Abstract
Objective
Cartilage lesions in the knee joint can lead to joint mechanics changes and cause knee pain. Bone marrow stimulation (BMS) promotes cartilage regeneration by perforating the subchondral bone just below the injury and inducing bone marrow cells. This study aimed to investigate whether systemic administration of granulocyte colony-stimulating factor (G-CSF) with BMS improves repair of chronic partial-thickness cartilage defects (PTCDs).
Design
Eighteen 6-month-old New Zealand white rabbits were divided into 3 groups: control (C, n = 6), BMS alone (n = 6), and BMS + G-CSF (n = 6). Partial cartilage defects with 5 mm diameter were created in the trochlear region of both knees; after 4 weeks, the BMS alone and BMS + G-CSF groups underwent BMS; G-CSF (50 µg/kg) or saline was administered subcutaneously for 5 days starting from 3 days before BMS. At 8 and 16 weeks after cartilage defect creation, the area of cartilage defects was macroscopically and histologically evaluated.
Results
International Cartilage Repair Society (ICRS) grades for macroscopic assessment were 0, 0.7, and 0.7 at 8 weeks and 0, 1.2, and 1.3 at 16 weeks in the C, BMS, and BMS + G-CSF groups, respectively. Wakitani scores for histological assessment were 9.8, 8.7, and 8.2 at 8 weeks and 9.5, 9, and 8.2 at 16 weeks in the C, BMS, and BMS + G-CSF groups, respectively. The BMS + G-CSF group showed significantly more repair than the C group, but there was no difference from the BMS group.
Conclusions
The effect of BMS and G-CSF on chronic PTCDs in mature rabbit knees was limited.
Keywords: partial-thickness cartilage defects, bone marrow stimulation, granulocyte colony-stimulating factor
Introduction
Cartilage lesions in the knee joint are common, can alternate joint mechanics, and might cause knee pain.1-3 Although accurate assessment of the true prevalence of cartilage lesions is difficult because they are often asymptomatic and unreported, 3 arthroscopic case series reported the incidence of lesions as 57% to 63%.4-7 Often, the cases were chronic with a relatively higher frequency of partial cartilage defects, where treatment tended to lead to unfavorable results compared to fresh cases.8-10 Contrary to the clinical situation, most animal studies have dealt with fresh full-thickness cartilage defects and osteochondral defects,11-17 and there are only few reports on chronic partial-thickness cartilage defects (PTCDs).
The treatment option for cartilage lesions includes bone marrow stimulation (BMS), 18 mosaic plasty, 19 and autologous chondrocyte implantation. 20 Among these, BMS is the most simple and minimally invasive treatment. The surgical procedure of BMS involves perforating a small hole through the subchondral bone and bleeding from the cancellous bone. The blood clots fill the cartilage defect and provide bone marrow mesenchymal stem cells (MSCs), which are believed to play an important role in cartilage repair. However, its indications are limited to small defects, and the resultant tissue is mainly composed of fibrous cartilage.21,22 To compensate for these shortcomings, systemic administration of bone marrow stimulants has been reported as a way to increase the efficacy of BMS in cartilage repair by increasing the outflow of MSCs from the bone marrow.23,24 Granulocyte colony-stimulating factor (G-CSF) is one of them, and Okano reported enhanced repair in freshly created osteochondral lesion in rats. 24 We also reported the potential of accelerated healing with G-CSF administration combined with BMS on fresh osteochondral defects in rabbit knee joints. 25
Considering clinical situations and the potential of G-CSF, the effect of G-CSF on chronic partial cartilage defect should be elucidated. This study aimed to investigate the effects of BMS and systemic administration of G-CSF on chronic PTCD.
Methods
Animals
A total of 18 male New Zealand white rabbits aged 24 to 26 weeks, weighing 2.8 to 3.6 kg, were used for the experiments. All animals were housed at the Laboratory Animal Center at the Graduate School of Medicine, Chiba University. All protocols for animal procedures were approved by the Ethics Committee of the Graduate School of Medicine, Chiba University, and followed the National Institutes of Health guidelines for the care and use of laboratory animals. The rabbits were randomly divided into 3 groups: control, BMS (PTCD + BMS), and BMS + G-CSF group (PTCD + BMS + G-CSF).
Chronic PTCD Creation and BMS Procedure
After general anesthesia, both lower extremities were disinfected with povidone iodine and draped under sterile conditions. A medial parapatellar approach was performed in each knee, and the skin incision was 30 mm longitudinally. The patella was laterally dislocated to allow full exposure of the articular surface, and a PTCD of 5 mm in diameter was created on the trochlea of the femur. A 5-mm-diameter rim was initially made using a standard skin biopsy punch, and a PTCD was prepared using a ring curette (Smith & Nephew, Andover, MA, USA) with careful removal of focal cartilage to reach at the depth of the calcified layer. No bleeding was observed from the defect. The knee was closed in sutured layers. A second surgery was performed 4 weeks after the initial surgery. After identifying original lesions, a ring curette (Smith & Nephew, Andover, MA, USA) was used to debride the calcified layer and repair tissues without damaging the subchondral bone plate in all the three groups. To perform BMS, 3 subchondral perforations were created using a 1.0-mm-diameter pin vice drill (Kaneko Mfg Corporation, Niigata, Japan). After confirming bleeding from perforation sites ( Fig. 1 ), the wound was closed in layers. G-CSF (GRAN; Kyowa Hakko Kirin, Tokyo, Japan) or saline was injected subcutaneously at a dose of 50 µg/kg (0.5 mL for saline) at a fixed time every day, starting 2 days before BMS. BMS was performed after the injection on day 3. Injection of G-CSF or saline was continued until day 5. All procedures were performed by a single surgeon (Y.O.). All rabbits were separately caged after surgery and walked without restrictions.
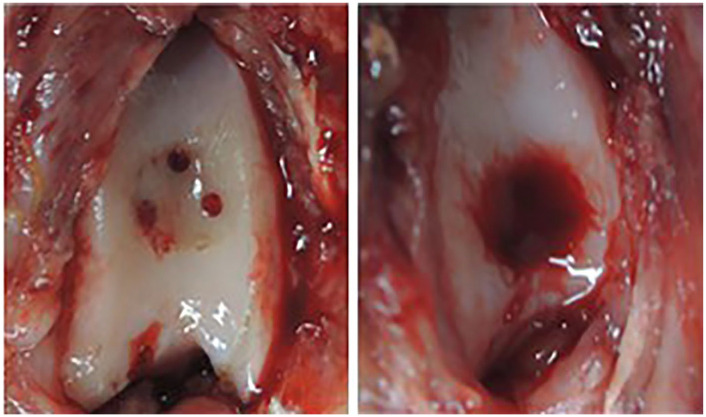
Figure 1.
Macroscopic appearance of bone marrow stimulation (BMS). After debridement 3 subchondral perforations were made (left) and fulfillment of cartilage defect with enough bleeding was confirmed prior to closure (right).
Measurement of Peripheral Blood
The number of leukocytes in the peripheral blood was measured at 3 points: immediately before G-CSF administration, on the day of BMS, and 1 week after G-CSF administration. Two rabbits from each group were evaluated using an automatic blood cell counter (ADVIA 2120i, Siemens Diagnostics, Tarrytown, NY, USA).
Macroscopic Evaluation
Three rabbits from each group were euthanized 8 and 16 weeks after the initial surgery, and bilateral knees were harvested from each rabbit. Macroscopic evaluation was performed by 2 blinded graders (Y.O. and S.W.). The International Cartilage Repair Society (ICRS) macroscopic cartilage evaluation score and grading (grade 1, normal, 12 points; grade 2, nearly normal, 8-11 points; grade 3, abnormal, 4-7 points; grade 4, severely abnormal, 0-3 points) were used to assess all cartilage repairs. 26
Histological Evaluation
After macroscopic scoring, the dissected distal femurs were fixed with 4% paraformaldehyde solution for 2 days at room temperature, decalcified with 10% ethylenediamine tetraacetic acid for 3 weeks, and then processed and embedded in paraffin blocks for histological evaluation. A 5-μm-thick section was prepared from the center of each defect using a microtome. Histological assessment was done with a single sagittal slice that passed through the circle center of cartilage injury as well as a BMS hole. Sections were histologically stained with hematoxylin-eosin and safranin-O and were blindly scored by 2 researchers (Y.O. and S.W.) using the Wakitani score. This score is composed of 4 items, cell morphology and matrix staining (metachromasia), surface regularity, thickness of cartilage, and integration of donor with host, with 0 point being normal and 14 points being no repair (cell morphology, 0-4 points; matrix staining, 0-3 points; surface regularity, 0-3 points; thickness of cartilage, 0-2 points; integration of donor with host, 0-2 points). 27
For immunohistochemical staining, a mouse monoclonal antibody against human type II collagen (#F-57, Kyowa Pharmaceuticals, Tokyo, Japan) was used. Before staining, specimens were deparaffinized in an enzyme solution of hyaluronidase (H3506; Sigma-Aldrich Corporation, St. Louis, MO, USA) dissolved to 0.05% in 100 mM acetate buffer (pH 5.0) at 37 °C, and pretreatment was performed by soaking for 60 minutes. The primary antibody was anti-COL2 mouse monoclonal (clone, II-4C11) antibody (F-57; Kyowa Pharma Chemical Co., Ltd., Takaoka, Toyama, Japan) diluted 500-fold and used as a primary antibody in an overnight reaction. As a secondary antibody, anti-mouse IgG goat polyclonal antibody (Histofine #424134; Nichirei Corporation, Tokyo, Japan) labeled with horse radish peroxidase was used and reacted with the specimens for 30 minutes at room temperature. Positive sites were visualized as brownish brown using 3,3′-diaminobenzidine tetrahydrochloride (K3468, DAKO, Glostrup, Denmark) as a chromogenic substrate; then, the nuclei were contrast-stained to a light purple color using hematoxylin.
Statistical Analysis
All statistical analyses were conducted using JMP 15 (SAS Institute Inc., Cary, NC, USA). Descriptive statistics were first calculated. The Wilcoxon rank-sum test was used to assess differences in the number of white blood cells in the peripheral blood at each time point, and the Kruskal-Wallis test was used to test for differences between various treatment groups postoperatively. A P value of <0.05 was regarded as significant.
Results
White Blood Cell Count in Peripheral Blood
There was a significant increase in the white blood cell count 3 days after the start of G-CSF treatment (on the day of BMS), and a downward trend was observed 1 week after the start of treatment. There was no significant increase in white blood cell count at any blood collection point in the group that did not receive G-CSF (saline injection) ( Fig. 2 ).
Figure 2.
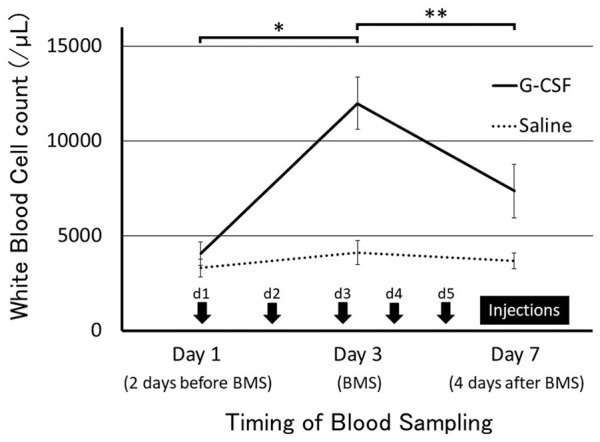
Timing of granulocyte colony-stimulating factor (G-CSF) injection and white blood cell count. Injections were given once a day from day 1 to day 5. Values are presented as mean ± SD. *P < 0.05, **P < 0.01.
Macroscopic Findings
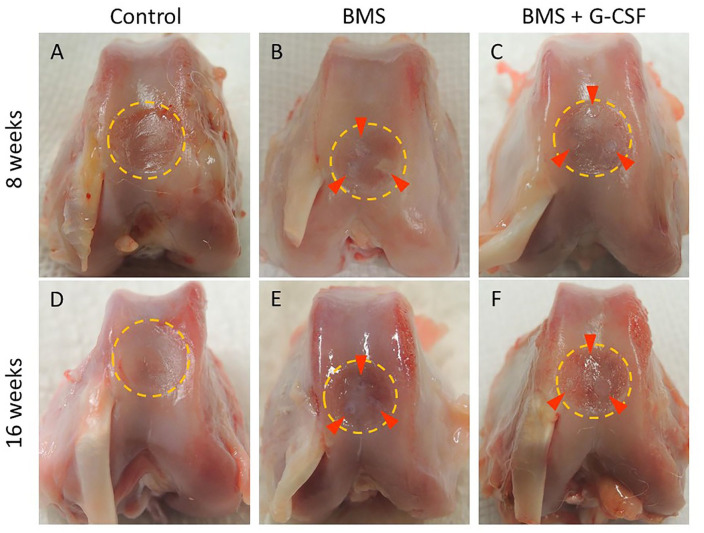
The macroscopic findings when performing BMS 4 weeks after the creation of the PTCD showed no white repair tissue in the chronic defect. At 8 weeks, the PTCD area was mostly left unchanged, and a small area adjacent to BMS holes was just filled with white tissue. At 16 weeks was nearly the same as that at 8 weeks. There was only a slight increase in covered area surrounding the bone holes ( Fig. 3 ). A significant difference in the ICRS score was observed between the BMS + G-CSF and control groups, but not between the BMS + G-CSF and BMS groups. Like the ICRS score at 8 weeks, a significant difference was found between the BMS + G-CSF and control groups, but not between the BMS + G-CSF and BMS groups (control group vs. BMS group [at 8 weeks]: 0 ± 0 vs. 0.67 ± 0.52, P = 0.047; control group vs. BMS + G-CSF group [at 8 weeks]: 0 ± 0 vs. 0.67 ± 0.52, P = 0.047; control group vs. BMS + G-CSF group [at 16 weeks]: 0 ± 0 vs. 1.3 ± 0.82; P = 0.003) ( Fig. 4 ).
Figure 3.
Macroscopic appearance of reparative tissue. A to C are 8 weeks after the creation of the partial-thickness chondral defect. D to F are 16 weeks after the creation of the partial-thickness chondral defect. Dotted yellow circles and red arrowheads indicate the defect area and BMS holes, respectively. BMS, bone marrow stimulation; G-CSF, granulocyte colony-stimulating factor.
Figure 4.
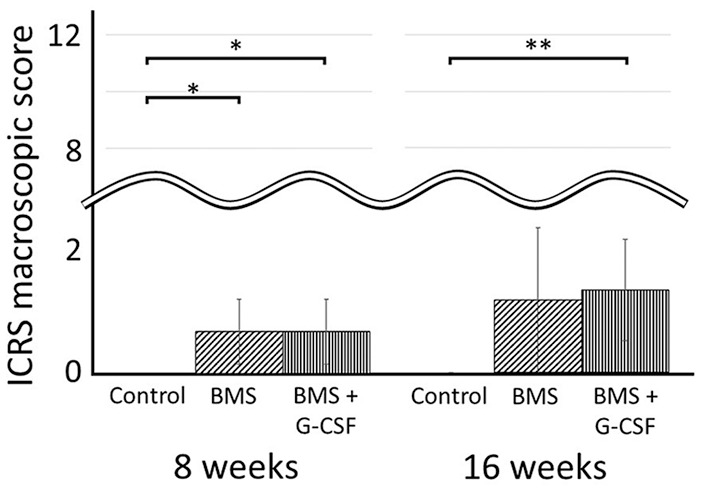
ICRS macroscopic scores at 8 and 16 weeks. Values are presented as mean ± SD. *P < 0.05, **P < 0.01. ICRS, International Cartilage Repair Society; BMS, bone marrow stimulation; G-CSF, granulocyte colony-stimulating factor.
Histological Findings
Histological images at 8 weeks after the creation of the initial cartilage injury are shown in Figures 5 and 6 . There was no apparent regeneration of the cartilage in all the 3 groups. Created bone holes of BMS were filled with fibrous tissue with abundant cells partly stained with safranin-O staining both in the BMS and BMS + G-CSF groups, which was indicative of endochondral ossification ( Fig. 6 ). Histological images at 16 weeks after the creation of the cartilage injury are shown in Figures 7 and 8 . There was no apparent regeneration of the cartilage injury in all the three groups. In some cases of BMS + G-CSF group reparative tissue was observed but amount of repair tissue was not consistent ( Fig. 7I ). In both the BMS and BMS+G-CSF groups, subchondral bone repair at BMS bone holes progressed and bone tissue emerged. Comparing to incomplete surface recovery of created bone holes of BMS in the BMS group, complete filling with bone nearly creating level surface to adjacent bone in the BMS + G-CSF group ( Fig. 8 ). Bone cyst formation was found only in 2 cases of BMS alone group at 16 weeks (data not shown as images). Wakitani scores are shown in Figure 9 . There was no significant difference between the BMS and BMS + G-CSF groups at either 8 or 16 weeks, whereas there was a significant difference between the control and BMS + G-CSF groups (control group vs. BMS + G-CSF group [at 8 weeks]: 9.8 ± 0.75 vs. 8.2 ± 0.41, P = 0.01; control group vs. BMS + G-CSF group [at 16 weeks]: 9.5 ± 0.84 vs. 8.2 ± 0.75, P = 0.003). Considering score of 0 being normal on Wakitani score, derived scores were far from normal cartilage.
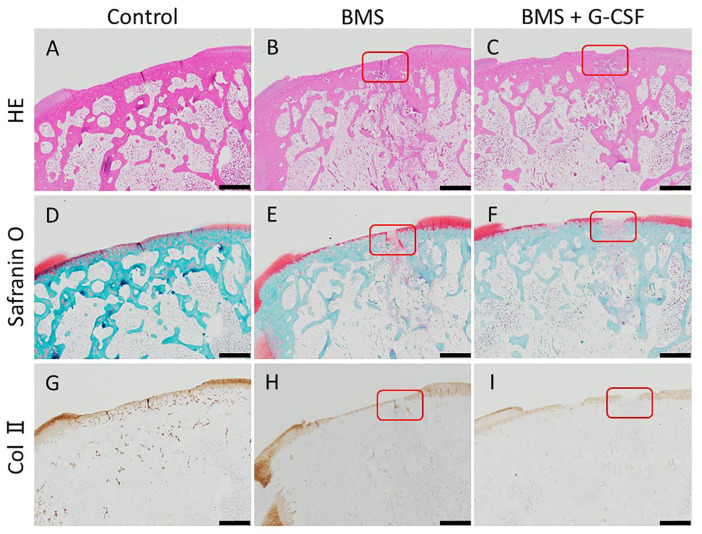
Figure 5.
Histological images in low magnification at 8 weeks. No apparent repair is observed in all the three groups. BMS holes are represented by red rectangles. (HE staining: A, C. Safranin-O staining: D, F. Collagen type II staining: G, I. Scale bars = 100 µm. HE, hematoxylin and eosin; Col II, collagen type II; BMS, bone marrow stimulation; G-CSF, granulocyte colony-stimulating factor).
Figure 6.
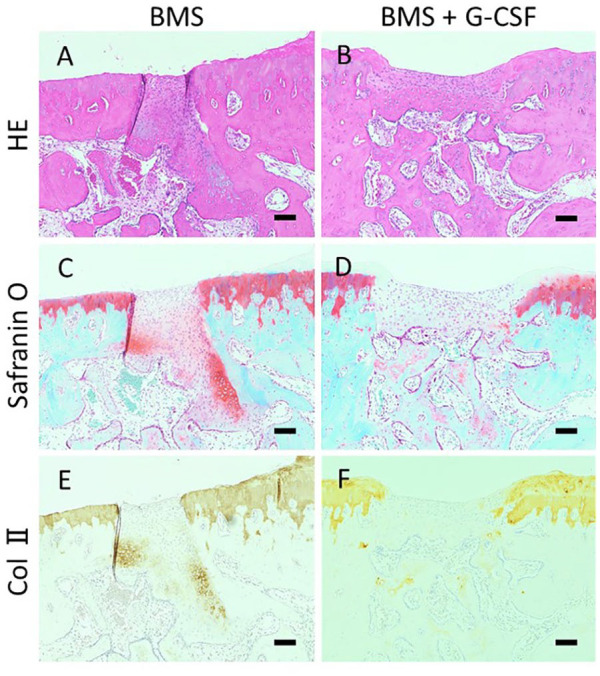
Histological images in high magnification at 8 weeks. The BMS hole in Figure 5 is magnified higher. Fillings of bone perforation with fibrous tissue with abundant cells were observed. Part of the tissue was stained with safranin-O and positive for type-II collagen. (HE staining: A, B. Safranin-O staining: C, D. Collagen type II staining: E, F. Scale bars = 100 µm. HE, hematoxylin and eosin; Col II, collagen type II; BMS, bone marrow stimulation; G-CSF, granulocyte colony-stimulating factor).
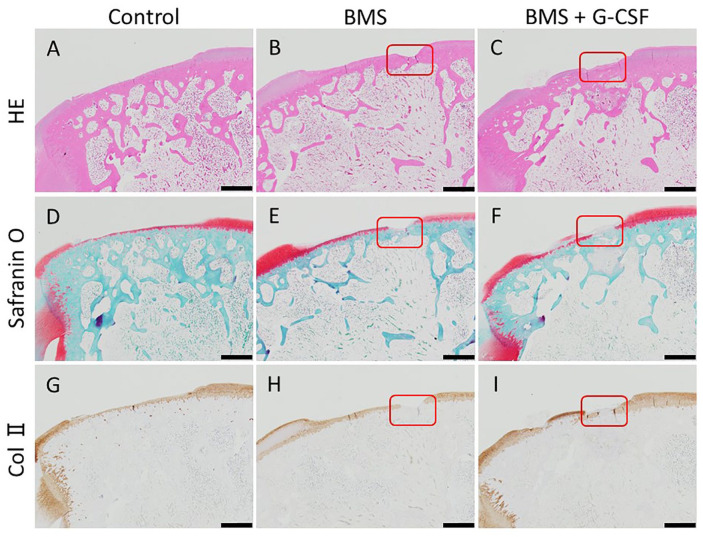
Figure 7.
Histological images in low magnification at 16 weeks. No apparent repair is observed in all the 3 groups but nearly half thickness repair tissue was found on the surface of the lesion in BMS + G-CSF in the presented case. BMS holes are represented by red rectangles. (HE staining: A, C. Safranin-O staining: D, F. Collagen type II staining: G, I. Scale bars = 100 µm. HE, hematoxylin and eosin; Col II, collagen type II; BMS, bone marrow stimulation; G-CSF, granulocyte colony-stimulating factor).
Figure 8.
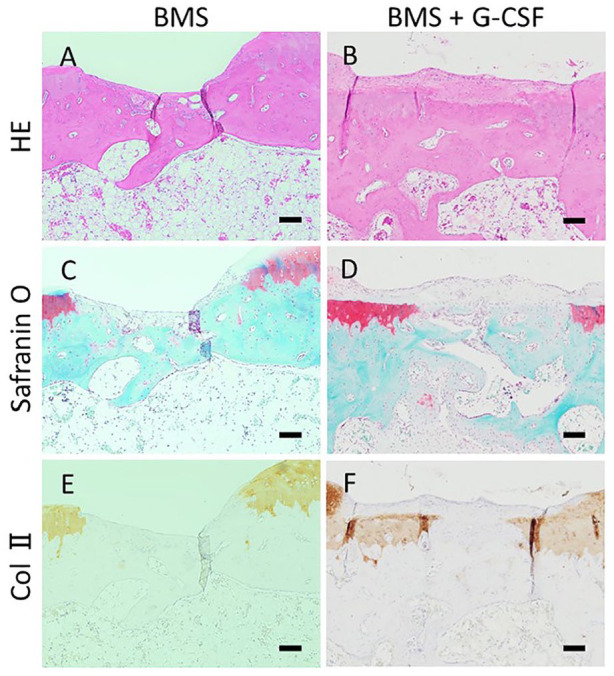
Histological images in high magnification at 16 weeks. The BMS hole in Figure 7 is magnified higher. BMS holes were incompletely filled with bony structure resulting in indented surface in BMS group (A, C, E) whereas filled with bony structure nearly reaching to the original surface above with accompanying fibrous tissue in BMS + G-CSF group (B, D, F). (HE staining: A, B. Safranin-O staining: C, D. Collagen type II staining: E, F. Scale bars = 100 µm. HE, hematoxylin and eosin; Col II, collagen type II; BMS, bone marrow stimulation; G-CSF, granulocyte colony-stimulating factor).
Figure 9.
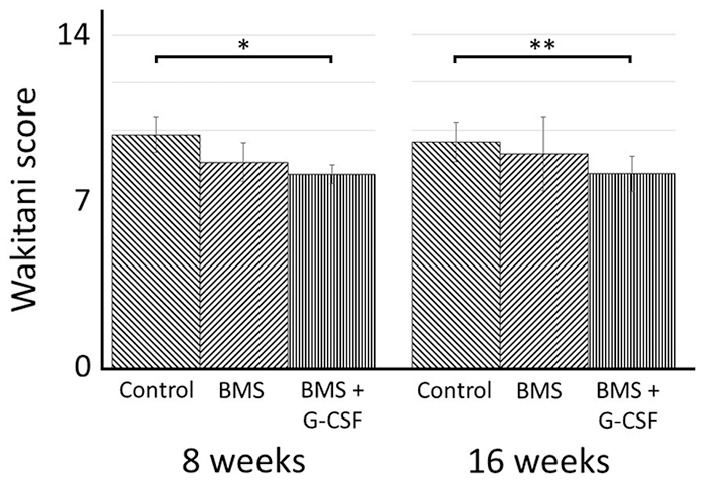
Histological scores (Wakitani scores) at 8 and 16 weeks. Values are presented as mean ± SD. *P < 0.01, **P < 0.05. BMS, bone marrow stimulation; G-CSF, granulocyte colony-stimulating factor.
Discussion
The present study showed that BMS combined with systemic administration of G-CSF did not produce better cartilage repair for chronic PTCDs than BMS alone. Compared with the control group, the BMS alone and BMS + G-CSF groups resulted in significantly better macroscopic and histological repair, but histologically, there was insufficient repair of the cartilage above the subchondral bone in either group. To the best of our knowledge, this is the first study to demonstrate that systemic administration of G-CSF has no obvious effect on chronic PTCD in vivo.
Contrary to the present study, Sasaki et al. 25 reported that cartilage repair of a rabbit knee was promoted by BMS with systemic administration of G-CSF. The possible explanation for this discrepancy is the timing of intervention, age of the rabbit, and depth of cartilage defect. 25 Chronic PTCD appeared as the prevailing type of injury in clinical practice, but none of previous animal studies have addressed this type of defects. Our model was the first to challenge this, but poor repair indicated the need for further modification to overcome this defect.
Age was another important factor. Mithoefer et al. 28 reported that aging and chronic defects are factors that lead to poor performance of BMS and may have contributed to the occurrence of poor repair of cartilage defects in the current study. We used 24- to 26-week-old rabbits that were sufficiently old to be skeletally mature compared with those in other studies, but Sasaki et al. used 12-week-old rabbits that were skeletally immature.25,29 Thus, the age difference might affect the effectiveness of G-CSF on cartilage defect repair.
Depth is another important factor in cartilage injury treatment. A previous study reported successful treatments for chronic osteochondral defects intervened with BMS and topical platelet-rich plasma (PRP), 30 BMS and freeze-dried chitosan/PRP, 31 and distraction and mesenchymal stem cells. 32 Although partial cartilage defect and osteochondral defect have not been directly compared, osteochondral defect appeared to have a possible advantage in that they could introduce bone marrow cells into injured site just by creating them. Therefore, the present study mimicked a more challenging situation.
There are several options for the treatment of cartilage injuries, and BMS is a simple and widely used treatment. Cartilage repaired by BMS is known to be a fibrous to hyaline-like tissue. MSCs play an important role in the process of cartilage repair.33-35 However, the small number of MSCs in the blood clots generated by BMS limits the effectiveness of BMS in clinical practice.36,37 In this study, we considered that G-CSF could recruit more MSCs by BMS. Because G-CSF has been shown to be a cytokine that promoted the mobilization of MSCs to both the bone marrow and peripheral blood, 23 G-CSF was also reported to promote the proliferation of MSCs in vivo. 25 However, better repair was not achieved. Although we did not assess the emergence of MSCs on the injured site, insufficient effect of G-CSF to recruit MSCs might cause the difference in results. Because direct addition of MSCs into joints or injured site resulted in enhanced cartilage repair, the administration of bone marrow–derived MSCs intra-articularly or onto cartilage defects directly was more effective than BMS alone.32,38,39 Systemic administration of several agents also resulted in better repair. Systemic administration of parathyroid hormone, 40 granulocyte macrophage CSF, 39 and G-CSF,24,25 and oral administration of losartan 13 promote repair of osteochondral lesions in animal models.
In this study, we could not achieve better repair of cartilage repair than BMS alone with the addition of G-CSF, which might indicate the difficulties in the treatment of chronic PTCD. Studies dealing with this type of cartilage defects, especially in aged animals, should be performed more intensely. Jansen et al. 41 reported that chronic cartilage injury leads to progressive cartilage deterioration and progression to osteoarthritis. With an aging population, osteoarthritis is a common and economically important disease that affects a large number of individuals.42,43 Moreover, chronic cartilage defects were one of the major causes of osteoarthritis of the knee joints.1-3 Ideally, through less-invasive techniques such as systemic administration of potential agents or multipotent cells, more effective methods should be sought. Mahmoud et al. 44 reported that intravenous administration of pluripotent cells (Muse cells) for osteochondral defects resulted in better repair than that of MSCs.
This study has several limitations. First, in the statistical analysis, the 2 knees obtained from each rabbit were independently treated. Generally, it is quite possible that there is a correlation between the treatment courses of the 2 knees obtained from a single rabbit. However, from an animal welfare perspective, using both knees to minimize the number of rabbits used in the experiment was necessary. Second, we have not measured cytokine levels in the joint or at the site of cartilage defect. It is unclear what cytokines are involved in the repair of the cartilage defect site. However, in the present study, an increase in the number of leukocytes in the peripheral blood was observed after the administration of G-CSF, and Okano et al. 24 also reported an increase in the number of leukocytes in the peripheral blood. Sasaki et al. 25 reported an increase in MSCs after systemic administration of G-CSF in vitro. Third, we do not optimize the BMS in terms of how many BMS holes should be drilled and what hole size is effective. Min et al. 35 reported that the number of MSCs varied depending on the size and number of BMS holes. In addition, depth of debridement might not be enough to remove all the calcified layer. Frisbie et al. 45 reported using horse knee that removal of calcified tissue was important to provide better environment for repair tissue to attach. However, all the model in the present study were created by an experienced single surgeon with the use of reliable instruments so that 3 groups of models were consistent enough and we believed we could examine the effect of G-CSF added to BMS.
Last, we did not employ any scaffold that might enhance cartilage repair.
Preoperative systemic administration of G-CSF is not technically challenging, as it does not require preoperative cell collection, culture, or invasion of the joint.
In the future, intra-articular injection or systemic drug administration that does not even require BMS would be ideal. We would like to explore treatments that are effective in a setting more similar to clinical practice, such as chronic PTCDs in this study.
Conclusion
We evaluated the effect of BMS with systemic administration of G-CSF on chronic PTCDs in mature rabbit knees and found that the intervention was not superior to BMS alone.
Footnotes
Acknowledgments and Funding: The authors would like to thank Enago (www.enago.jp) for the English language review. The author(s) received no financial support for the research, authorship, and/or publication of this article.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Ethical Approval: All protocols for animal procedures were approved by the Ethics Committee of the Graduate School of Medicine, Chiba University, and followed the National Institutes of Health guidelines for the care and use of laboratory animals.
ORCID iD: Yoshimasa Ono  https://orcid.org/0000-0002-8746-4224
https://orcid.org/0000-0002-8746-4224
References
- 1. Papaioannou G, Demetropoulos CK, King YH. Predicting the effects of knee focal articular surface injury with a patient-specific finite element model. Knee. 2010;17(1):61-8. [DOI] [PubMed] [Google Scholar]
- 2. Peña E, Calvo B, Martínez MA, Doblaré M. Effect of the size and location of osteochondral defects in degenerative arthritis. A finite element simulation. Comput Biol Med. 2007;37(3):376-87. [DOI] [PubMed] [Google Scholar]
- 3. Buckwalter JA. Articular cartilage injuries. Clin Orthop Relat Res. 2002;402:21-37. [DOI] [PubMed] [Google Scholar]
- 4. Solheim E, Krokeide AM, Melteig P, Larsen A, Strand T, Brittberg M. Symptoms and function in patients with articular cartilage lesions in 1,000 knee arthroscopies. Knee Surg Sports Traumatol Arthrosc. 2016;24(5):1610-6. [DOI] [PubMed] [Google Scholar]
- 5. Widuchowski W, Widuchowski J, Trzaska T. Articular cartilage defects: study of 25 124 knee arthroscopies. Knee. 2007; 14(3):177-82. [DOI] [PubMed] [Google Scholar]
- 6. Arøen A, Løken S, Heir S, Alvik E, Ekeland A, Granlund OG, et al. Articular cartilage lesions in 993 consecutive knee arthroscopies. Am J Sports Med. 2004;32(1):211-5. [DOI] [PubMed] [Google Scholar]
- 7. Curl WW, Krome J, Gordon ES, Rushing J, Smith BP, Poehling GG. Cartilage injuries: a review of 31 516 knee arthroscopies. Arthroscopy. 1997;13(4):456-60. [DOI] [PubMed] [Google Scholar]
- 8. Mithöfer K, Minas T, Peterson L, Yeon H, Micheli LJ. Functional outcome of knee articular cartilage repair in adolescent athletes. Am J Sports Med. 2005;33(8):1147-53. [DOI] [PubMed] [Google Scholar]
- 9. Kish G, Módis L, Hangody L. Osteochondral mosaicplasty for the treatment of focal chondral and osteochondral lesions of the knee and talus in the athlete. Rationale, indications, techniques, and results. Clin Sports Med. 1999;18(1):45-66, vi. [DOI] [PubMed] [Google Scholar]
- 10. Blevins FT, Steadman JR, Rodrigo JJ, Silliman J. Treatment of articular cartilage defects in athletes: an analysis of functional outcome and lesion appearance. Orthopedics. 1998;21(7):761-7. [DOI] [PubMed] [Google Scholar]
- 11. Spakova T, Amrichova J, Plsikova J, Harvanova D, Hornak S, Ledecky V, et al. A preliminary study comparing microfracture and local adherent transplantation of autologous adipose-derived stem cells followed by intraarticular injection of platelet-rich plasma for the treatment of chondral defects in rabbits. Cartilage. 2018;9(4):410-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Choi NH, Yang BS, Victoroff BN. Clinical and radiological outcomes after hamstring anterior cruciate ligament reconstructions: comparison between fixed-loop and adjustable-loop cortical suspension devices. Am J Sports Med. 2017;45(4):826-31. [DOI] [PubMed] [Google Scholar]
- 13. Utsunomiya H, Gao X, Deng Z, Cheng H, Nakama G, Scibetta AC, et al. Biologically regulated marrow stimulation by blocking TGF-β1 with losartan oral administration results in hyaline-like cartilage repair: a rabbit osteochondral defect model. Am J Sports Med. 2020;48(4):974-84. [DOI] [PubMed] [Google Scholar]
- 14. Jing L, Zhang J, Leng H, Guo Q, Hu Y. Repair of articular cartilage defects in the knee with autologous iliac crest cartilage in a rabbit model. Knee Surg Sports Traumatol Arthrosc. 2015;23(4):1119-27. [DOI] [PubMed] [Google Scholar]
- 15. Samuel S, Ahmad RE, Ramasamy TS, Manan F, Kamarul T. Platelet rich concentrate enhances mesenchymal stem cells capacity to repair focal cartilage injury in rabbits. Injury. 2018;49(4):775-83. [DOI] [PubMed] [Google Scholar]
- 16. Jia Z, Liu Q, Liang Y, Li X, Xu X, Ouyang K, et al. Repair of articular cartilage defects with intra-articular injection of autologous rabbit synovial fluid-derived mesenchymal stem cells. J Transl Med. 2018;16(1):123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Lee BH, Park JN, Lee EJ, Moon YW, Wang JH. Therapeutic efficacy of spherical aggregated human bone marrow–derived mesenchymal stem cells cultured for osteochondral defects of rabbit knee joints. Am J Sports Med. 2018;46(9):2242-52. [DOI] [PubMed] [Google Scholar]
- 18. Bedi A, Feeley BT, Williams RJ. Management of articular cartilage defects of the knee. J Bone Joint Surg S. 2010;92(4): 994-1009. [DOI] [PubMed] [Google Scholar]
- 19. Bentley G, Biant LC, Vijayan S, Macmull S, Skinner JA, Carrington RWJ. Minimum ten-year results of a prospective randomised study of autologous chondrocyte implantation versus mosaicplasty for symptomatic articular cartilage lesions of the knee. J Bone Joint Surg Br. 2012;94(4):504-9. [DOI] [PubMed] [Google Scholar]
- 20. McCormick F, Harris JD, Abrams GD, Frank R, Gupta A, Hussey K, et al. Trends in the surgical treatment of articular cartilage lesions in the United States: an analysis of a large private-payer database over a period of 8 years. Arthroscopy. 2014;30(2_suppl):222-6. [DOI] [PubMed] [Google Scholar]
- 21. Breinan HA, Martin SD, Hsu HP, Spector M. Healing of canine articular cartilage defects treated with microfracture, a type-i1 collagen matrix, or cultured autologous chondrocytes. J Orthop Res. 2000;18(5):781-9. [DOI] [PubMed] [Google Scholar]
- 22. Frisbie DD, Oxford JT, Southwood L, Trotter GW, Rodkey WG, Steadman JR, et al. Early events in cartilage repair after subchondral bone microfracture. Clin Orthop Relat Res. 2003;407(407):215-27. [DOI] [PubMed] [Google Scholar]
- 23. Deng J, Zou ZM, Zhou TL, Su YP, Ai GP, Wang JP, et al. Bone marrow mesenchymal stem cells can be mobilized into peripheral blood by G-CSF in vivo and integrate into traumatically injured cerebral tissue. Neurol Sci. 2011;32(4):641-51. [DOI] [PubMed] [Google Scholar]
- 24. Okano T, Mera H, Itokazu M, Okabe T, Koike T, Nakamura H, et al. Systemic administration of granulocyte colony-stimulating factor for osteochondral defect repair in a rat experimental model. Cartilage. 2014;5(2_suppl):107-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Sasaki T, Akagi R, Akatsu Y, Fukawa T, Hoshi H, Yamamoto Y, et al. The effect of systemic administration of G-CSF on a full-thickness cartilage defect in a rabbit model MSC proliferation as presumed mechanism: G-CSF for cartilage repair. Bone Joint Res. 2017;6(3):123-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Koh YG, Choi YJ, Kwon OR, Kim YS. Second-look arthroscopic evaluation of cartilage lesions after mesenchymal stem cell implantation in osteoarthritic knees. Am J Sports Med. 2014;42(7):1628-37. [DOI] [PubMed] [Google Scholar]
- 27. Wakitani S, Goto T, Pineda SJ, Young RG, Mansour JM, Caplan AI, et al. Mesenchymal cell-based repair of large, full-thickness defects of articular cartilage. J Bone Joint Surg Am. 1994;76(4):579-92. [DOI] [PubMed] [Google Scholar]
- 28. Mithoefer K, Hambly K, della Villa S, Silvers H, Mandelbaum BR. Return to sports participation after articular cartilage repair in the knee: scientific evidence. Am J Sports Med. 2009;37(Suppl 1):167S-176S. [DOI] [PubMed] [Google Scholar]
- 29. Kaweblum M, Aguilar MC, Blancas E, Kaweblum J, Lehman WB, Grant AD, et al. Histological and radiographic determination of the age of physeal closure of the distal femur, proximal tibia, and proximal fibula of the New Zealand white rabbit. J Orthop Res. 1994;12(5):747-9. [DOI] [PubMed] [Google Scholar]
- 30. Milano G, Passino ES, Deriu L, Careddu G, Manunta L, Manunta A, et al. The effect of platelet rich plasma combined with microfractures on the treatment of chondral defects: an experimental study in a sheep model. Osteoarthritis Cartilage. 2010;18(7):971-80. [DOI] [PubMed] [Google Scholar]
- 31. Dwivedi G, Chevrier A, Hoemann CD, Buschmann MD. Injectable freeze-dried chitosan-platelet-rich-plasma implants improve marrow-stimulated cartilage repair in a chronic-defect rabbit model. J Tissue Eng Regen Med. 2019;13(4):599-611. [DOI] [PubMed] [Google Scholar]
- 32. Harada Y, Nakasa T, Mahmoud EE, Kamei G, Adachi N, Deie M, et al. Combination therapy with intra-articular injection of mesenchymal stem cells and articulated joint distraction for repair of a chronic osteochondral defect in the rabbit. J Orthop Res. 2015;33(10):1466-73. [DOI] [PubMed] [Google Scholar]
- 33. Shapiro F, Koide S, Glimcher MJ. Cell origin and differentiation in the repair of full-thickness defects of articular cartilage. J Bone Joint Surg Am. 1993;75(4):532-53. [DOI] [PubMed] [Google Scholar]
- 34. Anraku Y, Mizuta H, Sei A, Kudo S, Nakamura E, Senba K, et al. Analyses of early events during chondrogenic repair in rat full-thickness articular cartilage defects. J Bone Miner Metab. 2009;27(3):272-86. [DOI] [PubMed] [Google Scholar]
- 35. Min BH, Choi WH, Lee YS, Park SR, Choi BH, Kim YJ, et al. Effect of different bone marrow stimulation techniques (BSTs) on MSCs mobilization. J Orthop Res. 2013;31(11):1814-9. [DOI] [PubMed] [Google Scholar]
- 36. Dhinsa BS, Adesida AB. Current clinical therapies for cartilage repair, their limitation and the role of stem cells. Curr Stem Cell Res Ther. 2012;7(2_suppl):143-8. [DOI] [PubMed] [Google Scholar]
- 37. Richter W. Mesenchymal stem cells and cartilage in situ regeneration. J Intern Med. 2009;266(4):390-405. [DOI] [PubMed] [Google Scholar]
- 38. Jin LH, Choi BH, Kim YJ, Park SR, Jin CZ, Min BH. Implantation of bone marrow-derived buffy coat can supplement bone marrow stimulation for articular cartilage repair. Osteoarthritis Cartilage. 2011;19(12):1440-8. [DOI] [PubMed] [Google Scholar]
- 39. Truong MD, Choi BH, Kim YJ, Kim MS, Min BH. Granulocyte macrophage—colony stimulating factor (GM-CSF) significantly enhances articular cartilage repair potential by microfracture. Osteoarthritis Cartilage. 2017;25(8):1345-52. [DOI] [PubMed] [Google Scholar]
- 40. Orth P, Cucchiarini M, Zurakowski D, Menger MD, Kohn DM, Madry H. Parathyroid hormone [1-34] improves articular cartilage surface architecture and integration and subchondral bone reconstitution in osteochondral defects in vivo. Osteoarthritis Cartilage. 2013;21(4):614-24. [DOI] [PubMed] [Google Scholar]
- 41. Jansen EJ, Emans PJ, van Rhijn LW, Bulstra SK, Kuijer R. Development of partial-thickness articular cartilage injury in a rabbit model. Clin Orthop Relat Res. 2008;466(2_suppl):487-94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Litwic A, Edwards MH, Dennison EM, Cooper C. Epidemiology and burden of osteoarthritis. Br Med Bull. 2013;105:185-99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Plotnikoff R, Karunamuni N, Lytvyak E, Penfold C, Schopflocher D, Imayama I, et al. Osteoarthritis prevalence and modifiable factors: a population study. BMC Public Health. 2015;15(1):1195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Mahmoud EE, Kamei N, Shimizu R, Wakao S, Dezawa M, Adachi N, et al. Therapeutic potential of multilineage-differentiating stress-enduring cells for osteochondral repair in a rat model. Stem Cells Int. 2017;2017:8154569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Frisbie DD, Morisset S, Ho CP, Rodkey WG, Steadman JR, McIlwraith CW. Effects of calcified cartilage on healing of chondral defects treated with microfracture in horses. Am J Sports Med. 2006;34(11):1824-31. [DOI] [PubMed] [Google Scholar]