Abstract
Objective
Bone morphogenetic protein 2 (BMP2) plays important roles in cartilage growth and development. Paradoxically, elevated levels of BMP2 leads to hypertrophic differentiation and osteoarthritis of cartilage. We examined the in vivo loss of BMP2 in cells expressing aggrecan of the mandibular condyle and knee.
Design
Three-week-old BMP2 flox/flox-CreER-positive mice and their Cre-negative littermates were treated with tamoxifen and raised until 3 or 6 months. We also investigated the direct effects of BMP2 on chondrocytes in vitro. Cells from the mandibular condyle of mice were treated with recombinant human BMP2 (rhBMP2) or rhNoggin (inhibitor of BMP2 signaling).
Results
Conditional deletion of BMP2 caused breakage of the cartilage integrity in the mandibular condyle of mice from both age groups, accompanied by a decrease in cartilage thickness, matrix synthesis, mineralization, chondrocyte proliferation, and increased expression of degeneration markers, while the effects at articular cartilage were not significant. In vitro results revealed that rhBMP2 increased chondrocyte proliferation, mineralization, and differentiation, while noggin induced opposite effects.
Conclusions
In conclusion, BMP2 is essential for postnatal maintenance of the osteochondral tissues of the mandibular condyle.
Keywords: temporomandibular joint, osteochondral tissues, mandibular condylar cartilage, bone morphogenetic protein 2
Introduction
Osteoarthritis (OA) is the most commonly occurring joint disease and affects the osteochondral tissues of the temporomandibular joint (TMJ).1-4 Chronic pain and functional limitation caused by cartilage degeneration impairs the quality of life for affected individuals, making OA a leading cause of disability and a significant cost to society.2-6 Currently, there are no disease-modifying treatments available for TMJ-OA, and the National Institute of Dental and Craniofacial Research (NIDCR) recommends palliative therapies which focus on decreasing pain.3,5-7 Due to the absence of effective clinical treatments, OA in joint cartilage typically progresses until total joint replacement becomes the only option. The greatest barrier to generating successful treatment strategies lies in our ability to understand the cellular and molecular mechanisms behind degeneration of the osteochondral tissues of the TMJ. 7
Bone morphogenetic proteins (BMPs) are multifunctional proteins and play critical roles during various stages of skeletal development and chondrogenesis.8-11 Specifically, bone morphogenetic protein 2 (BMP2) is known to be involved in condensation, chondrocyte proliferation and differentiation, and extracellular matrix synthesis of the osteochondral tissues of the knee.8-12 BMP2 is one of the candidate growth factor that has been used in osteochondral tissue engineering for repair and regeneration.8,10,12 BMP2 signaling is also associated with destructive arthritis of the knee and the TMJ.9,13 Our previous work has shown that BMP2 expression has been found to colocalize with damaged areas of the mandibular cartilage, suggesting that BMP2 may be directly involved in the development of OA, or potentially involved in the healing of OA lesions.14,15 Despite a wealth of literature on BMP2 signaling in the osteochondral tissues of knee, little is known about its role postnatally in the osteochondral tissues of the TMJ.
To determine the importance of postnatal BMP2 signaling in the TMJ, we characterized BMP2 expression in osteochondral tissues of wild-type mice (C57BL6/J mice). In order to define the role of BMP2 in TMJ homeostasis, we conditionally deleted BMP2 from aggrecan (ACAN) expressing cells of the cartilage. In our research, we report that BMP2 is distinctly expressed in the cartilage and the subchondral bone of the TMJ. We further delineated a unique role for BMP2 in the organization and function of the extracellular matrix in the cartilage. We have also examined the importance of BMP2 in the subchondral bone during joint maturation and homeostasis. Additionally, we showed that the conditional deletion of BMP2 lead to accelerated degeneration of the TMJ. Finally, to elucidate the underlying signaling mechanism we conducted in vitro experiments using triple transgenic reporter mice (Col1a1 X Col2a1 X Col10a1) and showed that exogenous BMP2 not only leads to proliferation and differentiation of chondrocytes but also increases the degree of mineralization.16,17
Material and Methods
In Vivo studies
BMP2 Conditional Deletion in Aggrecan Expressing Cells
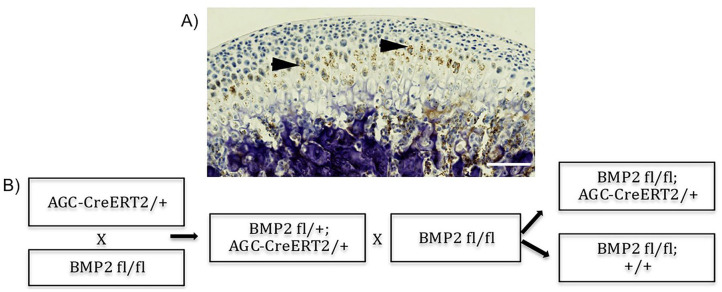
The Institutional Animal Care Committee at the University of Connecticut Health Center approved the experimental procedures used in this study (protocol number AP-200087-0823, year 2020). Prior to the development of a mouse model with conditional BMP2 deletion in chondrocytes (BMP2 cKO), we first performed in situ hybridization for BMP2 in sagittal sections of the mandibular condyle of adult wild-type mice. We concluded that BMP2 was expressed in the prehypertrophic and hypertrophic zones of the cartilage of the mandibular condyle ( Fig. 1A ), and thus decided to target our conditional deletion of BMP2 in ACAN expressing cells. ACAN is a proteoglycan that comprises the major matrix component of chondrocytes. 18 We have chosen mice as our mammalian model because mice can be genetically manipulated to study nearly any human disease or condition. We used BMP2 flox/flox mice 19 crossed with mice containing the CreERT2 tamoxifen-inducible recombinase specific to the ACAN promoter 20 ( Fig. 1B ). To activate Cre recombinase, 3-week-old BMP2 flox/flox-CreERT2-positive mice were treated for 5 consecutive days with intraperitoneal injections of tamoxifen (Sigma-Aldrich, St. Louis, MO) dissolved in corn oil, at a dose of 75 μg/kg body weight. We used mice from the same offspring lacking Cre (Cre-negative littermates) as controls. All animals were then raised for 3 or 6 months following treatment with tamoxifen. Only female mice were used as they have higher predilection for TMJ disorders. 7 We used 10 mice in each group. Prior to euthanization by CO2 asphyxiation, mice were injected with bone labels and a proliferation marker, as described below.
Figure 1.
Conditional bone morphogenetic protein 2 (BMP2) knockout mouse model. (A) In situ hybridization for Bmp2 performed in sagittal section of the mandibular condyle of wild-type (WT) mice. BMP2 is predominantly present in the prehypertrophic and hypertrophic zones. (B) Conditional BMP2 knockout mouse model. BMP2 was deleted in aggrecan expressing cells. Scale bar = 100 µm.
Injection of Fluorochrome Bone Labels and Cell Proliferation Marker
Mice were intraperitoneally injected with alizarin complexone and calcein at 3 days and 1 day before euthanization, respectively. These fluorochrome bone labels (Sigma-Aldrich) were dissolved in 2% sodium bicarbonate (pH 7.4), and injected at a dose of 3 μg/kg body weight. In addition, the cell proliferation marker EdU (5-ethyl-2′-deoxyuridine) from Life Technologies, Grand Island, NY, was injected at 2 days and 1 day before euthanization.
Micro-Computerized Tomography
Micro-computerized tomography (SCANCO Medical AG, Brüttisellen, Switzerland) was used to analyze the microstructure of the subchondral bone and calcified cartilage of the TMJ. Samples were scanned in 70% ethanol and serial tomographic projections were acquired at 55 kV and 145 µA, with a voxel size of 6 µm and 1000 projections per rotation and were collected at 300000 µs. In order to distinguish calcified tissue from non-calcified tissue an automated algorithm was used to segment the reconstructed grey scale images. Our region of interest (ROI) was the mushroom-shaped head of the mandibular condyle. Bone volume fraction (BVF) and tissue density within the ROI were determined.
Tissue Preparation and Histological Staining
Mandibles and knees were dissected and fixed in 10% buffered formalin for 48 hours at 4 °C. Samples were then transferred to 30% sucrose in phosphate-buffered saline (PBS) and kept at 4 °C overnight, and then embedded in frozen embedding resin (Shandon Cryomatrix, Thermo Scientific, Pittsburgh, PA).15-17 Mandibular condyles and knees were sectioned (5-7 µM) using a Leica CM1900 Cryostat (Leica, Inc., Nussloch, Germany) and transferred to slides using a previously described tape method. 21
Fluorochrome bone labeling (alizarin complexone and calcein) within the mandibular condyle was scanned using brightfield microscopy. The same sections were stained for tartrate resistant acid phosphatase (TRAP) activity using an ELF97 yellow fluorescent acid phosphatase substrate (Life Technologies) and the sections were reimaged. Sections were then stained for EdU (ClickiT EdU Alexa Fluor 555HCS kit) from Life Technologies, counterstained with DAPI (Thermo Fisher Scientific, Waltham, MA) and reimaged. An Axio Scan Z1 microscope (Carl Zeiss, Thornwood, NY) was used for brightfield and fluorescent imaging with chroma filters specific for each fluorophore.
Additional slides with sections from the mandibular condyle and sections of knees were stained for safranin O (IHC WORLD, LLC, Ellicott, MD). Immunostaining for matrix metallopeptidase 13 (MMP13) and ADAM metallopeptidase with thrombospondin type 1 motif 4 (ADAMTS4) were also performed in separate sections of the mandibular condyle.
In Vitro Studies
Micromass Chondrocyte Culture
We used 3-week-old transgenic mice with fluorescent markers (Col1a1 X Col2a1 X Col10a1) for cell culture experiments.5,6,14 Chondrocytes from the mandibular condyle were isolated and incubated at 37 °C in PBS and penicillin-streptomycin (P/S) (Thermo Fisher Scientific) with 3 mg/mL of collagenase D (Sigma-Aldrich) and 2 mg/mL of dispase (Thermo Fisher Scientific). Cells were released from the tissue following 3 rounds of enzyme incubation and transferred to media consisting of Dulbecco’s modified Eagle medium (DMEM) with glucose (4.5 g/L) and l-glutamine (Thermo Fisher Scientific), 50 U/mL P/S and 2% fetal bovine serum (Thermo Fisher Scientific). Cells were then centrifuged at 4 °C and resuspended in fresh media, described above, and counted using a cellometer (Nexelcom, Lawrence, MA).
Three wells (biological replicates) were plated for each treatment group. Cells were plated (50 µL) for micromass culture, with 50,000 cells per well, using a 6-well flat bottom culture dish (Corning, Corning, NY). Following 2.5 hours after plating the control cells received 3 mL of chondrogenic media, consisting of DMEM with glucose (4.5 g/L) and l-glutamine, 50 U/mL P/S, 50 µg/mL ascorbic acid, 40 µg/mL l-proline, 100 nM dexamethasone, 1 mM sodium pyruvate, 1% ITS+1 (Sigma-Aldrich), while treatment groups received recombinant human BMP2 (rhBMP2; R&D Systems, Minneapolis, MN) or rhNoggin (R&D Systems) in the chondrogenic media. The rhBMP2 stock solution was prepared at 200 µg/mL in 4 mM HCl with 0.1% bovine serum albumin (BSA). The rhNoggin stock solution was prepared at 250 µg/mL in PBS with 0.1% BSA. rhBMP2 and rhNoggin were each used at a working concentration of 100 ng/ml. Media was changed daily and cells were treated for a total of 14 days. Cells were then imaged in culture using a Zeiss Observer Z1 inverted microscope (Carl Zeiss) with appropriate fluorescent filter cubes (Chroma Technology, Bellows Falls, VT). For measurement of Col1a1 (GFP-topaz) we used YFP (ET500/20 Ex, ET535/30 Em), for Col2a1 (GFP-cyan) we used CFP (ET436/20 Ex, ET480/40 Em), and for Col10a1 (RFP-cherry) we used mCherry (HQ577/20 Ex, HQ640/40 Em).
Alkaline Phosphatase Staining
For staining of alkaline phosphatase (ALP), cells were first washed 3 times in PBS and fixed with 10% buffered formalin for 10 minutes. We used a Fast-Red ALP kit (Sigma-Aldrich) following the manufacturer’s instructions. We stained the cell cultures with the ALP reaction mixture for 20 minutes, rinsed 3 times in PBS, air dried and imagined using brightfield. Samples were then rehydrated and counterstained with Mayer’s hematoxylin and reimaged.
RNA Isolation and Relative Gene Expression
For RNA isolation, we washed the cells with cold PBS and used Trizol (Thermo Fisher Scientific) following the manufacturer’s protocol. We used 1 µg of RNA from each sample for reverse transcription using Superscript II (Thermo Fisher Scientific) and oligo dT 12-18 primer (Thermo Fisher Scientific). The sequences of primers (IDT, Coralville, IA) used in quantitative reverse-transcription polymerase chain reaction (qPCR) are found in Supplementary Table S1. We used Sybr Select Master Mix (Thermo Fisher Scientific) with a 20 µL reaction volume. The qPCR method used was as follows: 50 °C for 2 minutes, followed by a 95 °C step for 10 minutes, and 40 cycles of 95 °C for 15 seconds and 60 °C for 1 minute. Next, a dissociation curve was run with the temperature cycling from 95 °C to 65 °C and back to 95 °C. Melting curves were examined to determine product specificity. We used 96-well plates and samples were run in technical duplicate. GAPDH was used as a housekeeping gene to normalize the expression values. The delta-delta Ct method 22 was used for analysis. Relative transcription data is expressed as the fold-change ± standard error of the mean (SEM), versus the control group.
Histological and Cell Culture Quantification
The histopathologic grading was performed using an established protocol by the Osteoarthritis Research Society International (OARSI) for the grading of mouse cartilage degeneration (by safranin O staining). 23 Image quantifications were performed by using Adobe Photoshop (Adobe Inc., San Jose, CA). In the subchondral bone we examined mineralization by counting the number of red pixels (alizarin complexone staining) and green pixels (calcein staining), as well as TRAP activity (yellow pixels), and dividing each by the total number of pixels in the subchondral region. Cellular proliferation was determined by counting EdU- and DAPI-positive pixels in the proliferative zone of the mandibular condylar cartilage (MCC), and calculating the percentage of EdU positive pixels over DAPI-positive pixels. For in vitro experiments Col1a1 (green pixels) and Col10a1 (red pixels) positive cells were quantified by calculating the percentage of pixels per total area. Due to the weak expression of Col2a1 in these cultures, we were not able to quantify Col2a1-positive cells.
Statistics
Descriptive statistics were used to examine the distribution of BVF, tissue density, OARSI score, histological analysis, and gene expression. The one-sample Kolmogorov-Smirnov test was used to examine the data distributions for normality. In vivo outcomes were compared using an unpaired Student t test between the BMP2 cKO group and the control group. For cell culture studies using mandibular cells from transgenic mice (Col1a1 X Col2a1 X Col10a1), outcomes were compared between control cells (media alone), rhBMP2-treated cells, and rhNoggin-treated cells, using a one-way analysis of variance (ANOVA) test. All statistical tests were 2 sided and a P value <0.05 was considered statistically significant. Statistical analyses were performed using Graph Pad Prism (San Diego, CA).
Results
In Vivo results
Conditional Deletion of BMP2 in Chondrocytes Leads to Early Degeneration of the Mandibular Condyle
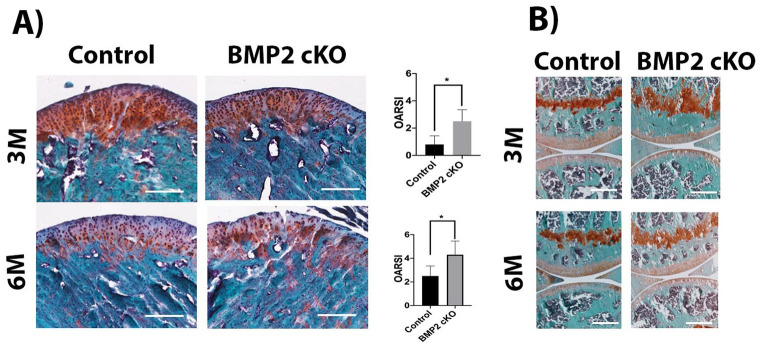
Safranin O staining revealed decreased cartilage thickness, altered cellular morphology (loss of zonal architecture) and decreased matrix synthesis in the mandibular condyle following deletion of BMP2 in ACAN-expressing cells (3 months following tamoxifen treatment) compared with Cre minus littermates ( Fig. 2A ). However, by 6 months following tamoxifen treatment the deletion of BMP2 led to breakage of the mandibular cartilage, while the Cre minus littermates presented with thinning of the cartilage but without noticeable degeneration ( Fig. 2A ). Histopathologic grading was performed using an established protocol by OARSI for the grading of mouse cartilage degeneration. BMP2 cKO at 3 and 6 months leads to clefting and fibrillation of the cartilage of TMJ ( Fig. 2A ).
Figure 2.
Safranin O staining and histopathologic grading (OARSI) performed in sagittal sections of mandibular condyles (A) and articular cartilage (B). Decreased cartilage thickness and early breakdown of the mandibular condyle in bone morphogenetic protein 2 (BMP2) conditional knockout mice. Scale bar = 200 µm.
Following conditional deletion of BMP2 in the knee, the effects in the articular cartilage (AC) were not was as severe as those observed in the mandibular condyle. A mild decrease in the AC thickness was observed in the knees of BMP2 cKO mice, at 3 months following tamoxifen treatment, in comparison to the Cre minus littermates. However, this alteration was not observed in BMP2 cKO animals at 6 months after tamoxifen treatment ( Fig. 2B ). Since the AC was not as severely affected by the conditional deletion of BMP2, we focused our studies on the changes observed in the mandibular condyle.
Conditional Deletion of BMP2 in Chondrocytes Leads to Decreased Mineralization of the Mandibular Condyle
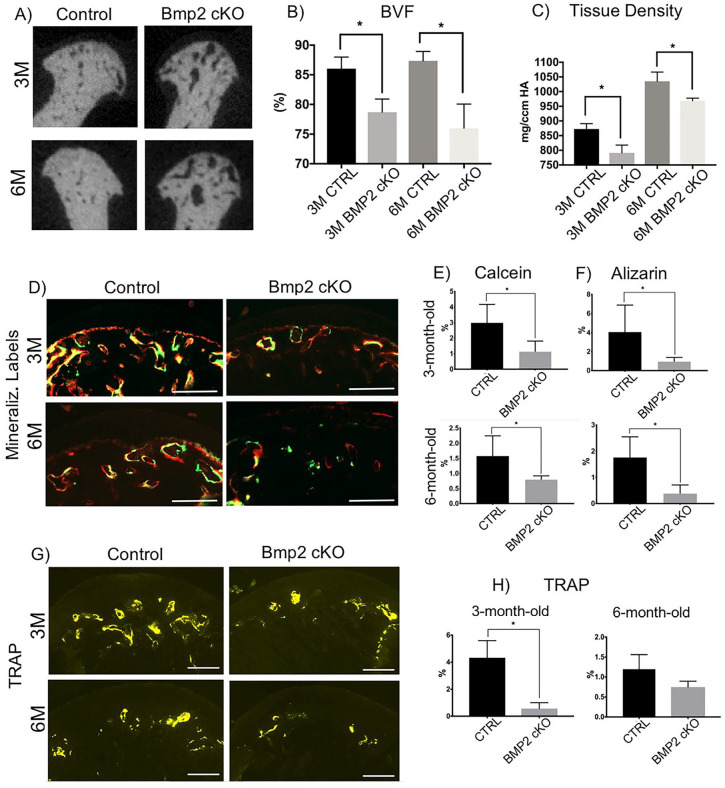
Using micro-CT, we compared the calcified tissue mass of the mandibular condyle in mice following conditional deletion of BMP2 compared to their Cre negative littermates ( Fig. 3A ). We found a significant decrease in the BVF and in the tissue density of BMP2 cKO mice compared with Cre-negative littermates, as shown in Figure 3B and C , respectively.
Figure 3.
Micro-computed tomography (micro-CT) analysis, mineralization labeling and TRAP staining in the mandibular condyles of BMP2 conditional knockout mice and controls. (A-C) Decreased bone volume and density at the subchondral region of the mandibular condyle in BMP2 conditional knockout mice. BMP, bone morphogenetic protein; BVF, bone volume fraction. *P < 0.05. Decreased mineral deposition (D-F) and bone remodeling (TRAP staining, G, H) at the subchondral region of the mandibular condyle in BMP2 conditional knockout mice. Scale bar = 200 µm. *P < 0.05.
Next, we examined mineral deposition and osteoclast activity in mice with conditional loss of BMP2 ( Figs. 3D-H ). Consistent with the micro-CT results, BMP2 cKO mice (3 and 6 months after tamoxifen) presented with significantly decreased mineralization compared with Cre-negative littermates, as illustrated by a significant reduction in the uptake of the fluorochrome markers calcein ( Fig. 3E ) and alizarin ( Fig. 3F ). There was also a substantial reduction in osteoclast activity, as demonstrated by a significant decrease in TRAP staining in the mandibular condyle of BMP2 cKO mice (3 and 6 months following tamoxifen) in comparison with their Cre-negative littermates ( Figs. 3G and H ).
Conditional Deletion of BMP2 in Chondrocytes Leads to Decreased Cell Proliferation and Increased Markers of Degeneration in the Mandibular Condyle
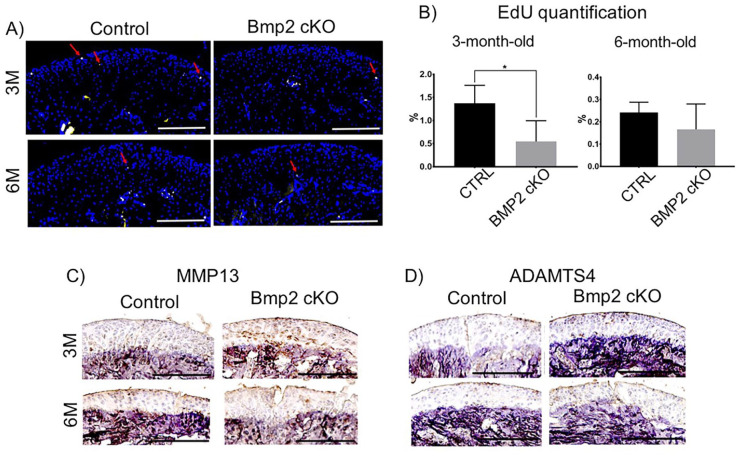
In order to further investigate the mechanisms by which the conditional deletion of BMP2 may cause early degeneration of the mandibular cartilage, we examined chondrocyte proliferation and performed immunostaining for markers of matrix degradation. We found a significant decrease in the chondrocyte proliferation (EdU-positive cells) relative to DAPI-stained cells, in the BMP2 cKO mice (3 months following tamoxifen) in comparison to their Cre-negative littermates ( Fig. 4A ). However, in the BMP2 cKO mice at 6 months following tamoxifen treatment, we did not observe a significant difference in chondrocyte proliferation compared with Cre-negative mice ( Fig. 4B ).
Figure 4.
5-Ethyl-2′-deoxyuridine (EdU) staining and immunohistochemistry for matrix metallopeptidase 13 (MMP13) and ADAM metallopeptidase with thrombospondin type 1 motif 4 (ADAMTS4) in sagittal sections of mandibular condyles bone morphogenetic protein 2 (BMP2) conditional knockout mice and controls. (A, B) Decreased chondrocyte proliferation at the mandibular condyle in BMP2 conditional knockout mice. Arrows represent EdU-positive cells. Scale bar = 200 µm. *P < 0.05. (C) Increased expression of MMP13 and ADAMTS in the mandibular condyle of BMP2 conditional knockout mice. Scale bar = 200 µm.
We carried out immunostaining to examine the expression of ADAMTS4 ( Fig. 4C ), a marker for proteoglycan and matrix degradation, and MMP13 ( Fig. 4D ), an enzyme that degrades collagen. We found an increase in ADAMTS4 expression in the group of mice with conditional deletion of BMP2, at 3 months following tamoxifen, compared to their Cre negative littermates. This difference in ADAMTS4 expression was not significant when comparing the BMP2 cKO mice (6 months following tamoxifen) versus their Cre-negative littermates ( Fig. 4C ). In addition, MMP13 expression was found to be significantly increased in the mandibular cartilage of mice with the conditional deletion of BMP2 (3 and 6 months following tamoxifen) in comparison with their Cre-negative littermates ( Fig. 4D ).
In Vitro Results
BMP2 Increases Markers for Chondrocyte Mineralization, Proliferation, and Differentiation in Micromass Cultures
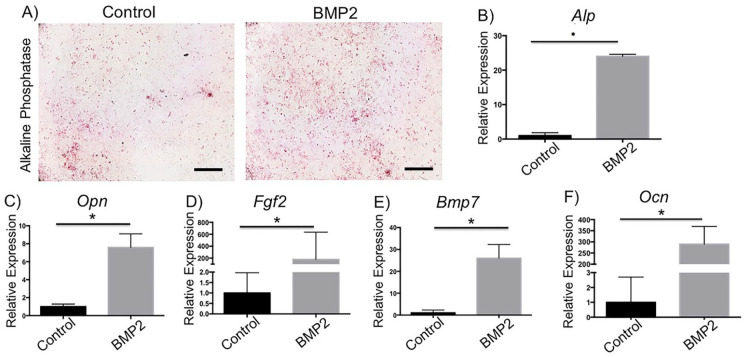
A series of in vitro experiments were performed using cells from transgenic mice (Col1a1 X Col2a1 X Col10a1) to confirm the direct effects of BMP2 signaling in chondrocytes. These mice have fluorescent markers; GFP-topaz for Col1a1 expression; GFP-cyan for Col2a1 expression; and RFP-cherry for Col10a1 expression. Micromass chondrocyte cultures from the mandibular condyle were treated with media alone (control), rhBMP2, or rhNoggin. We performed AP staining and found enhanced mineralization in rhBMP2-treated cells in comparison to control cells ( Fig. 5A ). Next, we performed qPCR to analyze the gene expression of factors essential for chondrocyte proliferation, differentiation, and mineralization. We found that rhBMP2-treated cells, compared with control cells, had significantly increased gene expression of alkaline phosphatase (Alpl, Fig. 5B ), osteopontin (Opn-has new name, Fig. 5C ), fibroblast growth factor 2 (Fgf2, Fig. 5D ), bone morphogenetic protein 7 (Bmp7, Fig. 5E ), and osteocalcin (Ocn-has new name, Fig. 5F ).
Figure 5.
Alizarin staining and gene expression in bone morphogenetic protein 2 (BMP2)-treated chondrocyte culture and controls. Increased mineralization and expression of mineralization markers in BMP2-treated chondrocyte culture. Cells were treated with rhBMP2 for 14 days, qPCR was conducted, and bar graphs show relative gene expression. *P < 0.05. Scale bar = 10×.
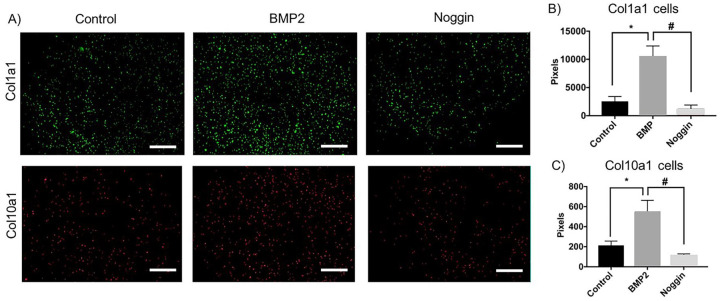
BMP2 Increases Col1a1 and Col10a1 in Chondrocyte Cultures While Noggin Induced an Opposite Effect
Last, using a chondrocyte cell culture model, we examined changes in collagen expression in response to treatment with rhBMP2 or with its antagonist rhNoggin ( Fig. 6A ). Treatment with rhBMP2 was found to increase the number of Col1a1-positive cells in comparison to the control cells ( Fig. 6B ), suggesting an enhancement of proliferation. In contrast, when cells were treated with rhNoggin there was a decrease in Col1a1-positive cells ( Fig. 6B ), suggesting that inhibition of BMP2 signaling reduces cellular proliferation (consistent with our in vivo studies). Moreover, we observed a similar pattern where rhBMP2 treatment resulted in an increase in Col10a1-positive cells compared to control cells, while an opposite effect was seen in rhNoggin-treated cells ( Fig. 6C ), suggesting that the BMP2 pathway promotes hypertrophic chondrocyte differentiation.
Figure 6.
Bone morphogenetic protein (BMP2) treatment increases Col1a1- and Col10a1-positive cells in chondrocyte culture while noggin has an opposite effect. Following 14 days of treatment with rhBMP2 or rhNoggin the number of green pixels (Col1a1-expressing cells) and red pixels (Col10a1-expressing cells) were quantified and are represented in bar graphs. * and #P < 0.05. Scale bar = 10×.
Discussion
In our research, we provide strong evidence that BMP2 plays a significant physiological role in the postnatal development and maintenance of the osteochondral tissues of the TMJ. Conditional deletion of BMP2 from ACAN-expressing cells in the cartilage of the TMJ results in postnatal deficits in osteochondral tissues and leads to progressive breakdown of the cartilage, which ultimately leads to accelerated TMJ degeneration. Additionally, our data reveal a significant role of BMP2 in the biology and integrity of the TMJ.
While prior research has identified the role BMP2 signaling plays in the homeostasis of the osteochondral tissues of the knee, we believe these are the first studies examining the role of BMP2 in postnatal TMJ biology. The osteochondral tissues of the TMJ and knee have distinct developmental origins.7,24,25 These tissues are composed of different types of cartilage, comprised of unique chondroprogenitor and chondrocyte populations, and have matrices that differ in molecular composition, structure, and mineral content.7,24,25 The cartilage of the TMJ is secondary cartilage (develops after bone formation), whereas the cartilage of the knee is primary cartilage (precedes bone formation).24-26 In addition, the cartilage of the TMJ is a fibrocartilage, while the cartilage of the knee is a hyaline cartilage.24-26 Finally, the chondrocytes of the MCC and knee AC exhibit different cellular characteristics, including differences in proliferation, differentiation, and matrix synthesis.25,26 Therefore, it is essential to study and interpret the role of BMP2 in each cartilage type separately.
In our initial examinations of BMP2 expression, in situ hybridization in 3-week-old mice allowed us to precisely detect Bmp2 expression in the cartilage and subchondral bone of the TMJ. This led us to generate a mouse model with conditional deletion of BMP2 in ACAN-expressing cells of the cartilage. Our research highlights the importance of local BMP2 activity in regulating the synthesis of the extracellular matrix within the cartilage. Additionally, we observed that local deletion of BMP2 had a deleterious effect on the subchondral bone. We observed decreased mineralization (bone labels) and decreased bone volume fraction, as well as decreased tissue density in the calcified bone and cartilage both at the 3- and 6-month time points after tamoxifen treatment. Interestingly, conditional deletion of BMP2 in the cartilage does also affect the subchondral bone and a plausible reason could be that ACAN-expressing cells have been shown to migrate from the cartilage to form subchondral bone.27,28 Furthermore, our in vitro results demonstrate that exogenous BMP2 is responsible for hypertrophic differentiation of chondrocytes (i.e., an increase in Col10a1) and mineralization (increased relative expression of osteocalcin-has new name, alkaline phosphatase, osteopontin-has new name, and FGF2). In addition, we observed that BMP2 mRNA was highly expressed in the prehypertrophic and hypertrophic chondrocytes of the postnatal TMJ cartilage suggesting an essential role of BMP2 in the proliferation, maturation, and mineralization of the calcified cartilage.
BMPs are multifunctional growth factors involved in numerous cellular and molecular signaling pathways. In addition to the well-characterized actions of BMP2 on osteoblasts (maturation and differentiation), BMP2 is also essential for osteoclast differentiation.29-31 In our research, we observed a decrease in TRAP staining with conditional deletion of BMP2 at the 3-month time point after tamoxifen treatment, compared to control mice. However, changes in TRAP at the 6-month time point after tamoxifen was not statistically different than the control. Studies have previously shown that treatment of osteoclasts in vitro with exogenous BMP2 not only enhances RANKL-stimulated differentiation of osteoclast precursors but also stimulates survival and resorptive activity of mature osteoclasts. 29 Thus, it seems likely that BMP2 is able to decrease TRAP activity in the BMP2 cKO mice used here, through a direct mechanism.
The role of BMP2 in cartilage biology has been a controversial topic in the field. 8 Interestingly, it has previously been observed in knee cartilage that BMP2 is necessary for postnatal maintenance and homeostasis, and selective deletion of BMP2 in growth differentiation factor 5 (GDF5)-expressing cells leads to an accelerated degeneration of the knee cartilage. 12 Similarly, in comparison to control mice the BMP2 cKO mice, at both the 3- and 6-month time points after tamoxifen, were found to have increased OARSI scores, decreased proliferation of chondrocytes, abnormal turnover of the extracellular matrix, and accelerated degeneration of the TMJ cartilage.
Many studies have also implicated BMP2 in the degeneration of knee cartilage.8,29 Hypertrophic differentiation and mineralization are well-known hallmarks of cartilage degeneration.14,32 Remarkably, we observed a greater degree of degeneration in the cartilage of the TMJ in the BMP2 cKO mice, when compared with the knee in these mice. This may be due to biological differences between the cartilage of the TMJ and the knee (i.e., different developmental origins; different extracellular matrix molecular composition and mineral content). Our results and published research have shown that exogenous BMP2 leads to mineralization, maturation, and hypertrophic differentiation of chondrocytes.32,33 Additionally, increased BMP2 protein expression has been observed in degenerated knee cartilage.8,10 However, it is unknown whether this increased BMP2 protein expression level led to degeneration, or alternatively if the cartilage degeneration occurred prior to the increased protein expression levels.
Increased expression of MMP13 and ADAMTS4 are key markers of osteochondral tissue degeneration.34-36 In our in vivo experiments, we observed an increase in the protein expression levels of MMP13 and ADAMTS4 in the cartilage of BMP2 cKO mice at 3 months after tamoxifen, compared with controls. While in the BMP2cKO mice at 6 month following tamoxifen, we did not find a significant difference in expression levels.
The OARSI score was significantly increased in BMP2 cKO mice at 6 months after tamoxifen, when compared to BMP2 cKO mice at 3 months after tamoxifen. In contrast, the cartilage degeneration markers (MMP13 and ADAMTS4) were decreased in BMP2 cKO mice at 6 months after tamoxifen, when compared to the 3 months after tamoxifen group. MMP13 is an important enzyme that targets connective tissue, including proteoglycans, and has a major affinity for type II collagen.35,36 In these studies, safranin O staining showed a significant decrease in the proteoglycan content with conditional deletion of BMP2, both at the 3- and 6-month timepoints after tamoxifen.35,36
One of the possible limitation of our study is that BMP2 and BMP4 coexist and may have functional redundancy. There is a possibility that BMP4 is compensating for the lack of BMP2. Therefore, our future study will focus on the conditional deletion of both BMP2 and BMP4 from the mature chondrocytes to completely understand the role of bone morphogenetic protein in the maintenance of the osteochondral tissues of the TMJ.
Conclusions
BMP2 is required for postnatal maintenance and maturation of the osteochondral tissues of the TMJ, as conditional deletion of BMP2 caused breakage of the mandibular condyle cartilage accompanied by a decrease in cartilage thickness, matrix synthesis, mineralization, chondrocyte proliferation, and increased expression of degeneration markers.
Supplemental Material
Supplemental material, sj-pdf-1-car-10.1177_1947603520980158 for BMP2 Is Required for Postnatal Maintenance of Osteochondral Tissues of the Temporomandibular Joint by Mara H. O’Brien, Eliane H. Dutra, Shivam Mehta, Po-Jung Chen and Sumit Yadav in CARTILAGE
Footnotes
Acknowledgments and Funding: We would like to thank Dr. David W. Rowe for generously providing us with the fluorescent reporter mice used for in vitro studies. We would also like to thank Li Chen for assisting with imaging of histological slides. The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: The research reported in this publication was supported by the National Institute of Dental and Craniofacial Research of the National Institute of Health under the award number KO8DE025914 to SY.
Author Contributions: Conceptualization and methodology: Mara H. O’Brien, Eliane H. Dutra, Po-Jung Chen, Sumit Yadav. Data collection: Mara H. O’Brien, Po-Jung Chen, Shivam Mehta. Data interpretation: Mara H. O’Brien, Eliane H. Dutra, Po-Jung Chen, Shivam Mehta, Sumit Yadav. Manuscript preparation: Mara H. O’Brien, Eliane H. Dutra, Po-Jung Chen, Shivam Mehta, Sumit Yadav. Manuscript approval: Mara H. O’Brien, Eliane H. Dutra, Po-Jung Chen, Shivam Mehta, Sumit Yadav. Funding: Sumit Yadav. Project administration: Sumit Yadav.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
ORCID iDs: Mara H. O’Brien  https://orcid.org/0000-0002-5646-2541
https://orcid.org/0000-0002-5646-2541
Sumit Yadav  https://orcid.org/0000-0002-2434-7995
https://orcid.org/0000-0002-2434-7995
Ethical Approval: The Institutional Animal Care Committee at the University of Connecticut Health Center approved the experimental procedures used in this study (protocol number AP-200087-0823, year 2020).
Supplementary material for this article is available on the Cartilage website at https://journals.sagepub.com/home/car.
References
- 1. Lories RJ, Luyten FP. The bone-cartilage unit in osteoarthritis. Nat Rev Rheumatol. 2011;7(1):43-9. [DOI] [PubMed] [Google Scholar]
- 2. Smith SB, Parisien M, Bair E, Belfer I, Chabot-Dore AJ, Gris P, et al. Genome-wide association reveals contribution of MRAS to painful temporomandibular disorder in males. Pain. 2019;160(3):579-91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Chen H, Slade G, Lim PF, Miller V, Maixner W, Diatchenko L. Relationship between temporomandibular disorders, widespread palpation tenderness, and multiple pain conditions: a case-control study. J Pain. 2012;13(10):1016-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Loeser RF, Goldring SR, Scanzello CR, Goldring MB. Osteoarthritis: a disease of the joint as an organ. Arthritis Rheum. 2012;64(6):1697-707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Gonzalez YM, Schiffman E, Gordon SM, Seago B, Truelove EL, Slade G, et al. Development of a brief and effective temporomandibular disorder pain screening questionnaire: reliability and validity. J Am Dent Assoc. 2011;142(10):1183-91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Lim PF, Smith S, Bhalang K, Slade GD, Maixner W. Development of temporomandibular disorders is associated with greater bodily pain experience. Clin J Pain. 2010;26(2_suppl):116-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Wadhwa S, Kapila S. TMJ disorders: future innovations in diagnostics and therapeutics. J Dent Educ. 2008;72(8):930-47. [PMC free article] [PubMed] [Google Scholar]
- 8. Gamer LW, Pregizer S, Gamer J, Feigenson M, Ionescu A, Li Q, et al. The role of Bmp2 in the maturation and maintenance of the murine knee joint. J Bone Miner Res. 2018;33(9):1708-17. [DOI] [PubMed] [Google Scholar]
- 9. Edwards CJ, Francis-West PH. Bone morphogenetic proteins in the development and healing of synovial joints. Semin Arthritis Rheum. 2001;31(1):33-42. [DOI] [PubMed] [Google Scholar]
- 10. Rountree RB, Schoor M, Chen H, Marks ME, Harley V, Mishina Y, et al. BMP receptor signaling is required for postnatal maintenance of articular cartilage. PLoS Biol. 2004;2(11):e355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Chen D, Zhao M, Mundy GR. Bone morphogenetic proteins. Growth Factors. 2004;22(4):233-41. [DOI] [PubMed] [Google Scholar]
- 12. Bau B, Haag J, Schmid E, Kaiser M, Gebhard PM, Aigner T. Bone morphogenetic protein-mediating receptor-associated Smads as well as common Smad are expressed in human articular chondrocytes but not up-regulated or down-regulated in osteoarthritic cartilage. J Bone Miner Res. 2002;17(12):2141-50. [DOI] [PubMed] [Google Scholar]
- 13. Shirakura M, Kram V, Robinson J, Sikka S, Kilts TM, Wadhwa S, et al. Extracellular matrix mediates BMP-2 in a model of temporomandibular joint osteoarthritis. Cells Tissues Organs. 2017;204(2_suppl):84-92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Bechtold TE, Saunders C, Mundy C, Um H, Decker RS, Salhab I, et al. Excess BMP signaling in heterotopic cartilage forming in Prg4-null TMJ discs. J Dent Res. 2016;95(3):292-301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Chen PJ, Dutra EH, Mehta S, O’Brien MH, Yadav S. Age-related changes in the cartilage of the temporomandibular joint. Geroscience. 2020;42(3):995-1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Dutra EH, O’Brien MH, Lima A, Nanda R, Yadav S. A morphometric and cellular analysis method for the murine mandibular condyle. J Vis Exp. 2018;(131):55998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. MH OB, Dutra EH, Lima A, Nanda R, Yadav S. PTH [1-34] induced differentiation and mineralization of mandibular condylar cartilage. Sci Rep. 2017;7(1):3226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Knudson CB, Knudson W. Cartilage proteoglycans. Semin Cell Dev Biol. 2001;12(2_suppl):69-78. [DOI] [PubMed] [Google Scholar]
- 19. Ma L, Martin JF. Generation of a Bmp2 conditional null allele. Genesis. 2005;42(3):203-6. [DOI] [PubMed] [Google Scholar]
- 20. Henry SP, Jang CW, Deng JM, Zhang Z, Behringer RR, de Crombrugghe B. Generation of aggrecan-CreERT2 knockin mice for inducible Cre activity in adult cartilage. Genesis. 2009;47(12):805-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kawamoto T. Use of a new adhesive film for the preparation of multi-purpose fresh-frozen sections from hard tissues, whole-animals, insects and plants. Arch Histol Cytol. 2003;66(2_suppl):123-43. [DOI] [PubMed] [Google Scholar]
- 22. Pfaffl MW. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001;29(9):e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Glasson SS, Chambers MG, Van Den Berg WB, Little CB. The OARSI histopathology initiative—recommendations for histological assessments of osteoarthritis in the mouse. Osteoarthritis Cartilage. 2010;18(Suppl 3):S17-S23. [DOI] [PubMed] [Google Scholar]
- 24. Benjamin M, Ralphs JR. Biology of fibrocartilage cells. Int Rev Cytol. 2004;233:1-45. [DOI] [PubMed] [Google Scholar]
- 25. Milam SB. Pathogenesis of degenerative temporomandibular joint arthritides. Odontology. 2005;93(1):7-15. [DOI] [PubMed] [Google Scholar]
- 26. Freemont AJ, Hoyland J. Lineage plasticity and cell biology of fibrocartilage and hyaline cartilage: its significance in cartilage repair and replacement. Eur J Radiol. 2006;57(1):32-6. [DOI] [PubMed] [Google Scholar]
- 27. Zhou X, von der Mark K, Henry S, Norton W, Adams H, de Crombrugghe B. Chondrocytes transdifferentiate into osteoblasts in endochondral bone during development, postnatal growth and fracture healing in mice. PLoS Genet. 2014;10(12):e1004820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Jing Y, Zhou X, Han X, Jing J, von der Mark K, Wang J, et al. Chondrocytes directly transform into bone cells in mandibular condyle growth. J Dent Res. 2015;94(12):1668-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Jensen ED, Pham L, Billington CJ, Jr, Espe K, Carlson AE, Westendorf JJ, et al. Bone morphogenic protein 2 directly enhances differentiation of murine osteoclast precursors. J Cell Biochem. 2010;109(4):672-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Canalis E, Economides AN, Gazzerro E. Bone morphogenetic proteins, their antagonists, and the skeleton. Endocr Rev. 2003;24(2_suppl):218-35. [DOI] [PubMed] [Google Scholar]
- 31. Cao X, Chen D. The BMP signaling and in vivo bone formation. Gene. 2005;357(1):1-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Dreier R. Hypertrophic differentiation of chondrocytes in osteoarthritis: the developmental aspect of degenerative joint disorders. Arthritis Res Ther. 2010;12(5):216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Shu B, Zhang M, Xie R, Wang M, Jin H, Hou W, et al. BMP2, but not BMP4, is crucial for chondrocyte proliferation and maturation during endochondral bone development. J Cell Sci. 2011;124(Pt 20):3428-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Haslauer CM, Elsaid KA, Fleming BC, Proffen BL, Johnson VM, Murray MM. Loss of extracellular matrix from articular cartilage is mediated by the synovium and ligament after anterior cruciate ligament injury. Osteoarthritis Cartilage. 2013;21(12):1950-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Li H, Wang D, Yuan Y, Min J. New insights on the MMP-13 regulatory network in the pathogenesis of early osteoarthritis. Arthritis Res Ther. 2017;19(1):248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Wang M, Sampson ER, Jin H, Li J, Ke QH, Im HJ, et al. MMP13 is a critical target gene during the progression of osteoarthritis. Arthritis Res Ther. 2013;15(1):R5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-pdf-1-car-10.1177_1947603520980158 for BMP2 Is Required for Postnatal Maintenance of Osteochondral Tissues of the Temporomandibular Joint by Mara H. O’Brien, Eliane H. Dutra, Shivam Mehta, Po-Jung Chen and Sumit Yadav in CARTILAGE