Abstract
Objective
The aim of this study is to investigate the role of Sirtuin1 (Sirt1) in the regulation of autophagy for human osteoarthritis (OA) chondrocytes.
Design
All cartilage samples were collected from human donors, including young group, aged group, and OA group. Primary chondrocytes were isolated and cultured with Sirt1 activator or inhibitor. Sirt1 expression in cartilage tissue and chondrocytes was evaluated, and the deacetylation activity of Sirt1 was determined. The alteration of autophagy activity after upregulating or downregulating Sirt1 was detected. Chondrocytes were treated with autophagy activator and inhibitor, and then the protein level of Sirt1 was examined. The interactions between Sirt1 and autophagy-related proteins Atg7, microtubule associated protein 1 light chain 3 (LC3), and Beclin-1 were determined by using immunoprecipitation.
Results
The assay of articular cartilage revealed that the expression of Sirt1 might be age-related: highly expressed in of younger people, and respectively decreased in the elderly people and OA patients. In vitro study was also validated this result. Further study confirmed that higher levels of Sirt1 significantly increased autophagy in aged chondrocytes, while the lower expression of Sirt1 reduced autophagy in young chondrocytes. Of note, the high levels of Sirt1 reduced autophagy in OA chondrocytes. When the chondrocytes were treated with autophagy activator or inhibitor, we found the expression of Sirt1 was not affected. In addition, we found that Sirt1 could interact with Atg7.
Conclusion
These results suggest that Sirt1 in human chondrocytes regulates autophagy by interacting with autophagy related Atg7, and Sirt1 may become a more important target in OA treatment.
Keywords: osteoarthritis, chondrocytes, sirtuin1, autophagy, Atg7
Introduction
Osteoarthritis (OA) is a prevalent debilitating joint disorder and is characterized by progressive degeneration of the articular cartilage.1,2 Its prominent risk factor is aging.3,4 As the newest report cited in 2018, 5 OA affects 240 million people globally, about 10% of men and 18% of women over 60 years of age. In China, Tang et al., 6 using data from the China Health and Retirement Longitudinal Study, reported an 8% prevalence of symptomatic knee OA and it increased with age until a plateau around age 70. In addition, according to the US Health information Brought to Life estimates, more than half of Americans ages 65 and older may show X-ray evidence of OA in at least one joint, and an estimated 70 million Americans will be at risk of developing it by 2030. These statistics suggest OA remains a public health problem and lacks effective treatment strategies. 5 Thus, OA is worthy of wide attention and studies for new and effective intervention and therapy are required.
Multiple studies showed that Sirtuin1 (Sirt1), an NAD+-dependent type III histone deacetylase, was involved in the development and progression of OA.4,7,8 In a previous study, Sirt1 expression decreased with the development of OA and the reduction of Sirt1 in chondrocytes may have caused chondrocyte hypertrophy and cartilage matrix loss. 9 Moreover, Matsuzaki et al. 10 found that loss of Sirt1 in chondrocytes led to the accelerated development of OA in mice under mechanical stress and during ageing, suggesting that Sirt1 had a preventive role against the development of OA. From these studies, we can clearly draw a conclusion that the expression or activity of Sirt1 in human OA is decreased. Thus, Sirt1 play a crucial protective role in human chondrocytes and prevents progression of OA. In addition, recent findings demonstrated that Sirt1 protects chondrocytes through several signaling pathways, including NF-κB signaling pathways, PI3K/AKT signaling pathway, and Wnt/β-catenin signaling pathway.11-13 For instance, Liu et al. 11 demonstrated that Sirt1 may regulate apoptosis and extracellular matrix degradation in resveratrol-treated OA chondrocytes via the Wnt/β-catenin signaling pathway.
Recent findings also suggested that autophagy, a mechanism of organelle recycling that promotes cell survival, is modulated during OA development.14,15 Autophagy is a cellular self-digestion process in response to various stress situations, which involves the uptake of intracellular contents for the degradation in the lysosomal system.16,17 Autophagy activity is essential for maintaining cellular homeostasis and viability through degradation of aggregated proteins, damaged organelles, and invading pathogens.18,19 Chondrocytes within articular cartilage show decreased autophagy in OA, leading to rapid cell death and cartilage degeneration. 20 Bouderlique et al. 21 demonstrated that autophagy protects from age-related OA by facilitating chondrocyte survival. Furthermore, Caramés et al. 22 found that cartilage structural damage of OA progressed in an age-dependent manner, subsequent to autophagy changes. However, it is not entirely clear what is the underlying mechanism of autophagy regulation in OA.
Sirt1 is involved in the regulation of acetylation-deacetylation in autophagy. 23 Some autophagy related proteins, LC3, Atg5, and Atg7, are deacetylated by Sirt1. 24 A recent study showed that Sirt1-mediated autophagy protected in endplate chondrocytes against apoptosis. 25 Given the key role of autophagy and Sirt1 in human chondrocytes of OA, we hypothesized that Sirt1 may play a key role in OA development via regulating autophagy. In this study, we investigated the quantitative expression and distribution of Sirt1 in normal and OA cartilage and to evaluate the effect of autophagy in cultured chondrocytes. We sought to understand whether there might be a connection between Sirt1 activity and the induction of autophagy in chondrocytes. To assess the role of Sirt1 in autophagy, we transiently increased or decreased the expression of Sirt1 in the chondrocytes, and then we observed the changes of autophagy activity.
Materials and Methods
Human Cartilage Samples
All cartilage samples were collected from human donors who underwent surgery at the Department of Orthopaedics, the First Affiliated Hospital of Anhui Medical University, Hefei, China. According to the records of specimen database information, the specimens were divided into 3 different groups, young group, aged group, and OA group, and each group consisted of 6 cartilage specimens. The specimens of young and aging cartilage were obtained from patients undergoing lower limb amputation in severe trauma. The tissues of OA cartilage were obtained from patients with early-stage OA undergoing total knee arthroplasty. Ethics approval was granted from the Hospital Ethics Committee of Anhui Medical University, and informed consent was given by all patients involved. The characteristics of the patients in this study are shown in Table 1 .
Table 1.
Characteristics of the patients in this study.
| Characteristics | OA cartilage (n=6) | Aged cartilage (n=6) | Young cartilage (n=6) |
|---|---|---|---|
| Age (mean±SD) | 62.0 ± 4.7 years | 60.5 ± 3.8 years | 27.5 ± 5.9 years |
| Gender (female/male) | 4/2 | 2/4 | 1/5 |
| Diagnosis | Osteoarthritis | Destructive limb injury | Destructive limb injury |
| Treatment | Total knee arthroplasty | Amputation | Amputation |
| OA grade | ΙΙ-ΙΙΙ | Ι-ΙΙ | Ι |
| Mankin score | 5-7 | 0-2 | 0 |
OA, osteoarthritis; SD, standard deviation.
Immunohistochemical Analysis
Cartilage tissue collected from osteoarthritic patients were graded according to disease severity based on Mankin score. Immunohistochemistry (IHC) was performed according to the indirect immunoperoxidase method. Briefly, after deparaffinization, hydration, and blockage of endogenous peroxidase, the cartilage specimens were incubated for 20 minutes with 10% nonfat milk in phosphate-buffered saline (PBS) and then individually incubated at 4°C overnight with anti-SirT1 (#8469, 1:400, CST). After rinsing, slides were washed, and sections were incubated with biotinylated goat anti-mouse secondary antibody for 30 minutes at room temperature, and then incubated with the Vectastain ABC-AP kit (Vector Laboratories, Burlingame, CA) for 30 minutes. Finally, sections were washed and incubated with 3,3-diaminobenzidine tetrahydrochloride (DAB) substrate for 2 to 8 minutes. For each specimen, 6 microscopic fields were examined under ×40 magnification. Visual impressions of the intensity of stain in the nucleus and percentage of Sirt1-positive chondrocytes were recorded by analyzing the digital photomicrographs using Image J software.
Isolation and Culture of Primary Chondrocytes
Chondrocytes were isolated from cartilage as described previously. 26 In brief, the cartilage tissue was incubated with trypsin (0.5 mg/mL, Sigma-Aldrich, St. Louis, MO) at 37°C for 10 minutes. After the trypsin solution was removed, the tissue slices were treated for 12 to 16 hours with type II collagenase (2 mg/mL, Sigma-Aldrich) in Dulbecco’s modified Eagle’s medium (DMEM; Gibco, Life Technologies) with 5% fetal calf serum. The isolated chondrocytes were recovered in DMEM supplemented with 10% fetal calf serum, L-glutamine, and antibiotics and allowed to attach to the culture flasks. The cells were incubated at 37°C in a humidified gas mixture containing 5% CO2 balanced with air. The chondrocytes were used in the experiments at confluency (2-3 weeks in primary culture). The chondrocytes between passages 3 and 5 were used for the analysis. Thus, our experiments herein utilized chondrocytes up to passage 4 (P4).
Assays for In Vitro Deacetylase Activity
The deacetylation activity of Sirt1 was determined with a deacetylation active testing kit (Sigma Aldrich) according to the manufacturer’s protocols. Briefly, nuclear proteins were extracted from chondrocytes with a Cytosol Fractionation kit (Bio Vision) to measure cellular Sirt1 deacetylase activity. To generate the standard curve, we prepared a serial dilution to yield solutions that covered the dynamic range of the assay of 0 to 50 µM. We made a reaction master mix, added 100 µL of assay buffer and 1 µg of PNC1, removed the samples from the incubator, and read the fluorescence with a microplate fluorimeter (excitation 360 nm, emission 460 nm). Experimental values were presented as pmol of converted substrate/µg of protein/min. The negative controls (10 mM NAM) were subtracted from each treatment to give the final values. The fluorescence intensities of Sirt1 deacetylase activity were normalized to protein levels measured in the chondrocyte samples.
Monodansylcadaverine (MDC) Staining
The analysis of autophagy was used by MDC staining as described by Munafó. 27 The chondrocytes were seeded on cover-slips overnight and then treated with autophagy activator Rapamycin (Sigma-Aldrich, Shanghai, China) or inhibitor 3-methyladenine (3-MA) (Sigma-Aldrich, Shanghai, China). After activated or inhibited autophagy, cells were incubated with 0.05 mM MDC (Sigma-Aldrich, Shanghai, China) in PBS at 37°C for 10 minutes, then were washed with 3 rinses with PBS and fixed with a solution of 4% paraformaldehyde for 30 minutes. Cover-slips were examined using a fluorescence microscopy (Olympus, XSZ-D2). To quantify autophagic cells after treatment, we counted the number of autophagic cells demonstrating MDC staining among 200 cells.
Sirt1 Activation and Inhibition
To activate Sirt1, we used resveratrol, which has been reported to induce Sirt1 activity.28-30 Inhibitors of Sirt1 such as sirtinol have been previously reported.31,32 Briefly, human knee articular chondrocytes were cultured with 10 µM resveratrol or 80 nM sirtinol for 12 hours.
Western Blot Analysis
The chondrocytes were isolated from confluent monolayer cultures. Before immunoblotting, protein was quantified by the Bradford method with a BCA detection kit (ThermoPierce) and adjusted to equal concentrations across different samples. Equal amounts of protein was separated by SDS-PAGE on precise10% polyacrylamide gels. After electrophoresis, proteins were transferred to a PVDF (Millipore), membranes were blocked with 5% nonfat milk in Tris-buffered saline containing 0.1% Tween-20 (TBST) at room temperature for 1 hour, and then incubated overnight at 4°C with primary antibodies: rabbit polyclonal LC3 antibody (NB100-2220, 1:2000, Novus), mouse polyclonal Anti-Sirt1 (#8469, 1:2000, CST), rabbit polyclonal anti-ATG7 (AB10511, 1:1800, Millipore), and rabbit polyclonal Beclin-1 (ab55878, 1:2000, Abcam). After incubation, membranes were washed in TBST buffer for5 minutes and probed with goat anti-mouse IgG antibody (AP124P, 1:40,000, Millipore) and goat anti-rabbit IgG antibody (401393, 1:50,000, Millipore) for1 hour at room temperature. They were washed with TBST 3 times for 5 minutes. Protein bands of interest were detected using the ECL Detection Kit (No. 34094 Thermo Pierce). The expression of GAPDH (ab9485, 1:1000, Abcam) was used as a loading control.
Immunoprecipitation
The chondrocytes were about 90% confluent, then harvested, washed, and centrifuged. Before immunoprecipitation, we calculated protein concentrations by using a BCA detection kit. We incubated lysates with 20 µL of protein A/G sepharose beads (Santa Cruz). After brief centrifugation to remove precleared beads, 0.5 to 2 µg of antibody against Atg or Sirt1 was added to each sample and incubated at 4°C overnight on a rotator. The immune complex was precipitated by incubating with 40 µL of protein sepharose beads at 4°C for 2 hours. The complex was centrifuged at 3000g for 3 minutes, beads were washed 3 times with 20 µL of RIPA buffer, and boiled for 5 minutes. The Sirt1 and Atgs are loaded on a 10% SDS-PAGE gel. Western blot was then performed again using anti-Sirt1 and anti-Atgs antibodies as described above.
Transmission Electron Microscopy (TEM)
TEM analysis was performed as described previously. 33 The chondrocytes were harvested and fixed in 2.5% glutaraldehyde in phosphate buffer, postfixed in 2% osmium tetroxide, and embedded in Luveak-812 (Nacalai Tesque, Japan). Ultrathin sections were stained with uranyl acetate for 10 minutes, then with lead citrate for 10 minutes, and evaluated in a JEM-1230 electron microscope (JEOL, Japan).
Statistical Analyses
All data and results presented in present study were repeated in at least 3 independent experiments. The data were expressed as mean ± SD. Data significance was evaluated by one-way ANOVA. Values of P < 0.05 were considered statistically significant.
Results
Sirt1 Expression Was Reduced in Aged and OA Cartilage
To determine the role of Sirt1 in the development of OA, Sirt1 expression was detected in the upper zone of human knee cartilage samples from young, aged, and OA donors. We found that Sirt1 expressed in the nucleus of chondrocytes in both normal (young and aged) and OA cartilage. The IHC staining results showed that Sirt1 expression was significantly higher in the young cartilage group compared to that in the aged and OA cartilage groups ( Fig. 1A.a-c ). The percentage of chondrocytes positive for Sirt1 was significantly decreased in both aged group (34.53 ± 5.56%) and OA group (27.18 ± 4.32%), compared with the young group (88.51 ± 4.60%) ( Fig. 1A.d ). Similarly, we found the protein levels of Sirt1 from lysates of these triple cartilage tissue was significantly higher in the young group, and it was rarely detected in aged and OA groups ( Fig. 1B ). Collectively, these results showed the expression of Sirt1 reduced in aged and OA samples.
Figure 1.
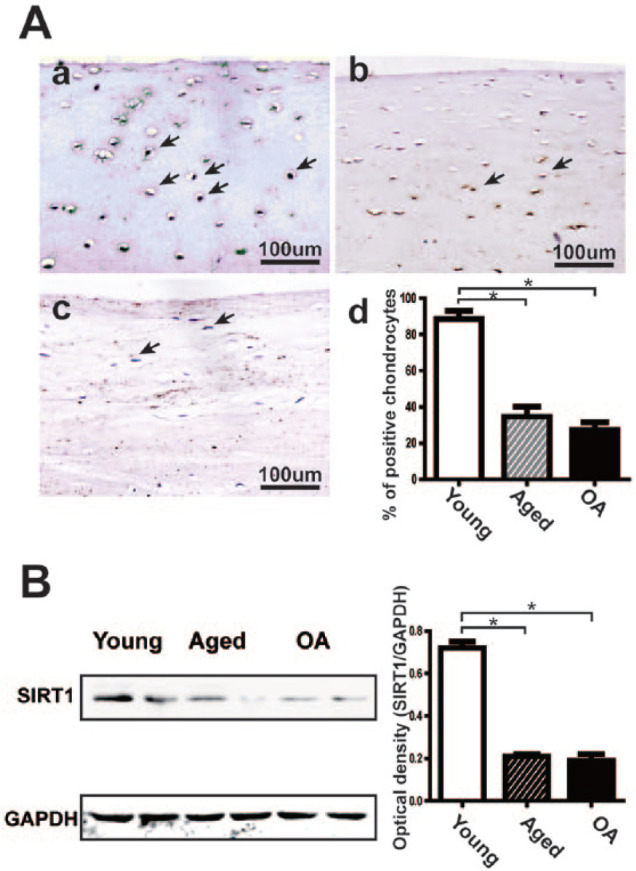
The expression of Sirt1in human articular cartilage tissue. (A) (a-c) The IHC staining of Sirt1 in the upper zone of articular cartilage from young, aged, and OA donors. Immunopositive chondrocytes are indicated by black arrows. The image representing 6 donors per group is shown. (d) Sirt1 quantitation expressed as percentage of Sirt1 immunopositive cells. The percentage of immunopositive chondrocytes was recorded by analyzing the digital photomicrographs on a computer loaded with ImageJ software. The results represent the means ± SD of OA and aged specimens. *P < 0.05 versus young. (B) Western blot analysis of Sirt1 expression in articular cartilage from young, aged, and OA donors. Immunoblotting results in Sirt1 and normalized against GAPDH. One of 3 experiments and each bar presents the means ± SD. *P < 0.05.
Resveratrol and Sirtinol Affected the Expression of Sirt1 in Chondrocytes
To investigate whether the activity of Sirt1 is affected by its activator or inhibitor, we pretreated cultured chondrocytes with resveratrol or sirtinol for 12 hours, and then analyzed the expression of Sirt1 by IHC. As shown in Figure 2A and B , the percentage of Sirt1 immunopositive cells in the young group was significantly higher compared to that in aged and OA groups, which is consistent with the result in cartilage tissue. Following the treatment of resveratrol, the percentage of Sirt1 immunopositive cells increased in all groups. In contrast, decreased percentage of Sirt1 was found following sirtinol stimulation. Furthermore, we examined the deacetylation activity of Sirt1 after treatments. The result showed sirtinol significantly inhibited the activity of Sirt1 in the young group, whereas resveratrol induced the activity of Sirt1 in OA and aged groups ( Fig. 2C ). Taken together, these results indicated that the expression of Sirt1 expression was affected by resveratrol or sirtinol in vitro.
Figure 2.
The expression of Sirt1 with different stimulators on chondrocytes. (A) Following the treatment of the chondrocytes with 10 µM resveratrol or 80 nM sirtinol, the expression of Sirt1 was detected in young, aged, and OA chondrocytes by IHC. Six donors were analyzed per group and representative images are shown. (B) To quantify autophagic cells after treatment, we counted the percentage of immunopositive chondrocytes. Values are the means ± SD. *P < 0.05 versus normal. (C) The changes of Sirt1deacetylation activity by Sirt1 activity assay kit and representative results after resveratrol or sirtinol treatment. Each bar represents the mean ± SD. *P < 0.05.
Sirt1 Was Involved in Autophagy Activity in Chondrocytes
To investigate the function of Sirt1 in chondrocytes autophagy, we detected the autophagy activity with the altered expression of Sirt1. TEM images of autophagy ultrastructure revealed a marked difference of autophagy activity among the 3 groups ( Fig. 3A ). The MDC results showed that the chondrocytes autophagy activity decreased in the young group when Sirt1 expression was inhibited by sirtinol, while it increased in the aged group. However, the chondrocytes autophagy activity decreased in the OA group when Sirt1 expression was upregulated by resveratrol ( Fig. 3B ). Beclin-1 is a key regulator of autophagy. Western blot results showed Beclin-1 was decreased after sirtinol treatment in the young group, and the Beclin-1/GAPDH expression was significantly increased in the aged group after resveratrol treatment, whereas it significantly decreased in the OA group after resveratrol treatment ( Fig. 3C ). In brief, these results indicated that Sirt1 can mediate autophagy activity in chondrocytes.
Figure 3.
Effect of Sirt1 regulator on autophagy in chondrocytes. (A) (a) TEM images demonstrating autophagic chondrocytes, a few autophagosomes (black arrows) with degraded organelles from the young chondrocyte group; (b) in the aged group, a few autophagic vacuoles (black arrows) but few autophagosomes were observed; and (c) autophagic chondrocyte death in the OA group; the cells including a large number of autophagic vacuoles (black arrows) contained cell fragments. (B) MDC staining was used to quantify autophagic chondrocytes. (C and D) We evaluated the effect of Sirt1 regulator on Beclin-1/glyceraldehyde 3-phosphate dehydrogenase (GAPDH) expression by western blot analysis. Densitometry revealed a significant decrease in Beclin-1/GAPDH expression from 0.92 ± 0.14 to 0.24 ± 0.11 in the young group with sirtinol and a significant increase by 3.63- to 4.27–fold in the aged group, a significant decrease in OA group with resveratrol. Values are the mean ± SD. *P < 0.05.
Autophagy Activity Did Not Affect the Expression of Sirt1 in Chondrocytes
To study the effect of autophagy activity on Sirt1 expression, we detected the protein level of Sirt1 in chondrocytes (young, aged, and OA) after treatment with rapamycin and 3-methyladenine (3-MA). Rapamycin is the most commonly used autophagy activator, while 3-MA is utilized to inhibit autophagy activity in most studies. The IHC staining results showed the percentage of Sirt1 immunopositive cells was not significantly altered in both rapamycin and 3-MA-treated chondrocytes, compared with untreated control ( Fig. 4A ). Further investigation of western blot showed no significant differences for Sirt1 expression after rapamycin or 3-MA treatment in young, aged, or OA groups, which was consist with the results of IHC ( Fig. 4B ). Together, these results demonstrated that the expression of Sirt1 was not affected by autophagy activator rapamycin and inhibitor 3-MA in vitro.
Figure 4.
Effect of autophagy regulator on Sirt1 expression in chondrocytes. (A and B) Following the treatment of the chondrocytes with 10 µM rapamycin or 3-MA, the expression of Sirt1 was detected in young, aged, and OA chondrocytes by IHC. The percentage of immunopositive chondrocytes was not significantly altered in the chondrocytes after treatment in the 3 groups. (C and D) We evaluated the effect of autophagy regulator on Sirt1/GAPDH expression by western blot analysis. The optical density of Sirt1/GAPDH was not significantly altered compared with no treatment (P < 0.05).
Sirt1-Mediated Chondrocytes Autophagy via Interacting with Atg7
As previously indicated that Sirt1 regulated autophagy in chondrocytes, we wanted to identify the underlying molecular mechanism. The interactions between Sirt1 and autophagy-related proteins Atg7, Beclin-1, and microtubule associated protein 1 light chain 3 (LC3) were determined by using immunoprecipitation. As shown in Figure 5A , Sirt1 was detected in Atg7 immunoprecipitates, indicating that these 2 proteins interact in chondrocytes. When we immunoprecipitated Sirt1, we could not detect the presence of LC3 and Beclin-1 ( Fig. 5B ). Collectively, these results suggest that Sirt1 forms a complex with autophagy protein Atg7 in chondrocytes.
Figure 5.

Sirt1 interacts with the autophagy-related proteins in chondrocytes. (A) Chondrocyte lysates were used for immunoprecipitation (IP) with an anti-Atg7 antibody, and the precipitates were immunoblotted with anti-Sirt1 antibodies. Rabbit IgG served as a negative control. Input represents Atg7 and Sirt1 expression in chondrocytes lysates. (B) Chondrocyte lysates were used for IP with an anti-Sirt1 antibody, and the precipitates were immunoblotted with anti-Atg7, anti-LC3, and anti-Beclin1 antibodies. Rabbit IgG served as a negative control. Input represents Atg7, LC3, Beclin1, and Sirt1 expression in chondrocyte lysates.
Discussion
In the present study, we found that the expression of Sirt1 in cartilage tissue samples of OA was significantly lower than the other groups, and this result was also validated in the cartilage cells in vitro. The cartilage cell activity continued to decline with increase of age, and this phenomenon was also reflected in chondrocyte expressions of Sirt1. Then, we investigated the effects of Sirt1 regulator on autophagy in chondrocytes in vitro. Moreover, our data indicate that Sirt1 forms a complex with autophagy protein Atg7 in human articular chondrocytes. These results suggest that Sirt1 regulates autophagy by interacting with autophagy-related Atg7 in human chondrocytes.
Sirt1, the mammalian homologue of Silent Information Regulator 2 (SIR2), is an important anti-aging molecule.4,7,8 As mentioned previously, OA are closely linked to ageing. In our study, Sirt1 was highly expressed in young human articular cartilage and chondrocyte, while decreased in aged and OA groups, suggesting that Sirt1 plays a protective role in the pathogenesis of OA. Sirt1 was the first member of Sirtuin family being discovered and is still the most studied for its involvement in the regulation of many biological processes, including cell differentiation, metabolism, senescence, apoptosis, inflammation, and stress resistance.34-37 For instance, Takayama et al. 38 demonstrated that inhibition of Sirt1 enhanced NO-induced apoptosis of human chondrocytes, which was mediated by activation of caspases 3 and 9. Moreover, Na et al. 39 showed that fisetin inhibited the IL-1β-induced inflammatory response and extracellular matrix degradation through activating Sirt1 in human OA chondrocytes. We also investigated the effect of activator resveratrol and inhibitor sirtinol on the expression of Sirt1, and the positive results indicated that resveratrol may have therapeutic potential for the treatment of OA by targeting Sirt1.
Furthermore, we speculated that Sirt1 activity was involved in autophagy activity in chondrocytes. Transiently augmenting Sirt1 activity is sufficient to activate autophagy in aged group chondrocytes; otherwise, the upregulation of Sirt1 dramatically decreases the expression of the Beclin-1 in OA group chondrocytes, and the inhibition of Sirt1 expression decreases the level of autophagy in young group chondrocytes. Thus, we could confirm that Sirt1 was involved in regulating the cartilage cell autophagy. Our observations are consistent with previous findings that Sirt1 could regulate autophagy. Ben Salem et al. 40 suggested that Sirt1 could protect cardiac cells against apoptosis induced by zearalenone or its metabolites alpha- and beta-zearalenol through an autophagy-dependent pathway. Shen et al. 41 showed that Sirt1 inhibition blunted autophagy induction in hepatocytes. In addition, Wang et al. 42 reported that Sirt1 could regulate vascular adventitial fibroblasts autophagy through the Akt/mTOR signaling pathway. Besides OA, a series of studies have indicated that autophagy played a protective role in various age-related diseases, such as diabetes, cancer, and neurodegeneration.43-45 Previous studies have indicated that the Sirt1 activator resveratrol can induce autophagy in a Sirt1-dependent manner.46,47 In our study, we did not consider the direct effect of resveratrol on autophagy. Further studies such as Sirt1 overexpression or knockdown on gene level are needed to discover the role of Sirt1 on autophagy. We further explored the effect of autophagy activity on Sirt1 expression. Results showed that the expression of Sirt1 was not affected by autophagy activator rapamycin and inhibitor 3-MA.
It is worth noting the opposite variations of beclin-1 expression in the aged and OA samples after resveratrol or sirtinol treatment. Beclin-1 expression was strongest in OA chondrocytes, while it was weakest in aged chondrocytes before treatment. TEM images of autophagy ultrastructure and MDC results also revealed a marked difference of autophagy activity among the 3 groups. When Sirt1 expression was regulated by resveratrol or sirtinol, the autophagy activity changed in all the 3 groups. It is now widely accepted that autophagy plays a complex role in human diseases where it can have both protective and injurious effects.48,49 The basal level of autophagy occurs to maintain cellular homeostasis and survival. 50 Blockade or uncontrolled activation of autophagy is associated with pathogenesis of aged and OA cartilage.21,51 We speculated that the elevation of Sirt1 expression could regulate autophagy homeostasis, and then serves as an adaptive and defensive mechanism for aged and OA chondrocytes.
We also observed that Sirt1 was involved in the regulation of cartilage cells autophagy via interacting with Atg7. Immunoprecipitation was used to determine the interactions between Sirt1 and autophagy-related proteins Atg7, LC3, and Beclin-1. It is known that autophagic process is mediated by a set of evolutionarily conserved Atg proteins. 52 Among these proteins, Beclin-1 (mammalian homologue of yeast Atg6) and LC3 (mammalian homologue of yeast Atg8) are commonly used as autophagy markers, which are involved in the biosynthesis of the autophagosome.53,54 Our results showed that Sirt1 did not interact with LC3 and Beclin-1 but formed a complex with autophagy protein Atg7 in chondrocytes. Nucleus-localized Sirt1 is known to induce the expression of autophagy pathway components through the activation of forkhead box O (FoxO) transcription factor family members.55,56 Ng et al. 55 reported that the perception of a linear Sirt1-FoxO axis in autophagy induction is complicated by recent findings that acetylated FoxO1 could bind to Atg7 in the cytoplasm and affect autophagy directly. It is also reported that phosphorylated FoxO1 located in the cytoplasm of immature NK cells (iNKs) interacts with Atg7, leading to induction of autophagy, and FoxO1-mediated autophagy is required for NK cell development and NK cell-induced innate immunity. 57 These studies are consistent with our observations.
Sirt1 regulates cellular metabolism in articular cartilage through the modulation of various substrates or signal pathways. Caramés et al. 58 demonstrated that aged and OA cartilage were associated with reduced expression of autophagy-related proteins including unc51-like autophagy activating kinase 1 (ULK1), Beclin-1, and LC3, and speculated that autophagy could play a protective role against chondrocytes death. Kang et al. 59 found that Sirt1 deacetylates PERK and attenuates the PERK-eIF-2α-CHOP axis of the unfolded protein response pathway and thereby promotes growth-plate chondrogenesis and longitudinal bone growth. Also, He et al. 60 showed that upregulation of Sirt1 expression may inhibit OA chondrocyte apoptosis and extracellular matrix degradation by increasing Bcl-2 expression and decreasing Bax, MMP1, and MMP13 expression, via downregulation of p38, JNK, and ERK phosphorylation.
We used agonists or inhibitors to change the basis of autophagy level, and we found that as autophagy regulator acts on chondrocytes, the Sirt1 protein expression showed no significant changes. Autophagy affects chondrocytes’ negative regulation of Sirt1.
Conclusions
The present study shows that Sirt1 is highly expressed in young human articular cartilage and reduced in aged and OA cartilage. Sirt1 regulates autophagy by interacting with autophagy-related Atg7 in human chondrocytes. Through increasing the activity of Sirt1 the autophagic cell death of OA chondrocytes could be inhibited. Therefore, we proposed Sirt1 may become a more important target in OA treatment.
Footnotes
Author Contributions: FXL, JC, and ZSY conceived and designed the study. FXL, FH, and WGM performed the experiments, analyzed the data, and drafted the manuscript. KPQ, PFX, and YFW read and analyzed documents. HW, JC, and ZSY revised the manuscript. All authors reviewed and approved the manuscript.
Acknowledgments and Funding: The authors wish to thank all participants in this study. This project was supported by the University Natural Science Foundation of Anhui (KJ2018A0665) and the Natural Science Foundation of China (No. 81601974).
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Ethics Approval: All cartilage samples were collected from human donors who undergo surgery at the Department of Orthopaedics, the First Affiliated Hospital of Anhui Medical University, Hefei, China. Ethics approval was granted by the Hospital Ethics Committee of Anhui Medical University (20170192).
Informed Consent: Informed consent was given by all patients involved.
ORCID iD: Zong-sheng Yin  https://orcid.org/0000-0002-3568-7071
https://orcid.org/0000-0002-3568-7071
References
- 1. Ripmeester EGJ, Timur UT, Caron MMJ, Welting TJM. Recent insights into the contribution of the changing hypertrophic chondrocyte phenotype in the development and progression of osteoarthritis. Front Bioeng Biotechnol. 2018;6:18. doi: 10.3389/fbioe.2018.00018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Dubey NK, Mishra VK, Dubey R, Syed-Abdul S, Wang JR, Wang PD, et al. Combating osteoarthritis through stem cell therapies by rejuvenating cartilage: a review. Stem Cells Int. 2018;2018:5421019. doi: 10.1155/2018/5421019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. van der Kraan P, Matta C, Mobasheri A. Age-related alterations in signaling pathways in articular chondrocytes: implications for the pathogenesis and progression of osteoarthritis—a mini-review. Gerontology. 2017;63(1):29-35. doi: 10.1159/000448711 [DOI] [PubMed] [Google Scholar]
- 4. Loeser RF, Collins JA, Diekman BO. Ageing and the pathogenesis of osteoarthritis. Nat Rev Rheumatol. 2016;12(7):412-20. doi: 10.1038/nrrheum.2016.65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Nelson AE. Osteoarthritis year in review 2017: clinical. Osteoarthritis Cartilage. 2018;26(3):319-25. doi: 10.1016/j.joca.2017.11.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Tang X, Wang S, Zhan S, Niu J, Tao K, Zhang Y, et al. The prevalence of symptomatic knee osteoarthritis in China: results from the China Health and Retirement Longitudinal Study. Arthritis Rheumatol. 2016;68(3):648-53. doi: 10.1002/art.39465 [DOI] [PubMed] [Google Scholar]
- 7. Liu-Bryan R. Inflammation and intracellular metabolism: new targets in OA. Osteoarthritis Cartilage. 2015;23(11):1835-42. doi: 10.1016/j.joca.2014.12.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Catana CS, Atanasov AG, Berindan-Neagoe I. Natural products with anti-aging potential: affected targets and molecular mechanisms. Biotechnol Adv. 2018;36(6):1649-56. doi: 10.1016/j.biotechadv.2018.03.012 [DOI] [PubMed] [Google Scholar]
- 9. Fujita N, Matsushita T, Ishida K, Kubo S, Matsumoto T, Takayama K, et al. Potential involvement of SIRT1 in the pathogenesis of osteoarthritis through the modulation of chondrocyte gene expressions. J Orthop Res. 2011;29(4):511-5. doi: 10.1002/jor.21284 [DOI] [PubMed] [Google Scholar]
- 10. Matsuzaki T, Matsushita T, Takayama K, Matsumoto T, Nishida K, Kuroda R, et al. Disruption of Sirt1 in chondrocytes causes accelerated progression of osteoarthritis under mechanical stress and during ageing in mice. Ann Rheum Dis. 2014;73(7):1397-404. doi: 10.1136/annrheumdis-2012-202620 [DOI] [PubMed] [Google Scholar]
- 11. Liu S, Yang H, Hu B, Zhang M. Sirt1 regulates apoptosis and extracellular matrix degradation in resveratrol-treated osteoarthritis chondrocytes via the Wnt/β-catenin signaling pathways. Exp Ther Med. 2017;14(5):5057-62. doi: 10.3892/etm.2017.5165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Yu F, Zeng H, Lei M, Xiao DM, Li W, Yuan H, et al. Effects of SIRT1 gene knock-out via activation of SREBP2 protein-mediated PI3K/AKT signaling on osteoarthritis in mice. J Huazhong Univ Sci Technolog Med Sci. 2016;36(5):683-90. doi: 10.1007/s11596-016-1645-0 [DOI] [PubMed] [Google Scholar]
- 13. Lei M, Wang JG, Xiao DM, Fan M, Wang DP, Xiong JY, et al. Resveratrol inhibits interleukin 1β-mediated inducible nitric oxide synthase expression in articular chondrocytes by activating SIRT1 and thereby suppressing nuclear factor-κB activity. Eur J Pharmacol. 2012;674(2-3):73-9. doi: 10.1016/j.ejphar.2011.10.015 [DOI] [PubMed] [Google Scholar]
- 14. Duarte JH. Osteoarthritis: autophagy prevents age-related OA. Nat Rev Rheumatol. 2015;11(12):683. doi: 10.1038/nrrheum.2015.145 [DOI] [PubMed] [Google Scholar]
- 15. Musumeci G, Castrogiovanni P, Trovato FM, Weinberg AM, Al-Wasiyah MK, Alqahtani MH, et al. Biomarkers of chondrocyte apoptosis and autophagy in osteoarthritis. Int J Mol Sci. 2015;16(9):20560-75. doi: 10.3390/ijms160920560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Baek SH, Kim KI. Epigenetic control of autophagy: nuclear events gain more attention. Mol Cell. 2017;65(5):781-5. doi: 10.1016/j.molcel.2016.12.027 [DOI] [PubMed] [Google Scholar]
- 17. Morel E, Mehrpour M, Botti J, Dupont N, Hamai A, Nascimbeni AC, et al. Autophagy: a druggable process. Annu Rev Pharmacol Toxicol. 2017;57:375-98. doi: 10.1146/annurev-pharmtox-010716-104936 [DOI] [PubMed] [Google Scholar]
- 18. Mizushima N. A brief history of autophagy from cell biology to physiology and disease. Nat Cell Biol. 2018;20(5):521-7. doi: 10.1038/s41556-018-0092-5 [DOI] [PubMed] [Google Scholar]
- 19. Mizushima N, Komatsu M. Autophagy: renovation of cells and tissues. Cell. 2011;147(4):728-41. doi: 10.1016/j.cell.2011.10.026 [DOI] [PubMed] [Google Scholar]
- 20. Rockel JS, Kapoor M. Autophagy: controlling cell fate in rheumatic diseases. Nat Rev Rheumatol. 2016;12(9):517-31. doi: 10.1038/nrrheum.2016.92 [DOI] [PubMed] [Google Scholar]
- 21. Bouderlique T, Vuppalapati KK, Newton PT, Li L, Barenius B, Chagin AS. Targeted deletion of Atg5 in chondrocytes promotes age-related osteoarthritis. Ann Rheum Dis. 2016;75(3):627-31. doi: 10.1136/annrheumdis-2015-207742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Carames B, Olmer M, Kiosses WB, Lotz MK. The relationship of autophagy defects to cartilage damage during joint aging in a mouse model. Arthritis Rheumatol. 2015;67(6):1568-76. doi: 10.1002/art.39073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Lee IH, Cao L, Mostoslavsky R, Lombard DB, Liu J, Bruns NE, et al. A role for the NAD-dependent deacetylase Sirt1 in the regulation of autophagy. Proc Natl Acad Sci U S A. 2008;105(9):3374-9. doi: 10.1073/pnas.0712145105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Hu X, Lu Z, Yu S, Reilly J, Liu F, Jia D, et al. CERKL regulates autophagy via the NAD-dependent deacetylase SIRT1. Autophagy. 2019;15:453-65. doi: 10.1080/15548627.2018.1520548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Zhang Z, Lin J, Tian N, Wu Y, Zhou Y, Wang C, et al. Melatonin protects vertebral endplate chondrocytes against apoptosis and calcification via the Sirt1-autophagy pathway. J Cell Mol Med. 2019;23:177-93. doi: 10.1111/jcmm.13903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Chang J, Wang W, Zhang H, Hu Y, Wang ML, Yin ZS. The dual role of autophagy in chondrocyte responses in the pathogenesis of articular cartilage degeneration in osteoarthritis. Int J Mol Med. 2013;32(6):1311-8. [DOI] [PubMed] [Google Scholar]
- 27. Munafo DB, Colombo MI. A novel assay to study autophagy: regulation of autophagosome vacuole size by amino acid deprivation. J Cell Sci. 2001;114(Pt 20):3619-29. [DOI] [PubMed] [Google Scholar]
- 28. Kuno A, Tanno M, Horio Y. The effects of resveratrol and SIRT1 activation on dystrophic cardiomyopathy. Ann N Y Acad Sci. 2015;1348(1):46-54. [DOI] [PubMed] [Google Scholar]
- 29. Ling L, Gu SH, Cheng Y. Resveratrol inhibits adventitial fibroblast proliferation and induces cell apoptosis through the SIRT1 pathway. Mol Med Rep. 2017;15(2_suppl):567-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Thompson AM, Martin KA, Rzucidlo EM. Resveratrol induces vascular smooth muscle cell differentiation through stimulation of SirT1 and AMPK. PLoS One. 2014;9(1):e85495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Ota H, Tokunaga E, Chang K, Hikasa M, Iijima K, Eto M, et al. Sirt1 inhibitor, sirtinol, induces senescence-like growth arrest with attenuated Ras-MAPK signaling in human cancer cells. Oncogene. 2006;25(2_suppl):176-85. [DOI] [PubMed] [Google Scholar]
- 32. Zhou XM, Zhang X, Zhang XS, Zhuang Z, Li W, Sun Q, et al. SIRT1 inhibition by sirtinol aggravates brain edema after experimental subarachnoid hemorrhage. J Neurosci Res. 2014;92(6):714-22. [DOI] [PubMed] [Google Scholar]
- 33. Chang J, Wang W, Zhang H, Hu Y, Yin Z. Bisphosphonates regulate cell proliferation, apoptosis and pro-osteoclastic expression in MG-63 human osteosarcoma cells. Oncology Lett. 2012;4(2_suppl):299-304. doi: 10.3892/ol.2012.723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Covington JD, Bajpeyi S. The sirtuins: markers of metabolic health. Mol Nutr Food Res. 2016;60(1):79-91. doi: 10.1002/mnfr.201500340 [DOI] [PubMed] [Google Scholar]
- 35. Chen B, Zang W, Wang J, Huang Y, He Y, Yan L, et al. The chemical biology of sirtuins. Chem Soc Rev. 2015;44(15):5246-64. doi: 10.1039/c4cs00373j [DOI] [PubMed] [Google Scholar]
- 36. Pillai VB, Sundaresan NR, Gupta MP. Regulation of Akt signaling by sirtuins: its implication in cardiac hypertrophy and aging. Circ Res. 2014;114(2_suppl):368-78. doi: 10.1161/CIRCRESAHA.113.300536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Carafa V, Rotili D, Forgione M, Cuomo F, Serretiello E, Hailu GS, et al. Sirtuin functions and modulation: from chemistry to the clinic. Clin Epigenetics. 2016;8:61. doi: 10.1186/s13148-016-0224-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Takayama K, Ishida K, Matsushita T, Fujita N, Hayashi S, Sasaki K, et al. SIRT1 regulation of apoptosis of human chondrocytes. Arthritis Rheum. 2009;60:2731-40. doi: 10.1002/art.24864 [DOI] [PubMed] [Google Scholar]
- 39. Na JY, Song K, Kim S, Kwon J. Rutin protects rat articular chondrocytes against oxidative stress induced by hydrogen peroxide through SIRT1 activation. Biochem Biophys Res Commun. 2016;473(4):1301-8. doi: 10.1016/j.bbrc.2016.04.064 [DOI] [PubMed] [Google Scholar]
- 40. Salem IB, Boussabbeh M, Da Silva JP, Guilbert A, Bacha H, Abid-Essefi S, et al. SIRT1 protects cardiac cells against apoptosis induced by zearalenone or its metabolites α- and β-zearalenol through an autophagy-dependent pathway. Toxicol Appl Pharmacol. 2017;314:82-90. doi: 10.1016/j.taap.2016.11.012 [DOI] [PubMed] [Google Scholar]
- 41. Shen C, Dou X, Ma Y, Ma W, Li S, Song Z. Nicotinamide protects hepatocytes against palmitate-induced lipotoxicity via SIRT1-dependent autophagy induction. Nutr Res. 2017;40:40-7. doi: 10.1016/j.nutres.2017.03.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Wang WR, Li TT, Jing T, Li YX, Yang XF, He YH, et al. SIRT1 Regulates the inflammatory response of vascular adventitial fibroblasts through autophagy and related signaling pathway. Cell Physiol Biochem. 2017;41(2_suppl):569-82. doi: 10.1159/000457878 [DOI] [PubMed] [Google Scholar]
- 43. Metaxakis A, Ploumi C, Tavernarakis N. Autophagy in age-associated neurodegeneration. Cells. 2018;7(5):E37. doi: 10.3390/cells7050037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Levy JMM, Towers CG, Thorburn A. Targeting autophagy in cancer. Nat Rev Cancer. 2017;17(9):528-42. doi: 10.1038/nrc.2017.53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Lee MS. Role of islet beta cell autophagy in the pathogenesis of diabetes. Trends Endocrinol Metab. 2014;25(12):620-7. doi: 10.1016/j.tem.2014.08.005 [DOI] [PubMed] [Google Scholar]
- 46. Li Z, Han X. Resveratrol alleviates early brain injury following subarachnoid hemorrhage: possible involvement of the AMPK/SIRT1/autophagy signaling pathway. Biol Chem. 2018;399(11):1339-50. doi: 10.1515/hsz-2018-0269 [DOI] [PubMed] [Google Scholar]
- 47. Morselli E, Maiuri MC, Markaki M, Megalou E, Pasparaki A, Palikaras K, et al. Caloric restriction and resveratrol promote longevity through the Sirtuin-1-dependent induction of autophagy. Cell Death Dis. 2010;1:e10. doi: 10.1038/cddis.2009.8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Patel AS, Morse D, Choi AM. Regulation and functional significance of autophagy in respiratory cell biology and disease. Am J Respir Cell Mol Biol. 2013;48(1):1-9. doi: 10.1165/rcmb.2012-0282TR [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Ryter SW, Choi AM. Autophagy in lung disease pathogenesis and therapeutics. Redox Biol. 2015;4:215-25. doi: 10.1016/j.redox.2014.12.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Kim KA, Shin D, Kim JH, Shin YJ, Rajanikant GK, Majid A, et al. Role of autophagy in endothelial damage and blood-brain barrier disruption in ischemic stroke. Stroke. 2018;49(6):1571-9. doi: 10.1161/STROKEAHA.117.017287 [DOI] [PubMed] [Google Scholar]
- 51. Filfan M, Sandu RE, Zavaleanu AD, GresiTa A, Glavan DG, Olaru DG, et al. Autophagy in aging and disease. Rom J Morphol Embryol. 2017;58(1):27-31. [PubMed] [Google Scholar]
- 52. Zhao C, Mei Y, Chen X, Jiang L, Jiang Y, Song X, et al. Autophagy plays a pro-survival role against methamphetamine-induced apoptosis in H9C2 cells. Toxicol Lett. 2018;294:156-65. doi: 10.1016/j.toxlet.2018.05.017 [DOI] [PubMed] [Google Scholar]
- 53. Qian HR, Yang Y. Functional role of autophagy in gastric cancer. Oncotarget. 2016;7(14):17641-51. doi: 10.18632/oncotarget.7508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Klionsky DJ, Baehrecke EH, Brumell JH, Chu CT, Codogno P, Cuervo AM, et al. A comprehensive glossary of autophagy-related molecules and processes (2nd edition). Autophagy. 2011;7(11):1273-94. doi: 10.4161/auto.7.11.17661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Ng F, Tang BL. Sirtuins’ modulation of autophagy. J Cell Physiol. 2013;228(12):2262-70. doi: 10.1002/jcp.24399 [DOI] [PubMed] [Google Scholar]
- 56. Giannakou ME, Partridge L. The interaction between FOXO and SIRT1: tipping the balance towards survival. Trends Cell Biol. 2004;14(8):408-12. doi: 10.1016/j.tcb.2004.07.006 [DOI] [PubMed] [Google Scholar]
- 57. Wang S, Xia P, Huang G, Zhu P, Liu J, Ye B, et al. FoxO1-mediated autophagy is required for NK cell development and innate immunity. Nat Commun. 2016;7:11023. doi: 10.1038/ncomms11023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Carames B, Taniguchi N, Otsuki S, Blanco FJ, Lotz M. Autophagy is a protective mechanism in normal cartilage, and its aging-related loss is linked with cell death and osteoarthritis. Arthritis Rheum. 2010;62(3):791-801. doi: 10.1002/art.27305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Hariharan N, Maejima Y, Nakae J, Paik J, Depinho RA, Sadoshima J. Deacetylation of FoxO by Sirt1 plays an essential role in mediating starvation-induced autophagy in cardiac myocytes. Circ Res. 2010;107(12):1470-82. doi: 10.1161/CIRCRESAHA.110.227371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Powell MJ, Casimiro MC, Cordon-Cardo C, He X, Yeow WS, Wang C, et al. Disruption of a Sirt1-dependent autophagy checkpoint in the prostate results in prostatic intraepithelial neoplasia lesion formation. Cancer Res. 2011;71(3):964-75. doi: 10.1158/0008-5472.CAN-10-3172 [DOI] [PMC free article] [PubMed] [Google Scholar]





