Abstract
Background
Repair of chondral injuries using cartilage chips has recently demonstrated clinical feasibility. Autologous platelet-rich plasma (PRP) is a potential promising technique for improving healing response during cartilage repair.
Purpose
To assess the cartilage repair tissue quality after autologous cartilage chips treatment (CC) with and without repeated local injections of PRP for the treatment of full-thickness focal chondral defects of the knee.
Materials and Methods
Two full-thickness chondral defects (Ø = 6 mm) were created in the medial and lateral trochlea facets of each knee in 6 skeletally mature Göttingen minipigs. The 2 treatment groups were (1) CC with 1 weekly PRP injection for 3 weeks (n = 12) and (2) CC alone (n = 12). The animals were euthanized after 6 months. Samples of whole blood and PRP were analyzed for concentrations of platelets and nucleated cells. The composition of the cartilage repair tissue was assessed using gross appearance assessment, histomorphometry, and semiquantitative scoring (ICRS II).
Results
Histological evaluation demonstrated no significant difference in the content of hyaline cartilage (CC + PRP: 18.7% vs. CC: 19.6%), fibrocartilage (CC + PRP: 48.1% vs. CC: 51.8%), or fibrous tissue (CC + PRP: 22.7% vs. CC: 21.8%) between the treatment groups. Macroscopic evaluation did not demonstrate any difference between groups.
Conclusions
PRP injections after CC in the treatment of full-thickness cartilage injuries demonstrated no beneficial effects in terms of macroscopic and histologic composition of cartilage repair tissue.
Keywords: platelet-rich plasma, injection, cartilage chips, particulated cartilage
Introduction
Many treatment modalities are available for articular cartilage injuries.1,2 The most frequently used treatment is a marrow stimulation technique known as microfracture (MFx). 3 However, studies have shown that marrow stimulation results in the formation of fibrocartilaginous and fibrous repair tissue rather than hyaline cartilage.4,5
Cartilage chips transplantation is emerging as a possible treatment alternative to marrow stimulation. In 2006, Lu et al. 6 reported that chondrocytes from cartilage explants migrated and produced a new extracellular matrix. Even though the repair mechanism is yet to be established, 7 the cartilage repair potential of cartilage chips has been confirmed in a number of studies.8-14 Two different methods have demonstrated clinical efficacy: particulated juvenile articular cartilage (PJAC; DeNovo NT) for chondral defects and autologous dual-tissue transplantation (ADTT) for osteochondral defects. The commercially available PJAC treatment consists of allogenic cartilage chips from juvenile donors, and these cartilage chips are embedded in fibrin glue and implanted in a chondral defect. Initial reports have been promising, noting significant clinical outcome improvements in patients after 2 years.11,15 In ADTT, a cartilage biopsy specimen is taken from a low weightbearing site in the affected knee, and a bone biopsy specimen is taken from the proximal tibia. First, the autologous bone graft is implanted in the bed of the osteochondral defect. Second, the cartilage biopsy specimen is chipped using a scalpel, embedded in fibrin glue, and implanted on top of the bone graft.16-18 Initial results are promising, 16 and an experimental study has established that the presence of autologous cartilage chips in a defect results in improved cartilage repair tissue. 17
Several enhancement principles for MFx have been described, such as biologic and polymer scaffolds and hydrogels.19-22 These may also function as enhancers for cartilage chips repair. Among the potential adjuvants, platelet-rich plasma (PRP), an endogenous source of anabolic growth factors, is an increasingly popular therapy for the treatment of musculoskeletal pathologies.23-25 PRP contains high concentrations of platelet and growth factors, such as platelet-derived growth factor and transforming growth factor–β.26,27 The potential advantages of using PRP as an adjuvant to cartilage chip transplantation are the capacity to promote cellular migration and adherence to the defects, and the presence of growth factors that are involved in maintaining chondrocyte viability. 28
The aim of the current study was to assess the cartilage repair tissue quality after autologous cartilage chips treatment (CC) with and without repeated local injections of PRP for the treatment of full-thickness focal chondral defects of the knee in large animal model. We hypothesized that PRP injection would increase the proportion of hyaline cartilage in the repair tissue.
Materials and Methods
Experimental Design
Six skeletally mature male Göttingen minipigs were included in the study. The average weight was 36.5 kg (range 32.2-41.1 kg), and the average age at surgery was 19.7 months (range 18.2-22.2 months).
Two chondral defects with a diameter of 6 mm were surgically applied to the trochlea of each knee: one defect in the medial trochlear facet and one in the lateral. There were 24 treated defects in the 6 animals. The defects of each knee were treated with either CC alone or CC and PRP (n = 12 per study group). Ultrasound-guided intra-articular PRP injections were administered per-operatively and once every week for 3 weeks (a total of four PRP injections). The animals were euthanized after 6 months. Follow-up consisted of a gross appearance assessment, histomorphometry, and semiquantitative scoring (International Cartilage Repair Society [ICRS] II).
The study complied with the Danish Law on Animal Experimentation and was approved by the Danish Ministry of Justice Ethical Committee, J.nr. 2012-15-2934-00301.
Anesthesia
The animal model and surgery have previously been described in detail.17,18,29 The animals were premedicated with a Zoletil mix 1 mL/10 kg (tiletamin 2.5 mg/mL, zolazepam 2.5 mg/mL, torbugesic 0.5 mg/mL, ketaminol 2.5 mg/mL, and rompun 2.5 mg/mL; Virbac, DK). General anesthesia and local anesthesia were achieved with Etomidate (Hypno-midate, 0.25 mL/kg, Janssen Pharmaceutica, Belgium), Sevo-flurane (3%, AbbVie, Denmark), fentanyl (0.175 mL/kg/h, Hameln Pharmaceuticals, Germany), and Xylocain (10 mL, 20 mg/mL, Astra Zeneca, Denmark). The animals received preoperative prophylactic antibiotics (Penicillinprokain 0.03 mL/kg, Ceva Sante Animale, France).
Surgery
Access to the knee joint was gained through the patellar ligament. The trochlea was exposed, and two 6-mm chondral defects were created using a skin biopsy punch and curette. The defects were thoroughly debrided, and the calcified cartilage layer was removed to improve graft integration to the subchondral bone, which was not violated. In the CC-only group, the cartilage removed in the debridement process was cut into 0.25 to 0.5 mm3 cartilage chips. The cartilage chips were placed in the chondral defect and embedded in fibrin glue (Tisseel Duo Quick, Baxter, Denmark).
The same procedure was performed in the CC and PRP group with the addition of a single PRP injection after closure of surgical incision, followed by ultrasound-guided intra-articular PRP injections once every week for 3 weeks (a total of 4 PRP injections). PRP injection treatment was randomly assigned to right and left knees. Postoperatively, 40 full-range motions of the knee were performed to ensure equal intraarticular distribution of the PRP. After 6 months, the minipigs were euthanized with an intravascular injection of pentobarbital (0.4 mL/kg) while under general anesthesia.
Preparation of PRP
Activated GPS III platelet factors (Biomet, Warsaw, IN) were used. Blood samples were taken by venipuncture on the day of surgery. For each subject, 26 mL of blood was collected in a 50-mL syringe containing 4 mL of anticoagulant ACD-A (adenosin-citrate-dextrose-acid). A 12-mL syringe containing 1.6 mL of ACD-A was used to collect 10.4 mL of blood to provide a base measurement.
The anticoagulated blood was centrifuged for 15 minutes at 3200 rpm (755VES-230 V, Biomet Biologics, LLC). The plasma was then withdrawn using a yellow cap. The system was then agitated for 30 seconds to resuspend the buffy coat, which contained most of the platelets. The PRP was then collected using a red cap in a 10-mL syringe.
Samples of both the whole blood and PRP were analyzed with an automated hematology analyzer using RNA fluorescent dyes, per the manufacturer’s instructions (Sysmex XE-5000, Sysmex Corp., Kobe, Japan), to determine the concentrations of platelets and nucleated cells. The GPS III system produced a leucocyte-rich PRP product.
Macroscopic Evaluation
Macroscopic evaluation was performed by an experienced investigator using the ICRS macroscopic evaluation score. 30 All the condyles were scored immediately after sacrificing the animals, and the characteristics of the lesion were recorded, describing color, surface texture, and the presence or absence of exposed subchondral bone. The score ranges from 1 to 12 with 12 been normal appearing cartilage.
Histology
The samples were dehydrated in ethanol of an increasing concentration (70% to 96%) and cleared in isopropanol and xylene. Finally, the samples were embedded in methylmethacrylate (MMA) and cut in 7-μm slices using a hard tissue microtome (Reichert Jung Polycot). All samples were stained with hematoxylin and eosin, safranin O, toluidine blue, and collagen type I and II.
Histomorphometry
The tissue composition of the repair tissue was quantitatively evaluated using histomorphometry, as described by Foldager et al. 31 Each defect was halved, and sections were cut for every 350 μm, resulting in a total of 7 sections per defect. All samples were stained with hematoxylin and eosin (H&E). 32
In each section, a region of interest was defined on the defect as the “repair tissue.” Using newCAST software (Visiopharm, Hørsholm, Denmark), a counting grid of 5 × 5 points was superimposed onto each section at 10× magnification. Using this software, 60% of the “repair tissue” was counted. Each point in the grid was counted according to the tissue type (hyaline cartilage, fibrocartilage, fibrous tissue, bone, or vascular tissue). Hyaline cartilage was defined as rounded cells located in lacunae within a hyaline matrix. Fibrocartilage was defined as rounded cells located in lacunae within a fibrous matrix. Fibrous tissue was defined as elongated cells located in a fibrous matrix. The sections were evaluated both with and without the use of polarized light microscopy to ensure the correct scoring of the tissue.
Semiquantitative Scoring
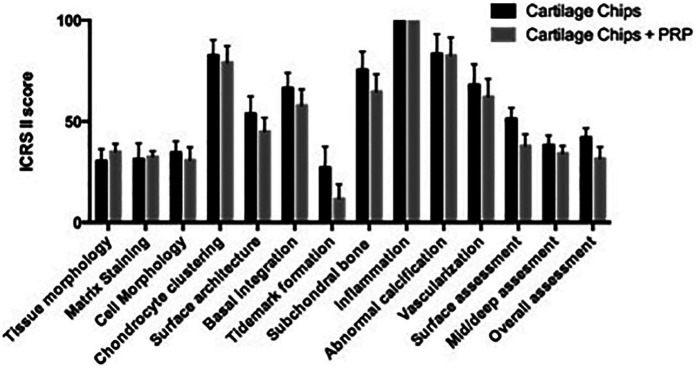
Each defect was evaluated using the ICRS II histological score. For the ICRS II score, the sections are evaluated on a visual analogue scale. There are 14 categories: tissue morphology, matrix staining, cell morphology, chondrocyte clustering, surface architecture, basal integration, formation of a tidemark, subchondral bone abnormalities, inflammation, abnormal calcification, vascularization, surface assessment, deep zone assessment, and overall assessment. 33
Statistics
The primary outcome was fraction of hyaline cartilage in repair tissue. The power analysis was based on the following assumptions based on previous studies.17,29
Hyaline cartilage formation in control group (CC alone): 20% (SD = 15%), and treatment group (CC + PRP): 40 % (SD: 20%). With these assumptions the sample size needed in each group was 12 defects.
The data were tested for normality using a histogram and QQ-plots. Because there were only 2 groups and no repeated measures for the outcome measures, Student’s t tests were applied. A P value <0.05 was considered significant. Statistical analysis was performed with STATA software version 11.2 (StataCorp, College Station, TX, USA).
Results
There were no postoperative complications, and all animals completed the follow-up period of 6 months.
PRP Analysis
Autologous PRP was generated from the blood of all the animals. The average fold increase in platelets was 8.6 ± 2.7. Leukocyte concentration also increased in PRP samples by an average fold change of 7.1 ± 1.9.
ICRS Macroscopic Score
At 6 months after the intervention, the repair tissues of the CC alone and the CC and PRP group were irregular and opaque. The condyles of both treatments showed differences in gross observation, ranging from samples with tissues that were slightly irregular, soft, and with a similar color to the surrounding cartilage to other samples with irregular tissue and bone exposure. The average ICRS score was 5.3 ± 1.2 for the CC and PRP group and 5.0 ± 1.3 for the CC group with no difference between groups. Both groups representing scores ranging exclusively in ICRS score group III: abnormal.
Histomorphometry and Histology
There were no significant differences found when comparing the fractions of hyaline cartilage (20.6% vs. 17.2%, P = 0.4), fibrocartilage (52.8% vs. 47.8%, P = 0.2), and fibrous tissue (22.9% vs. 24.8%, P = 0.4) in defects treated with CC and PRP compared with CC alone. Also, no differences were found when comparing the fractions of bone and vascular tissue (P > 0.05). The results are presented in Figure 1 .
Figure 1.
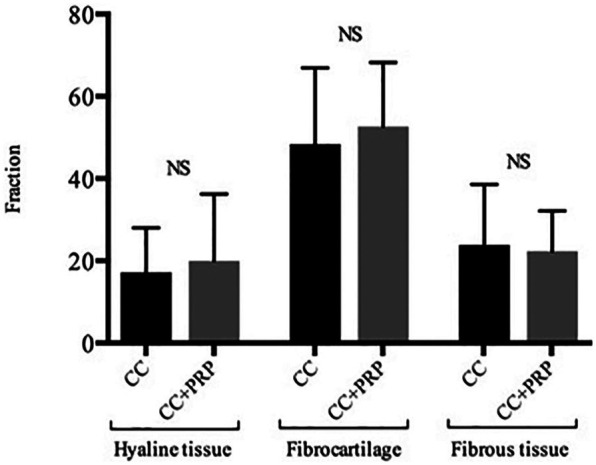
The fraction of hyaline tissue, fibrocartilage, and fibrous tissue in the cartilage repair tissue. CC, autologous cartilage chips transplantation; PRP, platelet-rich plasma; NS, nonsignificant. Error bars are standard error of mean (SEM).
The semiquantitative analysis of histological data using ICRS II score demonstrated no differences in any of the subscales ( Fig. 2 ). Histologic assessment of the osteochondral blocks revealed all stifles in both the CC and PRP and CC treatment groups had altered cartilage and bone histology. Observed changes included altered surface architecture, altered matrix staining, changes to the subchondral bone in form of irregularity of the subchondral bone plate as well as sporadic formation of small osteophytes in the defects. The CC and PRP group and the CC alone group had good filling of the defects. The repair tissue observed in the defects border area were predominantly fibrous or fibrocartilage tissue in all samples. Both treatment groups contained islands of viable hyaline cartilage tissue from cartilage chips were seen embedded in fibrocartilage tissue. In general, a mixture of hyaline cartilage, fibrocartilage, and fibrous tissue throughout the defects (Figs. 3, 4, and 5 ).
Figure 2.
International Cartilage Repair Society (ICRS) II scores. Error bars are standard error of mean (SEM).
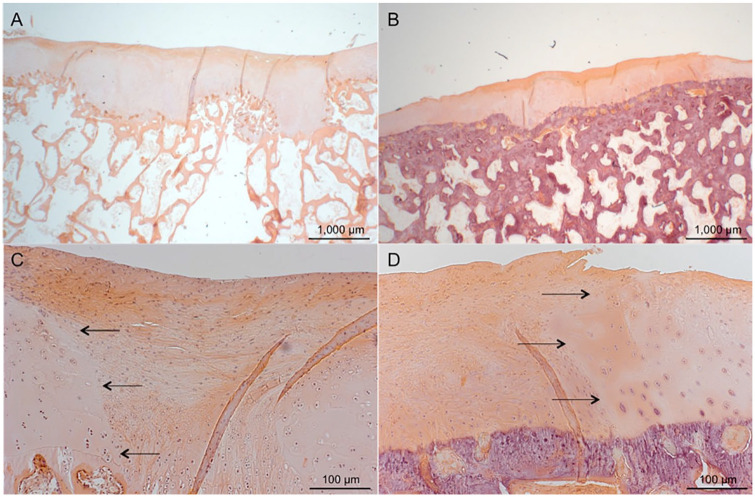
Figure 3.
Hematoxylin and eosin staining; scale bars 1000 μm (A and B) and 100 μm (C and D). (A and C) Histological section of defect treated with CC and PRP. The arrows mark the border between native cartilage and repair tissue. (B and D) A defect treated with CC alone. The defect is sufficiently filled, and the repair tissue is a mixture of fibrous tissue and fibrocartilage. Samples represent mean repair tissue quality. CC, autologous cartilage chips transplantation; PRP, platelet-rich plasma.
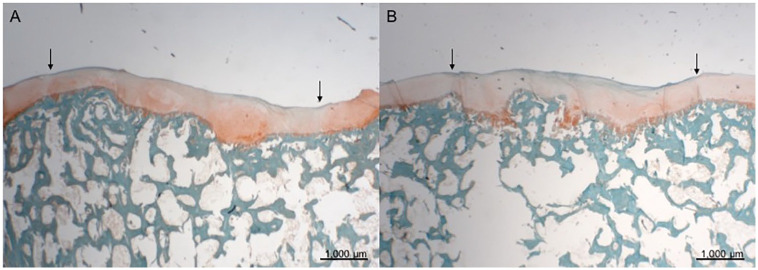
Figure 4.
Safranin O staining; scale bars 1000 μm. The arrows indicate the original defect area. (A) Treatment with CC and PRP. The filling of the defect is good. The repair tissue stains positive for glycosaminoglycans. An osteophyte in defect area is seen. (B) Treatment with CC alone. The filling of the defect is good. The repair tissue stains positive for glycosaminoglycans. Samples represent mean repair tissue quality. CC, autologous cartilage chips transplantation; PRP, platelet-rich plasma.
Figure 5.
Collagen type II staining; scale bars 1000 μm. (A) CC and PRP. (B) CC alone. The positive collagen type II staining is limited to a smaller area in the deepest part of the defect. Samples represent mean repair tissue quality. CC, autologous cartilage chips transplantation; PRP, platelet-rich plasma.
Discussion
The main finding of the present study is that repeated intraarticular PRP injections to cartilage chips transplantation treatment of full-thickness cartilage lesions did not improve the quality of the repair tissue.
To our knowledge, the present study is the first to investigate the contribution of PRP injections to hyaline cartilage regeneration of a particulated cartilage technique. The use of this adjuvant, however, did not contribute significantly to cartilage repair tissue quality when compared with particulated cartilage treatment alone after 6 months.
Because the investigated cartilage defects were solely full-thickness cartilage defects with no disruption of the subchondral bone, it is interesting to note the degree to which creating a chondral defect affects the underlying bone. We saw central osteophytes formation in both the CC and PRP–treated joints and CC alone joints although no significant differences were noted. McCauley et al. 34 described an association between full-thickness or near full-thickness cartilage defects with central osteophytes in naturally occurring disease in human knees. Similarly, Olive et al. 35 described central osteophytes deep within cartilage lesions in equine subjects. The finding of central osteophytes in the present study may indicate the natural progression of healing of bone and cartilage in areas where the immediate postoperative load could not be eliminated, leading to a bony healing response. The lack of subchondral and epiphyseal bone cysts in any defects is expected because both treatment groups had only a full-thickness cartilage defect that did not break the subchondral bone plate.
Another potential cause for lack of effect was that the PRP concentration was not adequate or correct for the promotion of chondral healing. However, in the current study, the preparation of PRP was performed using the protocol described by Wu et al. 36 In their research, Wu et al. successfully reconstructed an injectable cartilage graft by using autologous PRP and cultured chondrocytes without performing a platelet count, so there is evidence that PRP prepared with this protocol should at least be effective enough to allow for chondrocyte and chondral matrix growth. Moreover, the platelet concentrations in our PRP preparations were comparable to other studies using human PRP.37-39 After injection there are always an unknown model-related dilution of the PRP. This factor could reduce the effect of the PRP in the present study.
A limitation of our study could be that an optimal PRP concentration for chondral healing is not known; therefore, the PRP dosage is just chosen arbitrarily. But we did have documented platelet concentrations comparable to a standard human PRP preparation. Another limitation of the current study is that we only evaluated morphological parameters, and no functional data were analyzed. However, our aim was to describe the effect of PRP in the biological quality of the tissue generated after autologous particulated cartilage treatment, not its impact on joint function.
We chose repeated intra-articular injections over local application, which in theory would have guaranteed delivery of a higher concentration of platelets to the defects as opposed to the dissipated PRP given intraarticularly, however in a pilot trial we experienced that PRP compromised fibrin glue function, thus destabilizing the clot, and thereby risking the cartilage chips to detach from the defect area.
The strengths of the current study include the use of an easy and reproducible method and using the same anesthetic procedure, operative technique, and perioperative management protocol. We used a validated large animal model and that both quantitative histomorphometric and validated semiquantitative tissue repair evaluations were used.
Conclusion
In the present study, we were unable to demonstrate a beneficial effect of intraarticular administration of platelet-rich plasma as an adjuvant of cartilage chips transplantation to regenerate hyaline cartilage in acute full-thickness chondral lesions in a minipig model.
Footnotes
Acknowledgments and Funding: This study was financially supported by the Institute of Clinical Medicine, Aarhus University, Denmark and the Danish Rheumatism Association. The authors would like to thank laboratory technicians Anna Bay Nielsen and Anette Baatrup at the Orthopaedic Research Laboratory, Aarhus University Hospital, Denmark for their help and cooperation.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Ethical Approval: The study was approved by the Danish Ministry of Justice Ethical Committee, J.nr. 2012-15-2934-00301.
Animal Welfare: The study complied with the Danish Law on Animal Experimentation.
ORCID iDs: Kris Chadwick Hede  https://orcid.org/0000-0002-3309-5320
https://orcid.org/0000-0002-3309-5320
Martin Lind  https://orcid.org/0000-0002-7204-813X
https://orcid.org/0000-0002-7204-813X
References
- 1. Mithoefer K, Peterson L, Zenobi-Wong M, Mandelbaum BR. Cartilage issues in football—today’s problems and tomorrow’s solutions. Br J Sports Med. 2015;49(9):590-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Widuchowski W, Tomaszewski W, Widuchowski J, Czamara A. Current concepts in the treatment of cartilage lesions with special regard to the knee joint. Ortop Traumatol Rehabil. 2011;13(4):327-41. [DOI] [PubMed] [Google Scholar]
- 3. Gomoll AH, Farr J, Gillogly SD, Kercher J, Minas T. Surgical management of articular cartilage defects of the knee. J Bone Joint Surg Am. 2010;92(14):2470-90. [PubMed] [Google Scholar]
- 4. Gobbi A, Karnatzikos G, Kumar A. Long-term results after microfracture treatment for full-thickness knee chondral lesions in athletes. Knee Surg Sports Traumatol Arthrosc. 2014;22(9):1986-96. [DOI] [PubMed] [Google Scholar]
- 5. Steadman JR, Rodkey WG, Rodrigo JJ. Microfracture: surgical technique and rehabilitation to treat chondral defects. Clin Orthop Relat Res. 2001(391 Suppl):S362-9. [DOI] [PubMed] [Google Scholar]
- 6. Lu Y, Dhanaraj S, Wang Z, Bradley DM, Bowman SM, Cole BJ, et al. Minced cartilage without cell culture serves as an effective intraoperative cell source for cartilage repair. J Orthop Res. 2006;24(6):1261-70. [DOI] [PubMed] [Google Scholar]
- 7. Christensen BB. Autologous tissue transplantations for osteochondral repair. Dan Med J. 2016;63(4):B5236. [PubMed] [Google Scholar]
- 8. Adkisson HD, 4th, Martin JA, Amendola RL, Milliman C, Mauch KA, Katwal AB, et al. The potential of human allogeneic juvenile chondrocytes for restoration of articular cartilage. Am J Sports Med. 2010;38(7):1324-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Bonasia DE, Martin JA, Marmotti A, Amendola RL, Buckwalter JA, Rossi R, et al. Cocultures of adult and juvenile chondrocytes compared with adult and juvenile chondral fragments: in vitro matrix production. Am J Sports Med. 2011;39(11):2355-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Cole BJ, Farr J, Winalski CS, Hosea T, Richmond J, Mandelbaum B, et al. Outcomes after a single-stage procedure for cell-based cartilage repair: a prospective clinical safety trial with 2-year follow-up. Am J Sports Med. 2011;39(6):1170-9. [DOI] [PubMed] [Google Scholar]
- 11. Farr J, Tabet SK, Margerrison E, Cole BJ. Clinical, radiographic, and histological outcomes after cartilage repair with particulated juvenile articular cartilage: a 2-year prospective study. Am J Sports Med. 2014;42(6):1417-25. [DOI] [PubMed] [Google Scholar]
- 12. Liu H, Zhao Z, Clarke RB, Gao J, Garrett IR, Margerrison EE. Enhanced tissue regeneration potential of juvenile articular cartilage. Am J Sports Med. 2013;41(11):2658-67. [DOI] [PubMed] [Google Scholar]
- 13. Marmotti A, Bruzzone M, Bonasia DE, Castoldi F, Rossi R, Piras L, et al. One-step osteochondral repair with cartilage fragments in a composite scaffold. Knee Surg Sports Traumatol Arthrosc. 2012;20(12):2590-601. [DOI] [PubMed] [Google Scholar]
- 14. Riboh JC, Cole BJ, Farr J. Particulated articular cartilage for symptomatic chondral defects of the knee. Curr Rev Musculoskelet Med. 2015;8(4):429-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Bonasia DE, Martin JA, Marmotti A, Kurriger GL, Lehman AD, Rossi R, et al. The use of autologous adult, allogenic juvenile, and combined juvenile-adult cartilage fragments for the repair of chondral defects. Knee Surg Sports Traumatol Arthrosc. 2016;24(12):3988-96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Christensen BB, Foldager CB, Jensen J, Lind M. Autologous dual-tissue transplantation for osteochondral repair: early clinical and radiological results. Cartilage. 2015;6(3):166-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Christensen BB, Foldager CB, Olesen ML, Hede KC, Lind M. Implantation of autologous cartilage chips improves cartilage repair tissue quality in osteochondral defects: a study in Göttingen minipigs. Am J Sports Med. 2016;44(6):1597-604. [DOI] [PubMed] [Google Scholar]
- 18. Christensen BB, Foldager CB, Olesen ML, Vingtoft L, Rölfing JH, Ringgaard S, et al. Experimental articular cartilage repair in the Göttingen minipig: the influence of multiple defects per knee. J Exp Orthop. 2015;2(1):13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Erggelet C, Vavken P. Microfracture for the treatment of cartilage defects in the knee joint—a golden standard? J Clin Orthop Trauma. 2016;7(3):145-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Fortier LA, Potter HG, Rickey EJ, Schnabel LV, Foo LF, Chong LR, et al. Concentrated bone marrow aspirate improves full-thickness cartilage repair compared with microfracture in the equine model. J Bone Joint Surg Am. 2010;92(10):1927-37. [DOI] [PubMed] [Google Scholar]
- 21. Hoemann CD, Hurtig M, Rossomacha E, Sun J, Chevrier A, Shive MS, et al. Chitosan-glycerol phosphate/blood implants improve hyaline cartilage repair in ovine microfracture defects. J Bone Joint Surg Am. 2005;87(12):2671-86. [DOI] [PubMed] [Google Scholar]
- 22. Stanish WD, McCormack R, Forriol F, Mohtadi N, Pelet S, Desnoyers J, et al. Novel scaffold-based BST-CarGel treatment results in superior cartilage repair compared with microfracture in a randomized controlled trial. J Bone Joint Surg Am. 2013;95(18):1640-50. [DOI] [PubMed] [Google Scholar]
- 23. Kon E, Filardo G, Di Martino A, Marcacci M. Platelet-rich plasma (PRP) to treat sports injuries: evidence to support its use. Knee Surg Sports Traumatol Arthrosc. 2011;19(4):516-27. [DOI] [PubMed] [Google Scholar]
- 24. Manunta AF, Manconi A. The treatment of chondral lesions of the knee with the microfracture technique and platelet-rich plasma. Joints. 2013;1(4):167-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Papalia R, Balzani LD, Torre G, Tirindelli MC, Nobile C, Maffulli N, et al. Intraoperative application platelet rich fibrin, postoperative injections OF PRP or microfracture only for osteochondral lesions of the knee: a five-year retrospective evaluation. J Biol Regul Homeost Agents. 2016;30(4 Suppl 1):41-9. [PubMed] [Google Scholar]
- 26. Castillo TN, Pouliot MA, Kim HJ, Dragoo JL. Comparison of growth factor and platelet concentration from commercial platelet-rich plasma separation systems. Am J Sports Med. 2011;39(2_suppl):266-71. [DOI] [PubMed] [Google Scholar]
- 27. McCarrel TM, Minas T, Fortier LA. Optimization of leukocyte concentration in platelet-rich plasma for the treatment of tendinopathy. J Bone Joint Surg Am. 2012;94(19):e143(1-8). [DOI] [PubMed] [Google Scholar]
- 28. Mascarenhas R, Saltzman BM, Fortier LA, Cole BJ. Role of platelet-rich plasma in articular cartilage injury and disease. J Knee Surg. 2015;28(1):3-10. [DOI] [PubMed] [Google Scholar]
- 29. Christensen BB, Olesen ML, Lind M, Foldager CB. Autologous cartilage chip transplantation improves repair tissue composition compared with marrow stimulation. Am J Sports Med. 2017;45(7):1490-6. [DOI] [PubMed] [Google Scholar]
- 30. van den Borne MP, Raijmakers NJ, Vanlauwe J, Victor J, de Jong SN, Bellemans J, et al. International Cartilage Repair Society (ICRS) and Oswestry macroscopic cartilage evaluation scores validated for use in autologous chondrocyte implantation (ACI) and microfracture. Osteoarthritis Cartilage. 2007;15(12):1397-402. [DOI] [PubMed] [Google Scholar]
- 31. Foldager CB, Nyengaard JR, Lind M, Spector M. A stereological method for the quantitative evaluation of cartilage repair tissue. Cartilage. 2015;6(2_suppl):123-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Christensen BB, Foldager CB, Hansen OM, Kristiansen AA, Le DQ, Nielsen AD, et al. A novel nano-structured porous polycaprolactone scaffold improves hyaline cartilage repair in a rabbit model compared to a collagen type I/III scaffold: in vitro and in vivo studies. Knee Surg Sports Traumatol Arthrosc. 2012;20(6):1192-204. [DOI] [PubMed] [Google Scholar]
- 33. Mainil-Varlet P, Van Damme B, Nesic D, Knutsen G, Kandel R, Roberts S. A new histology scoring system for the assessment of the quality of human cartilage repair: ICRS II. Am J Sports Med. 2010;38(5):880-90. [DOI] [PubMed] [Google Scholar]
- 34. McCauley TR, Kornaat PR, Jee WH. Central osteophytes in the knee: prevalence and association with cartilage defects on MR imaging. AJR Am J Roentgenol. 2001;176(2_suppl):359-64. [DOI] [PubMed] [Google Scholar]
- 35. Olive J, D’Anjou MA, Girard C, Laverty S, Theoret CL. Imaging and histological features of central subchondral osteophytes in racehorses with metacarpophalangeal joint osteoarthritis. Equine Vet J. 2009;41(9):859-64. [DOI] [PubMed] [Google Scholar]
- 36. Wu W, Zhang J, Dong Q, Liu Y, Mao T, Chen F. Platelet-rich plasma—a promising cell carrier for micro-invasive articular cartilage repair. Med Hypotheses. 2009;72(4):455-7. [DOI] [PubMed] [Google Scholar]
- 37. Ehrenfest DMD, Rasmusson L, Albrektsson T. Classification of platelet concentrates: from pure platelet-rich plasma (P-PRP) to leucocyte- and platelet-rich fibrin (L-PRF). Trends Biotechnol. 2009;27(3):158-67. [DOI] [PubMed] [Google Scholar]
- 38. Lee GW, Son JH, Kim JD, Jung GH. Is platelet-rich plasma able to enhance the results of arthroscopic microfracture in early osteoarthritis and cartilage lesion over 40 years of age? Eur J Orthop Surg Traumatol. 2013;23(5):581-7. [DOI] [PubMed] [Google Scholar]
- 39. Weibrich G, Kleis WK, Hafner G, Hitzler WE, Wagner W. Comparison of platelet, leukocyte, and growth factor levels in point-of-care platelet-enriched plasma, prepared using a modified Curasan kit, with preparations received from a local blood bank. Clin Oral Implants Res. 2003;14(3):357-62. [DOI] [PubMed] [Google Scholar]