Abstract
Objective
Long non-coding RNA 01534 (LINC01534) is highly expressed in the tissues of patients with osteoarthritis (OA). This study investigated the mechanism of LINC01534 on abnormal metabolic dysfunction in OA chondrocytes induced by interleukin-1β (IL-1β).
Methods
The quantitative real-time polymerase chain reaction (qRT-PCR) was used to determine the expressions of LINC01534, aggrecan, collagen II, and matrix metalloproteinase (MMPs) in OA cartilage tissue or OA chondrocyte model induced by IL-1β. The expressions of aggrecan and collagen II in the chondrocyte were detected by Western blot. The levels of tumor necrosis factor–α (TNF-α), IL-8, IL-6, MMP-13, MMP-9, MMP-3, and prostaglandin E2 (PGE2) in chondrocyte were determined by enzyme-linked immunosorbernt assay. Bioinformatics, dual luciferin gene reporting, RNA pulldown, and Northern blot were used to determine the interaction between LINC01534 and miR-140-5p.
Results
The results showed that LINC01534 was upregulated in both OA cartilage tissue and OA chondrocyte model. In addition, silencing LINC01534 significantly alleviated the inhibitory effect of IL-1β on expressions of aggrecan and collagen II in chondrocytes, and significantly downregulated the expression of matrix metalloproteinases in IL-1β-induced chondrocytes. Meanwhile, silencing LINC01534 also significantly inhibited the productions of proinflammatory factors NO, PGE2, TNF-α, IL-6, and IL-8 in the IL-1β-induced chondrocytes. Furthermore, miR-140-5p was confirmed to be a direct target of LINC01534. More importantly, inhibition of miR-140-5p significantly reversed the inhibitory effect of silencing LINC01534 on abnormal matrix degradation in the IL-1β-induced chondrocyte model of OA.
Conclusion
Therefore, LINC01534 could promote the abnormal matrix degradation and inflammatory response of OA chondrocytes through the targeted binding of miR-140-5p.
Keywords: LINC01534, osteoarthritis, miR-140-5p, inflammatory response, extracellular matrix degradation
Introduction
Osteoarthritis (OA) is a chronic inflammatory disease of the joints, its main pathological manifestations are the degeneration of articular cartilage tissue, the disorder of metabolism of chondrocyte extracellular matrix and inflammatory reaction, so it is also known as degenerative osteoarthritis disease. 1 The pathogenesis of OA is related to a variety of factors, such as inflammation, obesity, age, gender, joint injury, and so on, but the etiology and pathogenesis have not been fully understood.2,3 Chondrocytes are the only cell type in articular cartilage. 4 The aggrecan and collagen II synthesized and secreted by chondrocytes are the major components of the extracellular matrix of cartilage and play important roles in maintaining the structural and functional integrity of articular cartilage. 5 In the pathogenesis of OA, chondrocytes have abnormal metabolic dysfunction, mainly manifested as inhibited synthesis of aggrecan and collagen II, and increased synthesis of matrix degrading enzyme matrix metalloproteinase (MMPs). 6 MMPs degrade most extracellular matrix proteins during organogenesis, growth and normal tissue turnover, of which MMP13 has the strongest ability to degrade type II collagen. 7 Therefore, MMPs can promote the degradation of OA cartilage extracellular matrix. 8 In addition, the large release of inflammatory factors such as interlwukin-1β (IL-1β), IL-6, and tumor necrosis factor–α (TNF-α) not only damages chondrocytes but also induces extracellular matrix degradation, which is also an important reason for promoting the development of OA.9,10 IL-1β and TNF-α can promote the decomposition of aggrecan and collagen II and the synthesis of MMPs and play a synergistic role in the pathogenesis of OA. 10 Furthermore, IL-1β stimulates chondrocytes to release NO, which enhances TNF-α-induced degradation of aggrecan. 11 Prostaglandin E2 (PGE2) is closely associated with inflammation and pain. 12 Therefore, pro-inflammatory factors such as IL-1β, TNF-α, NO, and PGE2 are involved in the pathogenesis of OA. 13
Studies have found that multiple long non-coding RNAs (lncRNAs) are involved in the pathogenesis of OA and can be potential targets for the treatment of OA. 14 LINC01534 is a newly discovered lncRNA, which has been confirmed to be highly expressed in OA tissue. 15 MicroRNA (miRNA) is a class of endogenous non-coding RNA molecules composed of 18 to 22 nucleotides, which is not only involved in tumorigenesis and development 16 but also closely related to the pathogenesis of OA. 16 Studies have reported that miR-140-5p is abnormally expressed in bladder cancer, non–small cell lung cancer, gastric cancer, glioma, and other tumor tissues17,18 and is also involved in the pathogenesis of rheumatoid arthritis and knee osteoarthritis. 19 As a competitive endogenous RNA (ceRNA), lncRNA can bind to miRNA to inhibit the function of miRNA, thus affecting the occurrence and development of OA. 20 However, the mechanism of LINC01534 and miR-140-5p in the progression of OA has not been reported. In this study, the effects of LINC01534 and miR-140-5p on abnormal metabolic dysfunction in OA chondrocytes were explored by establishing the OA chondrocyte model induced by IL-1β, so as to provide a certain theoretical basis for the treatment of OA.
Materials and Methods
Tissues Collection
A total of 25 patients (aged 32-59 years, 14 males and 11 females) with trauma or death were selected and normal knee cartilage tissue samples were obtained from the knees of patients with trauma or death. In addition, 25 patients (aged 30-60 years, 15 males and 10 females, Kellgren-Lawrence stage III 12 and IV 13) with OA were selected for arthroscopy surgery to obtain OA cartilage tissue. All the tissue samples were frozen rapidly by liquid nitrogen, and then stored at the −80°C in a cryogenic refrigerator. Ethical approval was obtained from the Ethics Committee of The Second Affiliated Hospital of Kunming Medical University. All patients or their families gave written informed consent.
Primary Chondrocyte Culture
First, trypsin was used to digest the cartilage tissue for 10 minutes. Next, the cartilage was digested overnight in Dulbecco’s modified Eagle medium (DMEM) medium (10% fetal bovine serum [FBS]) using collagenase II. The digested chondrocytes were separated and centrifuged at 2000 × g for 5 minutes. Chondrocytes were resuspended using DMEM medium (10% FBS) and 100 μg/mL streptomycin and 100 U/mL penicillin were selectively added to the medium. Subsequent experiments selected chondrocytes between the first and third generation, and used 10 ng/mL IL-1β induction to establish the OA chondrocyte model.
Cell Transfection
Si-LINC01534, miR-140-5p inhibitor (miR-140-5p-inh), miR-140-5p mimic, and their respective negative controls were purchased from Gene Pharma. Oligonucleotide or recombinant plasmids were transfected into chondrocytes using the Lipofectamine 2000 reagent.
The qRT-PCR Assay
Total RNA was extracted from OA cartilage tissue and chondrocytes exposed to IL-1β (10 ng/mL) for 24 hours using Trizol reagent (Invitrogen), and was reversely transcribed into cDNA using Super Script II First Strand Synthesis System (Invitrogen). Then, SYBR Premix Ex TaqTM II kit (Takara) was used for quantitative real-time polymerase chain reaction (qRT-PCR) amplification of primers MMP-13, MMP-9, MMP-3, aggrecan, collagen II, miR-140-5p, LINC01534 (Sangon Biotech), and template cDNA. β-Actin was used as the internal reference of mRNA and U6 as the internal reference of miRNA. The relative expression levels of each gene were calculated by 2−∆∆Ct method. The primers used in this study were as follows:
LINC01534-F: 5′-GCGACAGCTGTATAGACCCC-3′,
LINC01534-R: 5′-ACTCGAAAAGCCTCGTTCCG-3′;
MMP-13-F: 5′-GCCATTACCAGTCTCCGAGG-3′,
MMP-13-R: 5′-TACGGTTGGGAAGTTCTGGC-3′;
MMP-9-F: 5′-TCTATGGTCCTCGCCCTGAA-3′,
MMP-9-R: 5′-CATCGTCCACCGGACTCAAA-3′;
MMP-3-F: 5′-TGAGGACACCAGCATGAACC-3′,
MMP-3-R: 5′-ACTTCGGGATGCCAGGAAAG-3′;
aggrecan-F: 5′-CCCAAGACTACCAGTGGATCG-3′,
aggrecan-R: 5′-CGTTTGTAGGTGGTGGCTGTG-3′;
collagen II-F: 5′-ATGAGGGCGCGGTAGAGAC-3′,
collagen II-R: 5′-TCACAGACACAGATCCGGCA-3′;
miR-140-5p-F: 5′-CTGTGTCCTGCCAGTGGTTTT-3′,
miR-140-5p-R: 5′-CGGTATCCTGTCCGTGGTTCT-3′;
β-actin-F: 5′-GCATGGGTCAGAAGGATTCCT-3′,
β-actin-R: 5′-TCGTCCCAGTTGGTGACGAT-3′;
U6-F: 5′-CTCGCTTCGGCAGCACATATACT-3′,
U6-R: 5′-ACGCTTCACGAATTTGCGTGTC-3′.
Western Blot Assay
Total proteins in cells were extracted using RIPA (radioimmunoprecipitation assay) lysate and PMSF (phenylmethylsulfonyl fluoride) and quantified using BCA (bicichoninic acid) protein concentration kit (Beyotime). Total protein was separated by 12% SDS-PAGE (sodium dodecyl sulfate–polyacrylamide gel electrophoresis) and transferred to cellulose acetate membrane. 5% skim milk was then used to seal the cellulose acetate membrane for 2 hours. Next, the primary antibody was used to incubate the membrane under 4°C for overnight, and then the membrane was incubated with a second antibody for 1 hour at room temperature. GAPDH (glyceraldehyde 3-phosphate dehydrogenase) was used as the internal reference of the proteins. ECL (enhanced chemiluminiscence) reagent (Beyotime) and GelDocTM XR imaging system (Bio-Rad) were used to perform color imaging of the protein bands, and Quantity One software was used to analyze the grayscale value. The antibodies against aggrecan (1:1000, ab186414)), collagen II (1:1000, ab34712), and GAPDH (1:10000, ab181602) were obtained from Abcam (Cambridge, MA, USA).
ELISA Assay
The contents of IL-6, IL-8, TNF-α, PGE2, MMP-3, MMP-9, and MMP-13 in the cell supernatant were determined by ELISA (enzyme-linked immunosorbent assay) kits (ebioscience), and the specific steps were strictly in accordance with the instructions of the kits.
Determination of NO
NO production in cell supernatant was determined using NO detection kit (NanJing JianCheng Institute of Biological Engineering), and the specific steps were strictly in accordance with the instruction of the kit. After Griess reaction for 10 minutes, the absorbance value at 550 nm was determined, and the content of NO was calculated according to the standard nitrite curve.
Luciferase Reporter Gene Assay
The mutant LINC01534 (LINC01534-mut) or wild-type LINC01534 (LINC01534-wt) was inserted into the pGL3 vector (Promega). The LINC01534-mut) or LINC01534-wt vectors were transfected into chondrocytes with miR-140-5p mimic or miR-NC using Lipofectamine 2000 reagent. The luciferase activity was measured after transfection for 48 hours.
Pulldown Assay
A pH 7.5 buffer (1 mM EDTA [ethylenediaminetetraacetic acid], 0.5 M NaCl, 20 mM Tris-HCl) was used to dissolve the biotinylated DNA probe complementary to LINC01534 RNA. The streptavidin-coupled beads were incubated with the DNA probe. RNA isolated by protease K digestion was determined using Northern blot. The biomimetic miR-140-5p was transfected into the OA chondrocyte model and lytic the cells. Streptavidin-coupled beads were co-incubated with the lysis products of chondrocytes to form the biotin-miRNA-lncRNA complex. Binding RNA was extracted with Trizol reagent, and the enrichment of lncRNA LINC01534 was determined by qRT-PCR.
Northern Blot Assay
Samples were separated with 15% polyacrylamide-urea gel and transferred to nylon membrane (Millipore) with positive charge. After ultraviolet irradiation, the nylon membrane was incubated with the 100 pmol 3-digoxigenin (DIG) labelled probes of miR-140-5p at 42°C for the night. The extent of hybridization was determined using DIG light detection kit (Roche), and the specific steps were strictly in accordance with the instruction of the kit.
Statistical Analysis
All data are expressed as mean standard ± deviation (SD). SPSS 19.0 software was used for data analysis, and t test, analysis of variance (ANOVA) and Tukey’s post hoc were used for significance analysis. P < 0.05 indicated a significant difference.
Results
The mRNA Expressions of LINC01534 in Human OA Cartilage Tissues and OA Chondrocyte Model
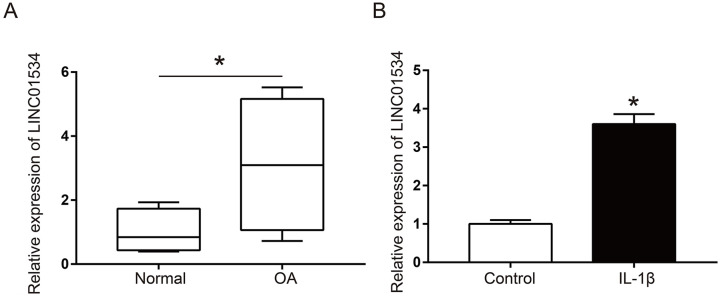
The mRNA expression of LINC01534 in human OA cartilage tissue (n = 25) and normal cartilage tissue (n = 25) were determined by qRT-PCR. The results found that LINC01534 expression in OA cartilage tissue was significantly higher than that in normal cartilage tissue ( Fig. 1A , P < 0.05), indicating that LINC01534 was highly expressed in OA cartilage tissue. In addition, the mRNA expression of LINC01534 in primary chondrocytes was determined by qRT-PCR. The results revealed that the mRNA expression of LINC01534 in the OA chondrocyte model induced by IL-1β was significantly higher than that in the control group ( Fig. 1B , P < 0.05), which suggested that LINC01534 was also highly expressed in the OA chondrocyte model.
Figure 1.
The mRNA expressions of LINC01534 in human osteoarthritis (OA) cartilage tissues and OA chondrocyte model. (A) Detection of the mRNA expression of LINC01534 in human OA cartilage (n = 25) and normal cartilage (n = 25) by quantitative real-time polymerase chain reaction (qRT-PCR). (B) Detection of the mRNA expression of LINC01534 in primary chondrocytes by qRT-PCR. *P < 0.05.
Effect of LINC01534 on the Metabolic Dysfunction of Chondrocytes Induced by IL-1β
The chondrocytes were transfected with LINC01534 siRNA to investigate the effect of LINC01534 on IL-1β-induced chondrocyte metabolic dysfunction. The results showed that LINC01534 expression was inhibited in primary chondrocytes transfected with si-LINC01534 ( Fig. 2A , P < 0.05), which indicated that si-LINC01534 successfully silenced the expression of INC01534 in the cells. In addition, the mRNA expressions of the main components of the chondrocyte extracellular matrix (aggrecan and collagen II) in the chondrocytes of the IL-1β group was significantly lower than that of the control group, suggesting that IL-1β inhibited the expression of the main components of the chondrocyte extracellular matrix ( Fig. 2B ). The mRNA expressions of aggrecan and collagen II in chondrocytes of the IL-1β + si-LINC01534 group were significantly higher than those in the IL-1β group ( Fig. 2B , P < 0.05), indicating that silencing LINC01534 alleviated the inhibitory effect of IL-1β on the major components of the extracellular matrix. Moreover, the mRNA expression levels of MMP-13, MMP-9, and MMP-3 in chondrocytes were determined by qRT-PCR. The results showed that IL-1β significantly upregulated the mRNA expressions of MMP-13, MMP-9, and MMP-3 in chondrocytes (P < 0.05), while silencing LINC01534 significantly inhibited the mRNA expressions of MMP-3, MMP-9, and MMP-13 in chondrocytes induced by IL-1β ( Fig. 2C , P < 0.05). At the same time, we also used Western blot to determine the protein expressions of aggrecan and collagen II, and the results showed the same expression trend as the results of qRT-PCR ( Fig. 2D and E , P < 0.05). Furthermore, the amount of MMP-13, MMP-9, and MMP-3 in the supernatant of chondrocytes were determined by ELISA. It was found that silencing LINC01534 also significantly inhibited the protein secretion of MMP-3, MMP-9, and MMP-13 in IL-1β-induced chondrocytes ( Fig. 2F-H , P < 0.05). The above results suggested that silencing LINC01534 could alleviate the abnormal metabolism dysfunction of IL-1β-induced chondrocytes, suggesting that LINC01534 could promote the degradation of chondrocyte extracellular matrix.
Figure 2.
Effect of LINC01534 on the metabolic dysfunction of chondrocytes induced by interleukin-1β (IL-1β). (A) Detection of the expression of LINC01534 in chondrocytes transfected with si-LINC01534 or siNC by quantitative real-time polymerase chain reaction (qRT-PCR). (B) Detection of the mRNA expressions of aggrecan and collagen II in chondrocytes by qRT-PCR. (C) Detection of the mRNA expressions of matrix metalloproteinase–3 (MMP-3), MMP-9, and MMP-13 in chondrocytes by qRT-PCR. (D, E) Detection of the protein expressions of MMP-3, MMP-9, and MMP-13 in chondrocytes by Western blot. (F, G) Detection of the contents of MMP-3, MMP-9, and MMP-13 in chondrocyte supernatant by enzyme-linked immunosorbent assay (ELISA). *P < 0.05.
Effect of LINC01534 on Inflammatory Responses of Chondrocytes Induced by IL-1β
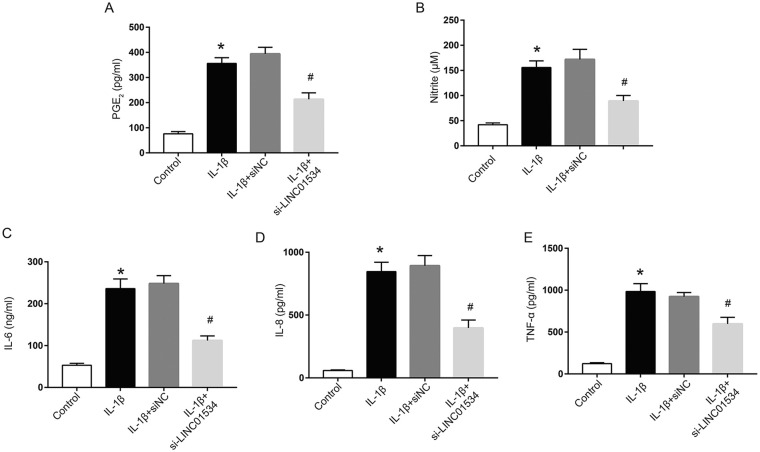
The secretions of inflammatory factors in chondrocyte supernatant were measured to investigate the role of LINC01534 in the inflammatory response of chondrocytes induced by IL-1β. The results showed that the levels of PGE2, NO, IL-6, IL-8, and TNF-α in the chondrocyte supernatant in the IL-1β group were significantly higher than those in the control group ( Fig. 3A-E , P < 0.05). In addition, silencing LINC01534 reduced the contents of PGE2, NO, IL-6, IL-8, and TNF-α in the chondrocyte supernatant induced by IL-1β, which indicated that silencing LINC01534 significantly suppressed the chondrocyte inflammatory response induced by IL-1β.
Figure 3.
Effect of LINC01534 on inflammatory responses of chondrocytes induced by interleukin-1β (IL-1β). (A-E) Determination of prostaglandin E2 (PGE2; A), NO (B), IL-6 (C), IL-8 (D), and tumor necrosis factor–α (TNF-α; E) in chondrocytes. *P < 0.05, #P < 0.05.
Identification of miR-140-5p as a Direct Target of LINC01534
First, we transfected si-LINC01534 or siNC into the OA chondrocyte model to determine the expression of miR-140-5p. The results showed that silencing LINC01534 significantly upregulated the expression of miR-140-5p in the OA chondrocyte model ( Fig. 4A , P < 0.05). In addition, we transfected the recombinant LINC01534 plasmid or pNC into the OA chondrocyte model to determine the expression of LINC01534 and miR-140-5p. The results showed that the recombinant plasmid LINC01534 significantly upregulated the expression of LINC01534 in the OA chondrocyte model ( Fig. 4B , P < 0.05), and inhibited the expression of miR-140-5p ( Fig. 4C , P < 0.05), which indicated that LINC01534 negatively regulated the expression of miR-140-5p in the OA chondrocyte model. In order to further explore the interaction between LINC01534 and miR-140-5p, bioinformatics was used to find that miR-140-5p might contain the binding site of LINC01534 ( Fig. 4D ). In addition, after co-transfection with miR-140-5p, the luciferase activity of cells in the LINC01534-wt group was significantly lower than that in the LINC01534-mut group ( Fig. 4E , P < 0.05). Furthermore, the results of pulldown and Northern blot analysis showed that the Bio-LINC01534 probe could pulldown miR-140-5p ( Fig. 4F ). Moreover, the results of pulldown and qRT-PCR showed that LINR01534 was remarkably enriched after Bio-miR-140-5p-wt transfection (P < 0.05), while Bio-miR-140-5p-mut transfection showed no such effect ( Fig. 4G ). These results indicated that miR-140-5p was the direct target of LINC01534 in the OA chondrocyte model.
Figure 4.
Identification of miR-140-5p as a direct target of LINC01534. (A) Determination of the expression of miR-140-5p in si-LINC01534 or siNC-transfected osteoarthritis (OA) chondrocyte model by quantitative real-time polymerase chain reaction (qRT-PCR). (B, C) Determination of the expression of LINC01534 or miR-140-5p in OA chondrocyte model transfected with recombinant LINC01534 plasmid or pNC by qRT-PCR. (D) Schematic diagram of binding sites of miR-140-5p and LINC01534. (E) Determination of the luciferase activity in OA chondrocytes transfected with LINC01534-wt or LINC01534-mut and miR-140-5p or miR-NC. (F) Determination of Bio-linc01534 probe binding to miR-140-5p by pulldown and Northern blot analysis. (G) Determination of the enrichment effect of Bio-miR-140-5p-wt or Bio-miR-140-5p-mut on LINR01534 by Pulldown and qRT-PCR. *P < 0.05, #P < 0.05.
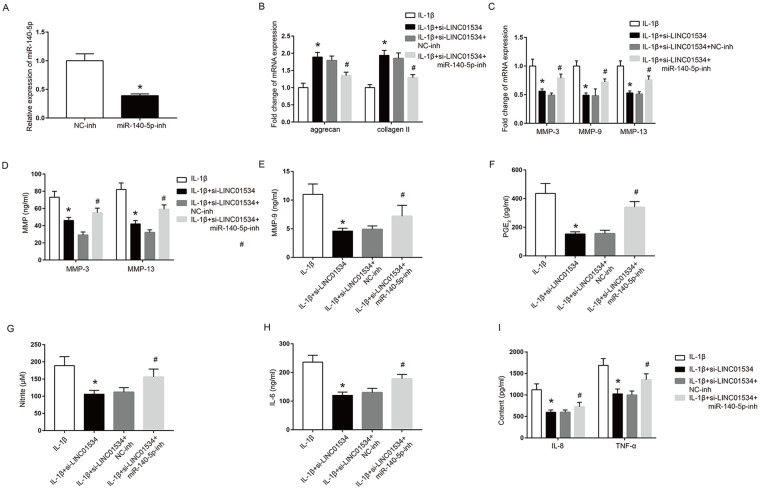
Effects of miR-140-5p on Abnormal Metabolic Dysfunction and Inflammatory Responses in the OA Chondrocyte Model Mediated by LINC01534
The miR-140-5p inhibitor was used to investigate the effect of miR-140-5p on abnormal metabolic dysfunction and inflammatory response inhibited by silenced LINC01534 in the OA chondrocyte model. The qRT-PCR results showed that the miR-140-5p inhibitor significantly inhibited the mRNA expression of miR-140-5p in the OA chondrocyte model ( Fig. 5A , P < 0.05). Additionally, miR-140-5p inhibitor significantly downregulated the mRNA expressions of aggrecan and collagen II promoted by silenced LINC01534 in the OA chondrocyte model. ( Fig. 5B , P < 0.05). Furthermore, miR-140-5p inhibitor also upregulated the mRNA and protein expressions of MMP-3, MMP-9 and MMP-13 inhibited by silenced LINC01534 in the OA chondrocyte model ( Fig. 5C-E , P < 0.05). Moreover, miR-140-5p inhibitor also significantly increased the contents of PGE2, NO, IL-6, IL-8, and TNF-α decreased by silenced LINC01534 in the OA chondrocyte model ( Fig. 5F-I , P < 0.05). The above results suggested that LLINC01534 could promote abnormal metabolic dysfunction and inflammatory responses in the IL-1β-induced chondrocyte model of OA by targeting miR-140-5p.
Figure 5.
Effects of miR-140-5p on abnormal metabolic dysfunction and inflammatory responses in the osteoarthritis (OA) chondrocyte model mediated by LINC01534. (A) Effect of miR-140-5p inhibitor on the mRNA expression of miR-140-5p in chondrocytes. (B) Effect of miR-140-5p inhibitor on the mRNA expressions of aggrecan and collagen II in chondrocytes. (C) The effect of miR-140-5p inhibitor on the mRNA expressions of matrix metalloproteinase–3 (MMP-3), MMP-9, and MMP-13 in chondrocytes. (D) Effect of miR-140-5p inhibitor on MMP-3 and MMP-13 contents in the supernatant of chondrocytes. (E) Effect of miR-140-5p inhibitor on MMP-9 content in the supernatant of chondrocytes. (F-I) Effect of miR-140-5p inhibitor on the contents of prostaglandin E2 (PGE2; F), NO (G), interleukin-6 (IL-6; H), IL-8, and tumor necrosis factor–α (TNF-α; I) in the supernatant of chondrocytes. *P < 0.05, #P < 0.05.
Discussion
OA is a degenerative disease characterized by reduction of chondrocytes and degeneration of articular cartilage tissue. 21 Studies have confirmed that a variety of long non-coding RNAs (lnc RNAs) are related to the pathogenesis of OA. 22 LINC01534 is a newly discovered LNC RNA, which has been confirmed to be highly expressed in OA tissue. 15 However, the specific mechanism of LINC01534 in the pathogenesis of OA remains unclear. In this study, the mRNA expression of LINC01534 in human OA cartilage tissue (n = 25) and normal cartilage tissue (n = 25) was determined by qRT-PCR. The results found that LINC01534 was highly expressed in OA cartilage tissue, which was consistent with the results reported in the literature. 15 Therefore, the results of this study suggested that LINC01534 might be involved in the pathogenesis of OA.
The immortalized chondrosarcoma cell line and the primary chondrocytes derived from the patient are often used for the in vitro study of OA, and the primary chondrocytes can better reflect the intracellular changes during the development of OA. 23 Currently, IL-1β is commonly used as an inducer in vitro experiments to simulate the inflammatory environment of OA. 24 In the present study, IL-1β induction was used to establish the OA chondrocyte model, and qRT-PCR results showed that LINC01534 was also highly expressed in the OA chondrocyte model. Studies have found that lncRNA can be used as a ceRNA to bind miRNA, thereby inhibiting the function of miRNA and affecting the pathogenesis of OA. 25 MiR-140-5p is not only abnormally expressed in various tumor tissues but also involved in the pathogenesis of rheumatoid arthritis and knee osteoarthritis.26-29 However, the interaction mechanism between LINC01534 and miR-140-5p in the development of OA is still unclear. This study found that LINC01534 negatively regulated the expression of miR-140-5p in the OA chondrocyte model. In order to further explore the interaction between LINC01534 and miR-140-5p, bioinformatics was used to find that miR-140-5p might contain the binding site of LINC01534. In addition, the direct interaction between LINC01534 and miR-140-5p was confirmed by luciferase activity determination and RNA pulldown assay. The results of this study suggested that miR-140-5p was a direct target of LINC01534 in the OA chondrocyte model.
In the process of the development of OA, abnormal metabolic dysfunction occurs in chondrocytes, and the synthesis of aggrecan and collagen II, the main components of chondrocyte extracellular matrix, are inhibited, while the synthesis of MMPs is increased, thus promoting the abnormal degradation of chondrocyte extracellular matrix.30-32 In addition, the large release of inflammatory factors such as IL-1β, IL-6, NO, PGE2, and TNF-α not only damages chondrocytes but also induces extracellular matrix degradation, which is also an important reason for promoting the development of OA.33-35 In this study, it was found that silencing LINC01534 alleviated the inhibitory effect of IL-1β on the major components of chondrocytes and significantly inhibited the synthesis of MMP-3, MMP-9, and MMP-13, while miR-140-5p inhibitor reversed this effect. Furthermore, this study also found that silencing LINC01534 significantly reduced the contents of PGE2, NO, IL-6, IL-8, and TNF-α in the chondrocyte supernatant induced by IL-1β, while miR-140-5p inhibitor showed the opposite effect. Zhao et al. 36 found that lncRNA PVT1 inhibited the expression of aggrecan and collagen II in IL-1β-induced OA chondrocytes by targeting miR-149, upregulated the secretions of MMPs, TNF-α, IL-8, IL-6, NO, and PGE2, and ultimately aggravated chondrocyte catabolism and inflammation. Therefore, the results of this study suggested that LLINC01534 could promote abnormal metabolic dysfunction and inflammatory responses in the IL-1β-induced chondrocyte model of OA by targeting miR-140-5p.
In conclusion, the present study assessed the molecular mechanism of LINC01534 on abnormal metabolic dysfunction and inflammation in OA chondrocytes induced by IL-1β. These results confirmed that LINC01534 could promote the abnormal matrix degradation and inflammatory response of OA chondrocytes through the targeted binding of miR-140-5p, which was expected to be a potential target for the treatment of OA.
Footnotes
Acknowledgments and Funding: We are thankful for the financial support from Epidemiological Investigation of Adolescent Scoliosis in Yunnan Province (2017FE467-066).
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Ethical Approval: Ethical approval was obtained from the Ethics Committee of The Second Affiliated Hospital of Kunming Medical University. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed Consent: Written informed consent was obtained from all individual participants included in the study.
Trial Registration: Not applicable.
ORCID iD: Zhihua Wang  https://orcid.org/0000-0002-6773-0281
https://orcid.org/0000-0002-6773-0281
References
- 1. Glyn-Jones S, Palmer AJ, Agricola R, Price AJ, Vincent T, Weinans H, et al. Osteoarthritis. Lancet. 2015;386(9991):376-87. [DOI] [PubMed] [Google Scholar]
- 2. Bartels EM, Juhl CB, Christensen R, Hagen KB, Danneskiold-Samsøe B, Dagfinrud H, et al. Aquatic exercise for the treatment of knee and hip osteoarthritis. Cochrane Database Syst Rev. 2016;(3):CD005523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Loeser RF, Collins JA, Diekman BO. Ageing and the pathogenesis of osteoarthritis. Nat Rev Rheumatol. 2016;12(7):412-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Varela-Eirin M, Loureiro J, Fonseca E, Corrochano S, Caeiro JR, Collado M, et al. Cartilage regeneration and ageing: targeting cellular plasticity in osteoarthritis. Ageing Res Rev. 2018;42:56-71. [DOI] [PubMed] [Google Scholar]
- 5. Hwang H, Kim H. Chondrocyte apoptosis in the pathogenesis of osteoarthritis. Int J Mol Sci. 2015;16(11):26035-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Nummenmaa E, Hämäläinen M, Moilanen T, Vuolteenaho K, Moilanen E. Effects of FGF-2 and FGF receptor antagonists on MMP enzymes, aggrecan, and type II collagen in primary human OA chondrocytes. Scand J Rheumatol. 2015;44(4):321-30. [DOI] [PubMed] [Google Scholar]
- 7. Pap T, Korb-Pap A. Cartilage damage in osteoarthritis and rheumatoid arthritis—two unequal siblings. Nat Rev Rheumatol. 2015;11(10):606-15. [DOI] [PubMed] [Google Scholar]
- 8. Nummenmaa E, Hämäläinen M, Pemmari A, Moilanen L, Nieminen R, Moilanen T, et al. Transient receptor potential ankyrin 1 (TRPA1) as a factor and drug target in osteoarthritis: TRPA1 mediates fibroblast growth factor 2 expression in chondrocytes. Osteoarthritis Cartilage. 2019;27(Suppl 1):S377. [Google Scholar]
- 9. Takano S, Uchida K, Miyagi M, Inoue G, Fujimaki H, Aikawa J, et al. Nerve growth factor regulation by TNF-α and IL-1β in synovial macrophages and fibroblasts in osteoarthritic mice. J Immunol Res. 2016;2016:5706359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Han PF, Wei L, Duan ZQ, Zhang ZL, Chen TY, Lu JG, et al. Contribution of IL-1β, 6 and TNF-α to the form of post-traumatic osteoarthritis induced by “idealized” anterior cruciate ligament reconstruction in a porcine model. Int Immunopharmacol. 2018;65:212-20. [DOI] [PubMed] [Google Scholar]
- 11. Zheng G, Zhan Y, Tang Q, Chen T, Zheng F, Wang H, et al. Monascin inhibits IL-1β induced catabolism in mouse chondrocytes and ameliorates murine osteoarthritis. Food Funct. 2018;9(3):1454-64. [DOI] [PubMed] [Google Scholar]
- 12. Fan W, Qian Y, Wang J, Yang X, Gui T, He B. Chondroprotective and anti-inflammatory activities of extracts from semen sojae germinatum on IL-1β-stimulated human osteoarthritis chondrocytes. Indian J Pharm Educ Res. 2016;50(3):397-402. [Google Scholar]
- 13. Damlar İ, Esen E, Tatli U. Effects of glucosamine-chondroitin combination on synovial fluid IL-1β, IL-6, TNF-α and PGE2 levels in internal derangements of temporomandibular joint. Med Oral Patol Oral Cir Bucal. 2015;20(3):e278-e283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Barter MJ, Ajekigbe B, Cheung K, Skelton AJ, Xu Y, Deehan D, et al. Identification and characterisation of long non-coding RNAS expressed and dysregulated in knee and hip osteoarthritic cartilage. Osteoarthritis Cartilage. 2018;26(Suppl 1):S28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Xiao K, Yang Y, Bian Y, Feng B, Li Z, Wu Z, et al. Identification of differentially expressed long noncoding RNAs in human knee osteoarthritis. J Cell Biochem. 2019;120(3):4620-33. [DOI] [PubMed] [Google Scholar]
- 16. Svoronos AA, Engelman DM, Slack FJ. OncomiR or tumor suppressor? The duplicity of microRNAs in cancer. Cancer Res. 2016;76(13):3666-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Fang Z, Yin S, Sun R, Zhang S, Fu M, Wu Y, et al. miR-140-5p suppresses the proliferation, migration and invasion of gastric cancer by regulating YES1. Mol Cancer. 2017;16(1):139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Cui Y, Yi L, Zhao JZ, Jiang YG. Long noncoding RNA HOXA11-AS functions as miRNA sponge to promote the glioma tumorigenesis through targeting miR-140-5p. DNA Cell Biol. 2017;36(10):822-28. [DOI] [PubMed] [Google Scholar]
- 19. Yin C, Suen W, Lin S, Wu X, Li G, Pan X. Dysregulation of both miR-140-3p and miR-140-5p in synovial fluid correlate with osteoarthritis severity. Bone Joint Res. 2017;6(11):612-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Liu Q, Hu X, Zhang X, Dai L, Duan X, Zhou C, et al. The TMSB4 pseudogene LncRNA functions as a competing endogenous RNA to promote cartilage degradation in human osteoarthritis. Mol Ther. 2016;24(10):1726-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Peffers M, Clegg P, Boothroyd E, Cremer A, Durtel D, Caron M, et al. BIG tasks for small RNAs; a new class of RNAs in the pathogenesis of osteoarthritis. Osteoarthritis Cartilage. 2016;24(Suppl 1):S372. [Google Scholar]
- 22. Chen WK, Yu XH, Yang W, Wang C, He WS, Yan YG, et al. lncRNAs: novel players in intervertebral disc degeneration and osteoarthritis. Cell Prolif. 2017;50(1):e12313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Haseeb A, Ansari MY, Haqqi TM. Harpagoside suppresses IL-6 expression in primary human osteoarthritis chondrocytes. J Orthop Res. 2017;35(2_suppl):311-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Yang X, Guan Y, Tian S, Wang Y, Sun K, Chen Q. Mechanical and IL-1β responsive miR-365 contributes to osteoarthritis development by targeting histone deacetylase 4. Int J Mol Sci. 2016;17(4):436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Le L, Ho P, Clark I. The role of microRNA 144 in osteoarthritis. Osteoarthritis Cartilage. 2018;26(Suppl 1):S161. [Google Scholar]
- 26. Tao SC, Yuan T, Zhang YL, Yin WJ, Guo SC, Zhang CQ. Exosomes derived from miR-140-5p-overexpressing human synovial mesenchymal stem cells enhance cartilage tissue regeneration and prevent osteoarthritis of the knee in a rat model. Theranostics. 2017;7(1):180-95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Lan H, Chen W, He G, Yang S. miR-140-5p inhibits ovarian cancer growth partially by repression of PDGFRA. Biomed Pharmacother. 2015;75:117-22. [DOI] [PubMed] [Google Scholar]
- 28. Lv J, Fan HX, Zhao XP, Lv P, Fan JY, Zhang Y, et al. Long non-coding RNA Unigene56159 promotes epithelial-mesenchymal transition by acting as a ceRNA of miR-140-5p in hepatocellular carcinoma cells. Cancer Lett. 2016;382(2_suppl):166-75. [DOI] [PubMed] [Google Scholar]
- 29. Zhang K, Chen J, Song H, Chen LB. SNHG16/miR-140-5p axis promotes esophagus cancer cell proliferation, migration and EMT formation through regulating ZEB1. Oncotarget. 2017;9(1):1028-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Gauci SJ, Stanton H, Little CB, Fosang AJ. Proteoglycan and collagen degradation in osteoarthritis. In: Grässel S, Aszódi A, eds. Cartilage. Cham, Switzerland: Springer; 2017:41-61. [Google Scholar]
- 31. Johnson CI, Argyle DJ, Clements DN. In vitro models for the study of osteoarthritis. Vet J. 2016;209:40-9. [DOI] [PubMed] [Google Scholar]
- 32. Tao K, Sr, Li R, Li H, Ke Y, Lin J. Inhibition of sterol regulatory element-binding protein-2 alleviates high-fat diet-induced deterioration of knee cartilage: an osteoarthritis animal model study. Osteoarthritis Cartilage. 2019;27(Suppl 1):S377. [Google Scholar]
- 33. Zheng W, Zhang H, Jin Y, Wang Q, Chen L, Feng Z, et al. Butein inhibits IL-1β-induced inflammatory response in human osteoarthritis chondrocytes and slows the progression of osteoarthritis in mice. Int Immunopharmacol. 2017;42:1-10. [DOI] [PubMed] [Google Scholar]
- 34. Luo Z, Zheng B, Jiang B, Xue X, Xue E, Zhou Y. Peimine inhibits IL-1β induced inflammatory response in mouse chondrocytes and ameliorates murine osteoarthritis. Food Func. 2019;10:2198-208. [DOI] [PubMed] [Google Scholar]
- 35. Mu Y, Hao W, Li S. Casticin protects against IL-1β-induced inflammation in human osteoarthritis chondrocytes. Eur J Pharmacol. 2019;842:314-20. [DOI] [PubMed] [Google Scholar]
- 36. Zhao Y, Zhao J, Guo X, She J, Liu Y. Long non-coding RNA PVT1, a molecular sponge for miR-149, contributes aberrant metabolic dysfunction and inflammation in IL-1β-simulated osteoarthritic chondrocytes. Biosci Rep. 2018;38(5):BSR20180576. [DOI] [PMC free article] [PubMed] [Google Scholar]