Abstract
Objective
Osteoarthritis (OA) is a global public health problem and a leading cause of morbidity and disability. Due to lack of sensitive and specific tools for early OA diagnosis and predicting prognosis, the availability of new reliable and sensitive biomarkers is a widely appreciated need to identify patients at risk for incident disease or disease progression. Accordingly, our study was conducted to validate the usefulness of disintegrin and metalloproteinase with thrombospondin motifs 5 (ADAMTS5) and follistatin-like protein 1 (FSTL1) to achieve this goal.
Design
Fifty-four male Wistar rats were randomized into 3 groups; 24 rats were subjected to medial meniscal tear (MMT) surgery on the right knee joint (OA group), 24 rats were subjected to sham surgery (sham group), and 6 healthy rats (negative control group). Six animals from each group were sacrificed every 2 weeks. At each time point, the right knee joint of each animal was visualized radiologically, a blood sample was collected, and cartilage tissues were isolated for histopathological and western blot analysis.
Results
We found that the expression levels of ADAMTS5 and FSTL1 significantly increased with OA progression, especially at weeks 4, 6, and 8 after surgery. Notably, the serum levels of ADAMTS5 and FSTL1 showed significant positive correlations with each other and with the studied inflammatory markers.
Conclusions
Our findings suggest that ADAMTS5 and FSTL1 can serve as important and informative serological markers of disease activity in OA. However, further research is needed to validate their use for improving the diagnosis and prognosis of OA in humans.
Keywords: osteoarthritis, ADAMTS5, FSTL1, biomarker
Introduction
Osteoarthritis (OA) is a multifactorial, chronic joint condition. The progressive destruction of articular cartilage is a prominent feature of OA, causing impaired movement, pain, and, eventually, disability. 1 Globally, there are more than 100 million people suffering from OA. 2 The prevalence of symptomatic, radiographic OA in adult men and women aged 60 years and older is reported to be 10% and 18%, respectively. As populations age, it is predicted that OA will become one of the major incentives of disability worldwide by the year 2020.2,3 The diagnosis is currently based on clinical symptoms in combination with radiography to visualize the degenerative changes in the joint architecture. These changes can be observed only in an advanced stage of the disease, at which point tissue damage is considered irreversible. 4 Radiologic evaluation of joints mainly assesses bone and is relatively insensitive to changes in cartilage tissue; a follow-up time for a year, and more likely for 2 years, is essential to define disease progress and therapeutic efficacy. Moreover, all radiography techniques illuminate the historical vision of the damage that occurred earlier, instead of estimating the current rate of disease progression. Furthermore, lack of sensitivity and specificity is limiting the diagnostic competence of the conventional serum inflammatory markers including tumor necrosis factor-α (TNF-α), an important proinflammatory cytokine in the innate immune response; C-reactive protein (CRP), an acute-phase protein that plays an important physiological role in complement system activation; and the erythrocytes sedimentation rate (ESR), a well-validated and inexpensive test for the evaluation of inflammation.5-7
Alternative methods are therefore needed to detect osteoarthritic changes in joints at an early phase of the disease in a quantitative, reliable, and sensitive manner. Molecular biomarkers could be considered as a promising option in this respect.4,8 An ideal biomarker needs to be disease specific, measurable using noninvasive techniques, sensitive to therapy, and should be able to reflect the actual disease activity and predict disease consequences. Most likely, all these requirements are not fully met by a single biomarker; however, using a combination of biomarkers can represent an acceptable alternative. 4
Recent studies in the field of OA biomarkers revealed that the desirable panels of biomarkers have to comprise markers of collagen synthesis and degradation, indicative of cartilage breakdown, and markers of bone and synovium breakdown, as well as inflammatory mediators.9-11
It is well known that in OA, an imbalance between the biosynthesis and degradation of the cartilage extracellular matrix components leads to the progressive destruction of the articular cartilage tissues and, ultimately, to irreversible damage to the articular surface. Proteoglycan and collagen represent the major component of the cartilage matrix. In the process of cartilage degeneration, the loss of proteoglycan occurs first, and subsequently, collagen fibrils catabolism pursue, leading to loss of cartilage structural firmness.12,13
ADAMTS5 (a disintegrin and metalloproteinase with thrombospondin motifs 5) is an enzyme involved in the devastation of the cartilage-specific proteoglycan aggrecan. In humans, ADAMTS5 is encoded by the ADAMTS5 gene. 14 Aggrecan is a fundamental motif of cartilage matrix that enables cartilage tissues to endure compression. 15 In OA, aggrecan destruction is a fundamental event in the early phases of the disease, but to date, the relative contribution of individual ADAMTS proteinases to cartilage damage during OA is not fully understood. 15
FSTL1 (follistatin-like protein 1), also known as TSC36, is an extracellular glycoprotein originally cloned from a mouse osteoblast cells as a transforming growth factor-β (TGF-β)-inducible gene. FSTL1 is widely expressed in human tissues and is induced by ischemic stress and proinflammatory mediators. Although its roles at the molecular level is still not fully explained, numerous reports revealed that FSTL1 is implicated in the pathogenesis of rheumatoid arthritis. 16 Despite the roles of FSTL1 on inflammation and immunity are complicated and argumentative, FSTL1 expression and its correlation with the clinical features of OA patients have not been completely assessed yet. 17
The present study was conducted to determine the usefulness of ADAMTS5 and FSTL1 as potential biomarkers of OA using medial meniscal tear (MMT) surgery in rats, which were previously used as a convenient model of OA.13,18-20 We also endeavored to conduct the correlation between these 2 proteins with OA progression and with a set of conventional OA-related inflammatory markers, including TNF-α, CRP, and ESR.5-7
Materials and Methods
Animal Model
Animal experiments were accomplished in accordance with the National Institutes of Health Guidelines (NIH 1985) for the Care and Use of Laboratory animals, and were approved by the Ethical Committee of the Faculty of Pharmacy, Al-Azhar University. Fifty-four, 16- to 17-week-old healthy male Wistar rats, weighing 150 to 160 g (El-Neil Pharmaceutical Company, Cairo, Egypt), were allowed to acclimatize for 2 weeks. Rats were housed (2 per cage) in a regulated environment (temperature, 20-22°C; humidity, 50 ± 5%; night/day cycle, 12 hours) with free access to standard diet pellets and sterile tap water ad libitum.
After 2 weeks of acclimatization, the animals were randomly divided into 3 groups. In the first group (the OA group), 24 rats were subjected to unilateral MMT surgery on the right knee joint.19,20 Briefly, each rat was anesthetized by intraperitoneal injection of pentobarbital (50 mg/kg), and the anterior portion of the right knee was shaved and sterilized. The surgery was achieved under a dissecting microscope. Using a surgical blade (size 11), the skin was incised along the ventral midline of the right knee, and then the joint cavity was opened through the standard medial parapatellar approach. The suture line, which passed over the patellar ligament and medial collateral ligament, was stretched for observation of the cavity. An ophthalmic surgical blade was used to incise the medial meniscotibial ligament. After that, forceps were used to probe the anterior half of the medial meniscus to confirm its clear displacement form the tibial plateau. After surgery, both the joint cavity and the skin were closed with absorbable and nonabsorbable fine sutures, respectively.
In the second group (the sham group), 24 rats were subjected to a careful surgical opening of the right knee joint without incision of the medial meniscotibial ligament to serve as sham operated controls. A third group comprising 6 rats was used as a healthy control group.
After surgery, 6 animals from each operated group were sacrificed every 2 weeks, that is, after 2, 4, 6, and 8 weeks. The 6 healthy control rats were sacrificed at the 8-week time point. At each time point, the severity of the posttraumatic osteoarthritic changes in the right knee joints was visualized radiologically before cervical decapitation under isoflurane anesthesia. Blood was then collected from the aorta. The whole right knee joint was resected and divided into 2 parts: the first part was kept in 10% neutral buffered formaldehyde for histopathological investigations. The cartilage tissues of the second part were homogenized in ice-cold Tris-HCl lysis buffer, pH 7.4, containing 1% protease inhibitor cocktail (Cell Signaling Technology, Inc., Danvers, MA) using a Potter-Elvehjem rotor stator homogenizer (glass/Teflon homogenizer) and kept at −70°C until use in western blot assays.
Radiological Examinations
At each time point, the severity of the posttraumatic osteoarthritic changes in the right knee joint was assessed radiologically by X-ray films (X-ray imaging apparatus, Hualun Medical Systems, China). Anterior-posterior and lateral knee radiographs were obtained in the relaxed position. The evaluation of radiologically defined OA was undertaken blindly by a radiologist and an orthopedic surgeon using the Kellgren-Lawrence (KL) system shown in the Atlas of Standard Radiographs of Arthritis. 21 Posttraumatic osteoarthritic changes are classified according to the 5-level scale and are scored as follows: 0 (normal); 1 (doubtful OA); 2 (minimal OA); 3 (moderate OA); or 4 (severe OA). 21
Histological Examinations
Tissue samples from the right knee were fixed in 10% neutral buffered formaldehyde at room temperature for 24 hours. Decalcification was carried out in a 12% neutral EDTA (ethylenediaminetetraacetic acid) solution with several changes of the solution over 4 weeks. Tissue specimens were cleared in xylene and were then embedded in paraffin at 56°C in a hot air oven for 24 hours. Tissue sections (4 µm) were prepared with a sledge microtome, and the obtained tissue sections were placed on glass slides, deparaffinized, and stained with hematoxylin and eosin (H&E). To reveal different macromolecules that make up the cells, other corresponding tissue sections were stained by Mallory’s trichrome stain. The stained slides were examined under an electric light microscope for the assessment of histopathological changes. 22
Biochemical Estimations
Quantitative estimations of serum ADAMTS5, FSTL1, and TNF-α levels were carried out using the corresponding rat-specific enzyme-linked immunosorbent assay (ELISA) kits (Biospes Co., Ltd., China) according to the manufacturer’s instructions. Serum CRP was assayed using an enhanced MISPA i2-based nephelometry kit (Agappe Diagnostics Switzerland GmbH). Part of the fresh blood sample was used to determine the ESR using an ESR Fast Detector.
Western Blotting
The proteins in each cartilage tissue homogenate were denatured at 95°C for 5 minutes in 2× Laemmli buffer followed by the addition of 5% (v/v) 2-mercaptoethanol. SDS-PAGE electrophoresis was achieved by running 30 µg of protein per lane at 50 volts through a stacking gel followed by running at 125 volts through 18% and 15% resolving gels for ADAMTS5 and FSTL1, respectively, for 2 hours; proteins were then transferred to a polyvinylidene difluoride (PVDF) membrane using a T-77 ECL semidry transfer unit (Amersham BioSciences UK, Ltd.) for 2 hours. Immunoblotting was performed by incubating the PVDF membrane in Tris-buffered saline (TBS) containing 0.1% Tween 20 and 5% nonfat milk for 1 hour at 4°C, followed by an overnight incubation at 4°C with a rabbit anti-ADAMTS5 polyclonal antibody (Boster Biological Technology Co., Ltd.) or a rabbit anti-FSTL1 polyclonal antibody (Bioss Inc., USA) at dilutions of 1:1500 and 1:1000, respectively. After washing 3 times with TBST buffer, each membrane was incubated for 1 hour at room temperature with an alkaline phosphatase-conjugated goat anti-mouse secondary antibody (Novus Biologicals, USA) at a dilution of 1:5000. After 4 washes with TBST, the membrane-bound antibodies were detected with a commercially available BCIP/NBT substrate detection kit (Genemed Biotechnologies, Inc., USA). Equivalent protein loading for each lane was confirmed by stripping and reblotting each membrane at 4°C with a rabbit polyclonal anti-β-actin antibody (Novus Biologicals, LLC, Littleton, CO) at a dilution of 1:5000. Each experiment was repeated 3 times to assure the reproducibility of results. Quantification was performed using ImageJ software and expressed as the band density relative to that of β-actin.
Statistical Analysis
The statistical analyses of the results were performed using GraphPad Prism software version 6.0 (GraphPad Software, Inc., San Diego, CA). All values are expressed as the mean ± SEM, and the variables were compared using one-way analysis of variance (ANOVA) followed by Tukey’s t test for multiple comparisons. Differences were considered statistically significant at P < 0.05. The correlations between continuous variables were analyzed using Pearson’s correlation.
Results
Radiological Findings
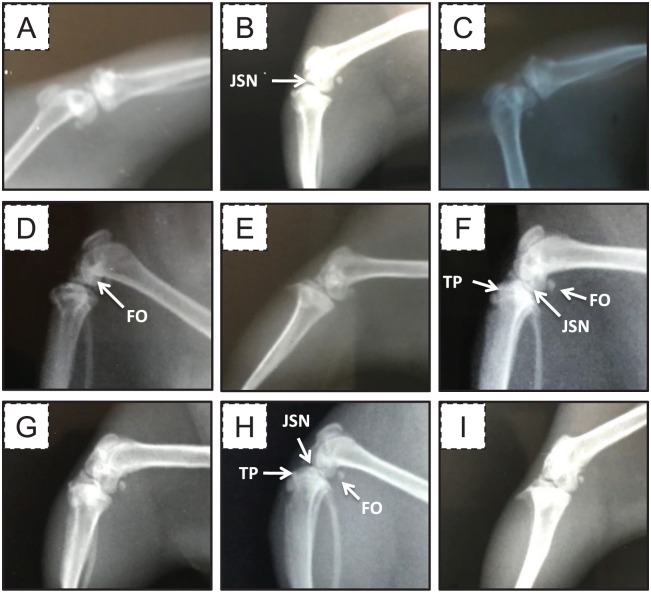
To confirm that the MMT-induced OA model works, the posttraumatic osteoarthritic changes in the right knee joint was radiologically visualized by X-ray films ( Fig. 1 ). We found that, according to the KL grading scheme, 21 the radiological examinations of the healthy and sham control groups showed signs of grade (0) OA, that is, normal tibial plateau and subchondral bone, at all time points. In contrast, the radiological examinations of the OA group at week 2 showed signs of grade (1) OA, that is, the joint space was slightly decreased, with joint space narrowing (JSN) and femur osteophyte formation. The radiological examinations of the OA group at week 4 showed signs of grade (2) OA, that is, osteophytes at the femoral and tibial plateaus, JSN in the tibiofemoral joint, bony subchondral sclerosis with cysts, and possible bony deformities. The radiological examinations of the OA group at week 6 showed signs of grades (2) and (3) OA, that is, femur osteophytes, reduced joint space, tibial plateau anterior and posterior osteophytes, bony subchondral sclerosis with cysts, and possible bony deformities. The radiological examinations of the OA group at the week 8 showed signs of grades (3) and (4) OA, that is, femur osteophytes, reduced joint space, tibial plateau anterior and posterior osteophytes with epiphyseal flattening, and marked JSN and deformity in the tibiofemoral joint.
Figure 1.
Representative X-ray films of the right knee joints from different groups. (A) The healthy control group, (B) the OA group at week 2, (C) the sham group at week 2, (D) the OA group at week 4, (E) the sham group at week 4, (F) the OA group at week 6, (G) the sham group at week 6, (H) the OA group at week 8, and (I) the sham group at week 8. FO = femur osteophytes; JSN = joint space narrowing; TP = tibial plateau.
Histopathological Findings
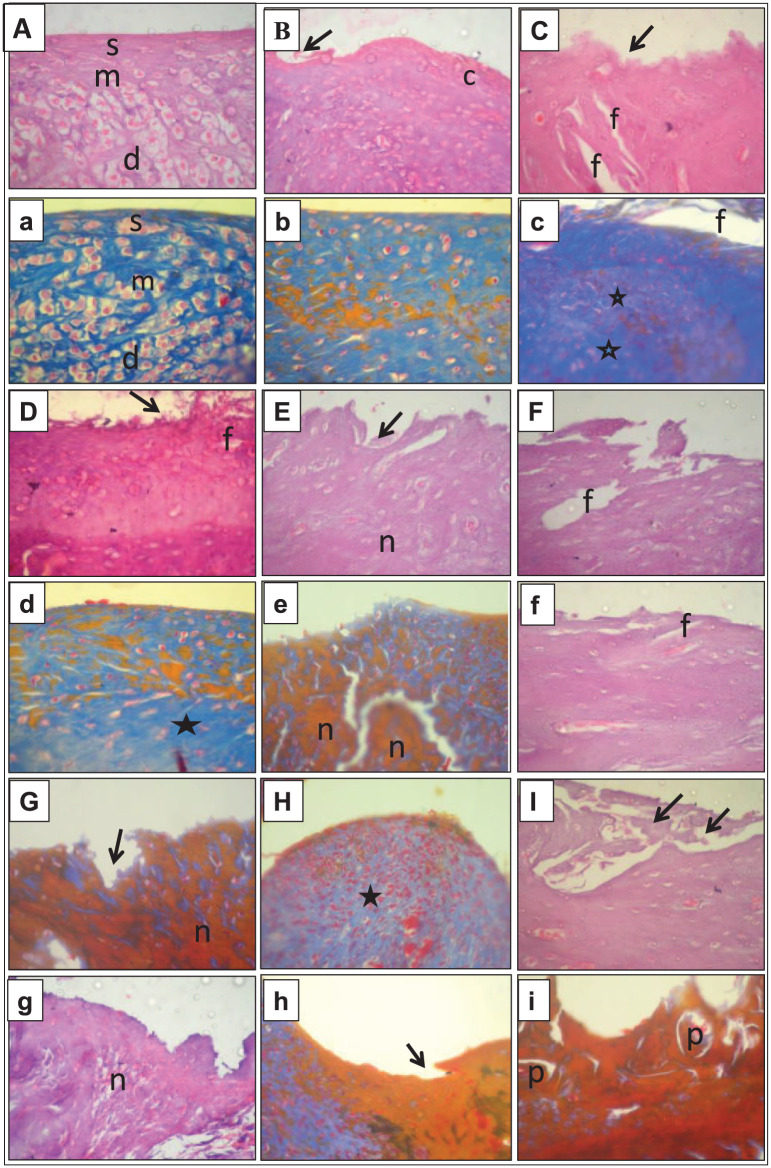
Histological examinations were carried out to evaluate the effect of the MMT surgery on the articular tissues. The stained tissue sections of the healthy control group showed normal articular cartilage with normal histological features; the cartilage surface was smooth, and the matrix and chondrocytes were organized into superficial (s), mid (m), and deep(d) zones—see Figure 2A and a . At week 2, the sham group showed minimal superficial fibrillation in the articular surface (arrow), accompanied by the necrosis of some chondrocytes (c), with unaffected mid and deep zones—see Figure 2B and b . At week 2, the articular surface in the OA group showed superficial fibrillation (arrow), with vertical fissuring (f) and a prominent increase in the matrix (star) extending to the mid and deep zones—see Figure 2C and c . At week 4, the articular surface in the sham group exhibited some superficial fibrillations (arrow) with surface discontinuity, cell necrosis, and an increase in the matrix staining (star)—see Figure 2D and d . Conversely, at week 4, the OA group showed marked erosion in the articular surface (arrow) with massive necrosis of the cells (n) extending to the deep zone—see Figure 2E and e . At week 6, the articular surface in the sham group had mild vertical fissuring (f) extending to the mid-zone—see Figure 2F and f . On the contrary, the articular surface in the OA group, at week 6, showed more extensive erosion (arrow) resulting in excavation, matrix loss with prominent fissuring, and more cellular necrosis (n) than that in the sham group—see Figure 2G and g. At week 8, the articular surface in the sham group had slight erosions with minimal cartilage matrix loss extending to the mid and deep zones—see Figure 2H and h . Conversely, at week 8, the articular surface in the OA group was deformed and completely eroded (arrow), showing signs of bone remolding and the presence of a bone plate (p)—see Figure 2I and i .
Figure 2.
Photomicrographs of articular cartilage from different groups. The capital letters refer to H&E staining, and the corresponding lowercase letters refer to Mallory’s trichrome staining at a magnification of 10×. (A and a) are examples from the healthy control group, (B and b) sham group week 2, (C and c) OA group week 2, (D and d) sham group week 4, (E and e) OA group week 4, (F and f) sham group week 6, (G and g) OA group week 6, (H and h) sham group week 8, and (I and i) OA group week 8. (c) = chondrocytes; (d) = deep zones; (f) = vertical fissuring; (m) = mid zones; (n) = necrosis; (p) = bone plate; (s) = superficial zones. Stars and arrows are used to point out the changes in the matrix staining and the articular surface, respectively.
Biochemical Estimations
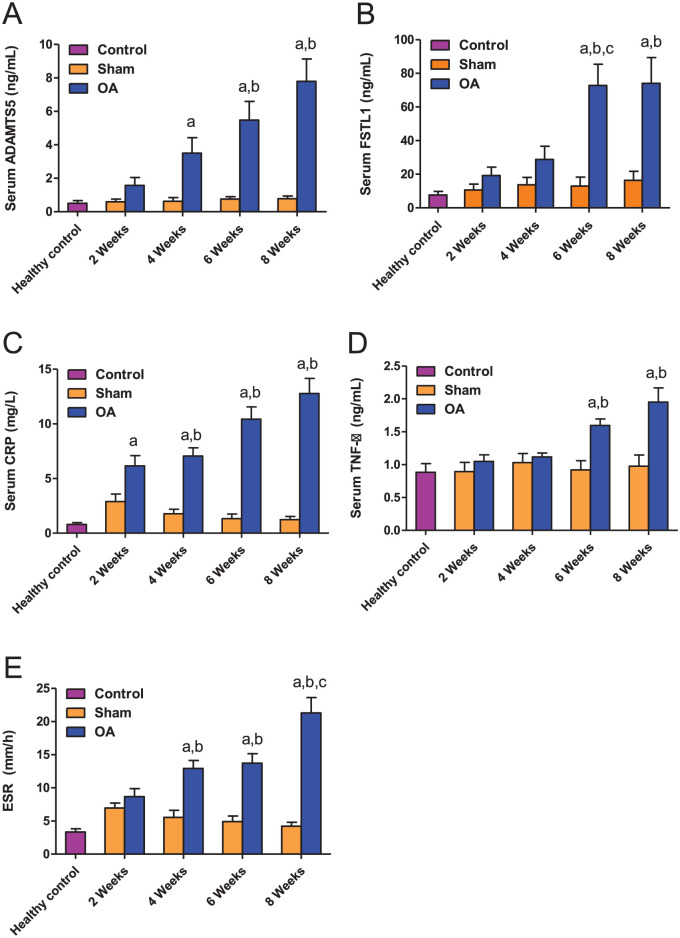
We found that the serum levels of ADAMTS5 showed a time-dependent increase in the OA group but not in the sham group, which presented stable low levels of ADAMTS5 in serum throughout the experiment ( Fig. 3A ). Significant increases in the serum levels of ADAMTS5 were reported in the OA group at weeks 4, 6, and 8 after surgery compared to the levels in the healthy controls. Compared to the ADAMTS5 levels in the sham group, the increased levels of ADAMTS5 in the OA group were only significant at weeks 6 and 8. The increase in the ADAMTS5 serum levels in the OA group at weeks 4, 6, and 8 was not statistically significant compared to the previous time point.
Figure 3.
Serum levels of ADAMTS5 (A), FSTL1 (B), CRP (C), and TNF-α (D), as well as ESR values (E), in the indicated groups. The data are presented as the means ± SEMs (n = 6 rats). The lowercase letters a, b, and c, indicate significant differences between the OA group and the healthy control group, the corresponding sham group, and the previous OA stage, respectively.
The circulating levels of FSTL1 were significantly higher in the OA group at weeks 6 and 8 after surgery compared to the healthy and sham control groups ( Fig. 3B ). We found that the FSTL1 serum levels significantly increased in the OA group at week 6 compared to the previous time points and remained high at week 8.
Also, our results established that the serum CRP levels showed a time-dependent increase in the OA group but not in the sham group, with the levels in the OA group significantly higher than in the sham group at weeks 4, 6, and 8 ( Fig. 3C ). The increase in CRP levels in the OA group was gradual and not statistically significant between consecutive time points.
Regarding TNF-α, serum levels in the OA and sham groups were relatively similar to those in the healthy group at weeks 2 and 4 postsurgery. However, in the OA group the levels significantly increased at weeks 6 and 8 compared to the corresponding sham group ( Fig. 3D ).
With regard to ESR, we observed significant increases in the OA group at weeks 4, 6, and 8 compared to healthy and sham controls, which presented similarly low ESR values at all the time points analyzed. At week 8, the ESR values in the OA group were significantly higher than those in the OA group at week 6 ( Fig. 3E ).
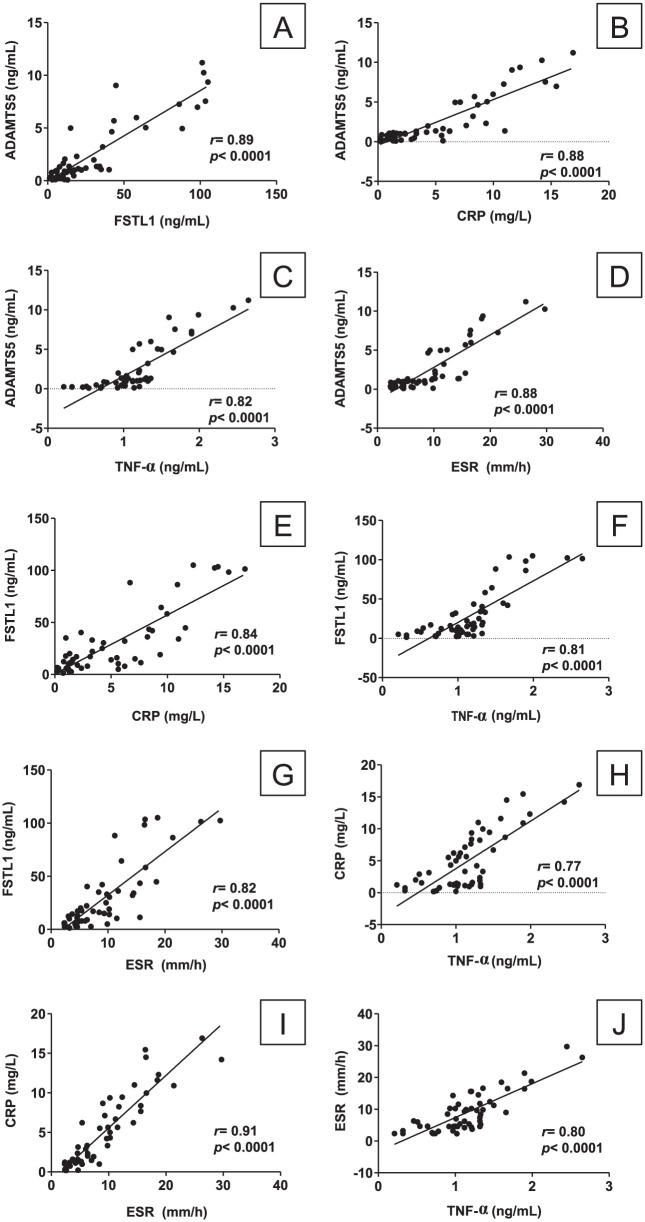
To show whether and how strongly each pairs of the assessed biomarkers are related, correlation statistics were performed. We found that the serum levels of ADAMTS5 showed a significant positive correlation with the circulating levels of FSTL1 (r = 0.89; P < 0.0001), CRP (r = 0.88; P < 0.0001), and TNF-α (r = 0.82; P < 0.0001), as well as with the ESR values (r = 0.88; P < 0.0001), as illustrated in Figure 4A to D . Additionally, the serum FSTL1 levels exhibited significant positive correlations with the serum CRP (r = 0.84; P < 0.0001) and TNF-α (r = 0.81; P < 0.0001) levels, as well as with the ESR values (r = 0.82; P < 0.0001), as illustrated in Figure 4E to G . Moreover, serum CRP levels showed significant positive correlations with the levels of TNF-α (r = 0.77; P < 0.0001) and ESR values (r = 0.91; P < 0.0001), as illustrated in Figure 4H and I . TNF-α exhibited a significant positive correlation with the ESR values (r = 0.80; P < 0.0001), as illustrated in Figure 4J . Together, these results showed that ADMST5 and FSTL1 serum levels increased as OA progressed and that this increase correlated with an increase in inflammatory markers.
Figure 4.
Pearson’s correlations between the continuous variables in the study: (A) between serum ADAMTS5 and serum FSTL1; (B) between serum ADAMTS5 and serum CRP; (C) between serum ADAMTS5 and serum TNF-α; (D) between serum ADAMTS5 and ESR; (E) between serum FSTL1 and serum CRP; (F) between serum FSTL1 and serum TNF-α; (G) between serum FSTL1 and ESR; (H) between serum CRP and serum TNF-α; (I) between serum CRP and ESR; (J) between serum TNF-α and ESR.
Western Blot Assessments of ADAMTS5 and FSTL1
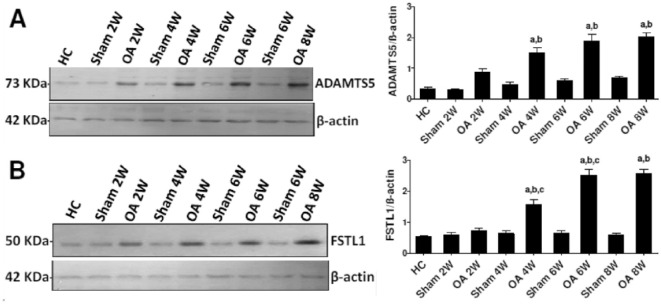
Next, we analyzed ADAMTS5 and FSTL1 protein levels in the cartilage of knee joints of rats by western blot. The results showed an increase in the levels of ADAMTS5 protein in the OA group over time ( Fig. 5A ), more detail are presented in the supplemental material published with this article. This increase was significant at weeks 4, 6, and 8 compared to both healthy controls and the corresponding sham group. There were no significant increases in the levels of ADAMTS5 protein in the sham group compared to the healthy controls or between the OA group at consecutive time points.
Figure 5.
Representative western blot analyses of ADAMTS5 (A) and FSTL1 (B) levels in cartilage tissue homogenates from different groups. β-Actin was used in parallel as the internal control. The panels on the right represent the corresponding quantification of each analysis as measured by ImageJ software and expressed as the relative band density to that of β-actin. The level of significance was accepted as P < 0.05, and all relevant results are graphically displayed as the mean ± SEM. The lowercase letters a, b, and c indicate significant differences between the OA group and the healthy control group, the corresponding sham group, and the previous OA stage, respectively.
Similarly, western blot analysis revealed a pronounced time-dependent increase in the level of FSTL1 protein in the OA groups ( Fig. 5B ). FSTL1 protein expression was significantly increased in the OA group at weeks 4, 6, and 8 compared to both the healthy controls and the corresponding sham group. There were no significant increases in the levels of FSTL1 protein in the sham groups compared to the healthy controls. In the OA group, FSTL1 levels at multiple exposures of different blots are presented in the supplemental material published with this article.
Discussion
Osteoarthritis is the most common progressive adult joint disorder.4,23 Disease progression is associated with synovial inflammation, cartilage degradation, JSN, and bony changes and affects many health outcomes.8,24,25 To supplement conventional biochemical and imaging techniques, there is an urgent medical need for the validation of novel biomarkers that identify patients at risk for progressive disease, identify molecular events that indicate early stages of the disease, and could be used as clinical surrogates that respond to candidate disease-modifying OA drug interventions expected to improve the clinical outcomes. 4
A previously published study reported that the increased breakdown of aggrecan is associated with the development of OA and that it could be mediated by members of the ADAMTS family of metalloproteinases. 26 One member in particular, ADAMTS5, is a major aggrecanase in cartilage metabolism and is involved in OA pathology.18,27 Work on ex vivo models supported a mechanism of cartilage destruction in which the production of ADAMTS5 in the synovium and the activation of ADAMTS zymogens in the articular tissues, rather than cytokine-induced ADAMTS5 expression by articular chondrocytes, might be responsible for the loss of cartilage proteoglycan in mice. 28
In the current study, there was a time-dependent increase in both the serum and tissue levels of ADAMTS5 in the OA groups compared to the control groups. This finding agrees with previous reports showing that ADAMTS proteins are upregulated in OA-affected cartilage and act as key downstream players in the inflammatory signal cascade.29,30 Our finding that ADAMTS5 levels in serum and cartilage tissue were markedly increased in the OA groups at weeks 4, 6, and 8 supports previous findings showing an increased expression of ADAMTS5 in knee cartilage from patients with late-stage OA but not in those with early-stage OA.31,32
The increased ADAMTS5 levels in OA could be due to enhanced aggrecanase activity against existing aggrecan in the joint cartilage matrix or against newly synthesized and secreted aggrecan in acute inflammatory arthritis. 33 Other investigators found that a single injection of lentivirus-mediated ADAMTS5 siRNA prevented the degradation of articular cartilage in rats. They suggested that silencing ADAMTS5 expression in articular cartilage might be a promising approach to inhibit the progression of OA. 18 Most animal studies have shown that ADAMTS5 mRNA expression is upregulated by catabolic cytokines. 34 This mechanism may explain the significant positive correlations we found between the levels of serum ADAMTS5 and those of the conventional inflammatory markers, TNF-α, CRP, and ESR. TNF-α, for example, is known to be involved in cartilage degradation and is a potent inhibitor of cartilage matrix synthesis. 13
FSTL1, an extracellular glycoprotein, has been reported to be implicated in the pathogenesis of OA. 16 In the present study, both the serum and tissue levels of FSTL1 were noticeably increased in the OA groups but not in the healthy control or sham groups. FSTL1 levels exhibited a positive correlation with the severity of OA, as assessed by our histopathological and radiological results at different time points. These findings are in agreement with those of other investigators who showed that FSTL1 expression is induced in mice exacerbates collagen-induced model of arthritis and is correlated with disease activity,35,36 while its neutralization suppresses collagen-induced arthritis. 37 However, conflicting data have shown that FSTL1 could have a potential preventive effect on joint destruction by hindering the production of matrix metalloproteinases and cytokines both in vitro in synovial cells and in vivo mouse models. 16
In the context of OA, the increase in FSTL1 expression fits with the hypothesis that FSTL1 is a leading regulatory cytokine that stimulates the release of proinflammatory mediators by cells in arthritic joints. 35 Other reports have suggested that FSTL1 has robust proinflammatory characteristics that can exacerbate OA. 38
The effects of FSTL1 on cytokine expression were recorded in a different study, in which FSTL1 overexpression increased IL-6, IL-8, and MCP-1 synthesis in activated macrophages and stromal cells, suggesting that FSTL1 has a role in regulating the expression of these cytokines. 35 TGF-β has been reported to synergize with IL-6, leading to the overexpression of FSTL1, which in turn leads to the aggravation of synovial inflammation.16,35 The mechanism by which FSTL1 acts to accomplish this effect is currently unclear; however, the numerous proinflammatory effects of FSTL1 that have been observed suggest that it may act as an adjuvant to amplify the release of proinflammatory mediators. This hypothesis is supported by previous data showing that FSTL1 can promote T-cell responses and induce the expression of IFN-γ. 36 Transfection of FSTL1 into macrophages results in an upregulation of proinflammatory cytokines, whereas the neutralization of endogenous FSTL1 recovers inflammation, further indicating that FSTL1 may act as a proinflammatory molecule.36,37
Also, FSTL1 has been described as a new proinflammatory mediator that causes and aggravates OA by promoting the expression of IL-1b, TNF-α, and IL-6 and by enhancing the activity of the interferon signaling pathways. 16 This proposed role of FSTL1 is supported in our study by the significant positive correlations between FSTL1 levels and the evaluated inflammatory markers, including, TNF-α, CRP, and ESR, as well as the histological and radiological assessments in the OA groups, as previously documented in several studies.36,37,39
Conclusion
Our findings proposed that ADAMTS5 and FSTL1 can serve as important and informative serological markers of disease activity in OA. However, supplementary clinical researches are in demand to validate the use of these biomarkers for improving the diagnosis and prognosis of OA in humans. Ultimately, further understanding of their contribution to the disease (by directly breaking down cartilage or stimulating inflammation) could point to new approaches for treating OA.
Supplemental Material
Supplemental material, CART-18-0176-Suplimantary_materials for Validation of the Diagnostic and Prognostic Values of ADAMTS5 and FSTL1 in Osteoarthritis Rat Model by Bakheet E. M. Elsadek, Ahmed A. Abdelghany, Mohamed A. Abd EL-Aziz, Hafez R. Madkor, Ahmed Abd Elrady Ahmed, Sary Kh. Abd-Elghaffar and Amer Alkot Mostafa Elsadek in CARTILAGE
Footnotes
Supplementary material for this article is available on the Cartilage website at https://journals.sagepub.com/home/car
Acknowledgments and Funding: The authors are deeply thankful to the Nature Research Editing Service for the language and scientific editing. The authors are also grateful to the nursing staff in the Department of Orthopaedic and Trauma Surgery, Faculty of Medicine, Al-Azhar University, Assiut, Egypt, for their assistance in the animal model design. The author(s) received no financial support for the research, authorship, and/or publication of this article.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Ethical Approval: Animal experiments were approved by the Ethical Committee of the Faculty of Pharmacy, Al-Azhar University.
Animal Welfare: Animal experiments were accomplished in accordance with the National Institutes of Health Guidelines (NIH 1985) for the Care and Use of Laboratory animals.
ORCID iD: Bakheet E. M. Elsadek  https://orcid.org/0000-0001-5444-0245
https://orcid.org/0000-0001-5444-0245
References
- 1. DeGroot J, Bank RA, Tchetverikov I, Verzijl N, TeKoppele JM. Molecular markers for osteoarthritis: the road ahead. Curr Opin Rheumatol. 2002;14:585-9. [DOI] [PubMed] [Google Scholar]
- 2. Murray C, Marshall M, Rathod T, Bowen CJ, Menz HB, Roddy E. Population prevalence and distribution of ankle pain and symptomatic radiographic ankle osteoarthritis in community dwelling older adults: a systematic review and cross-sectional study. PLoS One. 2018;13:e0193662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Woolf AD, Pfleger B. Burden of major musculoskeletal conditions. Bull World Health Organ. 2003;81:646-56. [PMC free article] [PubMed] [Google Scholar]
- 4. Attur M, Krasnokutsky-Samuels S, Samuels J, Abramson SB. Prognostic biomarkers in osteoarthritis. Curr Opin Rheumatol. 2013;25:136-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Adler N, Schoeniger A, Fuhrmann H. Effects of transforming growth factor-β and interleukin-1β on inflammatory markers of osteoarthritis in cultured canine chondrocytes. Am J Vet Res. 2017;78:1264-72. [DOI] [PubMed] [Google Scholar]
- 6. Adler N, Schoeniger A, Fuhrmann H. Polyunsaturated fatty acids influence inflammatory markers in a cellular model for canine osteoarthritis. J Anim Physiol Anim Nutr (Berl). 2018;102:e623-e632. [DOI] [PubMed] [Google Scholar]
- 7. Price AK, de Godoy MRC, Harper TA, Knap KE, Joslyn S, Pietrzkowski Z, et al. Effects of dietary calcium fructoborate supplementation on joint comfort and flexibility and serum inflammatory markers in dogs with osteoarthritis. J Anim Sci. 2017;95:2907-16. [DOI] [PubMed] [Google Scholar]
- 8. Henrotin Y, Gharbi M, Mazzucchelli G, Dubuc JE, De Pauw E, Deberg M. Fibulin 3 peptides Fib3-1 and Fib3-2 are potential biomarkers of osteoarthritis. Arthritis Rheum. 2012;64:2260-7. [DOI] [PubMed] [Google Scholar]
- 9. Gu HY, Yang M, Guo J, Zhang C, Lin LL, Liu Y, et al. Identification of the biomarkers and pathological process of osteoarthritis: weighted gene co-expression network analysis. Front Physiol. 2019;10:275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hosnijeh FS, Bierma-Zeinstra SM, Bay-Jensen AC. Osteoarthritis year in review 2018: biomarkers (biochemical markers). Osteoarthritis Cartilage. 2019;27:412-23. [DOI] [PubMed] [Google Scholar]
- 11. Zhang R, Guo H, Yang X, Li Z, Zhang D, Li B, et al. Potential candidate biomarkers associated with osteoarthritis: evidence from a comprehensive network and pathway analysis. J Cell Physiol. Epub 2019 Feb 28. [DOI] [PubMed] [Google Scholar]
- 12. Henrotin Y, Sanchez C, Bay-Jensen AC, Mobasheri A. Osteoarthritis biomarkers derived from cartilage extracellular matrix: current status and future perspectives. Ann Phys Rehabil Med. 2016;59:145-8. [DOI] [PubMed] [Google Scholar]
- 13. Xu Y, Liu Q, Liu ZL, Lim L, Chen WH, Lin N. Treatment with SiMiaoFang, an anti-arthritis Chinese herbal formula, inhibits cartilage matrix degradation in osteoarthritis rat model. Rejuvenation Res. 2013;16:364-76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Abali O, Gokce EC, Cemil B, Erdogan B, Yonezawa T, Demircan K. Early induction of ADAMTS-1, -4, -5 and -9 in IL-stimulated mouse astrocytes. Turk Neurosurg. 2014;24:519-24. [DOI] [PubMed] [Google Scholar]
- 15. Demircan K, Topcu V, Takigawa T, Akyol S, Yonezawa T, Ozturk G, et al. ADAMTS4 and ADAMTS5 knockout mice are protected from versican but not aggrecan or brevican proteolysis during spinal cord injury. Biomed Res Int. 2014;2014:693746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Chaly Y, Fu Y, Marinov A, Hostager B, Yan W, Campfield B, et al. Follistatin-like protein 1 enhances NLRP3 inflammasome-mediated IL-1β secretion from monocytes and macrophages. Eur J Immunol. 2014;44:1467-79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ni S, Miao K, Zhou X, Xu N, Li C, Zhu R, et al. The involvement of follistatin-like protein 1 in osteoarthritis by elevating NF-κB-mediated inflammatory cytokines and enhancing fibroblast like synoviocyte proliferation. Arthritis Res Ther. 2015;17:91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Chu X, You H, Yuan X, Zhao W, Li W, Guo X. Protective effect of lentivirus-mediated siRNA targeting ADAMTS-5 on cartilage degradation in a rat model of osteoarthritis. Int J Mol Med. 2013;31:1222-8. [DOI] [PubMed] [Google Scholar]
- 19. Yates MP, Settle SL, Yocum SA, Aggarwal P, Vickery LE, Aguiar DJ, et al. IGFBP-5 metabolism is disrupted in the rat medial meniscal tear model of osteoarthritis. Cartilage. 2010;1:43-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Yu DG, Nie SB, Liu FX, Wu CL, Tian B, Wang WG, et al. Dynamic alterations in microarchitecture, mineralization and mechanical property of subchondral bone in rat medial meniscal tear model of osteoarthritis. Chin Med J (Engl). 2015;128:2879-86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kellgren JH, Lawrence JS. Radiological assessment of osteo-arthrosis. Ann Rheum Dis. 1957;16:494-502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Banchroft JD, Stevens A, Turner DR. Theory and practice of histological techniques. 4th ed. London: Churchill Livingstone; 1996. [Google Scholar]
- 23. Huang K, Wu LD. Aggrecanase and aggrecan degradation in osteoarthritis: a review. J Int Med Res. 2008;36:1149-60. [DOI] [PubMed] [Google Scholar]
- 24. Felson DT. Identifying different osteoarthritis phenotypes through epidemiology. Osteoarthritis Cartilage. 2010;18:601-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. van den Berg WB. Osteoarthritis year 2010 in review: pathomechanisms. Osteoarthritis Cartilage. 2011;19:338-41. [DOI] [PubMed] [Google Scholar]
- 26. Kashiwagi M, Tortorella M, Nagase H, Brew K. TIMP-3 is a potent inhibitor of aggrecanase 1 (ADAM-TS4) and aggrecanase 2 (ADAM-TS5). J Biol Chem. 2001;276:12501-4. [DOI] [PubMed] [Google Scholar]
- 27. Gendron C, Kashiwagi M, Lim NH, Enghild JJ, Thøgersen IB, Hughes C, et al. Proteolytic activities of human ADAMTS-5: comparative studies with ADAMTS-4. J Biol Chem. 2007;282:18294-306. [DOI] [PubMed] [Google Scholar]
- 28. Wylie JD, Ho JC, Singh S, McCulloch DR, Apte SS. Adamts5 (aggrecanase-2) is widely expressed in the mouse musculoskeletal system and is induced in specific regions of knee joint explants by inflammatory cytokines. J Orthop Res. 2012;30:226-33. [DOI] [PubMed] [Google Scholar]
- 29. Cawston TE, Wilson AJ. Understanding the role of tissue degrading enzymes and their inhibitors in development and disease. Best Pract Res Clin Rheumatol. 2006;20:983-1002. [DOI] [PubMed] [Google Scholar]
- 30. Song RH, Tortorella MD, Malfait AM, Alston JT, Yang Z, Arner EC, et al. Aggrecan degradation in human articular cartilage explants is mediated by both ADAMTS-4 and ADAMTS-5. Arthritis Rheum. 2007;56:575-85. [DOI] [PubMed] [Google Scholar]
- 31. Bau B, Gebhard PM, Haag J, Knorr T, Bartnik E, Aigner T. Relative messenger RNA expression profiling of collagenases and aggrecanases in human articular chondrocytes in vivo and in vitro. Arthritis Rheum. 2002;46:2648-57. [DOI] [PubMed] [Google Scholar]
- 32. Plaas A, Osborn B, Yoshihara Y, Bai Y, Bloom T, Nelson F, et al. Aggrecanolysis in human osteoarthritis: confocal localization and biochemical characterization of ADAMTS5-hyaluronan complexes in articular cartilages. Osteoarthritis Cartilage. 2007;15:719-34. [DOI] [PubMed] [Google Scholar]
- 33. Larsson S, Lohmander LS, Struglics A. Synovial fluid level of aggrecan ARGS fragments is a more sensitive marker of joint disease than glycosaminoglycan or aggrecan levels: a cross-sectional study. Arthritis Res Ther. 2009;11:R92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Fosang AJ, Rogerson FM. Identifying the human aggrecanase. Osteoarthritis Cartilage. 2010;18:1109-16. [DOI] [PubMed] [Google Scholar]
- 35. Chaly Y, Marinov AD, Oxburgh L, Bushnell DS, Hirsch R. FSTL1 promotes arthritis in mice by enhancing inflammatory cytokine/chemokine expression. Arthritis Rheum. 2012;64:1082-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Clutter SD, Wilson DC, Marinov AD, Hirsch R. Follistatin-like protein 1 promotes arthritis by up-regulating IFN-gamma. J Immunol. 2009;182:234-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Miyamae T, Marinov AD, Sowders D, Wilson DC, Devlin J, Boudreau R, et al. Follistatin-like protein-1 is a novel proinflammatory molecule. J Immunol. 2006;177:4758-62. [DOI] [PubMed] [Google Scholar]
- 38. Kawabata D, Tanaka M, Fujii T, Umehara H, Fujita Y, Yoshifuji H, et al. Ameliorative effects of follistatin-related protein/TSC-36/FSTL1 on joint inflammation in a mouse model of arthritis. Arthritis Rheum. 2004;50:660-8. [DOI] [PubMed] [Google Scholar]
- 39. Tanaka M, Ozaki S, Osakada F, Mori K, Okubo M, Nakao K. Cloning of follistatin-related protein as a novel autoantigen in systemic rheumatic diseases. Int Immunol. 1998;10:1305-14. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, CART-18-0176-Suplimantary_materials for Validation of the Diagnostic and Prognostic Values of ADAMTS5 and FSTL1 in Osteoarthritis Rat Model by Bakheet E. M. Elsadek, Ahmed A. Abdelghany, Mohamed A. Abd EL-Aziz, Hafez R. Madkor, Ahmed Abd Elrady Ahmed, Sary Kh. Abd-Elghaffar and Amer Alkot Mostafa Elsadek in CARTILAGE