Abstract
Objective
Anterior cruciate ligament reconstruction (ACLR) has not been shown to decrease the risk for development of post-traumatic osteoarthritis. Magnetic resonance imaging (MRI) T2 mapping can be used to assess cartilage compositional changes. This study tests whether (1) worse cartilage arthroscopic status at ACLR is reflected by higher cartilage T2 values in matched study regions 6 weeks and 1 year after ACLR, and (2) increasing cartilage T2 values between 6 weeks and 1 year after ACLR are associated with worsening patient-reported outcomes.
Design
Twenty-two participants with ACLR and 26 controls underwent 3T MRI. T2 values in medial and lateral femoral and tibial cartilage were measured at 6 weeks and 1 year after ACLR and compared with arthroscopic grades, Knee injury and Osteoarthritis Outcome Scores (KOOS), and control T2 values.
Results
Most (59%-86%) cartilage study regions examined by arthroscopy demonstrated intact articular surfaces. Average T2 value increased in 3 of 4 study regions between 6 weeks and 1 year after ACLR (P = .001-.011). T2 value increased (P < .013) even for participants whose cartilage had intact articular surfaces at ACLR. Participants with ACLR who showed greater increases in cartilage T2 values had less improvement to KOOS Quality of Life (P = .009, ρ = −0.62).
Discussion
Cartilage status assessed arthroscopically at ACLR and by MRI T2 maps 6 weeks later was healthier than cartilage status assessed by MRI T2 maps at 1-year follow-up. Progressive T2 elevations were observed over the first year after ACLR even in patients with arthroscopically intact cartilage at the time of surgery and were associated with reduced improvement in knee quality of life suggesting preosteoarthritis.
Keywords: anterior cruciate ligament reconstruction, magnetic resonance imaging, arthroscopy, T2 mapping, preosteoarthritis
Introduction
Anterior cruciate ligament (ACL) injury and ACL reconstruction (ACLR) surgery are known risk factors for the development of symptomatic radiographic knee osteoarthritis (OA) within 10 to 15 years following surgery.1,2 Notably, the estimated lifetime risk for knee OA following ACLR more than doubles to 34% for young adults with concomitant ACL and meniscal tear injuries. 3 In OA, radiographic changes to joint morphology are typically preceded by compositional degeneration of the cartilage, including derangement of the extracellular matrix in preosteoarthritis. 4 Detection of early compositional changes to cartilage extracellular matrix is important to patient care because it may support application of therapeutic interventions prior to development of gross morphologic damage.
Magnetic resonance imaging (MRI) T2 mapping provides a means for noninvasive detection of subsurface changes to the structure and composition of the collagen extracellular matrix. 5 Compromise of the cartilage extracellular matrix permits water to move more freely within the matrix and prolongs MRI T2 relaxation time.5,6 A systematic review and meta-analysis of T2 mapping in participants with ACLR but without substantial radiographic changes showed that cartilage T2 values were “significantly prolonged in participants at risk for knee OA in all analyzed compartments.” 7 Moreover, a direct comparison of preoperative T2 in nearly 900 cartilage regions with intraoperative cartilage morphology International Cartilage Repair Society (ICRS) 8 grades by Soellner et al. 9 showed that mean cartilage T2 relaxation time significantly increased with increasing defect severity, with the highest level of correlation observed in the central medial femur condyle region. Patients with ACL reconstruction are among those at high risk of developing OA, especially those with concomitant meniscus injury.2,3,10 In fact, at a mid-term follow-up of 40 patients at 6 years after ACLR, Snoj et al. 11 detected T2 elevations in tibiofemoral cartilage related to meniscal insufficiency.
Other prior studies have reported “early” cartilage T2 changes observed sometime between “baseline” MR imaging, acquired weeks to months prior to ACLR and follow-up imaging, at 6 months or 1 year post-reconstruction.12-14 In these studies, T2 changes that happened prior to surgery cannot be differentiated from those that occurred following surgery, nor can they be related to cartilage status at the time of surgery. Thus, it remains unclear whether and how cartilage status at the time of ACLR influences future joint health or how the postsurgical knee environment (including altered biomechanics 15 and inflammatory cytokines from the surrounding synovium) 16 affects cartilage composition. As early osteoarthritic changes can be clinically occult and second-look arthroscopy is not usually feasible, an understanding of how cartilage arthroscopic status at the time of ACLR relates to cartilage T2 changes within the first year after ACLR may be useful to clinical decisions regarding advancement of physical activity or additional interventions.
This study has 2 aims. The first is to determine whether worse cartilage arthroscopic status within central weight-bearing study regions at the time of ACLR is reflected by higher T2 value for the same regions at 6 weeks and 1 year after surgery. The second is to test the hypothesis that increasing cartilage T2 value between 6 weeks and 1 year after ACLR is associated with worsening patient-reported outcomes (PROs) over the same time.
Method
Study Population
All participants provided written informed consent for these Stanford University institutional review board (IRB)–approved studies (IRB-27369) and were recruited in accordance with IRB-approved protocols for this prospective cohort study. Two groups of participants were enrolled: patients with ACLR and controls.
Participants with ACLR
Twenty-two consecutive participants aged >18 years presenting with clinical indication for ACLR determined by history, physical signs, preoperative clinical imaging, where available, and interoperative findings (9 men/13 women; mean age = 33 ± 9 years, range = 22-60 years; mean body mass index (BMI) = 24.2 ± 3.5 kg/m2, range = 17.5-30.5 kg/m2) who completed 6-week and 1-year postsurgical research MRIs and PROs were included. Exclusion criteria included inflammatory arthritis, gout/pseudogout, concomitant knee injury aside from meniscus tear and medial collateral ligament (MCL) tear not requiring surgery, and pregnant or intending to become pregnant. Standard-of-care arthroscopic ACLR was performed within an average of 4.9 ± 4.5 months (median = 12 weeks) after ACL injury (9 bone patellar tendon bone autografts, 7 hamstring autografts, and 6 allografts). Arthroscopic meniscus evaluation detected 1 participant with isolated tear of the medial posterior horn, 11 participants with isolated lateral meniscal tear (5 posterior horn, 2 radial, 2 vertical, 2 free-edge), and 2 participants with tears in both menisci (medial undersurface tear plus delaminating tear of lateral posterior horn; medial complex tear plus lateral vertical tear). Medial tears were repaired (n = 2) or resected (n = 1). Lateral tears were repaired (n = 4), resected (n = 7), or rasped (n = 1) and one 5 mm stable tear was left untreated.
Controls
Twenty-six participants whose self-reported health histories included no known or suspected past or present knee injury were enrolled as controls and included 13 men and 13 women with the mean age of 25 ± 6 (range = 20-49) years and mean BMI of 23.0 ± 4.0 (range = 16.8-37.0) kg/m2. Controls were scanned at 1 time point (16 right knees/10 left).
Arthroscopic Assessment
During ACLR surgery, targeted standard arthroscopic assessments were conducted in the central weight-bearing regions of the medial and lateral knee compartments. Visual landmarks consisting of the top of the notch, the medial and lateral borders of the condyle, and the posterior border of the condyle when the knee is flexed at 90° were used to define the central weight-bearing regions and the mid-sagittal plane of the femoral condyles and tibial plateaus. Arthroscopic grades were assigned to the area by the treating surgeons (CRC or JLD) using a modified Outerbridge scale: grade 0 = surface-intact and firm; grade 1 = surface-intact and soft; grade 2 = surface not-intact, partial thickness injury involving less than 50% of the depth; grade 3 = surface not-intact, partial thickness injury involving greater than 50% of the depth; and grade 4 = full-thickness injury.17,18 Cartilage grading at arthroscopy was performed in a systematic fashion by the principal investigator (PI, CRC) or by an experienced collaborating orthopaedic surgeon trained by the PI (JLD), and closely subscribing to the described Outerbridge classifications. Of the 22 participants with ACLR, arthroscopic grades were recorded for the centers of the medial and lateral femoral condyles and medial and lateral tibia plateaus in 21, 20, 18, and 22 participants, respectively.
MRI T2 Mapping Assessment
T2 maps were calculated from an interleaved series of oblique-sagittal T2-weighted MR images (oriented sagittal to the joint) acquired at 8 echo times (TE range = 7-62 ms; TE interval range = 7.4-7.6 ms), using a 2-dimensional (2D) fast spin echo multiecho, multislice sequence (2D FSE MESE, CartiGram; GE Healthcare) and an 8-channel extremity coil (Invivo, Inc.) on a 3-tesla MRI scanner (MR750 Discovery; GE Healthcare). Other T2 sequence parameters included 1500 ms repetition time, 12 cm field of view, bandwidth 62.5 kHz, 26-30 slices, 3 mm thick slices, no gap, acquisition matrix 384 × 256 zero-filled to 512 × 512, 13-minute scantime. T2 maps were generated using MRIMapper software (Beth Israel Deaconess & MIT, Boston, MA, 2006).
Regions of interest (ROIs) were manually segmented from single slices in the center of the medial and lateral compartments by 1 expert individual (AAW) with 20 years of cartilage segmentation experience who was blinded to arthroscopic scores at the time of T2 map processing. The ROIs were drawn to evaluate full-thickness cartilage in central weight-bearing regions of femoral condyles (medial femoral condyle [MFC] and lateral femoral condyle [LFC]) as well as the center of the medial and lateral tibial (MT and LT) plateaus, Figure 1 . The location of the ROIs was chosen to match the arthroscopic study regions.14,19,20
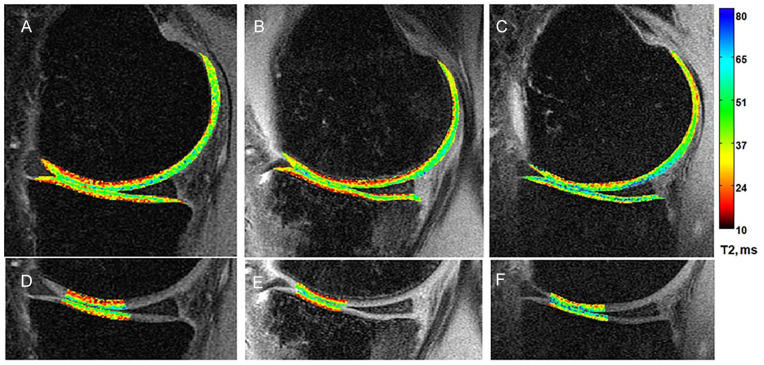
Figure 1.
Sample T2 maps from (A) a 28-year uninjured male control and a 32-year-old man at (B) 6 weeks and (C) 1 year after anterior cruciate ligament reconstruction. At just 6 weeks after surgery, the T2 map of the participant with anterior cruciate ligament reconstruction appears similar to that of the control. At 1 year after surgery, higher T2 values (more green-blue) are seen compared with the earlier time point. Femoral and tibial cartilage regions included in quantitative analyses are shown in panels D to F.
Patient-Reported Outcomes
As recommended by Ingelsrud et al. 21 for an ACLR population, the Knee injury and Osteoarthritis Outcome Scores (KOOS) subscales 22 of Sports and Recreational Function (Sport/Rec) and Knee-related Quality of Life (QOL) were assessed at both time points. Change in the KOOS Sports/Rec and QOL subscales was compared with change in T2 value in each study region.
Statistics
Normality of all data sets was assessed by Shapiro-Wilk tests. T2 value differences between participants with ACLR and controls were assessed with t tests (or Mann-Whitney U tests for non-normally distributed data). T2 value differences by arthroscopic status were assessed with analysis of variance (ANOVA) tests, or Kruskal-Wallis tests for non-normal distributions, comparing intact articular surface at the time of surgery (scope grades 0,1) with those with surface defects (scope grade 2+) and controls. Longitudinal changes in T2 values between 6 weeks and 1 year following ACLR were assessed with paired t-tests. Longitudinal T2 changes by meniscus tear status (tear/no tear) and treatment type (intact/repair/resection) were assessed with ANOVA (or Kruskal-Wallis tests for non-normal distributions). T2 value and longitudinal change by sex and longitudinal T2 change by age (20-29 vs. 30-42 years) were assessed with t-tests. Individual participants’ longitudinal changes in KOOS scores were correlated to changes in T2 value with Spearman’s rho correlations. Statistical analyses were conducted with SPSS (IBM v25).
Results
Arthroscopic Modified Outerbridge Cartilage Grading
Most participants with ACLR had arthroscopically intact articular cartilage surfaces (grade 0 and grade 1) in all targeted study regions at arthroscopy. The distribution of arthroscopic grades for each targeted study region is shown in Table 1 .
Table 1.
Intraoperative Modified Outerbridge Grade Distribution by Study Region.
| ROI | N | Grade 0 | Grade 1 | Grade 2 | Grade 3 | Grade 4 | % Intact Surface (Grade 0 or 1) |
|---|---|---|---|---|---|---|---|
| MFC | 21 | 1 | 17 | 2 | 1 | 0 | 86 |
| LFC | 20 | 4 | 12 | 4 | 0 | 0 | 80 |
| MT | 18 | 4 | 10 | 4 | 0 | 0 | 78 |
| LT | 22 | 3 | 10 | 7 | 2 | 0 | 59 |
ROI = regions of interest; MFC = medial femoral condyle; LFC = lateral femoral condyle; MT = medial tibial; LT = lateral tibial.
T2 Value by Arthroscopic Status at 6 Weeks and 1 Year Following ACLR
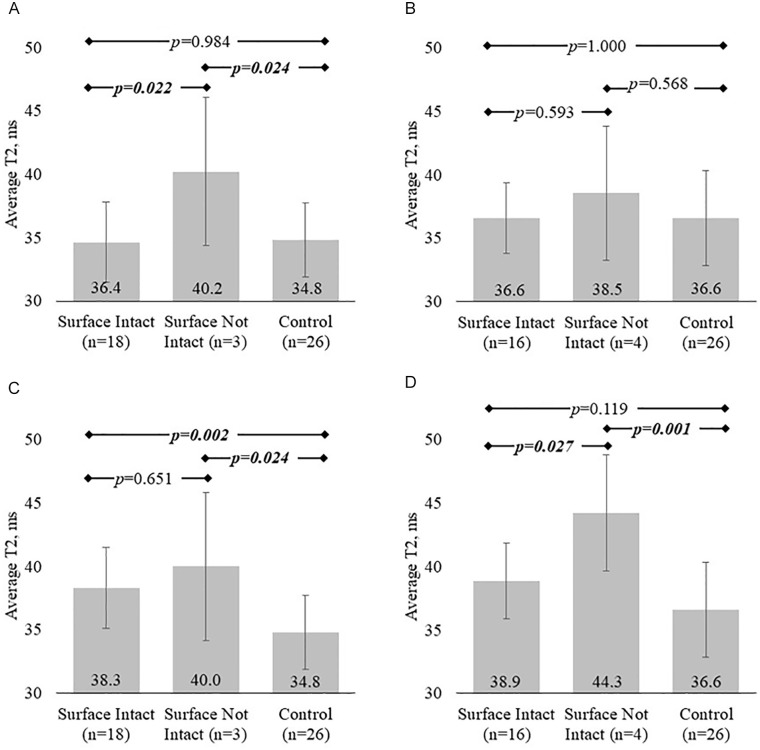
All ACLR participants underwent MRI scans of their injured knee at both 6 weeks (mean = 6.2 ± 1.0, range = 4-8) and 1 year (mean = 12.2 ± 0.8, range = 11-14 months) after ACLR. Cartilage T2 values differed by arthroscopic status in femoral study regions at both time points (6-week MFC ANOVA, P = .025; 1-year MFC, LFC ANOVA, P = .001, .001). Post hoc assessments indicated that participants with defects on the MFC or LFC surfaces at arthroscopy and those with intact MFC surfaces had significantly elevated T2 values in the corresponding study regions compared with controls by 1 year after ACLR ( Fig. 2 ). There was also a trend for similar elevations laterally. T2 values did not vary with arthroscopic status in the tibial study regions at either time point (P > .054).
Figure 2.
Average cartilage T2 values grouped by arthroscopic and injury status for each study region. (A) At 6 weeks after ACLR, only participants with surface defects in the MFC have elevated T2 values compared with controls. (C) However, T2 values in surface-intact MFC cartilage increase overtime to become significantly higher than controls at 1 year. Laterally, significant cartilage T2 differences with injury and arthroscopic status do not manifest until 1 year after surgery. (B, D) Lateral femoral cartilage T2 increases between 6 weeks and 1 year following ACLR lead to elevated T2 values in surface-not-intact and a trend for elevation in surface-intact cartilage at 1 year after ACLR. ACLR = anterior cruciate ligament reconstruction; MFC = medial femoral condyle; LFC = lateral femoral condyle. Boldfaced and italicized values indicate a significant difference.
Longitudinal T2 Change by Arthroscopic Status
Among participants with ACLR determined to have intact MFC and LFC articular surfaces at arthroscopy (scope grades 0,1; n = 18, 16), T2 values in those regions increased 10.5% and 6.3% between 6 weeks and 1 year (mean difference = 3.6 ± 3.3 and 2.3 ± 3.3 ms, 95% confidence interval [CI] = 2.0-5.3 and 0.6-4.0, and paired t test P < .001 and .013). In the MT and LT study regions, T2 values among participants with intact cartilage surfaces did not change significantly between 6 weeks and 1 year (grades 0,1; n = 14, 13; P > .164). Among participants with surface defects on the LT study region (grades 2+; n = 9), T2 value increased 8.3% over time (mean difference = 2.6 ± 2.5 ms, 95% CI = 0.6-4.5, and paired t test P = .015). There were no changes over time in T2 values in the remaining study regions (MFC, LFC, and MT) with surface disruptions (grade 2+, n = 3, 4, 4; P > .176).
T2 Values and T2 Changes in Pooled Participants with ACLR
No significant differences were detected between mean T2 values measured across all participants with ACLR compared with controls in any study region (P > .212) at 6 weeks after surgery. However, by 1 year after surgery, the mean T2 value of participants with ACLR was higher in all study regions compared with controls (P < .049; Table 2 ).
Table 2.
T2 Values and T2 Changes by Study Region for Controls and Pooled Participants with ACLR.
| ROI | Controls | ACLR 6 Weeks |
ACLR 1 Year |
P
ACLR 6 Weeks Vs. Control ACLR 1 Year Vs. Control ACLR 6 Weeks Vs. ACLR 1 Year |
|---|---|---|---|---|
| n | 26 | 22 | 22 | |
| MFC, ms | 34.8 ± 2.9 (30-40) |
35.4 ± 3.9 (29-46) |
38.3 ± 3.4 (33-45) |
.544 <.001 .001 |
| LFC, ms | 36.6 ± 3.8 (30-45) |
37.2 ± 3.4 (32-46) |
40.0 ± 4.0 (33-50) |
.580 .004 .004 |
| MT, ms | 35.2 ± 3.7 (29-47) |
36.5 ± 3.3 (29-42) |
37.8 ± 3.5 (32-47) |
.212 .017 .13 |
| LT, ms | 29.9 ± 3.5 (23-36) |
30.4 ± 3.8 (25-38) |
32.1 ± 3.9 (25-39) |
.657 .049 .011 |
ACLR = anterior cruciate ligament reconstruction; ROI = regions of interest; MFC = medial femoral condyle; LFC = lateral femoral condyle; MT = medial tibial; LT = lateral tibial.
Boldfaced values indicate significant difference.
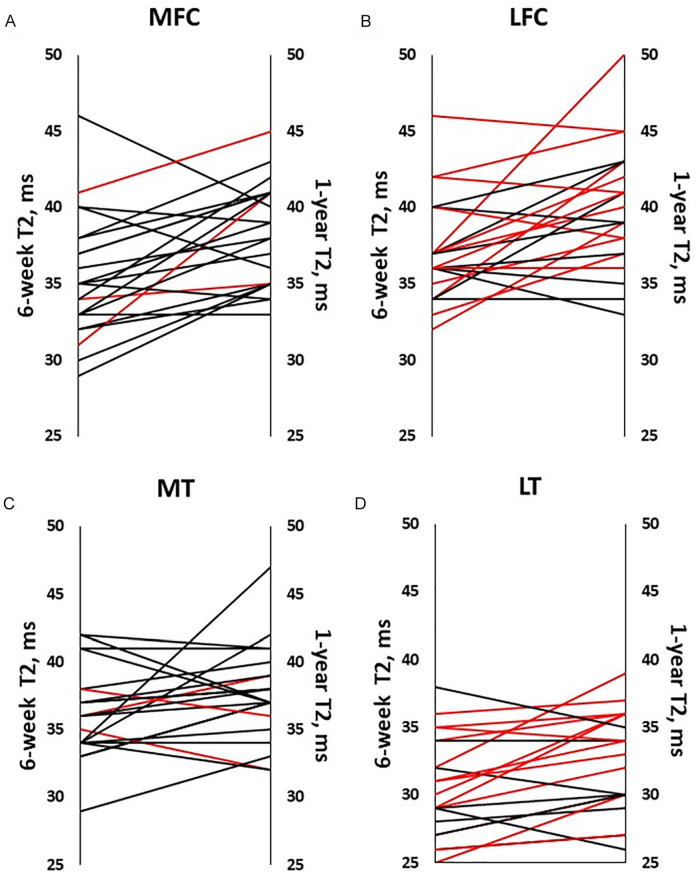
Mean T2 values for participants with ACLR increased between 6 weeks and 1 year after surgery in MFC, LFC, and LT cartilage ( Table 2 ). However, in LT cartilage, change in T2 value over time was affected by tearing of the lateral meniscus. Participants with torn lateral menisci at the time of arthroscopy (n = 13) experienced significantly greater LT cartilage T2 increases compared with participants with intact menisci (n = 9; P = .004) ( Fig. 3D ). There were no significant differences in average T2 values in the LFC among participants dichotomized by torn lateral meniscus status (P = .215, Fig. 3B ) or in medial cartilage dichotomized by torn medial meniscus status (P > .305, Fig. 3A and C ).
Figure 3.
Individuals’ T2 values at 6 weeks and 1 year following ACLR. T2 value averaged across all participants with ACLR increased between 6 weeks and 1 year following surgery in (A) the MFC, P = .001; (B) LFC, P = .004; (D) LT, P = .011, but not in the (C) MT, P = .130. Red lines indicate meniscal tears of the same (medial or lateral) compartment. In LT cartilage, (D) T2 increased more in participants with torn menisci treated by repair or resection than in participants with intact lateral menisci (P = .003, .046). ACLR = anterior cruciate ligament reconstruction; MFC = medial femoral condyle; LFC = lateral femoral condyle; LT = lateral tibial; MT = medial tibial.
Regarding meniscus tear treatment, while participants with ACLR who received repair (n = 4) or resection (n = 7) of lateral meniscus tears demonstrated larger LT T2 increases over time compared with participants with intact lateral menisci (P = .003, P = .046), post hoc pairwise assessments did not detect a difference in magnitude of T2 increase between repair versus resection treatments (P = .13). By contrast, in LFC cartilage, larger T2 increases were seen only participants who underwent lateral meniscus repair (P = .005). There were insufficient numbers of medial meniscus tears (1 resection, 2 repairs) to evaluate effects on articular cartilage.
Only 3 ACLR participants included in these analyses were aged 40 years or older at the time of surgery (40, 42, and 60 years, respectively), whereas 10 participants were in their 30s and 9 were in their 20s. Average longitudinal T2 change did not differ between participants in their 20s compared with participants aged 30 to 42 years (n = 12) in any study region (P > .34). Neither T2 value at 6 weeks, or 1 year, nor change in T2 value between 6 weeks and 1 year varied with sex in any of the study regions (P > .12).
Longitudinal KOOS Change and Correlation to T2 Change
Average KOOS Sports/Rec subscores increased 42.1 ± 24.4 points (mean ± SD; 95% CI = 29.5-54.6, n = 17, paired t-test P < .001), between 6 weeks and 1 year after ACLR. Average KOOS QOL subscores increased 24.6 ± 18.2 points (mean ± SD; 95% CI = 15.3-34.0, n = 17, paired t-test P < .001). In addition, there was a correlation between the change in QOL KOOS score over time and T2 change in MFC cartilage over the same time, where participants with greater increases in MFC cartilage T2 value showed less QOL improvement (P = .009, ρ = −0.62). No other significant correlations between change in KOOS scores and change in T2 values were detected (P > .151).
Discussion
MRI T2 mapping detected evidence of progressive compositional degeneration of articular knee cartilage between 6 weeks and 1 year after ACLR. While 80% to 86% of femoral cartilage study regions were found to be intact at surgery, T2 increases were observed even in these regions. Furthermore, greater T2-detected cartilage compositional degeneration was associated with less improvement in patient-reported quality of life suggesting preosteoarthritis.
Few studies have directly compared arthroscopic assessments of cartilage status with MRI T2 values of the same cartilage region9,20,23,24 or related intraoperative cartilage status to subsequent longitudinal cartilage T2 change. In this study, we were not able to show how arthroscopic status at the time of surgery related to cartilage T2 values or T2 changes over the subsequent year because the cohort was skewed toward participants with arthroscopically intact cartilage. Accordingly, it was not possible to assess if worse cartilage arthroscopic status was reflected by higher T2 values. Rather, the observed T2 increases indicate that the cartilage was not able to maintain a healthy composition under new loading and/or biological conditions present in the post-ACLR joint within the first year after surgery.
T2 increases observed in participants with arthroscopically intact cartilage, and persistent elevations in those with surface defects, suggest that MFC cartilage, in particular, was subject to compositional degeneration within the first year after surgery. Known changes to knee joint alignment and kinematics following ACLR that lead to altered loading especially in the medial compartment, including persistent external rotation and anterior tibial translation,25-29 may have contributed to the medial T2 changes observed here. In the lateral femur, the cartilage T2 response to ACLR appeared more muted 1 year after ACLR compared with the medial side, but the small number of participants with arthroscopically detected cartilage damage in either compartment prohibits definitive comparisons between the compartments. That LFC cartilage T2 value did not differ from controls at 6 weeks after ACLR, regardless of arthroscopic status, was unexpected because the lateral femoral condyle is generally thought to sustain more direct impact at the time of ACL injury compared with the medial femoral condyle. 18 The lack of a detectable T2 difference here may be the result of the unbalanced distribution of lateral femoral arthroscopic grades.
Several studies have reported associations between MRI cartilage compositional changes within months following ACLR surgery and worse knee outcomes up to 2 years later. Su et al. 30 found higher cartilage T1ρ in ACLR injured knees 6 months after reconstruction was associated with worse KOOS and Marx activity scores at 1 year. Recently, Li et al. 31 observed that participants with ACLR with significantly higher cartilage T1ρ in the medial tibia of their ACLR knees 6 months after ACLR demonstrated asymmetric postural stability 2 years after ACLR. In addition, change in average T2 value over the first 6 months following ACLR was shown to correlate to average T2 change over 2 years and 6-month change in the spatial distribution of T2 across the cartilage correlated with 2-year cartilage thinning. 14 Together, these studies highlight that evaluation of cartilage composition with MRI within the first 6 months after ACLR may help identify patients on track to exhibit poorer symptomatic, functional, and structural outcomes 1 and 2 years after ACLR.
In both Su et al. 30 and Li et al., 31 the baseline MRI evaluation was acquired prior to ACLR and the changes in T2 and T1ρ values were assessed by comparing preoperative imaging with imaging obtained 6 months after ACLR. Although arthroscopic assessments in the current work were collected on some knees still in an acute injury phase, cartilage compositional changes were assessed exclusively after surgery, as the baseline MRI here was acquired 6 weeks after ACLR. That subtle difference is relevant to understanding the evolution of pre-OA following ACLR, specifically in the postsurgical knee where cartilage is exposed to a different mechanical27,32,33 and biological environment34-37 compared with freshly injured and unreconstructed joints. Although the longitudinal cartilage T2 increases observed in this study occurred in the context of the postsurgical joint, effects of the initial injury contribute to the sum total of the post-ACLR environment.15,16 Thus, a postoperative baseline scan takes into account changes attributable to the initial injury and the postoperative environment, which are both important to long-term evaluations (i.e., 2-5 years follow-up) of cartilage health. 38
Participants in this study reported KOOS score improvements between 6 weeks and 1 year after ACLR that substantially exceeded minimal important changes (MICs) established by Ingelsrud et al. 21 for ACLR. The average KOOS Sports/Rec increase in the current study was 42.1 points (compared with the previously established MIC of 12.1 points) and the average QOL increase was 24.6 points (compared with an MIC of 18.3 points). 21 Although overall increases were observed in both of the primary PROs recommended for ACLR, 21 at the individual level, worsening cartilage composition to the medial femoral condyle, evidenced by larger T2 increases, was associated with less improvement in QOL score. While the existence of a causal relationship between cartilage composition and PROs cannot be confirmed by this study, it does support T2 mapping as a clinically relevant metric of joint health.
Several limitations should be considered in interpreting this study. MRI data from controls were only collected at one time; hence, the test-retest reliability of T2 values and the degree of T2 change attributable to natural progression over 1 year in participants with ACLR cannot be estimated from these analyses. Notably, the directions of individual T2 changes observed among participants with ACLR primarily indicate increases. In fact, the majority of participants (64%-77%) showed increases in every study region ( Fig. 3 ). Due to the small number of participants with arthroscopically detected surface defects in the study regions, there was insufficient power to definitively relate higher T2 value to more severe arthroscopic status. While broadly consistent with those of Snoj et al. 11 in which T2 elevations in anterofemoral cartilage at 6 years follow-up were associated with meniscal insufficiency detected at arthroscopy, our subanalyses of T2 changes with respect to meniscal tear and repair status and age should be also interpreted with caution because of the small sample sizes. Although no power analyses were performed a priori, all available data were utilized. Importantly, post hoc power analyses indicate that longitudinal T2 increases of 10.5% and 6.3% in MFC and LFC cartilage with intact surfaces in sample sizes of 18 and 16 were detected with 95% and 75% powers, respectively. Post hoc analyses further show that a sample of size of 17 participants for whom both PROs and T2 values were measured longitudinally achieves 78% power to detect the observed regression slope of 0.62 compared with the null hypothesis correlation of 0.0, using a 2-sided hypothesis test with a significance level of .05.
Many factors potentially affect the observed T2 changes within the first year after ACLR. It is possible that cartilage not adequately visualized at arthroscopy such as the posterolateral tibial plateau may have harbored lesions contributing to T2 changes and KOOS outcomes. The degree to which damage in one region of cartilage affects T2 values in other regions of cartilage is unknown. It is quite possible that a change in loading patterns due to (1) joint damage not assessed as part of this study, or (2) arising from altered kinematics and kinetics following ACLR, (3) biologic factors or (4) a combination of these and other factors may have contributed to variations in T2 values within our study regions and in PROs. Although a previous study did not find associations between tibiofemoral T2 values and femoral tunnel inclination, graft inclination, nor anterior tibial translation measures at a mid-term follow-up after ACLR, 3 these factors cannot be ruled out as potentially affecting T2 within the first year of surgery. Additional patient and treatment factors not accounted for in the current analyses that may have contributed to T2 and/or KOOS outcomes include the presence of bone marrow lesions or effusions; surgical techniques, including graft type and tensioning; biomechanics and alignment; and differences in rehabilitation strategies. Furthermore, patient activity prior to MRI scans was not controlled for in this study. While beyond the scope of this work to identify and assess the impacts of these many factors, the findings of this study highlight the need for additional research into these areas that may yield potential therapeutic targets for early intervention.
In conclusion, this study showed progressive cartilage compositional degeneration over the first year after ACLR even in patients with arthroscopically intact cartilage at surgery. This study also showed that subtle and progressive cartilage injury can be detected and quantitatively monitored with a noninvasive and commercially available T2 sequence. Identification of such changes after ACLR is important because increased cartilage T2 values may signal preosteoarthritis where patients are at risk for diminished postsurgical quality-of-life improvements. Further research into potential mechanisms contributing to these early cartilage matrix changes may yield therapeutic targets to prevent or delay joint degeneration after ACLR.
Footnotes
The reported work was performed at Stanford University.
Acknowledgments and Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was funded with grant support from NIH RO1 AR052784 (principal investigator, Chu) and DOD W81XWH-18-1-0590 (principal investigator, Chu).
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Ethical Approval: All participants provided written informed consent for these Stanford University institutional review board approved studies (IRB-27369) and were recruited in accordance with IRB-approved protocols for this prospective cohort study.
ORCID iD: Ashley A. Williams  https://orcid.org/0000-0002-5845-7957
https://orcid.org/0000-0002-5845-7957
References
- 1. Lohmander LS, Englund PM, Dahl LL, Roos EM. The long-term consequence of anterior cruciate ligament and meniscus injuries: osteoarthritis. Am J Sports Med. 2007;35(10):1756-69. [DOI] [PubMed] [Google Scholar]
- 2. Oiestad BE, Holm I, Aune AK, Gunderson R, Myklebust G, Engebretsen L, et al. Knee function and prevalence of knee osteoarthritis after anterior cruciate ligament reconstruction: a prospective study with 10 to 15 years of follow-up. Am J Sports Med. 2010;38(11):2201-10. [DOI] [PubMed] [Google Scholar]
- 3. Suter LG, Smith SR, Katz JN, Englund M, Hunter DJ, Frobell R, et al. Projecting lifetime risk of symptomatic knee osteoarthritis and total knee replacement in individuals sustaining a complete anterior cruciate ligament tear in early adulthood. Arthritis Care Res (Hoboken). 2017;69(2_suppl):201-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Chu CR, Williams AA, Coyle CH, Bowers ME. Early diagnosis to enable early treatment of pre-osteoarthritis. Arthritis Res Ther. 2012;14(3):212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Mosher TJ, Dardzinski BJ. Cartilage MRI T2 relaxation time mapping: overview and applications. Semin Musculoskelet Radiol. 2004;8(4):355-68. [DOI] [PubMed] [Google Scholar]
- 6. Menezes NM, Gray ML, Hartke JR, Burstein D. T2 and T1rho MRI in articular cartilage systems. Magn Reson Med. 2004;51(3):503-9. [DOI] [PubMed] [Google Scholar]
- 7. Atkinson HF, Birmingham TB, Moyer RF, Yacoub D, Kanko LE, Bryant DM, et al. MRI T2 and T1rho relaxation in patients at risk for knee osteoarthritis: a systematic review and meta-analysis. BMC Musculoskelet Disord. 2019;20(1):182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Roos EM, Engelhart L, Ranstam J, Anderson AF, Irrgang JJ, Marx RG, et al. ICRS recommendation document: patient-reported outcome instruments for use in patients with articular cartilage defects. Cartilage. 2011;2(2_suppl):122-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Soellner ST, Goldmann A, Muelheims D, Welsch GH, Pachowsky ML. Intraoperative validation of quantitative T2 mapping in patients with articular cartilage lesions of the knee. Osteoarthritis Cartilage. 2017;25(11):1841-9. [DOI] [PubMed] [Google Scholar]
- 10. Shelbourne KD, Gray T. Results of anterior cruciate ligament reconstruction based on meniscus and articular cartilage status at the time of surgery. Five- to fifteen-year evaluations. Am J Sports Med. 2000;28(4):446-52. [DOI] [PubMed] [Google Scholar]
- 11. Snoj Z, Zupanc O, Salapura V. Retrospective quantitative cartilage and semi-quantitative morphological evaluation at 6 years after ACL reconstruction. Arch Orthop Trauma Surg. 2016;136(7):967-74. [DOI] [PubMed] [Google Scholar]
- 12. Pedoia V, Su F, Amano K, Li Q, McCulloch CE, Souza RB, et al. Analysis of the articular cartilage T1rho and T2 relaxation times changes after ACL reconstruction in injured and contralateral knees and relationships with bone shape. J Orthop Res. 2017;35(3):707-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Su F, Hilton JF, Nardo L, Wu S, Liang F, Link TM, et al. Cartilage morphology and T1rho and T2 quantification in ACL-reconstructed knees: a 2-year follow-up. Osteoarthritis Cartilage. 2013;21(8):1058-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Williams A, Winalski CS, Chu CR. Early articular cartilage MRI T2 changes after anterior cruciate ligament reconstruction correlate with later changes in T2 and cartilage thickness. J Orthop Res. 2017;35(3):699-706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hart HF, Culvenor AG, Collins NJ, Ackland DC, Cowan SM, Machotka Z, et al. Knee kinematics and joint moments during gait following anterior cruciate ligament reconstruction: a systematic review and meta-analysis. Br J Sports Med. 2016;50(10):597-612. [DOI] [PubMed] [Google Scholar]
- 16. Hunt ER, Jacobs CA, Conley CE, Ireland ML, Johnson DL, Lattermann C. Anterior cruciate ligament reconstruction reinitiates an inflammatory and chondrodegenerative process in the knee joint. J Orthop Res. 2021;39(6):1281-8. [DOI] [PubMed] [Google Scholar]
- 17. Outer bridge RE. The etiology of chondromalacia patellae. J Bone Joint Surg Br. 1961;43-B:752-7. [DOI] [PubMed] [Google Scholar]
- 18. Potter HG, Jain SK, Ma Y, Black BR, Fung S, Lyman S. Cartilage injury after acute, isolated anterior cruciate ligament tear: immediate and longitudinal effect with clinical/MRI follow-up. Am J Sports Med. 2012;40(2_suppl):276-85. [DOI] [PubMed] [Google Scholar]
- 19. Williams A, Qian Y, Chu CR. UTE-T2 * mapping of human articular cartilage in vivo: a repeatability assessment. Osteoarthritis Cartilage. 2011;19(1):84-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Chu CR, Williams A, Tolliver D, Kwoh CK, Bruno S, III, Irrgang JJ. Clinical optical coherence tomography of early articular cartilage degeneration in patients with degenerative meniscal tears. Arthritis Rheum. 2010;62(5):1412-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ingelsrud LH, Terwee CB, Terluin B, Granan LP, Engebretsen L, Mills KAG, et al. Meaningful change scores in the knee injury and osteoarthritis outcome score in patients undergoing anterior cruciate ligament reconstruction. Am J Sports Med. 2018;46(5):1120-8. [DOI] [PubMed] [Google Scholar]
- 22. Roos EM, Lohmander LS. The Knee injury and Osteoarthritis Outcome Score (KOOS): from joint injury to osteoarthritis. Health Qual Life Outcomes. 2003;1:64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Apprich S, Welsch GH, Mamisch TC, Szomolanyi P, Mayerhoefer M, Pinker K, et al. Detection of degenerative cartilage disease: comparison of high-resolution morphological MR and quantitative T2 mapping at 3.0 Tesla. Osteoarthritis Cartilage. 2010;18(9):1211-7. [DOI] [PubMed] [Google Scholar]
- 24. Kijowski R, Blankenbaker DG, Munoz Del Rio A, Baer GS, Graf BK. Evaluation of the articular cartilage of the knee joint: value of adding a T2 mapping sequence to a routine MR imaging protocol. Radiology. 2013;267(2_suppl):503-13. [DOI] [PubMed] [Google Scholar]
- 25. Tashman S, Zandiyeh P, Irrgang JJ, Musahl V, West RV, Shah N, et al. Anatomic single- and double-bundle ACL reconstruction both restore dynamic knee function: a randomized clinical trial-part II: knee kinematics. Knee Surg Sports Traumatol Arthrosc. 2021;29(8):2676-83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Li AK, Ochoa JK, Pedoia V, Amano K, Souza RB, Li X, et al. Altered tibiofemoral position following ACL reconstruction is associated with cartilage matrix changes: a voxel-based relaxometry analysis. J Orthop Res. 2020;38(11):2454-63. [DOI] [PubMed] [Google Scholar]
- 27. Andriacchi TP, Favre J, Erhart-Hledik JC, Chu CR. A systems view of risk factors for knee osteoarthritis reveals insights into the pathogenesis of the disease. Ann Biomed Eng. 2015;43(2_suppl):376-87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Barenius B, Ponzer S, Shalabi A, Bujak R, Norlen L, Eriksson K. Increased risk of osteoarthritis after anterior cruciate ligament reconstruction: a 14-year follow-up study of a randomized controlled trial. Am J Sports Med. 2014;42(5):1049-57. [DOI] [PubMed] [Google Scholar]
- 29. Ushio T, Okazaki K, Osaki K, Takayama Y, Sagiyama K, Mizu-Uchi H, et al. Degenerative changes in cartilage likely occur in the medial compartment after anterior cruciate ligament reconstruction. Knee Surg Sports Traumatol Arthrosc. 2019;27(11):3567-74. [DOI] [PubMed] [Google Scholar]
- 30. Su F, Pedoia V, Teng HL, Kretzschmar M, Lau BC, McCulloch CE, et al. The association between MR T1rho and T2 of cartilage and patient-reported outcomes after ACL injury and reconstruction. Osteoarthritis Cartilage. 2016;24(7):1180-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Li AK, Pedoia V, Tanaka M, Souza RB, Ma CB, Li X. Six-month post-surgical elevations in cartilage T1rho relaxation times are associated with functional performance 2 years after ACL reconstruction. J Orthop Res. 2020;38 (5):1132-40. [DOI] [PubMed] [Google Scholar]
- 32. Chu CR, Andriacchi TP. Dance between biology, mechanics, and structure: a systems-based approach to developing osteoarthritis prevention strategies. J Orthop Res. 2015;33(7):939-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Wellsandt E, Khandha A, Capin J, Buchanan TS, Snyder-Mackler L. Operative and nonoperative management of anterior cruciate ligament injury: differences in gait biomechanics at 5 years. J Orthop Res. 2020;38(12):2675-84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Bigoni M, Turati M, Gandolla M, Sacerdote P, Piatti M, Castelnuovo A, et al. Effects of ACL reconstructive surgery on temporal variations of cytokine levels in synovial fluid. Mediators Inflamm. 2016;2016:8243601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Chmielewski TL, Trumble TN, Joseph AM, Shuster J, Indelicato PA, Moser MW, et al. Urinary CTX-II concentrations are elevated and associated with knee pain and function in subjects with ACL reconstruction. Osteoarthritis Cartilage. 2012;20(11):1294-301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Pietrosimone B, Blackburn JT, Harkey MS, Luc BA, Hackney AC, Padua DA, et al. Greater mechanical loading during walking is associated with less collagen turnover in individuals with anterior cruciate ligament reconstruction. Am J Sports Med. 2016;44(2_suppl):425-32. [DOI] [PubMed] [Google Scholar]
- 37. Pietrosimone B, Loeser RF, Blackburn JT, Padua DA, Harkey MS, Stanley LE, et al. Biochemical markers of cartilage metabolism are associated with walking biomechanics 6-months following anterior cruciate ligament reconstruction. J Orthop Res. 2017;35(10):2288-97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Eckstein F, Wirth W, Lohmander LS, Hudelmaier MI, Frobell RB. Five-year followup of knee joint cartilage thickness changes after acute rupture of the anterior cruciate ligament. Arthritis Rheumatol. 2015;67(1):152-61. [DOI] [PubMed] [Google Scholar]
- 39. Snoj Z, Zupanc O, Strazar K, Salapura V. A descriptive study of potential effect of anterior tibial translation, femoral tunnel and anterior cruciate ligament graft inclination on clinical outcome and degenerative changes. Int Orthop. 2017;41(4):789-96. [DOI] [PubMed] [Google Scholar]