Abstract
Objective
Osteoarthritis (OA) commonly affects weight-bearing joints and is characterized by articular cartilage breakdown combined with osteophyte formation at the joint margins and chronic nonspecific inflammation of synovium. Understanding the profile of inflammation in a patient population is an essential starting point to predict or prevent OA progression. The aim of this study was to identify the profile of selected biomolecules in synovial fluid (SF) and investigate the correlation according to gender, age, and severity of the disease within patients from among the general knee OA population.
Design
In our study SF samples were aspirated from the knees of 65 OA patients (46 patients with early knee OA and 19 patients with end-stage knee OA according to the Kellgren-Lawrence grading scale). The concentration of interleukins (IL-6, IL-8), matrix metalloproteinases (MMP-1, MMP-3, MMP-13), MMPs inhibitors (TIMP-1, TIMP-2), cartilage oligomeric matrix protein (COMP), and adiponectin was analyzed using a multiplex ELISA-based approach.
Conclusions
Our results indicate significant linear correlation of MMP-13 and COMP concentration with age (P < 0.05), but not with OA severity. In fact, 3 of the examined biomolecules, MMP-3 (P < 0.01), TIMP-1 (P < 0.01), and COMP (P < 0.05) significantly correlate with the grade of knee OA and might be associated with OA severity.
Keywords: osteoarthritis, cytokines, synovial fluid, biomarker, inflammation
Introduction
Osteoarthritis (OA), a degenerative joint disorder, is the most prevalent form of arthritis. 1 It commonly affects weight-bearing joints and is characterized by loss and damage of articular cartilage, joint space narrowing, osteophyte formation, subchondral sclerosis, and synovial inflammation.2,3 In pathogenesis of OA, the inflammation is playing a pivotal role and may be a potent contributor to chronic pain.4-6 Inflammation is part of the innate immune system and is initiated when it recognizes invading pathogens or molecules from tissue injury through pattern recognition receptors.7,8 Macrophages, neutrophils, and synovial fibroblasts respond to and maintain local inflammation environment. The inflammatory response promoted by damage associated molecular patterns and inflammatory cytokines includes activation of macrophages in the joint, which leads to significant modulation of local cytokines, growth factors, and matrix metalloproteinases (MMPs). 9
There are several risk factors for OA pathogenesis, which include age, physical trauma, and obesity. 10 Current methods of evaluating disease progression primarily include radiological investigation, which reflects disease severity by grading the joint degeneration. Other imaging methods are magnetic resonance imaging (MRI), optical coherence tomography (OCT), and ultrasound (US), which permit visualization of joint structures and can evaluate disease onset and progression. 11 In clinical practice, OA is diagnosed radiographically when clinical signs of pain and decrease of mobility have already appeared. However, studies have shown that radiographic changes over time are not significant and occur in only a subset of patients. Therefore, it is necessary to develop sensitive and predictive biochemical assays that could ultimately be an alternative for the current radiographic approach and prompt punctual, more targeted, and personalized treatments.12-14 It seems that using of biochemical markers (biomarkers) will be a better choice for identification of OA severity. Their concentrations are linked to tissue metabolism and can be measured in all biological fluids like blood, urine, or synovial fluid (SF). 15 The SF is the most applicable biofluid to investigate progression of OA because of its direct and intimate relationship with different tissues of knee joint. SF composition may be more informative than systemic markers as they relate to structural changes and damage in knee joint/tissue environment. 16 Biomarkers can represent not only effector molecules (such as cytokines and enzymes) but also extracellular matrix constituents (such as precursors or degradation products of collagen and proteoglycans).
It is important to examine potential biochemical parameters not only to predict and diagnose knee OA and its severity but also to evaluate changes in markers concentrations and their relationship with other variable factors such as age, gender, and daytime variations, among others. The goal of this study was to evaluate the significance of measuring the SF levels of biomolecules (cytokines, enzymes, inhibitors of enzymes) in OA patients and to correlate these levels with the parameters of disease severity and demographics of patients in an attempt to provide more insight regarding their role in the pathogenesis and diagnosis of OA
Materials and Methods
Ethics Statement
This study was approved by the Institutional Ethics Board of Louis Pasteur University Hospital, Kosice, Slovakia. Samples were taken with patients’ written consent according to the guidelines of the local ethics committee.
Sample Collection and Processing
Inclusion criteria were age ≥18 years for all participants and clinical and radiographic findings of knee OA. Standardized clinical assessment and radiographic images of patients were used to prove evidence of grade 1, 2, or 3, 4 OA of the knee joint according to Kellgren-Lawrence (KL) grading scale. 17 Radiographs were realized at a fixed flexion, where images can provide reliable information about the joint space in patients with moderate knee OA, and interpreted by experienced radiologist and orthopedist. Early knee OA was considered to be present when OA was defined as KL grade ≤2, and end-stage knee OA was considered to be present when the OA was defined as KL grade ≥3.
Arthrocentesis of the affected knee was performed on the patients to collect SF samples prior to the first therapeutic platelet-rich plasma (PRP) injection (early OA, KL grade 1-2) or prior to total knee arthroplasty (TKA; end-stage OA, KL grade 3-4). Knee pain and function prior to PRP injection and surgery (TKA), respectively, was assessed using the 11-point pain intensity Numeric Rating Scale (0 = no pain; 11 = worst pain). SF samples were successfully aspirated from the knee of 65 OA patients (37 females, 28 males) without the use of lavage into vacutainers without additives and stored at 4 °C until next processing. The mean volume of obtained SF was 1.8 ± 1.4 mL in the case of early OA and 4.2 ± 2.3 mL in the case of patients with end-stage OA. The samples were aliquoted and stored at −80 °C until further analysis after initial centrifugation at 1,000 × g for 30 minutes at 4 °C in order to remove debris. For standardization of the protocol, all SF samples were subjected to only one freeze-thaw event and no enzyme (hyaluronidase) treatment was performed on the SF prior to the assessment, because this can increase sample to sample variability.
Analysis of Biomolecules
In order to determine the concentrations of interleukin (IL)-6, IL-8, MMP-1, MMP-3, MMP-13, TIMP-1, and TIMP-2 proteins, SF was analyzed using the multiplex ELISA Quantibody Human Cytokine Antibody Array (RayBiotech, Baria, Prague, Czech Republic) described previously in our in vitro study. 18 The Quantibody array was custom made for our study using the above-mentioned 7 biomolecules. Cryopreserved SF samples (200 µL) were sent to Baria s.r.o. (official distributor of RayBiotech) for human cytokine antibody array screening for selected cytokines, which utilizes a validated, quantitative, multiplex ELISA (enzyme-linked immunosorbent assay). Intra-assay coefficients of variation (CV) were calculated for each slide, based on duplicate positive controls, and the average CV detected was 6.6%.
Assays were performed according to the manufacturer’s instructions, and data were obtained from experiments in triplicate. Concentration of COMP and Adiponectine was measured by using commercially available ELISA kits for each biomolecule (Abcam, Cambridge, UK). Data were obtained from experiments in duplicate. The average intra-assay CV for COMP was 4.2% and 3% for Adiponectin. SF samples were diluted 1:1 in all cases of measurements.
Statistical Analysis
Statistical analysis was performed using statistical program GraphPad Prism5. Demographic data for patients were compared by the Fisher’s test and the Mann-Whitney U test, where appropriate. All measurements were carried out in duplicate or triplicate and data are shown as mean, standard deviation (SD), or median and interquartile range (IQR). Test of normality was used to analyze the concentration of biomolecules in SF (early vs. end-stage OA, male vs. female). Because some of the biomolecule concentrations were not normally distributed, both Student’s t test and Mann-Whitney U tests were utilized. T test and Mann-Whitney U test were performed to compare the concentration of biomolecules between groups (male vs. female; early vs. end-stage OA). Pearson’s/Spearman’s rank correlation coefficient (normally/not normally distributed data) was used to examine correlation between biomolecule concentration and age. A value of P < 0.05, P < 0.01, P < 0.001 was considered as statistically significant, *, **, ***, respectively. The strength of correlation was interpreted via suggestion by Mukaka. 19 Correlation coefficient 0.1 to 0.3 was considered weak, 0.3 to 0.5 was considered moderate, and 0.5 to 1.0 was a strong correlation.
Results
Demographics of the Participants
Sixty-five participants were randomly recruited in this study, whereas 46 patients were diagnosed with early OA and 19 patients were diagnosed with end-stage OA. Radiographic assessment of severity was performed using the KL grading system. 17 SF was aspirated prior to the first therapeutic platelet-rich plasma (PRP) injection (early OA) and prior to the total knee arthroplasty (end-stage OA). Demographic characteristics of OA patients are shown in Table 1 . Mean age was 56.8 (±12.98) years and 69.9 (±5.64) for patients with early OA and end-stage OA, respectively. The age distribution between the 2 patient groups were notably different. Understandably, patients with end-stage OA were older. There was remarkable difference in sex between 2 groups. A mean score of NRS for patients in early OA group was significantly lower (5.27 ± 1.18) in comparison patients in end-stage OA group (6.32 ± 1.20), P = 0.0022. Differences in the concentration of selected biomolecules between male and female were also monitored. There were significant differences only in the concentration of adiponectin (mean: 3,883 ng/mL vs. 2,302 ng/mL, P = 0.0484) while its concentration was higher in female. The level of other biomolecules (IL-6, IL-8, MMP-1, MMP-3, MMP-13, TIMP-1, TIMP-2, and COMP) was not significantly different according to gender ( Table 2 ).
Table 1.
Demographic Characteristics of Patients with Early and End-Stage OA.
| Characteristic | Early OA (n = 46) | End-Stage OA (n = 19) | P |
|---|---|---|---|
| Age (year) | <0.0001 | ||
| Mean age (SD) | 56.8 (12.98) | 69.9 (5.64) | |
| Median age (range) | 58 (27-82) | 68 (63-85) | |
| Gender | 0.2786 | ||
| Male | 22 | 6 | |
| Female | 24 | 13 | |
| NRS score (SD) | 5.27 (1.18) | 6.32 (1.20) | 0.0022 |
| PRP | Yes | No | |
| TKA | No | Yes |
OA = osteoarthritis; n = number of patients; NRS = 11-point pain intensity Numeric Rating Scale (0 = no pain; 11 = worst pain); PRP = platelet-rich plasma; TKA = total knee arthroplasty
P values were calculated by using an unpaired, 2-tailed Mann-Whitney U test with 95% confidence interval or Fisher’s test, respectively. *P < 0.05 and **P < 0.01 was considered statistically significant.
Table 2.
Differences in Concentration of Biomolecules in SF between Female and Male OA Patient Groups.
| Marker | Female, Median (IQR) | Male, Median (IQR) | P |
|---|---|---|---|
| IL-6, pg/mL | 32.55 (114.05) | 55.63 (920.87) | 0.2681 |
| IL-8, pg/mL | 7.115 (14.9) | 5.94 (17.41) | 0.9085 |
| MMP-1, pg/mL | 8346 (21102.7) | 7813 (13586) | 0.9290 |
| MMP-3, pg/mL | 31764 (28177) | 30788 (23373) | 0.790 |
| Marker | Female, Mean ± SD | Male, Mean ± SD | p |
| MMP-3, pg/mL | 28130 ± 3213 | 26330 ± 3053 | 0.6885 |
| TIMP-1, pg/mL | 14830 ± 659.2 | 13610 ± 756.0 | 0.2260 |
| TIMP2, pg/mL | 8138 ± 1092 | 7737 ± 1122 | 0.8003 |
| Adiponectin, ng/mL | 3883 ± 523.7 | 2302 ± 552.2 | 0.0484* |
| COMP, ng/mL | 3442 ± 325.6 | 3802 ± 411.2 | 0.4929 |
Values are expressed as median and interquartile ranges (IQR) or mean and standard deviation (SD). P values were calculated by using a Mann-Whitney U test or Student’s t test with 95% confidence interval. *P < 0.05 was considered statistically significant.
OA = osteoarthritis; IL = interleukin; MMP = matrix metalloproteinase; TIMP = tissue inhibitor of metalloproteinase; COMP = cartilage oligomeric matrix protein.
Comparison of Biomolecule Levels with Age
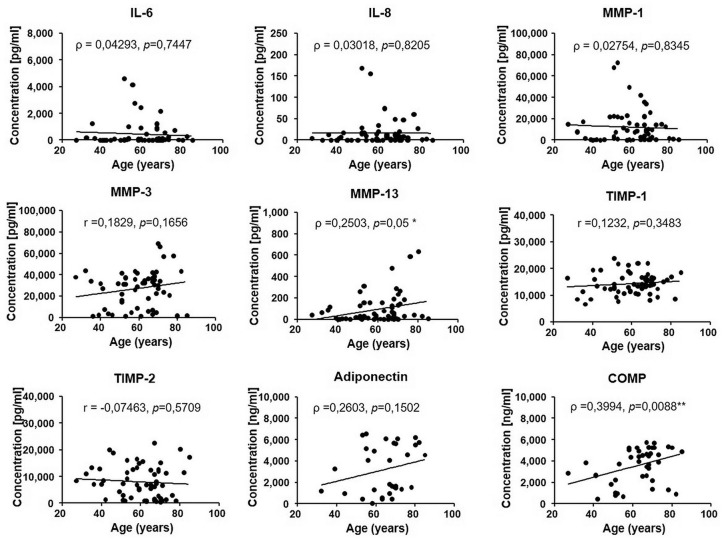
Because OA is an age-related disorder, we performed the correlation analysis between concentration of biomolecules and age. Pearson’s (r) or Spearman’s (ρ) rank correlation coefficients between age and measured biomolecules are shown in Figure 1 .The levels of MMP-13 and COMP protein in SF were positively correlated with age of patients (ρ = 0.2503, P = 0.05, and r = 0.3994, P = 0.0088, respectively). The correlation of MMP-13 with age was weak while correlation of COMP with age was moderate. There was no significant correlation between age and the concentration of IL-6, IL-8, MMP-1, MMP-3, TIMP-1, TIMP-2, and adiponectin. Our results showed significant linear correlations between age and concentration only in the case of 2 markers, MMP-13 and COMP, suggesting their clinically relevant role in the pathophysiology of knee OA.
Figure 1.
Correlation between age and concentration of studied biomolecules in SF, r = Pearson’s coefficient, ρ = Spearman’s coefficient. P value is based on the Pearson’s/Spearman’s correlation test. *P < 0.05 and **P < 0.01 considered statistically significant.
Comparison of Biomolecule Levels with the Grade of OA
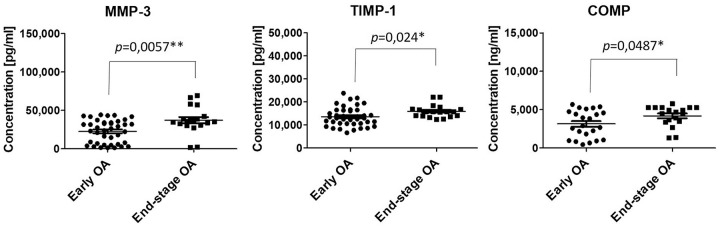
Changes in concentration of markers between early and end-stage OA were also evaluated in order to monitor the severity of disease. In the case of IL-6, IL-8, MMP-1, MMP-13, TIMP-2, and adiponectin there were no significant differences in concentration according to the grade of OA. On the other hand, concentrations of MMP-3, TIMP-1, and COMP were significantly lower (25,054 pg/mL vs. 35,705 pg/mL, 13,520 pg/mL vs. 15,920 pg/mL, 3,324 pg/mL vs. 4,601 pg/mL, respectively) in group of patients with early OA compared to the group of patients with end-stage OA ( Table 3 , Fig. 2 ). It follows that severity of OA had an impact on the concentration of degradative enzyme MMP-3, TIMP-1 (an inhibitor of MMPs), and also COMP.
Table 3.
Synovial Fluid Concentrations of the Biological Markers in Patients with Early and End-Stage OA a .
| Marker | Early OA (KL Grade ≤2), Median (IQR) | End-Stage OA (KL Grade ≥3), Median (IQR) | P |
|---|---|---|---|
| IL-6, pg/mL | 55.63 (595.2) | 32.73 (142.47) | 0.5998 |
| IL-8, pg/mL | 4.80 (17.68) | 8.086 (10.44) | 0.5127 |
| MMP-1, pg/mL | 3670 (14898.0) | 9844 (18028) | 0.0861 |
| MMP-3, pg/mL | 25054 (28063.0) | 35705 (11358) | 0.0057** |
| MMP-13, pg/mL | 21.46 (16.86) | 58.42 (184.33) | 0.1978 |
| Adiponectin, ng/mL | 4350 (4439) | 1661 (4286) | 0.4856 |
| COMP, ng/mL | 3324 (3450) | 4601 (1806) | 0.0487* |
| Marker | Early OA (KL Grade ≤2), Mean ± SD | End-Stage OA (KL Grade ≥3), Mean ± SD | P |
| TIMP-1, pg/mL | 13520 ± 640.4 | 15920 ± 628.2 | 0.0240* |
| TIMP2, pg/mL | 8796 ± 919.4 | 6148 ± 1398 | 0.1144 |
OA = osteoarthritis; IL = interleukin; MMP = matrix metalloproteinase; TIMP = tissue inhibitor of metalloproteinase; COMP = cartilage oligomeric matrix protein.
Values are expressed as median and interquartile ranges (IQR) or mean and standard deviation (SD). P values were calculated by using a Mann-Whitney U test or Student’s t test with 95% confidence interval. *P < 0.05 and **P < 0.01 was considered statistically significant.
Figure 2.
Concentration of biomolecules measured in synovial fluid from patients with early (•) and end-stage OA (■). The data are presented as mean ± SD scatterplots, where each point represents an average of 3 measurements. *P < 0.05 and **P < 0.01 was considered statistically significant.
Correlation Analysis of the Biomolecule Concentrations Measured in Early and End-Stage OA Samples
Our results have demonstrated that IL-8 moderately correlate with IL-6, MMP-3, and TIMP-1 (ρ = 0.3526, P = 0.024; ρ = 0.4013, P = 0.01; and ρ = 0.3097, P = 0.049, respectively) and strongly correlate with MMP-1 (ρ = 0.579, P = 0.000073) in the group of patients with early OA. In contrast, IL-8 concentration showed negative correlation with TIMP-2 concentration and this correlation was strong (ρ = −0.6897, P = 0.0000006095). There were also considerable positive correlations between the IL-6 and MMP-1, IL-6, and MMP-13 (ρ = 0.5288, P = 0.0003789; ρ = 0.649, P = 0.000004499) and between MMP-1 and MMP-3 and MMP-1 and MMP-13 (ρ = 0.5801, P = 0.0001; ρ = 0.5192, P = 0.0005). Adiponectin, TIMP-1, and COMP did not show any correlation with the rest of biomolecules ( Table 4 ).
Table 4.
Spearman’s Rank Correlation Coefficients for Early OA a .
| IL6 | IL8 | MMP1 | MMP3 | MMP13 | TIMP1 | TIMP2 | Adiponectin | COMP | |
|---|---|---|---|---|---|---|---|---|---|
| IL6 | ρ = 1.000 | ||||||||
| IL8 | ρ = 0.3526, P ≤ 0.05 | ρ = 1.000 | |||||||
| MMP1 | ρ = 0.5288, P ≤ 0.001 | ρ = 0.5790, P ≤ 0.001 | ρ = 1.000 | ||||||
| MMP3 | ρ = 0.2734,P = 0.088 | ρ = 0.4013, P ≤ 0.05 | ρ = 0.5801, P ≤ 0.001 | ρ = 1.000 | |||||
| MMP13 | ρ = 0.649, P ≤ 0.001 | ρ = 0.1706, P = 0.286 | ρ = 0.5192, P ≤ 0.001 | ρ = 0.1831, P = 0.258 | ρ = 1.000 | ||||
| TIMP1 | ρ = −0.1476, P = 0.357 | ρ = 0.3097, P ≤ 0.05 | ρ = 0.1481, P = 0.355 | ρ = 0.2253, P = 0.162 | ρ = −0.2221, P = 0.163 | ρ = 1.000 | |||
| TIMP2 | ρ = 0.0960, P = 0.551 | ρ = −0.6897, P ≤ 0.001 | ρ = −0.2833, P = 0.073 | ρ = −0.2195, P = 0.174 | ρ = 0.06475, P = 0.688 | ρ = −0.0199, P = 0.902 | ρ = 1.000 | ||
| Adiponectin | ρ = −0.3559, P = 0.176 | ρ = −0.0529, P = 0.846 | ρ = −0.4706, P = 0.066 | ρ = −0.3029, P = 0.254 | ρ = −0.4296, P = 0.097 | ρ = 0.0118, P = 0.966 | ρ = −0.1882, P = 0.485 | ρ = 1.000 | |
| COMP | ρ = −0.0609, P = 0.778 | ρ = −0.1787, P = 0.403 | ρ = −0.0530, P = 0.806 | ρ = −0.1047, P = 0.634 | ρ = −0.2336, P = 0.272 | ρ = 0.0670, P = 0.756 | ρ = 0.2278, P = 0.284 | ρ = −0.1235, P = 0.649 | ρ = 1.0000 |
OA = osteoarthritis; IL = interleukin; MMP = matrix metalloproteinase; TIMP = tissue inhibitor of metalloproteinase; COMP = cartilage oligomeric matrix protein.
Values are the Spearman’s rank correlation coefficient (ρ). P < 0.05 was considered statistically significant.
There were less significant correlations between biomolecule levels in the end-stage OA group in comparison with early OA group. In contrast to significant correlation of IL-8 with several biomolecules, like IL-6, MMP-1, MMP-3, TIMP-1, and TIMP-2 in early OA group ( Table 4 ), concentration of IL-8 did not show any significant correlation with other biomolecules in the end-stage OA group ( Table 5 ). In this group of participants there were strongly positive and statistically significant correlations between IL-6 and MMP-1, IL-6, and MMP-3 (ρ = 0.6406, P = 0.003; ρ = 0.6713, P = 0.002) and between MMP-1 and MMP-3, TIMP-1 and TIMP-2 (ρ = 0.7544, P = 0.0001901; ρ = 0.5789, P = 0.009).
Table 5.
Spearman’s Rank Correlation Coefficients for Late OA a .
| IL6 | IL8 | MMP1 | MMP3 | MMP13 | TIMP1 | TIMP2 | Adiponectin | COMP | |
|---|---|---|---|---|---|---|---|---|---|
| IL6 | ρ = 1.000 | ||||||||
| IL8 | ρ = 0.3657, P = 0.136 | ρ = 1.000 | |||||||
| MMP1 | ρ = 0.6406, P ≤ 0.01 | ρ = 0.4047, P = 0.096 | ρ = 1.000 | ||||||
| MMP3 | ρ = 0.6713, P ≤ 0.01 | ρ = 0.443, P = 0.066 | ρ = 0.7544, P ≤ 0.001 | ρ = 1.000 | |||||
| MMP13 | ρ = 0.3314, P = 0.166 | ρ = 0.1818, P = 0.47 | ρ = 0.1430, P = 0.559 | ρ = 0.1483, P = 0.545 | ρ = 1.000 | ||||
| TIMP1 | ρ = −0.1036, P = 0.673 | ρ = −0.1053, P = 0.677 | ρ = −0.0386, P = 0.875 | ρ = −0.0842, P = 0.732 | ρ = 0.2896, P = 0.229 | ρ = 1.000 | |||
| TIMP2 | ρ = 0.0667, P = 0.786 | ρ = −0.095, P = 0.708 | ρ = −0.0649, P = 0.792 | ρ = −0.2789, P = 0.247 | ρ = 0.3695, P = 0.120 | ρ = 0.5789, P ≤ 0.01 | ρ = 1.000 | ||
| Adiponectin | ρ = 0.1340, P = 0.621 | ρ = −0.1000, P = 0.712 | ρ = 0.0618, P = 0.820 | ρ = 0.3929, P = 0.132 | ρ = 0.3299, P = 0.212 | ρ = 0.3355, P = 0.204 | ρ = −0.1810, P = 0.502 | ρ = 1.000 | |
| COMP | ρ = −0.1453, P = 0.565 | ρ = −0.0047, P = 0.985 | ρ = −0.3859, P = 0.114 | ρ = −0.3330, P = 0.177 | ρ = 0.2086, P = 0.406 | ρ = 0.0861, P = 0.734 | ρ = −0.0975, P = 0.700 | ρ = 0.1635, P = 0.545 | ρ = 1.0000 |
OA = osteoarthritis; IL = interleukin; MMP = matrix metalloproteinase; TIMP = tissue inhibitor of metalloproteinase; COMP = cartilage oligomeric matrix protein.
Values are the Spearman’s rank correlation coefficient (ρ). P < 0.05 was considered statistically significant.
Discussion
OA is a slowly progressive joint disease leading to pain, stiffness, reduced motion, swelling, and disability. It is characterized by articular cartilage breakdown combined with osteophyte formation at the joint margins and chronic nonspecific inflammation of synovium. 20 Inflammatory process triggers both anabolic and catabolic events such as production of chemokines, cytokines, matrix metalloproteinases, tissue inhibitors of metalloproteinase and all of these molecules can also be detected in SF of patients with OA. 21 In order to successfully identify patients at risk for progressive joint damage, there is a need for early diagnostic tools to detect molecular events leading to cartilage destruction. The routinely used diagnostic techniques for joint diseases are radiographs and other types of joint imaging, 11 but they do not have the capacity to measure dynamic changes in the joint. Radiographic changes occur very late during the disease pathogenesis pathway and have poor sensitivity for monitoring disease progression.22,23
In the study by Haraden et al., 24 a subset of 6 SF biomarkers, including VEGF, MMP-3, TIMP-1, sICAM-1, sVCAM-1, and MCP-1, was related to synovial inflammation in OA, as well as radiographic and symptom severity. They also observed association of these biomarkers with activated macrophages and neutrophils. Our preliminary study provides a profile of 9 synovial inflammatory and noninflammatory mediators in knee OA and identifies cytokines of potential clinical relevance in accordance to 3 variable factors: OA grade, gender, and age. In this study, we measured and analyzed the concentration of interleukins (IL-6, IL-8), matrix metalloproteinases (MMP-1, MMP-3, MMP-13), MMPs inhibitors (TIMP-1, TIMP-2), COMP, and adipokine (adiponectin) in SF of patients with early and end-stage OA.
Interleukins are proteins that participate on regulation of the cellular immune response. Pro-inflammatory cytokines have been implicated in the pathophysiology of OA, including IL-1β, TNF-α, IL-6, IL-15, IL-17, IL-18, IL-21, and IL-8. 25 The main cytokines that cause degradation in the synovia are the IL-1, IL-6, IL-17, and TNF-α. 26 IL-6 is a 184 amino acid residue protein, which has been shown in a number of studies to play a pro-inflammatory role in the pathophysiology of OA. IL-8 (also known as CXCL8) is a potent chemokine in the immune system. 27 Study by Kaneko et al. observed increased levels of IL-6 and IL-8 in OA serum and SF, 28 and the other group of researchers demonstrated that patients with end-stage knee OA have significantly higher levels of IL-6 in SFs as compared with control donors, 29 supporting the hypothesis that inflammatory processes are involved in OA. Similarly, another study has shown that the levels of IL-6 in the SF from patients with OA and those with symptomatic cartilage defects are identical, and this level is significantly higher than those of healthy volunteers. 30 On the other hand, there are several studies that have been shown no correlation of IL-8 with OA grade, BMI, or age in SF samples from OA patients.31,32 Based on our results, we detected no significant difference of IL-6 and IL-8 concentration in early OA SF compared with end-stage OA SF. Nor were any remarkable differences found in concentration levels of these 2 inflammatory cytokines according to age or gender.
Pérez-García et al. 33 reported synovial fibroblasts activation by inflammatory mediators present in the joint, release dis-integrin and metalloprotease with thrombospondin motifs (ADAMTS) metalloproteinases, which contribute to the maintenance of cartilage destruction in osteoarthritic patients. Mainly ADAMTS 4, 5, 7, and 12 have been implicated in the breakdown of cartilage in OA.34,35 ADAMTS 4 and 5 degrading aggrecan and ADAMTS 7 and 12 degrading COMP. Fragments of COMP have been detected in the cartilage, SF, and serum of patients with posttraumatic and primary OA and rheumatoid arthritis (RA). 36 It was also demonstrated that elevated levels of cartilage degradation product—COMP—persist in SF from patients with joint injuries for a long time after the initial injury. 37 Cartilage oligomeric matrix protein (COMP) is a 524 kDa noncollagenous homopentameric protein present in cartilage. 38 It is well established that COMP can be used as a marker of cartilage turnover 39 ; however, it remains unclear whether COMP is sensitive enough to evaluate knee OA patients. Since COMP is synthesized not only by cartilage but also by synovial cells, tendon fibroblasts, and osteoblasts, the increase may be due to cartilage destruction or synovial inflammation. Arellano et al. highlighted the importance to consider daytime variation of COMP levels in SF to avoid false diagnosis, because they have demonstrated a differences in COMP concentration during the day. 40 Among various biological markers associated with OA, MMPs play a primary role in cartilage degradation in human joint disease.41,42 Increased levels of MMPs was found in OA cartilage at the site of cartilage destruction and specific digested parts of MMPs were present in SF samples from OA patients. 43 Inactivation of the MMPs involves specific TIMPs, which are naturally occurring inhibitors present in SF during inflammation. 44 Each TIMP binds with differential rates of interaction and affinity to a MMP. TIMP-1 inhibits MMP-1, MMP-3, and MMP-9. 45 TIMP-2 inhibits proMMP-2. 46 Our results showed significant linear correlations of concentration of MMP-13 and COMP with age, but when we compared the levels of MMP-13 and COMP between patients with early OA and end-stage OA, MMP-13 concentration did not correlate with OA severity. Within the present study, significant differences in the concentration of MMP-3, TIMP-1, and COMP were detected in the SF between early and end-stage OA. Several studies have shown that concentration of COMP in SF was positively correlated with age and severity of disease, too.40,47 Chen et al. detected MMP-3 in synovial tissue. 48 The expression of MMP-3 in the OA group was significantly higher than in the normal synovium. Their results demonstrated that expression of MMP-3 was positively related to the severity of OA. Heard et al. investigated protein expression levels of MMPs and TIMPs in SF from normal, early OA, and OA joints. They showed significant difference in MMP-3 expression between normal and early OA samples and also MMP-1, -2, -3, -8, and -13 expression levels were significantly higher in advanced OA samples when compared to early OA samples. They also showed that advanced OA sample expression levels were elevated when compared to early OA samples for TIMP-1 and -3. 49 Based on results present here and previous studies it would appear that the regulation of MMPs by TIMPs may be important in the progression of disease. Adiponectin is a 28- to 30-kDa collagen-like protein. Its effect and role have been intensely studied in inflammatory and anti-inflammatory processes. 50 Results of the study of Cuzdan et al. supported that there is a positive correlation between plasma concentration of adiponectin levels and the Kellgren-Lawrence grading scores. 51 On the other hand, some data suggest that the adiponectin expression level is negatively correlated with the radiographic severity of OA and might be playing a protective role in the pathogenesis of OA.52,53 However, the effects of adiponectin in OA process were still controversial. In our study, we did not observe any significant correlations between adiponectin concentration and age, severity of disease, and concentration of other analyzed biomolecules. The higher concentration of adiponectin in female group may be due to the higher percentage of body fat in comparison with men. 54 Similar conclusions have also been reached by Francin et al. 55 They reported that adiponectin (adipose-derived protein) is not detected in healthy cartilage but is upregulated in damaged tissue. They also showed that the increase in adiponectin production depends on the percentage of body fat of the patients and is not strongly related to the grade of cartilage destruction.
The strength of our study was the availability of a relatively large number of SF samples reflecting patients from among the general knee OA population. We measured soluble biomolecules in the SF, because SF biomarkers provide a more proximal indicator of disease state than serum biomarkers. One possible limitation of our study is single sampling of biomolecules in SF. Our study was also limited by lacking of control group with healthy volunteers, in order to detect true differences in levels of cytokine or growth factor in SF from healthy knee and OA knee.
Although many studies have compared expression of different proteins levels in SF, to our knowledge, this is the first study in a Slovak population that compares and correlates an extensive profile of synovial inflammatory mediators in SF from OA knee with other important variables such as age, sex, and severity of disease. Further study should be also performed with randomized and larger cohort groups, including age-, gender-, and BMI-matched patients to convincingly resolve the role of the cytokine network in knee OA diagnosis to reach concrete conclusions. We are preparing a placebo-controlled prospective multicenter trial to evaluate the effect of PRP treatment on the level of larger number of cytokines by multiplex assay. Our perspective underscores the significant potential of the use of the multiplex approach with the close cooperation of clinical orthopedists and biochemists in order to improve the management of OA pathologies based on standardized multimarker testing. However, in this preliminary study we have indicated that TIMP-1, MMP-3, and COMP have the potential of being utilized as a marker although studies are being planned to identify an optimal combination of already well-established biomarkers to give most comprehensive and precise information on OA pathology in a given patient instead of searching for novel molecules.
Footnotes
Acknowledgment and Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This research was supported by VEGA Grant 1/0229/20 from the Ministry of Education, Science, Research and Sport of the Slovak Republic, by the Slovak Research and Development Agency under Contract No. APVV17-0118, No. APVV0684-12, and by grants OPVaV-2012/2.2/08-RO-MEDIPARK, ITMS: 26220220185, and ITMS: 313011D103.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Ethical Approval: This study was approved by the Institutional Ethics Board of Louis Pasteur University Hospital, Kosice, Slovakia.
Informed Consent: Samples were taken with patients’ written consent according to the guidelines of the local ethics committee.
ORCID iD: Timea Spakova  https://orcid.org/0000-0001-6787-1925
https://orcid.org/0000-0001-6787-1925
References
- 1. Lawrence RC, Helmick CG, Arnett FC, Deyo RA, Felson DT, Giannini EH, et al. Estimates of the prevalence of arthritis and selected musculoskeletal disorders in the United States. Arthritis Rheum. 1998;41(5):778-99. [DOI] [PubMed] [Google Scholar]
- 2. Bijlsma JWJ, Berenbaum F, Lafeber FPJG. Osteoarthritis: an update with relevance for clinical practice. Lancet. 2011;377(9783):2115-26. doi: 10.1016/S0140-6736(11)60243-2 [DOI] [PubMed] [Google Scholar]
- 3. Nalbant S, Martinez JAM, Kitumnuaypong T, Clayburne G, Sieck M, Schumacher HR., Jr. Synovial fluid features and their relations to osteoarthritis severity: new findings from sequential studies. Osteoarthritis Cartilage. 2003;11(1):50-4. [DOI] [PubMed] [Google Scholar]
- 4. Berenbaum F. Osteoarthritis as an inflammatory disease (osteoarthritis is not osteoarthrosis!). Osteoarthritis Cartilage. 2013;21(1):16-21. doi: 10.1016/j.joca.2012.11.012 [DOI] [PubMed] [Google Scholar]
- 5. Kraus VB, McDaniel G, Huebner JL, Stabler TV, Pieper CF, Shipes SW, et al. Direct in vivo evidence of activated macrophages in human osteoarthritis. Osteoarthritis Cartilage. 2016;24(9):1613-21. doi: 10.1016/j.joca.2016.04.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Sokolove J, Lepus CM. Role of inflammation in the pathogenesis of osteoarthritis: latest findings and interpretations. Ther Adv Musculoskelet Dis. 2013;5(2_suppl):77-94. doi: 10.1177/1759720X12467868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Lee MS, Kim YJ. Pattern-recognition receptor signaling initiated from extracellular, membrane, and cytoplasmic space. Mol Cells. 2007;23(1):1-10. [PubMed] [Google Scholar]
- 8. Feldman N, Rotter-Maskowitz A, Okun E. DAMPs as mediators of sterile inflammation in aging-related pathologies. Ageing Res Rev. 2015;24(Pt A):29-39. doi: 10.1016/j.arr.2015.01.003 [DOI] [PubMed] [Google Scholar]
- 9. Huang WC, Sala-Newby GB, Susana A, Johnson JL, Newby AC. Classical macrophage activation up-regulates several matrix metalloproteinases through mitogen activated protein kinases and nuclear factor-κB. PLoS One. 2012;7(8):e42507. doi: 10.1371/journal.pone.0042507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Dieppe PA, Lohmander LS. Pathogenesis and management of pain in osteoarthritis. Lancet. 2005;365(9463):965-73. [DOI] [PubMed] [Google Scholar]
- 11. Braun HJ, Gold GE. Diagnosis of osteoarthritis: imaging. Bone. 2012;51(2_suppl):278-88. doi: 10.1016/j.bone.2011.11.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Mobasheri A, Henrotin Y. Biomarkers of (osteo)arthritis. Biomarkers. 2015;20(8):513-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Mobasheri A. Osteoarthritis year 2012 in review: biomarkers. Osteoarthritis Cartilage. 2012;20(12):1451-64. doi: 10.1016/j.joca.2012.07.009 [DOI] [PubMed] [Google Scholar]
- 14. Mobasheri A, Bay-Jensen AC, van Spil WE, Larkin J, Levesque MC. Osteoarthritis year in review 2016: biomarkers (biochemical markers). Osteoarthritis Cartilage. 2017;25(2_suppl):199-208. doi: 10.1016/j.joca.2016.12.016 [DOI] [PubMed] [Google Scholar]
- 15. Glyn-Jones S, Palmer AJR, Agricola R, Price AJ, Vincent TL, Weinans H, et al. Osteoarthritis. Lancet. 2015;386(9991):376-87. doi: 10.1016/S0140-6736(14)60802-3 [DOI] [PubMed] [Google Scholar]
- 16. Felson DT. The current and future status of biomarkers in osteoarthritis. J Rheumatol. 2014;41(5):834-6. doi: 10.3899/jrheum.140094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kellgren JH, Lawrence JS. Radiological assessment of osteo-arthrosis. Ann Rheum Dis. 1957;16(4):494-502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Spakova T, Plsikova J, Harvanova D, Lacko M, Stolfa S, Rosocha J. Influence of kartogenin on chondrogenic differentiation of human bone marrow-derived MSCs in 2D culture and in co-cultivation with OA osteochondral explant. Molecules. 2018;23(1):E181. doi: 10.3390/molecules23010181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Mukaka MM. Statistics corner: a guide to appropriate use of correlation coefficient in medical research. Malawi Med J. 2012;24(3):69-71. [PMC free article] [PubMed] [Google Scholar]
- 20. Krasnokutsky S, Attur M, Palmer G, Samuels J, Abramson SB. Current concepts in the pathogenesis of osteoarthritis. Osteoarthritis Cartilage. 2008;16(Suppl 3):S1-S3. doi: 10.1016/j.joca.2008.06.025 [DOI] [PubMed] [Google Scholar]
- 21. Wojdasiewicz P, Poniatowski ŁA, Szukiewicz D. The role of inflammatory and anti-inflammatory cytokines in the pathogenesis of osteoarthritis. Mediators Inflamm. 2014;2014:561459. doi: 10.1155/2014/561459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Rousseau JC, Delmas PD. Biological markers in osteoarthritis. Nat Clin Pract Rheumatol. 2007;3(6):346-56. [DOI] [PubMed] [Google Scholar]
- 23. Rousseau JC, Garnero P. Biological markers in osteoarthritis. Bone. 2012;51(2_suppl):265-77. doi: 10.1016/j.bone.2012.04.001 [DOI] [PubMed] [Google Scholar]
- 24. Haraden CA, Huebner JL, Hsueh MF, Li YJ, Kraus VB. Synovial fluid biomarkers associated with osteoarthritis severity reflect macrophage and neutrophil related inflammation. Arthritis Res Ther. 2019;21(1):146. doi: 10.1186/s13075-019-1923-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kapoor M, Martel-Pelletier J, Lajeunesse D, Pelletier JP, Fahmi H. Role of proinflammatory cytokines in the pathophysiology of osteoarthritis. Nat Rev Rheumatol. 2011;7(1):33-42. doi: 10.1038/nrrheum.2010 [DOI] [PubMed] [Google Scholar]
- 26. Schaible HG, von Banchet GS, Boettger MK, Brauer R, Gajda M, Richter F, et al. The role of proinflammatory cytokines in the generation and maintenance of joint pain. Ann N Y Acad Sci. 2010;1193:60-9. doi: 10.1111/j.1749-6632.2009.05301.x [DOI] [PubMed] [Google Scholar]
- 27. Mabey T, Honsawek S. Cytokines as biochemical markers for knee osteoarthritis. World J Orthop. 2015;6(1):95-105. doi: 10.5312/wjo.v6.i1.95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kaneko S, Satoh T, Chiba J, Ju C, Inoue K, Kagawa J. Interleukin-6 and interleukin-8 levels in serum and synovial fluid of patients with osteoarthritis. Cytokines Cell Mol Ther. 2000;6(2_suppl):71-9. [DOI] [PubMed] [Google Scholar]
- 29. Beekhuizen M, Gierman LM, van Spil WE, Van Osch GJ, Huizinga TWJ, Saris DBF, et al. An explorative study comparing levels of soluble mediators in control and osteoarthritic synovial fluid. Osteoarthritis Cartilage. 2013;21(7):918-22. doi: 10.1016/j.joca.2013.04.002 [DOI] [PubMed] [Google Scholar]
- 30. Tsuchida AI, Beekhuizen M, Rutgers M, van Osch GJ, Bekkers JE, Bot AGJ, et al. Interleukin-6 is elevated in synovial fluid of patients with focal cartilage defects and stimulates cartilage matrix production in an in vitro regeneration model. Arthritis Res Ther. 2012;14(6):R262. doi: 10.1186/ar4107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Rubenhagen R, Schuttrumpf JP, Sturmer KM, Frosch KH. Interleukin-7 levels in synovial fluid increase with age and MMP-1 levels decrease with progression of osteoarthritis. Acta Orthop. 2012;83(1):59-64. doi: 10.3109/17453674.2011.645195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Pierzchala AW, Kusz DJ, Hajduk G. CXCL8 and CCL5 expression in synovial fluid and blood serum in patients with osteoarthritis of the knee. Arch Immunol Ther Exp (Warsz). 2011;59(2_suppl):151-5. doi: 10.1007/s00005-011-0115-4 [DOI] [PubMed] [Google Scholar]
- 33. Pérez-García S, Gutiérrez-Cañas I, Seoane IV, Fernández J, Mellado M, Leceta J, et al. Healthy and osteoarthritic synovial fibroblasts produce a disintegrin and metalloproteinase with thrombospondin Motifs 4, 5, 7, and 12: induction by IL-1β and fibronectin and contribution to cartilage damage. Am J Pathol. 2016;186(9):2449-61. doi: 10.1016/j.ajpath.2016.05.017 [DOI] [PubMed] [Google Scholar]
- 34. Zhang Q, Huang M, Wang X, Xu X, Ni M, Wang Y. Negative effects of ADAMTS-7 and ADAMTS-12 on endplate cartilage differentiation. J Orthop Res. 2012;30(8):1238-43. doi: 10.1002/jor.22069 [DOI] [PubMed] [Google Scholar]
- 35. Malfait AM, Liu RQ, Ijiri K, Komiya S, Tortorella MD. Inhibition of ADAM-TS4 and ADAM-TS5 prevents aggrecan degradation in osteoarthritic cartilage. J Biol Chem. 2002;277(25):22201-8. [DOI] [PubMed] [Google Scholar]
- 36. Lorenzo P, Aspberg A, Saxne T, Önnerfjord P. Quantification of cartilage oligomeric matrix protein (COMP) and a COMP neoepitope in synovial fluid of patients with different joint disorders by novel automated assays. Osteoarthritis Cartilage. 2017;25(9):1436-42. doi: 10.1016/j.joca.2017.04.004 [DOI] [PubMed] [Google Scholar]
- 37. Verma P, Dalal K. Serum cartilage oligomeric matrix protein (COMP) in knee osteoarthritis: a novel diagnostic and prognostic biomarker. J Orthop Res. 2013;31(7):999-1006. doi: 10.1002/jor.22324 [DOI] [PubMed] [Google Scholar]
- 38. Tseng S, Reddi AH, Di Cesare PE. Cartilage oligomeric matrix protein (COMP): a biomarker of arthritis. Biomark Insights. 2009;4:33-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Sharif M, Saxne T, Shepstone L, Kirwan JR, Elson CJ, Heinegård D, et al. Relationship between serum cartilage oligomeric matrix protein levels and disease progression in osteoarthritis of the knee joint. Br J Rheumatol. 1995;34(4):306-10. [DOI] [PubMed] [Google Scholar]
- 40. Arellano RD, Aguilar LS, Argüello R, Hernadez F, Gonzalez FF, Moran J. Cartilage oligomeric matrix protein levels in synovial fluid in patients with primary knee osteoarthritis and healthy controls: a preliminary comparative analysis with serum cartilage oligomeric matrix protein. Arch Rheumatol. 2017;32(3):189-96. doi: 10.5606/ArchRheumatol.2017.6220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Burrage PS, Mix KS, Brinckerhoff CE. Matrix metalloproteinases: role in arthritis. Front Biosci. 2006;11:529-43. [DOI] [PubMed] [Google Scholar]
- 42. Takaishi H, Kimura T, Dalal S, Okada Y, D’Armiento J. Joint diseases and matrix metalloproteinases: a role for MMP-13. Curr Pharm Biotechnol. 2008;9(1):47-54. [DOI] [PubMed] [Google Scholar]
- 43. Gupta K, Shukla M, Cowland JB, Malemud CJ, Haqqi TM. Neutrophil gelatinase-associated lipocalin is expressed in osteoarthritis and forms a complex with matrix metalloproteinase 9. Arthritis Rheum. 2007;56(10):3326-35. [DOI] [PubMed] [Google Scholar]
- 44. Brew K, Dinakarpandian D, Nagase H. Tissue inhibitors of metalloproteinases: evolution, structure and function. Biochim Biophys Acta. 2000;1477(1-2):267-83. [DOI] [PubMed] [Google Scholar]
- 45. Brew K, Nagase H. The tissue inhibitors of metalloproteinases (TIMPs): An ancient family with structural and functional diversity. Biochim Biophys Acta. 2010;1803(1):55-71. doi: 10.1016/j.bbamcr.2010.01.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Bernardo MM, Fridman R. TIMP-2 (tissue inhibitor of metalloproteinase-2) regulates MMP-2 (matrix metalloproteinase-2) activity in the extracellular environment after pro-MMP-2 activation by MT1 (membrane type 1)-MMP. Biochem J. 2003;374(Pt 3):739-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. El-Arman MM, El-Fayoumi G, El-Shal E, El-Boghdady I, El-Ghaweet A. Aggrecan and cartilage oligomeric matrix protein in serum and synovial fluid of patients with knee osteoarthritis. HSS J. 2010;6(2_suppl):171-6. doi: 10.1007/s11420-010-9157-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Chen JJ, Huang JF, Du WX, Tong PJ. Expression and significance of MMP3 in synovium of knee joint at different stage in osteoarthritis patients. Asian Pac J Trop Med. 2014;7(4):297-300. doi: 10.1016/S1995-7645(14)60042-0 [DOI] [PubMed] [Google Scholar]
- 49. Heard BJ, Martin L, Rattner JB, Frank CB, Hart DA, Krawetz R. Matrix metalloproteinase protein expression profiles cannot distinguish between normal and early osteoarthritic synovial fluid. BMC Musculoskelet Disord. 2012;13:126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Fantuzzi G. Adiponectin and inflammation: consensus and controversy. J Allergy Clin Immunol. 2008;121(2_suppl):326-30. [DOI] [PubMed] [Google Scholar]
- 51. Cuzdan Coskun N, Ay S, Evcik FD, Oztuna D. Adiponectin: is it a biomarker for assessing the disease severity in knee osteoarthritis patients? Int J Rheum Dis. 2017;20(12):1942-9. doi: 10.1111/1756-185X.12790 [DOI] [PubMed] [Google Scholar]
- 52. Yusuf E, Ioan-Facsinay A, Bijsterbosch J, Klein-Wieringa I, Kwekkeboom J, Slagboom PE, et al. Association between leptin, adiponectin and resistin and long-term progression of hand osteoarthritis. Ann Rheum Dis. 2011;70(7):1282-4. doi: 10.1136/ard.2010.146282 [DOI] [PubMed] [Google Scholar]
- 53. Zheng S, Xu J, Xu S, Zhang M, Huang S, He F, et al. Association between circulating adipokines, radiographic changes, and knee cartilage volume in patients with knee osteoarthritis. Scand J Rheumatol. 2016;45(3):224-9. doi: 10.3109/03009742.2015.1083053 [DOI] [PubMed] [Google Scholar]
- 54. Jackson AS, Stanforth PR, Gagnon J, Rankinen T, Leon AS, Rao DC, Skinner JS, Bouchard C, Wilmore JH. The effect of sex, age and race on estimating percentage body fat from body mass index: the Heritage Family Study. Int J Obes Relat Metab Disord. 2002;26(6):789-96. [DOI] [PubMed] [Google Scholar]
- 55. Francin PJ, Abot A, Guillaume C, Moulin D, Bianchi A, Gegout-Pottie P, et al. Association between adiponectin and cartilage degradation in human osteoarthritis. Osteoarthritis Cartilage. 2014;22(3):519-26. doi: 10.1016/j.joca.2014.01.002 [DOI] [PubMed] [Google Scholar]