Abstract
Objective
Osteoarthritis (OA) is a prevalent chronic multifactorial degenerative disease characterized by joint tissue inflammation, osteophyte formation, subchondral bone sclerosis, and articular cartilage degradation. Low-intensity pulsed ultrasound (LIPUS), a noninvasive ultrasound technique, is widely used to attenuate diseases. The aim of this study was to investigate whether LIPUS can ameliorate OA, and to explore its underlying molecular mechanism.
Design
The OA model was established in a C57BL/6 mouse by the anterior cruciate ligament transaction method. OA was assessed using arthritis scoring and weightbearing parameters. Chondrocyte proliferation was detected by a CCK-8 assay. The levels of interleukin-6 (IL-6), IL-8 and tumor necrosis factor-α (TNF-α) in synovial fluid of the mice were measured by enzyme-linked immunosorbent assay.
Results
In OA mice, the arthritis score and weightbearing abilities were dramatically improved by LIPUS treatment. LIPUS also remarkably declined the levels of inflammatory cytokines IL-6, IL-8, and TNF-α in synovial fluid of OA mice. Moreover, LIPUS promoted chondrocyte proliferation and differentiation by activating focal adhesion kinase (FAK) signaling. Inhibition of FAK significantly blocked LIPUS-mediated cell proliferation and differentiation in vitro, as well as inflammation condition in OA mice.
Conclusion
LIPUS alleviates OA through promoting chondrocytes proliferation and differentiation by activating FAK, which could act as an intervening target for OA treatment.
Keywords: low-intensity pulsed ultrasound, osteoarthritis, chondrocytes, focal adhesion kinase
Introduction
Osteoarthritis (OA) is a prevalent and chronic multifactor-related joint degenerative disease, which clinically presents with joint tissue inflammation, osteophyte formation, subchondral bone sclerosis, and articular cartilage degradation. 1 The incidence rate of OA increases with age and can shorten the time an adult spends working due to pain and disability. 2 Signaling pathways are involved in the pathogenesis of OA, including Mmp13, HIF-2α, syndecan-4, hedgehog signal pathway, and Wnt/β-catenin.3-8 Accordingly, strategies aimed at relieving synovial inflammation and proteolytic degradation of cartilage for OA treatment have been developed. However, novel and more effective therapeutic approaches for the delay or treatment of OA are needed.
Low-intensity pulsed ultrasound (LIPUS) is a noninvasive ultrasound technique characterized by low intensity,9,10 As a safe and effective therapy for healing fracture, LIPUS is authorized by the Food and Drug Administration in 1994. The effective impaction of LIPUS on fracture healing is also endorsed by lots of studies.11-13 It is reported that LIPUS is benefited to facilitate fracture healing via stimulating callus remodeling, and vascular formation. 14 Moreover, LIPUS stimulation was reported to augment mesenchymal stem cells (MSCs) toward the site of fracture and thereby contributes to fracture healing. 15
Focal adhesion kinase (FAK), a nonreceptor tyrosine kinase, is reported to be activated by growth factor receptors and integrins. 16 Mitogen-activated protein kinase (MAPK) is the downstream signaling effector of FAK. Additionally, mitogen-activated protein kinase 38 (p38) is an important component of MAPK, which mediates bone cartilage degeneration. 17 FAK signaling plays a vital role in cell proliferation, apoptosis, and differentiation. The IGF-1 receptor, which is activated and stabilized by FAK, takes part in the proliferation of leukemic cells. 18 Interestingly, LIPUS effectively aids in avoiding cartilage damage through the activation of integrin/FAK/MAPK pathway in early-stage OA. 19 The aim of this study was to investigate whether LIPUS can attenuate OA condition and explored the role of chondrocytes and FAK in this process.
Methods
Animal Experiments
Male C57BL/6 wild-type mice (8-10 weeks old) that were healthy and pathogen-free were obtained from the Laboratory Animal Center of Shanghai. The study was approved by the Animal Ethics Committee of our hospital. To generate an OA model, an intraperitoneal injection of 2% (w/v) pentobarbital (40 mg/kg) was used to anesthetize the mice, and the anterior cruciate ligament transaction operation was performed as previously described.20,21 Briefly, mice were placed on the test bench and hair was removed from the hind legs. Then, 5 μL of 100 mg/mL iodoacetate was injected through the skin around the joint. An arthrotomy was performed and the patella was dislocated laterally and the knee placed in full flexion. The anterior cruciate ligament was visualized and transected with a blade. The joint was irrigated with sterile saline and closed. A sham group included mice who had undertaken the same surgery of arthrotomy with no process performed to the medial ligament in the right knee joint.
Low-Intensity Pulsed Ultrasound Therapy
The mice in OA/LIPUS group and sham/LIPUS groups were treated with LIPUS (Ito Corporation, Tokyo, Japan) with the following parameters: on-off ratio of 20%, free mode, 20 minutes 3 MHz irradiation, 40 mW/cm2 irradiation intensity, once per day for 8 consecutive weeks. All parameters were the same for the OA/LIPUS group and sham/LIPUS group, but without ultrasound output for the sham group. For FAK inhibitor treatment, mice were treated with 30 mg/kg TAE226 by oral administration combined with LIPUS treatment for 8 weeks.
OA Status Evaluation
The macroscopic changes in joint morphology were observed on the operated knee joint of all mice to apply the in vivo arthritis scoring system. An arthritis score was recorded weekly on the knee joints, and the average scores were calculated. 22 The weightbearing tolerance of mice was measured with an incapacitance tester (Linton Instrumentation, Norfolk, UK).
Cell Culture and LIPUS Treatment
Chondrocytes, including C28/I2 cells and CHON-001 cells (American Type Culture Collection, ATCC), were seeded and cultured in Dulbecco’s modified Eagle medium (DMEM) supplemented with 10% fetal bovine serum at 37°C with 5% CO2 in a humidified atmosphere environment. For FAK inhibition, chondrocytes were maintained in DMEM containing TAE226 (Sigma-Aldrich, St Louis, MO, USA) for 10 minutes. Then, chondrocytes were plated in 6-well plates for 24 hours prior to LIPUS treatment (1.5 MHz and a pulsed-wave mode intensity of 50 or 100 mW/cm2) applied via a sterilized transducer (Osteotron D2; Ito Co., Tokyo, Japan), and placed on the surface of culture medium so that the distance between the transducer and the cells was approximately 3 to 4 mm. The control cells were cultured in the same medium without LIPUS treatment.
Cell Proliferation
Cell proliferation was detected by a Cell Counting Kit-8 (CCK-8) assay. Briefly, chondrocytes were seeded in 96-well plates at a density of 5 × 104/mL for 24 hours. Then, chondrocytes were treated with LIPUS and/or TAE226. After treatment of 24, 48, 72, and 96 hours, cells were incubated in 10 μL CCK-8 (Sangon Biotech, Shanghai, China), and the optical density values at 450 nm were assessed via a plate reader (Thermo Fisher Scientific Oy, Vantaa, Finland).
Western Blot Analysis
RIPA protein extraction reagent was used to lyse chondrocytes on ice, followed by the BCA Protein Assay Kit (Beyotime, Shanghai, China) to measure protein concentrations. Protein fractions were separated on a 10% SDS/PAGE gel and then transferred onto a polyvinylidene fluoride membrane (Millipore, Bedford, MA, USA). The membrane was blocked with 5% non-fat milk, and then incubated with primary antibodies against OPN, BMP2, Coll II, FAK, p-FAK, p38, p-p38, and ACTIN (Abcam, Cambridge, UK) at 4°C for 24 hours. Afterward, the membrane was washed and maintained with a secondary antibody (peroxidase-linked IgG) for 1 hour at room temperature. An ECL detection system (Amersham Biosciences, Freiburg, Germany) was used to visualize the protein bands.
The Measurement of IL-6, IL-8, and TNF-α
The levels of interleukin-6 (IL-6), IL-8, and tumor necrosis factor-α (TNF-α) in the serum and synovial fluid were measured by the enzyme-linked immunosorbent assay (ELISA) kits (R&D Systems, Minneapolis, MN, USA) in accordance with the manufacturers’ instructions. The 450 nm absorbance was measured on a microplate reader (Bio-Rad, Hercules, CA, USA).
Statistical Analysis
The total data of the results were expressed as the mean ± SD. SPSS 20.0 (IBM Corp, Armonk, NY, USA) was employed to carry out statistical analysis. Differences between groups were evaluated with the Student t test or one-way analysis of variance analysis. P < 0.05 was considered statistically significant.
Results
LIPUS Alleviates OA Condition
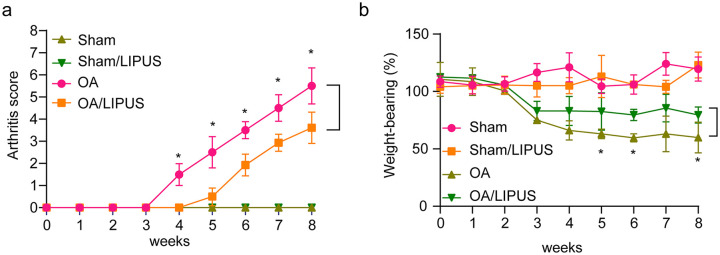
After the OA mice model was established, the arthritis score and weightbearing ability of each mouse in four groups were assessed every week. Figure 1a and b shows that the arthritis score was higher and the weightbearing was markedly lower in OA group mice than that in the sham group mice. Additionally, the arthritis score was significantly decreased and the weightbearing ability was remarkably increased, following LIPUS treatment compared with the untreated OA group mice. Moreover, LIPUS treatment had minimal effects on joint function in untreated mice. These data demonstrate that LIPUS treatment significantly alleviates OA in mice.
Figure 1.
Low-intensity pulsed ultrasound (LIPUS) alleviates osteoarthritis (OA) in mice. (a) The arthritis score of the mice in the sham group, sham/LIPUS group, OA group, and OA/LIPUS group were evaluated. (b) The weightbearing tolerance of mice in the sham group, sham/LIPUS group, OA group, and OA/LIPUS group was measured with an incapacitance tester. Compared with OA group, *P < 0.05.
LIPUS Induces Chondrocyte Proliferation and Differentiation
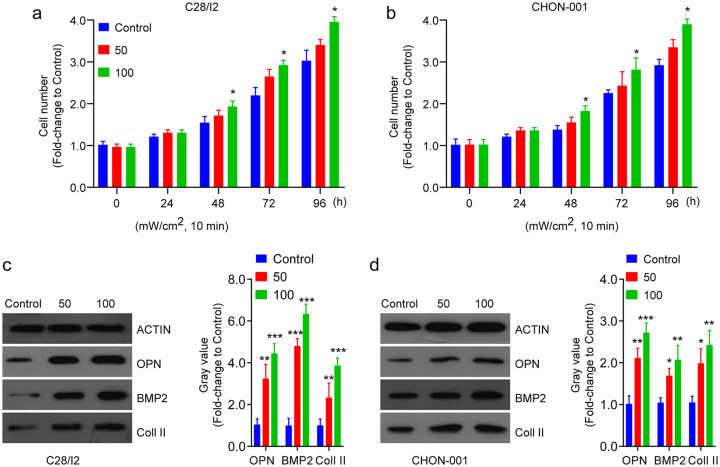
To assess the effect of LIPUS on chondrocytes in vitro, cell proliferation and differentiation assays were performed on C28/I2 cells and CHON-001 cells. Cells were treated by LIPUS with either a lower dose of 50 mW/cm2 or a higher dose of 100 mW/cm2 for 10 minutes. The cell number of chondrocytes (both C28/I2 cells and CHON-001 cells) was increased after LIPUS treatment when compared with the control cells ( Fig. 2a and b ). Then, we analyzed whether LIPUS treatment affects cell differentiation. As is shown in Figure 2c and d , the expression levels of OPN, BMP2, and Coll II protein in C28/I2 cells and CHON-001 were significantly increased following LIPUS treatment.
Figure 2.
Low-intensity pulsed ultrasound (LIPUS) induces chondrocytes proliferation and differentiation. (a, b) The cell proliferation of C28/I2 and CHON-001 cells after different dose of LIPUS treatment was detected by a CCK-8 assay. (c, d) The cell differentiation-related proteins were assessed by Western blot analysis in C28/I2 and CHON-001 cells after different dose of LIPUS treatment. Compared with control, *P < 0.05, **P < 0.01, ***P < 0.001.
LIPUS Promotes Chondrocyte Proliferation and Differentiation by Activating FAK
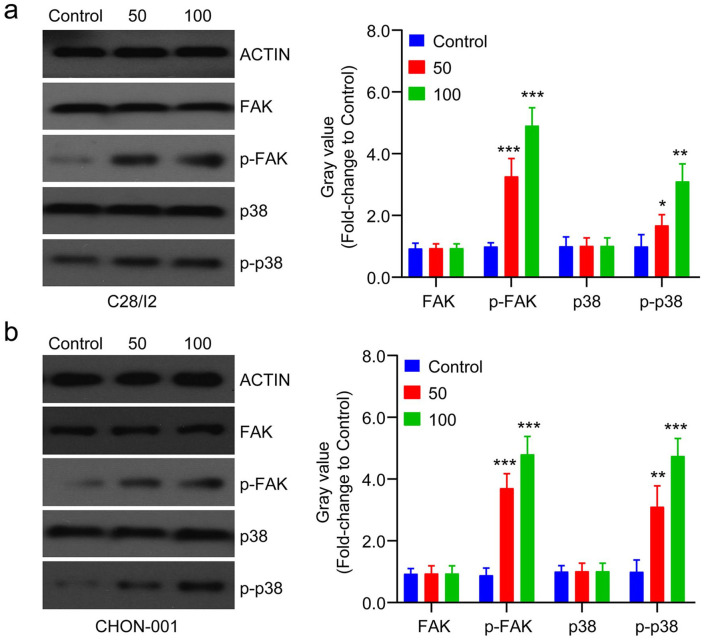
Previous research showed that FAK activation was involved in cell differentiation. 23 The molecular mechanism of chondrocyte proliferation and differentiation induced by LIPUS was investigated. LIPUS induction significantly activated FAK signaling leading to its downstream activation of p38 in C28/I2 and CHON-001 cells, shown by the increased expression levels of p-FAK and p-p38 ( Fig. 3a and b ). The data demonstrates that LIPUS promotes chondrocyte proliferation and differentiation by activating FAK signaling.
Figure 3.
Low-intensity pulsed ultrasound (LIPUS) promotes chondrocyte proliferation and differentiation by focal adhesion kinase (FAK) signaling. (a, b) The protein levels of FAK and the downstream p38 pathway were assessed by Western blot in C28/I2 and CHON-001 cells after different dose of LIPUS treatment. Compared with control, *P < 0.05, **P < 0.01, ***P < 0.001.
Inhibition of FAK Reverses LIPUS-Induced Cell Proliferation and Differentiation
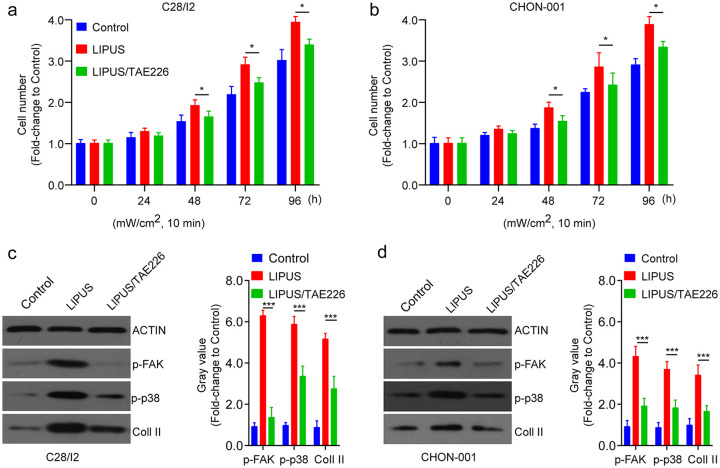
Next, cells were treated with a FAK inhibitor, TAE226, and then treated by LIPUS. Compared with LIPUS-treated C28/I2 cells, cell proliferation was significantly inhibited in untreated C28/I2 and CHON-001 cells ( Fig. 4a and b ). In addition, cell differentiation was remarkably reduced by TAE226 treatment, showed by the decreased expression levels of p-FAK, p-p38, and Coll II ( Fig. 4c and d ). The findings demonstrate that inhibition of FAK reversed LIPUS-induced chondrocyte proliferation and differentiation.
Figure 4.
Inhibition of focal adhesion kinase (FAK) reverses low-intensity pulsed ultrasound (LIPUS)-induced cell proliferation and differentiation. (a, b) The cell proliferation of C28/I2 and CHON-001 cells after different dose of LIPUS treatment in the presence or absence of TAE226 was detected by CCK-8 assay. (c, d) The cell differentiation-related proteins were assessed by Western blot analysis in C28/I2 and CHON-001 cells after different dose of LIPUS treatment in the presence or absence of TAE226. Compared with control, *P < 0.05, **P < 0.01, ***P < 0.001.
Inhibition of FAK Reverses LIPUS-Mediated Improvements of OA
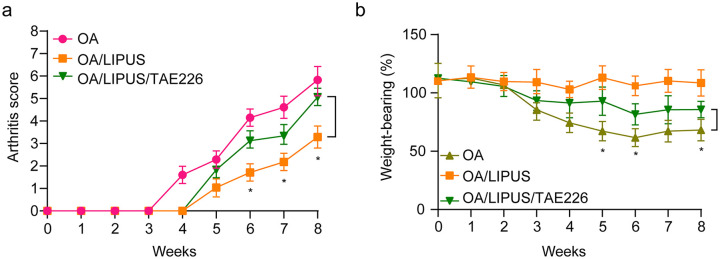
To identify the effects of TAE226 on OA condition in vivo, the OA mice were administrated with TAE226 and followed by LIPUS treatment. Compared with OA, LIPUS treatment significantly alleviated OA symptoms by reducing arthritis score ( Fig. 5a ) and increasing weightbearing ability ( Fig. 5b ). However, the arthritis score and weightbearing ability of the mice in OA group was significantly reduced and increased after TAE226 treatment, respectively ( Fig. 5b ). Hence, inhibition of FAK reversed LIPUS-mediated improvements of OA.
Figure 5.
The effect of focal adhesion kinase (FAK) inhibitor and low-intensity pulsed ultrasound (LIPUS) on osteoarthritis (OA) in mice. (a) The arthritis score of the mice in the OA group, OA/LIPUS group, and OA/LIPUS/TAE226 group were evaluated. (b) The weightbearing tolerance of mice in the OA group, OA/LIPUS group, and OA/LIPUS/TAE226 group was measured with an incapacitance tester. Compared with OA group, *P < 0.05, ***P < 0.001.
Inhibition of FAK Reverses LIPUS-Mediated Inflammation Condition in OA Mice
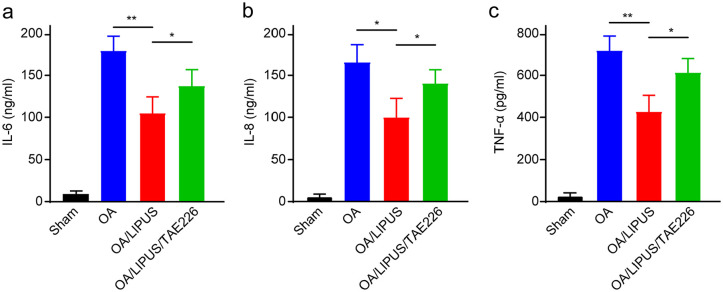
We next detected the effects of FAK inhibition on LIPUS-mediated inflammatory response in OA. As is shown in Figure 6 , the levels of IL-6, IL-8, and TNF-α were significantly increased in synovial fluid of OA mice. Meanwhile, LIPUS remarkably declined the inflammatory response by reducing the levels of IL-6, IL-8, and TNF-α. In addition, inhibition of FAK by TAE226 reversed the LIPUS-induced reduction of inflammation in OA mice ( Fig. 6a-c ). Taken together, the results indicate that FAK plays pivotal role in LIPUS-mediated inflammatory response in OA mice.
Figure 6.
The effect of low-intensity pulsed ultrasound (LIPUS) on inflammation condition in osteoarthritic (OA) mice. The levels of interleukin-6 (IL-6) (a), interleukin-8 (IL-8) (b), and tumor necrosis factor-α (TNF-α) (c) in the synovial fluid of the mice were measured by enzyme-linked immunosorbent assay kits. Compared with the indicated group, *P < 0.05, **P < 0.01.
Discussion
The development and progression of OA is a complex process involving multiple factors that influence chondrocyte homoeostasis. LIPUS influences multiple signaling pathways and their physiological and biological influence on numerous human diseases. To explore the therapeutic effects of LIPUS on OA disease, we studied its in vivo efficacy using an OA mouse model, and further demonstrated its potential therapeutic mechanisms. The biomarkers of arthritis score and weight-bearing ability in the OA mouse model revealed that LIPUS dramatically moderates the status of OA and has strong protective effects against OA.
LIPUS is widely used as a therapeutic method in clinical treatments. For example, LIPUS could promote angiogenesis, callus formation and remodeling, as well as accelerate bone fractures healing. 24 The influences of LIPUS on physiological process, including proliferation and differentiation, are significantly visible in different cell types. It was reported that LIPUS suppresses the proliferation of preadipocytes in rat visceral organs. 10 LIPUS exposure was demonstrated to improve the proliferation of human umbilical cord-derived MSCs and amnion-derived MSCs.25,26 The living cells are receptive to the acoustic pressure wave produced by LIPUS, leading to a series of biochemical reaction at the cellular level.27,28 Likewise, according to our study, LIPUS has remarkable effects on improving proliferation and differentiation of chondrocytes in OA mice. In addition, LIPUS shows an anti-inflammatory effect on arthritic knee joints. 29 Similarly, our present study found that LIPUS alleviated OA inflammatory response by decreasing the level of inflammatory cytokines IL-6, IL-8, and TNF-α.
It has been reported that LIPUS enhances the proliferation of hAD-MSCs by activation of ERK1/2 and PI3K-Akt signaling pathways. 26 Moreover, the proliferation and migration of HaCaT cells were promoted by LIPUS via JNK and PI3K/AKT signaling. 30 Recently, it has been demonstrated that LIPUS could balance osteoblast differentiation and is consistent with our study as LIPUS remarkably promoted chondrocyte cell proliferation and differentiation via modulating the expression of collagen II. Additionally, the FAK/p38 signaling pathway was activated by LIPUS, indicating that LIPUS promoted chondrocytes proliferation by activating FAK/p38 signaling pathway. In particular, synovial fluid and serum levels of TNF-α and IL-6 have been linked to OA severity, knee cartilage loss, and the narrowing of joint space. 31 In the present study, LIPUS remarkably alleviated inflammatory response by decreasing the levels of IL-6, IL-8, and TNF-α in serum and synovial fluid trough activation of FAK signaling pathway in OA mice.
In conclusion, LIPUS alleviates OA by promoting weightbearing ability. In addition, LIPUS treatment significantly promotes chondrocytes proliferation and differentiation by activating FAK/p38 signaling pathway. Meanwhile, inhibition of FAK reverses the effects of LIPUS on chondrocytes in vitro and in mice. The results indicate that the therapeutic effects of LIPUS on OA are mediated through FAK-mediated chondrocyte proliferation and differentiation and inflammatory response.
Footnotes
Acknowledgments and Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Ethical Approval: The study was approved by the Animal Ethics Committee of our hospital (No.202004).
Animal Welfare: The present study followed international, national, and/or institutional guidelines for humane animal treatment and complied with relevant legislation.
References
- 1. Li G, Yin J, Gao J, Cheng TS, Pavlos NJ, Zhang C, et al. Subchondral bone in osteoarthritis: insight into risk factors and microstructural changes. Arthritis Res Ther. 2013;15:223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Pereira D, Severo M, Santos RA, Barros H, Branco J, Lucas R, et al. Knee and hip radiographic osteoarthritis features: differences on pain, function and quality of life. Clin Rheumatol. 2016;35:1555-64. [DOI] [PubMed] [Google Scholar]
- 3. Zhu M, Chen M, Zuscik M, Wu Q, Wang YJ, Rosier RN, et al. Inhibition of beta-catenin signaling in articular chondrocytes results in articular cartilage destruction. Arthritis Rheum. 2008;58:2053-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Echtermeyer F, Bertrand J, Dreier R, Meinecke I, Neugebauer K, Fuerst M, et al. Syndecan-4 regulates ADAMTS-5 activation and cartilage breakdown in osteoarthritis. Nat Med. 2009;15:1072-6. [DOI] [PubMed] [Google Scholar]
- 5. Lin AC, Seeto BL, Bartoszko JM, Khoury MA, Whetstone H, Ho L, et al. Modulating hedgehog signaling can attenuate the severity of osteoarthritis. Nat Med. 2009;15:1421-5. [DOI] [PubMed] [Google Scholar]
- 6. Zhu M, Tang D, Wu Q, Hao S, Chen M, Xie C, et al. Activation of beta-catenin signaling in articular chondrocytes leads to osteoarthritis-like phenotype in adult beta-catenin conditional activation mice. J Bone Miner Res. 2009;24:12-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Saito T, Fukai A, Mabuchi A, Ikeda T, Yano F, Ohba S, et al. Transcriptional regulation of endochondral ossification by HIF-2α during skeletal growth and osteoarthritis development. Nat Med. 2010;16:678-86. [DOI] [PubMed] [Google Scholar]
- 8. Yang S, Kim J, Ryu JH, Oh H, Chun CH, Kim BJ, et al. Hypoxia-inducible factor-2α is a catabolic regulator of osteoarthritic cartilage destruction. Nat Med. 2010;16:687-693. [DOI] [PubMed] [Google Scholar]
- 9. Claes L, Willie B. The enhancement of bone regeneration by ultrasound. Prog Biophys Mol Biol. 2007;93:384-98. [DOI] [PubMed] [Google Scholar]
- 10. Xu T, Gu J, Li C, Guo X, Tu J, Zhang D, et al. Low-intensity pulsed ultrasound suppresses proliferation and promotes apoptosis via p38 MAPK signaling in rat visceral preadipocytes. Am J Transl Res. 2018;10:948-56. [PMC free article] [PubMed] [Google Scholar]
- 11. Sun S, Sun L, Kang Y, Tang L, Qin YX, Ta D. Therapeutic effects of low-intensity pulsed ultrasound on osteoporosis in ovariectomized rats: intensity-dependent study. Ultrasound Med Biol. 2020;46:108-21. [DOI] [PubMed] [Google Scholar]
- 12. Tang L, Kang Y, Sun S, Zhao T, Cao W, Fan X, et al. Inhibition of MSTN signal pathway may participate in LIPUS preventing bone loss in ovariectomized rats. J Bone Miner Metab. 2020;38:4-26. [DOI] [PubMed] [Google Scholar]
- 13. Chen D, Xiang M, Gong Y, Xu L, Zhang T, He Y, et al. LIPUS promotes FOXO1 accumulation by downregulating miR-182 to enhance osteogenic differentiation in hPDLCs. Biochimie. 2019;165:219-28. [DOI] [PubMed] [Google Scholar]
- 14. Cheung WH, Chow SK, Sun MH, Qin L, Leung KS. Low-intensity pulsed ultrasound accelerated callus formation, angiogenesis and callus remodeling in osteoporotic fracture healing. Ultrasound Med Biol. 2011;37:231-8. [DOI] [PubMed] [Google Scholar]
- 15. Wei FY, Leung KS, Li G, Qin J, Chow SK, Huang S, et al. Low intensity pulsed ultrasound enhanced mesenchymal stem cell recruitment through stromal derived factor-1 signaling in fracture healing. PLoS One. 2014;9:e106722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Sakurama K, Noma K, Takaoka M, Tomono Y, Watanabe N, Hatakeyama S, et al. Inhibition of focal adhesion kinase as a potential therapeutic strategy for imatinib-resistant gastrointestinal stromal tumor. Mol Cancer Ther. 2009;8:127-34. [DOI] [PubMed] [Google Scholar]
- 17. Ding L, Guo D, Homandberg GA. The cartilage chondrolytic mechanism of fibronectin fragments involves MAP kinases: comparison of three fragments and native fibronectin. Osteoarthritis Cartilage. 2008;16:1253-62. [DOI] [PubMed] [Google Scholar]
- 18. Andersson S, D’Arcy P, Larsson O, Sehat B. Focal adhesion kinase (FAK) activates and stabilizes IGF-1 receptor. Biochem Biophys Res Commun. 2009;387:36-41. [DOI] [PubMed] [Google Scholar]
- 19. Xia P, Shen S, Lin Q, Cheng K, Ren S, Gao M, et al. Low-intensity pulsed ultrasound treatment at an early osteoarthritis stage protects rabbit cartilage from damage via the integrin/focal adhesion kinase/mitogen-activated protein kinase signaling pathway. J Ultrasound Med. 2015;34:1991-9. [DOI] [PubMed] [Google Scholar]
- 20. Yoshioka M, Coutts RD, Amiel D, Hacker SA. Characterization of a model of osteoarthritis in the rabbit knee. Osteoarthritis Cartilage. 1996;4:87-98. [DOI] [PubMed] [Google Scholar]
- 21. Saito M, Sasho T, Yamaguchi S, Ikegawa N, Akagi R, Muramatsu Y, et al. Angiogenic activity of subchondral bone during the progression of osteoarthritis in a rabbit anterior cruciate ligament transection model. Osteoarthritis Cartilage. 2012;20:1574-82. [DOI] [PubMed] [Google Scholar]
- 22. Thwin MM, Douni E, Aidinis V, Kollias G, Kodama K, Sato K, et al. Effect of phospholipase A2 inhibitory peptide on inflammatory arthritis in a TNF transgenic mouse model: a time-course ultrastructural study. Arthritis Res Ther. 2004;6:R282-R294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Sun C, Yuan H, Wang L, Wei X, Williams L, Krebsbach PH, et al. FAK Promotes osteoblast progenitor cell proliferation and differentiation by enhancing Wnt signaling. J Bone Miner Res. 2016;31:2227-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Nakamura T, Fujihara S, Yamamoto-Nagata K, Katsura T, Inubushi T, Tanaka E. Low-intensity pulsed ultrasound reduces the inflammatory activity of synovitis. Ann Biomed Eng. 2011;39:2964-71. [DOI] [PubMed] [Google Scholar]
- 25. Yoon JH, Roh EY, Shin S, Jung NH, Song EY, Lee DS, et al. Introducing pulsed low-intensity ultrasound to culturing human umbilical cord-derived mesenchymal stem cells. Biotechnol Lett. 2009;31:329-35. [DOI] [PubMed] [Google Scholar]
- 26. Ling L, Wei T, He L, Wang Y, Wang Y, Feng X, et al. Low-intensity pulsed ultrasound activates ERK1/2 and PI3K-Akt signalling pathways and promotes the proliferation of human amnion-derived mesenchymal stem cells. Cell Prolif. 2017;50. doi: 10.1111/cpr.12383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Padilla F, Puts R, Vico L, Raum K. Stimulation of bone repair with ultrasound: a review of the possible mechanic effects. Ultrasonics. 2014;54:1125-45. [DOI] [PubMed] [Google Scholar]
- 28. Hu B, Zhang Y, Zhou J, Li J, Deng F, Wang Z, et al. Low-intensity pulsed ultrasound stimulation facilitates osteogenic differentiation of human periodontal ligament cells. PLoS One. 2014;9:e95168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Nakamura T, Fujihara S, Katsura T, Yamamoto K, Inubushi T, Tanimoto K, et al. Effects of low-intensity pulsed ultrasound on the expression and activity of hyaluronan synthase and hyaluronidase in IL-1β-stimulated synovial cells. Ann Biomed Eng. 2010;38:3363-70. [DOI] [PubMed] [Google Scholar]
- 30. Leng X, Shang J, Gao D, Wu J. Low-intensity pulsed ultrasound promotes proliferation and migration of HaCaT keratinocytes through the PI3K/AKT and JNK pathways. Braz J Med Biol Res. 2018;51:e7862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Robinson WH, Lepus CM, Wang Q, Raghu H, Mao R, Lindstrom TM, et al. Low-grade inflammation as a key mediator of the pathogenesis of osteoarthritis. Nat Rev Rheumatol. 2016;12:580-92. [DOI] [PMC free article] [PubMed] [Google Scholar]