline and hypertrophic cartilage were also assessed in first-trimester (n = 3), term (n = 3), and BM-MSC (n = 2) derived pellets that were stained with antibodies reactive with types I, II, or X collagen. After 21 days of culture, all pellets expressed type II collagen in a diffuse distribution throughout the cell pellet ( Fig. 7 ), and there was limited or no evidence of the hypertrophy marker type X collagen in any of the pellets. However, consistent with hematoxylin and eosin staining, there was a marked absence or little staining of type I collagen in both first-trimester and term MSC derived pellets compared with a marked pattern of type I collagen staining on the outer edge of the BM-MSC derived pellets. No staining was observed in negative control sections incubated with relevant immunoglobulin G (IgG) antibodies.
Figure 7.
Collagen distribution at day 21 of chondrogenic culture. Representative immunohistochemical detection of types I, II, and X collagen in sections of bone marrow mesenchymal stem/stromal cell (BM-MSC), first-trimester MSC, and term MSC pellet cultures at day 21 of chondrogenic culture. Scale bars represent 200 µm. Controls were treated with immunoglobulin G (IgG) isotype matched antibodies.
Discussion
The placental mesenchyme arises from the somatic mesoderm of the embryo at around 4 weeks of gestation, and consequently MSCs can be isolated from first-trimester placentae only a short time later (6-12 weeks of gestation) and exhibit a relatively plastic phenotype.13,25 In this work, we sought to assess the chondrogenic potential of first-trimester MSCs in order to determine whether the early embryonic nature of these cells may provide a unique in vitro model to better understand and recapitulate the developmental pathways involved in generating articular cartilage. Placental and BM-MSCs exhibited different global gene expression profiles prior to and during chondrogenic culture but endpoint structural analysis showed that cells from all sources formed toluidine blue–positive cell pellets that were GAG rich, expressed type II collagen, and did not strongly express the hypertrophic maker, type X collagen. However, placental MSCs did not express fibrocartilage marker type I collagen. Together, these data highlight important differences between BM-MSC chondrogenesis and placental MSC chondrogenesis and demonstrate that placental MSCs may be a superior cell source for therapeutic cartilage generation.
The source of MSCs is known to influence their differentiation capacity. 10 Therefore, to fully interpret our chondrogenic differentiation data, it was important to first consider the state of the undifferentiated MSCs being used as starting material. The data in this article point to several key differences between undifferentiated placental MSCs and BM-MSCs that may have important implications for chondrogenic differentiation.
The first key difference was that our 3 starting MSC populations had different global profiles of stromal/stem cell–related gene expression. Placental MSCs had a global tendency to express greater levels of pluripotency-associated genes than BM-MSCs, and also expressed a very different profile of MSC related genes to BM-MSCs. This supports previous reports that placental MSCs are more plastic than adult MSC populations. 26 When first-trimester and term MSC populations were directly compared, no statistical differences in basal (day 0) gene expression were observed; however, the global patterns of MSC-associated gene expression differed considerably between these populations. This has the potential to contribute to the previously reported increased plasticity in first-trimester MSCs in comparison with term MSCs. 13 Primary placental cell isolates are notoriously heterogeneous, and it is possible that future studies using increased biological replicates may be able to confirm the potential differences highlighted here in a statistically significant manner.
The second key difference between MSC populations was that both first-trimester and term MSCs expressed high basal levels of several early chondrogenesis-associated genes (ABCB1, SOX-9, BMP4, GDF5, GDF6, and GDF7), which were comparable to the levels seen in BM-MSCs that had undergone 21 days of chondrogenic micromass culture. In chondrogenic culture, expression of the chondrogenesis-associated genes ABCB1 and BMP4, showed a significant increase over the culture period in first-trimester MSCs, but not term MSCs, supporting the notion that first-trimester MSCs have a greater response to chondrogenic cues than their term counterparts. Understanding the molecular mechanisms that underlie the more rapid differentiation kinetics of first-trimester MSCs may allow us to streamline the production of cartilage for transplantation in the clinic in the future.
Chondrogenic potential varies considerably based on induction protocols used, as well as the source of MSC.1,27 For example, TGFβ receptor expression is crucial for chondrogenesis in adipose-derived MSCs; however, despite showing TGFΒ1 gene expression during chondrogenesis, there are no current studies reporting TGFβ receptor expression in undifferentiated placental MSC populations.28,29 Although BM-MSCs are the most commonly studied MSC, we have shown BM-MSCs to have reduced chondrogenic capacity compared with both placental cell sources, in terms of proteoglycan staining measured by both toluidine blue and Alcian blue staining, and greater GAG content over the 21-day culture period. In particular, term MSCs produced significantly greater GAG than BM-MSCs at day 14 (with a nonsignificant trend persistent at 21 days). These results are similar to those observed with other perinatal-derived MSC sources such as amnion and umbilical cord derived cells, suggesting that donor age is an important factor for a robust chondrogenic response. 30
A simultaneous advantage and limitation of this study was the use of a commercial proprietary chondrogenesis induction kit from Invitrogen. The advantage of using this kit, guided by the protocol published by Solchaga et al. 8 is that it will allow future studies to benchmark chondrogenesis of novel sources of MSCs using identical protocols and standardized kits; an important contribution to aid in the reproducibility of studies. However, the limitation is that we do not know what specific agents were included as media supplements. One may assume that growth factors such as TGFβ would be included as well as dexamethasone but further studies are essential to examine the role of individual growth factors such as bone morphogenetic protein (BMPs) or TGFβ1 versus TGFβ3 would have in enhancing induction, 31 particularly in light of our data that show induction-associated BMP gene expression changes in the placental MSCs.
The undesirable production of type I collagen as well as type X collagen has been one of the major challenges that limit MSC-based hyaline cartilage regeneration approaches in which a wide range of differentiation protocols yield more fibrocartilage-like tissues. 1 In this study, one notable difference was the absence of type I collagen labeling at the pellet periphery in both types of placental MSC pellets at day 21, despite no significant differences in COL1A1 gene expression between groups at any time point. This was a striking difference compared to the BM-MSC pellets that demonstrated dense and intense type I collagen labeling. It is interesting to note that proteoglycan toluidine blue staining was much darker in BM-MSC pellets and that these pellets appeared more structurally compact, whereas placental MSC pellet section integrity was poor. Although wet weight and pellet diameters were not measured in this study, the increased GAG content and apparent size of the placental MSC pellets could be a result of unconfined pellet swelling due to reduced type I collagen confinement as observed in the BM-MSC pellets. The lack of confinement in the placental MSC pellets may have contributed to poor pellet integrity and mechanical testing of these pellets would be an important further consideration. More work is required to test the GAG production of placental MSCs within scaffolds that are both chondro-inducive and provide a degree of swelling confinement similar to native tissue. All MSC pellets demonstrated consistent distribution of type II collagen and minimal type X collagen. Term MSCs have previously shown to downregulate type X collagen on chondrogenesis, 5 and the data in our study confirm this despite patient cell source variability.
In conclusion, using standard commercial differentiation protocols, solutions, and BM-MSCs, we have shown than MSCs derived from both first-trimester and term pregnancies are strongly chondrogenic. Even though first-trimester MSCs showed greater early chondrogenic gene response following induction, term MSCs showed greater GAG production and reduced type I collagen compared with both first trimester MSCs and BM-MSCs. These findings suggest that placental MSCs derived from earlier in development do not provide any additional advantage in terms of cartilage production, but that MSCs across gestation provide a novel cell source that could potentially minimize the production of fibrocartilage tissue for joint resurfacing and repair.
Supplemental Material
Supplemental material, sj-pdf-1-car-10.1177_19476035211044822 for The Chondrogenic Potential of First-Trimester and Term Placental Mesenchymal Stem/Stromal Cells by Joanna L. James, Anandita Umapathy, Sonia Srinivasan, Claire N. Barker, Anna Brooks, James Hearn, Ashika Chhana, Eloise Williams, Hilary Sheppard and Sue R. McGlashan in CARTILAGE
Footnotes
Supplementary material for this article is available on the Cartilage website at http://cart.sagepub.com/supplemental.
Acknowledgments and Funding: The antibody against type II collagen (II-II6B3), developed by T.F. Linsenmayer, was obtained from the DSHB developed under the auspices of the NICHD and maintained by The University of Iowa, Department of Biology, Iowa City, IA, USA. We acknowledge support from Satya Amirapu (Histology Unit, School of Medical Sciences, University of Auckland), Lulu Zuo, and Farqad Abdulqader for histology processing and immunohistochemistry optimization. The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: The University of Auckland School of Medicine Performance Based Research Fund, the University of Auckland School of Medicine Graduate Student Fund.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Ethical Approval: The collection and use of both first-trimester and term placentae for this project was approved by the Northern Regional Health and Disability Ethics Committee (reference number NTX/12/06/057/AM06).
ORCID iDs: James Hearn  https://orcid.org/0000-0002-3988-2659
https://orcid.org/0000-0002-3988-2659
Sue R. McGlashan  https://orcid.org/0000-0002-8666-2456
https://orcid.org/0000-0002-8666-2456
References
- 1. Somoza RA, Welter JF, Correa D, Caplan AI. Chondrogenic differentiation of mesenchymal stem cells: challenges and unfulfilled expectations. Tissue Eng Part B Rev. 2014;20(6):596-608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Johnstone B, Hering TM, Caplan AI, Goldberg VM, Yoo JU. In vitro chondrogenesis of bone marrow-derived mesenchymal progenitor cells. Exp Cell Res. 1998;238(1):265-72. [DOI] [PubMed] [Google Scholar]
- 3. Mueller MB, Fischer M, Zellner J, Berner A, Dienstknecht T, Prantl L, et al. Hypertrophy in mesenchymal stem cell chondrogenesis: effect of TGF-beta isoforms and chondrogenic conditioning. Cells Tissues Organs. 2010;192(3):158-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Dahlin RL, Ni M, Meretoja VV, Kasper FK, Mikos AG. TGF-β3-induced chondrogenesis in co-cultures of chondrocytes and mesenchymal stem cells on biodegradable scaffolds. Biomaterials. 2014;35(1):123-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bernardo ME, Emons JAM, Karperien M, Nauta AJ, Willemze R, Roelofs H, et al. Human mesenchymal stem cells derived from bone marrow display a better chondrogenic differentiation compared with other sources. Connect Tissue Res. 2007;48(3):132-40. [DOI] [PubMed] [Google Scholar]
- 6. Cheung JO, Grant ME, Jones CJP, Hoyland JA, Freemont AJ, Hillarby MC. Apoptosis of terminal hypertrophic chondrocytes in an in vitro model of endochondral ossification. J Pathol. 2003;201(3):496-503. [DOI] [PubMed] [Google Scholar]
- 7. Cowell HR, Hunziker EB, Rosenberg L. The role of hypertrophic chondrocytes in endochondral ossification and in the development of secondary centers of ossification. J Bone Joint Surg Am. 1987;69(2_suppl):159-61. [PubMed] [Google Scholar]
- 8. Solchaga LA, Penick KJ, Welter JF. Chondrogenic differentiation of bone marrow-derived mesenchymal stem cells: tips and tricks. Methods Mol Biol. 2011;698:253-78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Hung BP, Hutton DL, Kozielski KL, Bishop CJ, Naved B, Green JJ, et al. Platelet-derived growth factor BB Enhances osteogenesis of adipose-derived but not bone marrow-derived mesenchymal stromal/stem cells. Stem Cells. 2015;33(9):2773-84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Wegmeyer H, Bröske AM, Leddin M, Kuentzer K, Nisslbeck AK, Hupfeld J, et al. Mesenchymal stromal cell characteristics vary depending on their origin. Stem Cells Dev. 2013; 22(19):2606-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Billing AM, Hamidane HB, Dib SS, Cotton RJ, Bhagwat AM, Kumar P, et al. Comprehensive transcriptomic and proteomic characterization of human mesenchymal stem cells reveals source specific cellular markers. Sci Rep. 2016;6:21507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Oldershaw RA. Cell sources for the regeneration of articular cartilage: the past, the horizon and the future. Int J Exp Pathol. 2012;93(6):389-400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Park S, Koh SE, Hur CY, Lee WD, Lim J, Lee YJ. Comparison of human first and third trimester placental mesenchymal stem cell. Cell Biol Int. 2013;37(3):242-9. [DOI] [PubMed] [Google Scholar]
- 14. Zhang X, Mitsuru A, Igura K, Takahashi K, Ichinose S, Yamaguchi S, et al. Mesenchymal progenitor cells derived from chorionic villi of human placenta for cartilage tissue engineering. Biochem Biophys Res Commun. 2006;340(3):944-52. [DOI] [PubMed] [Google Scholar]
- 15. Kusuma GD, Brennecke SP, O’Connor AJ, Kalionis B, Heath DE. Decellularized extracellular matrices produced from immortal cell lines derived from different parts of the placenta support primary mesenchymal stem cell expansion. PLoS One. 2017;12(2_suppl):e0171488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Park YB, Seo S, Kim JA, Heo JC, Lim YC, Ha CW. Effect of chondrocyte-derived early extracellular matrix on chondrogenesis of placenta-derived mesenchymal stem cells. Biomed Mater. 2015;10(3):035014. [DOI] [PubMed] [Google Scholar]
- 17. Hsu SH, Huang TB, Cheng SJ, Weng SY, Tsai CL, Tseng CS, et al. Chondrogenesis from human placenta-derived mesenchymal stem cells in three-dimensional scaffolds for cartilage tissue engineering. Tissue Eng Part A. 2011; 17(11-12):1549-60. [DOI] [PubMed] [Google Scholar]
- 18. Muiños-López E, Hermida-Gómez T, Fuentes-Boquete I, de Toro-Santos J, Blanco FJ, Diaz-Prado SM. Human amniotic mesenchymal stromal cells as favorable source for cartilage repair. Tissue Eng Part A. 2017;23(17-18):901-12. [DOI] [PubMed] [Google Scholar]
- 19. Ishii R, Kami D, Toyoda M, Makino H, Gojo S, Ishii T, et al. Placenta to cartilage: direct conversion of human placenta to chondrocytes with transformation by defined factors. Mol Biol Cell. 2012;23:3511-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Abumaree MH, Al Jumah MA, Kalionis B, Jawdat D, Al Khaldi A, Altalabani AA, et al. Phenotypic and functional characterization of mesenchymal stem cells from chorionic villi of human term placenta. Stem Cell Rev. 2013;9(1):16-31. [DOI] [PubMed] [Google Scholar]
- 21. Dominici M, Le Blanc K, Mueller I, Slaper-Cortenbach I, Marini F, Krause D, et al. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8(4):315-7. [DOI] [PubMed] [Google Scholar]
- 22. Eom J, Feisst V, Ranjard L, Loomes K, Damani T, Jackson-Patel V, et al. Visualization and quantification of mesenchymal cell adipogenic differentiation potential with a lineage specific marker. J Vis Exp. 2018;(133):57153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Leggate J, Allain R, Isaac L, Blais BW. Microplate fluorescence assay for the quantification of double stranded DNA using SYBR green I dye. Biotechnol Lett. 2006;28(19):1587-94. [DOI] [PubMed] [Google Scholar]
- 24. Farndale RW, Buttle DJ, Barrett AJ. Improved quantitation and discrimination of sulphated glycosaminoglycans by use of dimethylmethylene blue. Biochim Biophys Acta. 1986;883(2_suppl):173-7. [DOI] [PubMed] [Google Scholar]
- 25. Boss AL, Chamley LW, James JL. Placental formation in early pregnancy: how is the centre of the placenta made? Hum Reprod Update. 2018;24(6):750-60. [DOI] [PubMed] [Google Scholar]
- 26. Guillot PV, Gotherstrom C, Chan J, Kurata H, Fisk NM. Human first-trimester fetal MSC express pluripotency markers and grow faster and have longer telomeres than adult MSC. Stem Cells. 2007;25(3):646-54. [DOI] [PubMed] [Google Scholar]
- 27. Lam ATL, Reuveny S, Oh SK. Human mesenchymal stem cell therapy for cartilage repair: review on isolation, expansion, and constructs. Stem Cell Res. 2020;44:101738. [DOI] [PubMed] [Google Scholar]
- 28. Oberbauer E, Steffenhagen C, Feichtinger G, Hildner F, Hacobian A, Danzer M, et al. A luciferase-based quick potency assay to predict chondrogenic differentiation. Tissue Eng Part C Methods. 2016;22(5):487-95. [DOI] [PubMed] [Google Scholar]
- 29. Rothweiler R, Basoli V, Duttenhoefer F, Kubosch D, Schmelzeisen R, Johnstone B, et al. Predicting and promoting human bone marrow MSC chondrogenesis by way of TGFβ receptor profiles: toward personalized medicine. Front Bioeng Biotechnol, 2020;8:618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. James JL, McGlashan SR, Chamley LW. Stem cells derived from the placental villi. In: Atala A, Cetrulo KJ, Taghizadeh RR, Murphy SV, Cetrulo CL. editors. Perinatal stem cells. Academic Press; 2018. p. 187-200. [Google Scholar]
- 31. Teunissen M, Verseijden F, Riemers FM, van Osch G, Tryfonidou MA. The lower in vitro chondrogenic potential of canine adipose tissue-derived mesenchymal stromal cells (MSC) compared to bone marrow-derived MSC is not improved by BMP-2 or BMP-6. Vet J. 2021;269:105605. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-pdf-1-car-10.1177_19476035211044822 for The Chondrogenic Potential of First-Trimester and Term Placental Mesenchymal Stem/Stromal Cells by Joanna L. James, Anandita Umapathy, Sonia Srinivasan, Claire N. Barker, Anna Brooks, James Hearn, Ashika Chhana, Eloise Williams, Hilary Sheppard and Sue R. McGlashan in CARTILAGE