Abstract
Background
Long noncoding RNA (lncRNA) OIP5 antisense RNA 1 (OIP5-AS1) is an oncogenic lncRNA; however, its role in osteoarthritis (OA) pathology still remains unknown.
Materials and Methods
qRT-PCR was performed to measure the expressions of OIP5-AS1, miR-29b-3p and progranulin (PGRN) mRNA in OA cartilage tissues and normal cartilage tissues. Chondrocyte cell lines, CHON-001 and ATDC5, were treated with different doses of interleukin-1β (IL-1β) to induce the inflammatory response. Overexpression plasmids, microRNA mimics, microRNA inhibitors and small interfering RNAs were constructed and transfected into CHON-001 and ATDC5 cells. CCK-8 assay was used for determining the cell viability and Transwell assay was used for monitoring cell migration. Western blot was applied to measure the expressions of apoptosis-related proteins. Enzyme-linked immunosorbent assay (ELISA) was adopted to measure the contents of inflammatory factors. StarBase and TargetScan were used to predict the binding sites between OIP5-AS1 and miR-29b-3p, miR-29b-3p and 3′-UTR of PGRN respectively, which were verified by dual luciferase reporter assay.
Results
OIP5-AS1 and PGRN mRNA were downregulated while miR-29b-3p was upregulated in OA tissues and models. The up-regulated OIP5-AS1 facilitated the proliferation and migration of CHON-001 and ATDC5 cells, while ameliorated the apoptosis and inflammatory response. However, miR-29b-3p had opposite effects. PGRN was identified as a target gene of miR-29b-3p, which could be indirectly suppressed by OIP5-AS1 knockdown.
Conclusion
Downregulation of OIP5-AS1 induced by IL-1β could inhibit the proliferation and migration abilities of CHON-001 and ATDC5 cells and facilitate the apoptosis and inflammation response via regulating miR-29b-3p/PGRN axis.
Keywords: OIP5-AS1, miR-29b-3p, PGRN, OA, proliferation, migration, apoptosis, inflammation
Introduction
Osteoarthritis (OA) is a common chronic disease of joints, characterized by progressive articular cartilage destruction, subchondral bone remodeling, osteophyte formation, and inflammatory changes of synovium. The major clinical manifestations include deformity, pain, and dysfunction in joints. Since OA pathogenesis still remains unclear and there is a lack of effective treatment strategies to slow down, prevent, or even reverse the course of such disease, OA has become one of the major causes of pains and disabilities among the middle-aged and the elderly. 1 Previous studies have confirmed that degeneration and apoptosis of chondrocytes in OA patients are associated with joint destruction, which play a critical role in OA development. 2 Therefore, it is of great importance to investigate the molecular mechanisms of chondrocyte viability in OA.
Long noncoding RNAs (lncRNAs) are a type of newly found noncoding RNAs consisting of more than 200 nucleotides. LncRNAs are reported to play an important role in the development of various diseases, including OA, and their abnormal expressions may lead to changes of cellular biological behaviors.3,4 At present, accumulative evidence suggest that lncRNAs play a crucial role in the progression of OA by influencing cell proliferation, apoptosis, and inflammation. 5 OIP5 antisense RNA 1 (OIP5-AS1), as a member of lncRNAs, regulates the development of multiple diseases, including tumors.6-8 However, whether OIP5-AS1 participates in the development of OA and the related mechanisms still remain unclear.
MicroRNAs (miRNAs), a class of non-coding RNAs consisting of 18-24 nucleotides, can bind to 3′-untranslated region (3′-UTR) of the target gene by complementary base pairing to regulate their expressions. 9 Numerous studies have shown that miRNAs are involved in cell differentiation, apoptosis, proliferation, lipid metabolism, inflammation, immune responses, oncogenesis, and many other important cellular activities.10-16 More and more miRNAs are found to be expressed abnormally in OA and closely correlated with the development of the disease.17,18 Studies have indicated that miR-29b-3p, can facilitate the development of OA by promoting apoptosis and suppressing proliferation of chondrocytes. 19 However, the potential mechanisms of miR-29b-3p in the progression of OA require further studies.
Progranulin (PGRN), an autocrine growth factor, is widely expressed in various tissues and plays a pivotal role in inflammatory responses, early embryogenesis, tissue repair, oncogenesis, neurodegenerative disorders and many other pathophysiological activities.20-24 PGRN may inhibit the progression of OA via tumor necrosis factor–α (TNF-α) and β-catenin signaling pathways, while miR-29b-3p might be its upstream molecule.19,25 Recent studies suggest that lncRNA can regulate miRNAs expressions as a competing endogenous RNA (ceRNA), thereby regulating the expressions of the downstream proteins. However, whether OIP5-AS1 plays a role in OA as a ceRNA remains unknown.
This study was designed to discuss the function of OIP5-AS1 and its mechanism in OA. We confirmed the function of OIP5-AS1 in promoting chondrocytes proliferation and migration, inhibiting apoptosis and inflammation, and the specific mechanism of regulating miR-29b-3p/PGRN axis. This study provides a new insight into the therapeutic strategies of OA by exploring the functions of OIP5-AS1 in OA.
Materials and Methods
Specimen Collection
OA cartilages were collected from 35 OA patients undergoing total knee replacement (TKR) in Honghui Hospital from 2015 to 2017. Normal joint cartilages were also acquired from traumatic patients without OA or rheumatoid arthritis. Cartilage tissues were kept in liquid nitrogen at −196°C immediately after surgical resection. Informed consent forms were signed by all the patients. This study was approved by the Ethics Committee of Honghui Hospital and the Ethics Committee of Xi’an Jiaotong University. Table 1 shows the clinical characteristics of OA patients.
Table 1.
Clinical Features of Patients with Osteoarthritis.
| Clinicopathological Features | Number |
|---|---|
| Age (years) | |
| <60 | 9 |
| ≥60 | 26 |
| Gender | |
| Male | 19 |
| Female | 16 |
| Disease duration in months | |
| <60 | 21 |
| ≥60 | 14 |
| Kellgren-Lawrence stage | |
| III | 15 |
| IV | 20 |
Cell Culture and Treatment
Human chondrocyte cell line CHON-001, mouse chondrocyte cell line ATDC5 and human embryonic kidney cell line HEK293 were purchased from the Cell Bank of the Chinese Academy of Sciences (Shanghai, China). CHON-001 cells and HEK293 cells were cultured in Dulbecco’s modified Eagle’s medium (DMEM; Gibco, Grand Island, NY), supplemented with 0.1 mg/mL G-418 (Gibco, Grand Island, NY) and 10% fetal bovine serum (FBS; Gibco, Gibco, Grand Island, NY). ATDC5 cells were cultured in Dulbecco’s modified Eagle’s medium/Ham nutrient mixture F12 (DMEM/F12; Sigma-Aldrich, St. Louis, MO) containing 10% FBS and 100 U/mL penicillin–100 μg/mL streptomycin solution (Gibco, Life Technologies, Carlsbad, CA). All the cells were kept in a humidified atmosphere with 5% CO2 at 37°C.
Interleukin-1β (IL-1β; Sigma-Aldrich) was dissolved in double-distilled H2O according to the manufacturer’s instructions, with a storage concentration of 5 mg/mL. Then IL-1β was diluted to 1, 10, or 100 μg/mL using serum-free DMEM/F12 medium, respectively. In this study, CHON-001 and ATDC5 cells were treated with 1, 10, or 100 μg/mL IL-1β, respectively, for 12 hour to induce cellular injuries and expressions of inflammatory cytokines.
qRT-PCR
Total RNA was extracted from chondrocytes and cartilage tissues with TRIzol reagent (Invitrogen, Carlsbad, CA, USA). Then 1μg RNA was reversely transcribed into cDNA using first-strand cDNA synthesis kit (Tiangen Biotech, Beijing, China; Cat#KR105) according to the manufacturer’s instructions. The relative quantities of OIP5-AS1, miR-29b-3p, and PGRN mRNA were determined by using SYBR Premix Dimer Eraser kit (Takara Shiga, Japan; Cat#DRR420A) and analyzed by ABI 7500 Real-Time PCR system (Applied Biosystems, Carlsbad, CA). The result was expressed as U6/GAPDH relative folds and calculated by the 2−ΔΔCt method. The following primers were used:
Cell Transfection
The OIP5-AS1-specific small interfering RNA (siRNA) sequence (si-OIP5-AS1) and sequence-scrambled siRNA (si-NC), miR-29b-3p mimics and miR-29b-3p inhibitors were obtained from RiboBio (Guangzhou, China). PGRN-overexpression plasmids (pcDNA-PGRN) were constructed by GenePharma (Shanghai, China), and empty pcDNA plasmids were used as normal control (NC). All the oligonucleotides (ODN) and plasmids were transfected into cells using Lipofectamine 3000 (Invitrogen, Carlsbad, CA) according to the manufacturer’s instructions.
Cell Viability Experiment
Cell Counting Kit-8 (CCK-8, Dojindo Laboratories, Kumamoto, Japan) was used to detect cell viability according to the manufacturer’s instructions. After IL-1β treatment or transfection, CHON-001 or ATDC5 cells were inoculated to 96-well plates (1 × 104 cells/well) and cultured for 24, 48, 72, and 96 hours, respectively. Afterward, 10 μL CCK-8 solution was added, followed by incubation at 37°C for 1 hour. Optical density (OD) at 450 nm was measured by Microplate Reader (Bio-Tek Instruments, Winooski, VT).
Transwell Assay
Transwell Matrigel Chambers (8 µm pore size; Corning, Beijing, China) were used to detect the migration ability of chondrocytes. Chondrocytes in the logarithmic phase were harvested, and the cell concentration was adjusted to 5 × 104/mL with serum-free DMEM. Then, the cells were placed into the upper compartments of Transwell Chambers, while 600 μL 10% FBS-containing medium was added in each lower compartment. After cultured at 37°C in 5% CO2 for 24 hours, cells that adhered to the bottom of the membrane were fixed in 4% paraformaldehyde for 30 minutes and stained with 0.1% crystal violet for 15 minutes. After rinsing with distilled water, 4 microscopic fields (400×) were randomly selected, and the number of stained cells was counted.
Western Blot
After IL-1β treatment or transfection, the total proteins of CHON-001 or ATDC5 cells were extracted with RIPA Lysis (Thermo Fisher Scientific). Pierce BCA Protein Assay Kit (Thermo Fisher Scientific) was used to detect the concentration of total protein. Total proteins of the same concentration were used for SDS-PAGE electrophoresis, transferred to nitrocellulose (NC) membranes (Millipore, Bedford, MA) and incubated overnight together with primary antibodies, including rabbit anti-PGRN antibody (1:1000, ab108608; Abcam, Cambridge, UK), anti-Bax antibody (1:1000, ab32503, Abcam) and anti-GAPDH (1:2000, ab181602, Abcam). Then, the membranes and goat anti-rabbit IgG H&L (HRP) secondary antibody (ab205718, Abcam) were incubated at room temperature for 1 hour. Then the membranes were immersed in 200 μL Immobilon Western Chemiluminescent HRP Substrate (Millipore) and protein signals were recorded by the Bio-Rad ChemoDox XRS System (Bio-Rad Laboratories).
ELISA
After the transfected CHON-001 and ATDC5 cells were treated with IL-1β, IL-6, IL-8, and TNF-α concentrations in the culture supernatants were detected by enzyme linked immunosorbent assay (ELISA) with human IL-6 ELISA kit (ab178013, Abcam), IL-8 ELISA kit (ab46032, Abcam), TNF-α ELISA kit (ab181421, Abcam) and mouse IL-6 ELISA kit (RAB0308, Sigma-Aldrich), IL-8 ELISA kit (KMC2222, Bio-Medical Assay Corporation, Beijing, China) and TNF-α ELISA kit (BMS607-3FIVE, Thermo Fisher Scientific).
Dual-Luciferase Reporter Assay
The plasmid sequence containing wild type or mutant type OIP5-AS1 or PGRN 3′-UTR was co-transfected with miR-29b-3p mimics or controls. Cells were transfected using Lipofectamine 3000 (Invitrogen) as per the manufacturer’s instructions. After being transfected for 48 hours, cells were lysed by dual-luciferase reporter system (Promega, Madison, WI) to determine luciferase activity. Luciferase activity was calculated as firefly luciferase intensity/ranilla luciferase intensity ratio.
Statistical Analysis
The data obtained were statistically analyzed by SPSS software 22.0 (IBM Corp., Armonk, NY). All the data were expressed as mean ± SD. Student’s t test was carried out for statistical analysis. Each experiment was repeated 3 times by using 3 independent samples. P < 0.05 was considered as statistically significant.
Results
IL-1β Induced the Inflammation Response of CHON-001 and ATDC5 Cells
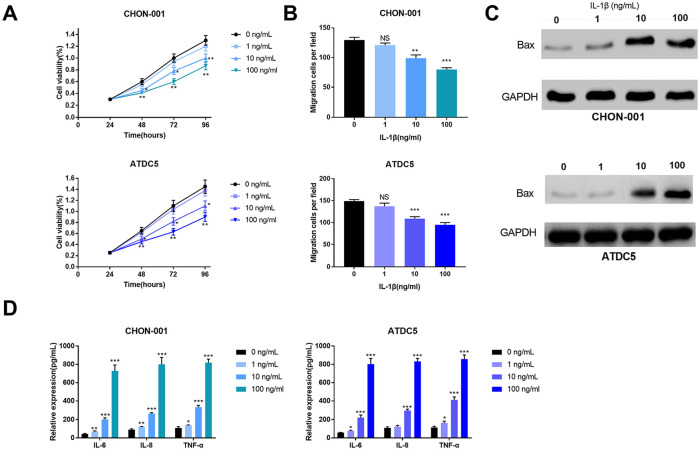
In order to establish in vitro OA models, IL-1β at different concentrations (1, 10, and 100 ng/mL) was used to simulate the inflammation response of CHON-001 and ATDC5 cells. CCK-8 results showed that cell viability was reduced in the groups of 10 and 100 ng/mL IL-1β ( Fig. 1A ). Transwell assay indicated that cell migration was evidently decreased in the cells simulated by 10 and 100 ng/mL IL-1β ( Fig. 1B ). Western blot suggested that the expression levels of apoptosis-related protein Bax was significantly increased ( Fig. 1C ). Furthermore, in cell culture supernate, the levels of inflammatory cytokines IL-6, IL-8, and TNF-α in IL-1β treatment groups were remarkably upregulated compared with those in the control group ( Fig. 1D ). Besides, the above effects became more significant with the increase of IL-1β concentrations. Therefore, 100 ng/mL IL-1β was used as the simulant in subsequent experiments.
Figure 1.
Interleukin-1β (IL-1β) induced the inflammatory injury of CHON-001 and ATDC5 cells treated with 0, 1, 10, and 100 ng/mL IL-1β. (A) The viability of chondrocytes was measured by CCK-8 assay. (B) The migration of chondrocytes was measured by Transwell assay. (C) The expression levels of apoptosis-related protein Bax in chondrocytes were detected by Western blot. (D) The levels of IL-6, IL-8, and TNF-α in chondrocytes were determined by ELISA.
OIP5-AS1 Expression and PGRN Expression Were Downregulated, while miR-29b-3p Expression Was Upregulated in OA Tissues
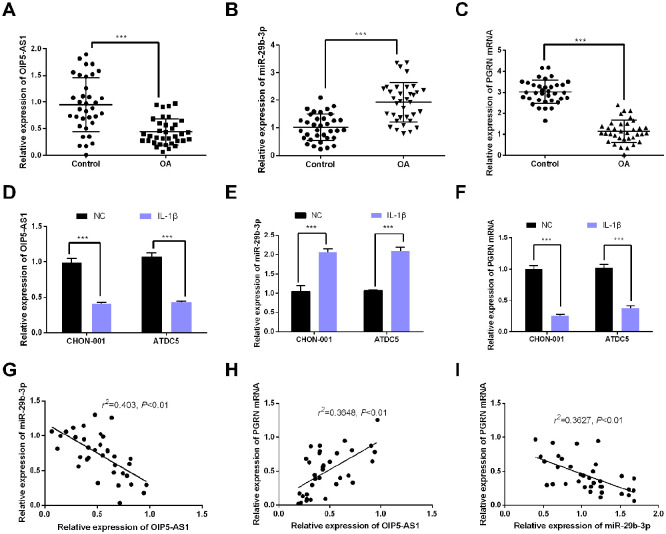
In order to verify the role of OIP5-AS1 in the pathological mechanisms of OA, the expressions of OIP5-AS1, miR-29b-3p, and PGRN mRNA in OA cartilage tissues were detected by qRT-PCR. The results indicated that the OIP5-AS1 expression and PGRN expression were obviously reduced in OA, while miR-29b-3p expression was significantly upregulated ( Fig. 2A-C ), suggesting that they play a role in OA pathogenesis. We also determined the levels of OIP5-AS1, miR-29b-3p and PGRN mRNA in CHON-001 and ATDC5 cells treated with 100ng/mL IL-1β. The results were consistent with the results of tissues: OIP5-AS1 expression and PGRN expression were decreased in OA models, while miR-29b-3p expression was increased ( Fig. 2D-F ). In addition, Spearman correlation analysis suggested that the OIP5-AS1 expression was negatively correlated with the miR-29b-3p expression but positively with the PGRN expression, while the miR-29b-3p expression was negatively correlated with PGRN ( Fig. 2G-I ), indicating that there were regulatory relationships among these 3 molecules.
Figure 2.
OIP5-AS1 and PGRN expressions were downregulated and the miR-29b-3p expression was upregulated in OA tissues. (A-C) The OIP5-AS1, miR-29b-3p, and PGRN mRNA expressions in osteoarthritic (OA) cartilage and normal cartilage was detected by qRT-PCR (***P < 0.001). (D-F) The OIP5-AS1, miR-29b-3p, and PGRN mRNA expressionsin OA models was detected by qRT-PCR. The models were established using CHON-001 and ATDC5 cells treated with 100 ng/mL IL-1β. (G) Correlation analysis indicated that the OIP5-AS1 expression was negatively correlated with the miR-29b-3p expression. (H) Correlation analysis indicated that the OIP5-AS1 expression was positively correlated with the PGRN mRNA expression. (I) Correlation analysis suggested that the miR-29b-3p expression was negatively correlated with the PGRN mRNA expression.
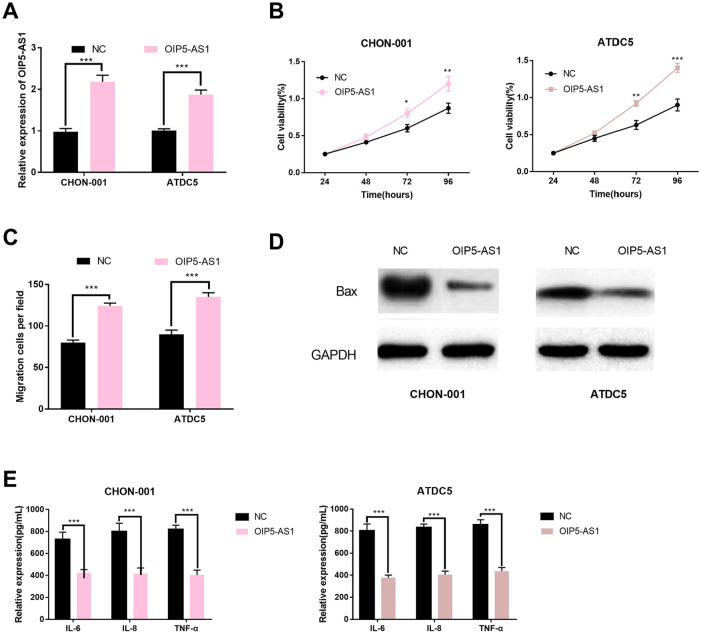
OIP5-AS1 Overexpression Promoted the Viability and Migration of Chondrocytes and Inhibited the Apoptosis and Inflammation
To further examine the influence of OIP5-AS1 on chondrocytes, OIP5-AS1-overexpression plasmids was transfected into CHON-001 and ATDC5 cell lines. qRT-PCR confirmed successful establishment of the models ( Fig. 3A ). The viability of chondrocytes was detected by CCK-8, and the results indicated that viability was promoted following the upregulation of OIP5-AS1 ( Fig. 3B ). Then we further explored the influence of OIP5-AS1 on the migration of chondrocytes. Our findings suggested that the migration of chondrocytes was enhanced after OIP5-AS1 was upregulated ( Fig. 3C ). The expression of Bax in si-OIP5-AS1 group was detected by Western blot, and it was found that the expression level of Bax was decreased ( Fig. 3D ). The expression levels of inflammatory cytokines were also detected. The results showed that the levels of IL-6, IL-8, and TNF-α in OIP5-AS1 overexpression group were significantly down-regulated compared with the control group ( Fig. 3E ). Therefore, we concluded that OIP5-AS1 was a protective factor during OA progression. It promoted the viability and migration of chondrocytes and inhibit the apoptosis and inflammation responses, and its downregulation in OA tissues could aggravate the disease.
Figure 3.
OIP5-AS1 overexpression promoted the viability and migration of chondrocytes and inhibited the apoptosis and inflammatory responses. (A) The OIP5-AS1 expression in osteoarthritis (OA) models following transfection was detected by qRT-PCR. (B) The viability of OA models following transfection was detected by CCK-8 kit. (C) The migration of OA models following transfection was detected by Transwell assay. (D) The expression levels of apoptosis-related protein Bax in OA models following transfection were detected by Western blot. (E) The expressions of IL-6, IL-8, and TNF-α in OA models following transfection were determined by ELISA.
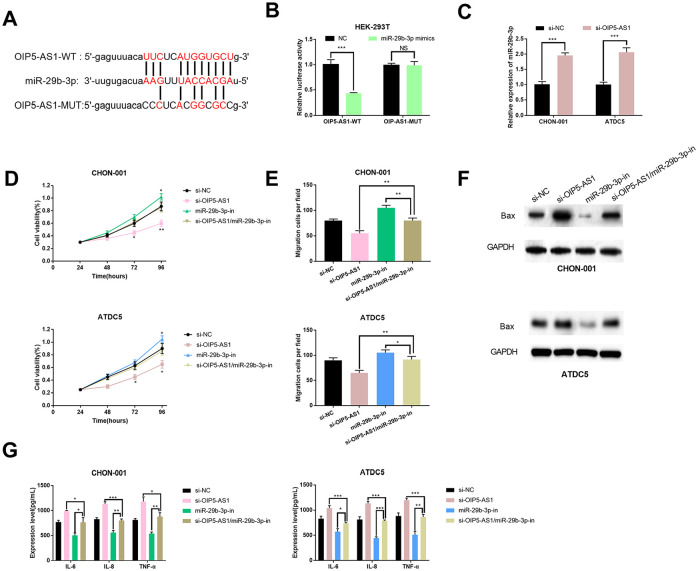
MiR-29b-3p Was a Target of OIP5-AS1
According to the information on StarBase, we predicated that miR-29b-3p may bind to OIP5-AS1 ( Fig. 4A ) and found the negative correlation between them ( Fig. 2D ). Aiming to further verify the relationship between them, we performed a dual luciferase reporter assay. The results proved that miR-29b-3p mimics reduced the luciferase activity of OIP5-AS1-WT in HEK-293T cells but had no influence on the luciferase activity of OIP5-AS1-MUT ( Fig. 4B ). After OIP5-AS1 was knocked down, the miR-29b-3p expression in chondrocytes was significantly increased compared with that in the control group ( Fig. 4C ). We further explored whether OIP5-AS1 played a role in cellular activities via miR-29b-3p. CCK-8 assay and Transwell assay indicated that the inhibition of cell proliferation by si-OIP5-AS1 could be counteracted by miR-29b-3p inhibitors ( Fig. 4D and E ). After co-transfection with miR-29b-3p inhibitors and si-OIP5-AS1, Western blot showed that the expression of Bax was significantly lower compared with si-OIP5-AS1 group ( Fig. 4F ). Additionally, after co-transfection with miR-29b-3p inhibitors and si-OIP5-AS1, ELISA indicated that the expressions of inflammatory cytokines IL-6, IL-8, and TNF-α were remarkably reduced compared with si-OIP5-AS1 group ( Fig. 4G ). Therefore, we conclude that OIP5-AS1 may regulate the proliferation, migration, apoptosis, and inflammation response of chondrocytes via miR-29b-3p.
Figure 4.
miR-29b-3p was a target gene of OIP5-AS1. (A) The potential binding sites between OIP5-AS1 with miR-29b-3p. (B) Luciferase activity was determined after HEK-293T cells were transfected with wild type OIP5-AS1 (OIP5-AS1-WT) and mutant type OIP5-AS1 (OIP5-AS1-MUT). (C) The miR-29b-3p expression in si-OIP5-AS1 group was detected by qRT-PCR. Osteoarthritis (OA) models were established by using CHON-001 and ATDC5 cells treated with 100 ng/mL IL-1β and transfected with si-NC, si-OIP5-AS1, si-OIP5-AS1/miR-29b-3p-in, and miR-29b-3p-in. (D) The viability of OA models was detected by CCK-8 assay. (E) The migration of OA models following transfection was determined by Transwell assay. (F) The expression levels of apoptosis-related protein Bax in OA models following transfection were determined by Western blot. (G) The expressions of IL-6, IL-8, and TNF-α in OA models following transfection were measured by ELISA.
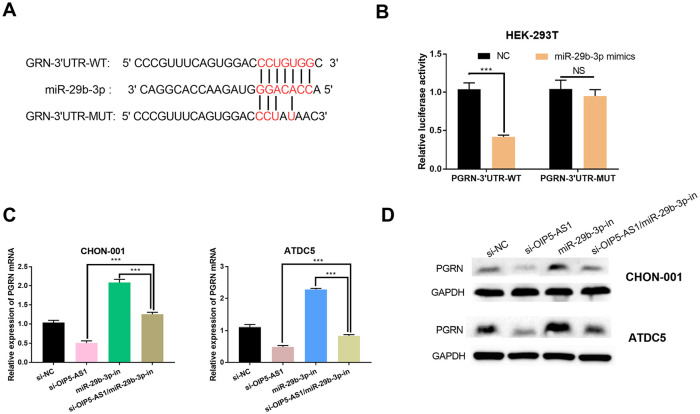
OIP5-AS1 Regulated PGRN Expressions via miR-29b-3p
In this study, we also explored whether OIP5-AS1 regulated PGRN expressions by competitively sponging miR-29b-3p in chondrocytes. With TargetScan database, we predicted the binding of miR-29b-3p to PGRN 3′-UTR ( Fig. 5A ). Dual luciferase reporter assay confirmed that miR-29b-3p mimics reduced the luciferase activity of HEK293 cells in PGRN-3′-UTR-WT group ( Fig. 5B ). After transfection with si-NC, si-OIP5-AS1, miR-29b-3p-in, and si-OIP5-AS1/miR-29b-3p-in, PGRN protein and mRNA levels in OA models were detected by western blot and qRT-PCR. It was found that PGRN protein and mRNA expressions were decreased after OIP5-AS1 knockdown but increased after miR-29b-3p inhibition, and the inhibition of PGRN expressions by miR-29b-3p could be ameliorated by OIP5-AS1 knockdown ( Fig. 5C and D ). Therefore, we concluded that OIP5-AS1 could indirectly regulate PGRN via regulating miR-29b-3p to affect the proliferation, migration, and apoptosis and inflammatory response of chondrocytes.
Figure 5.
OIP5-AS1 regulated the PGRN expression via miR-29b-3p. (A) The potential bindings sites of miR-29b-3p with PGRN 3′-UTR. (B) Luciferase activity was determined after HEK-293T cells were transfected with wild type PGRN-3′-UTR (PGRN-3′-UTR-WT) and mutant type PGRN-3′-UTR (PGRN-3′-UTR-MUT) (***P < 0.001). (C) The expression levels of PGRN mRNA in osteoarthritis (OA) models transfected with si-NC, si-OIP5-AS1, si-OIP5-AS1/miR-29b-3p-in, and miR-29b-3p-in, respectively, were determined by qRT-PCR. (D) The expression levels of PGRN protein in OA models transfected with si-NC, si-OIP5-AS1, si-OIP5-AS1/miR-29b-3p-in, and miR-29b-3p-in, respectively, were determined by qRT-PCR.
Discussion
OA is a degenerative and chronic disease in joints mainly characterized by cartilage destruction. Chondrocyte degeneration is an important factor in cartilage destruction. Proper regulation of the proliferation, apoptosis, autophagy, secretion and other functions of chondrocytes is the key to the prevention and treatment of OA. In this study, we investigated the role of OIP5-AS1 in OA. It was found that the OIP5-AS1 expression in OA specimens was significantly downregulated compared with normal specimens. It was also discovered that the OIP5-AS1 expression was downregulated in IL-1β-induced OA models. These results demonstrated that the downregulation of OIP5-AS1 may play a critical role in the development of OA.
IL-1β was produced by macrophages, chondrocytes, synovial cells and many other cells, which plays an important role in the development of OA. 26 IL-1β can suppress the synthesis of proteoglycan and collagen in cartilage and upregulate the expression of proteolytic enzyme ADAMTS-4 and matrix metalloproteinase-13 (MMP-13), thus resulting in the degradation of cartilage tissues. 27 On the other hand, IL-1β can facilitate the chondrocyte in synthesis and secretion of multiple pro-inflammatory cytokines, such as IL-6, IL-8, TNF-α, and so on. These factors can further lead to the biological dysfunction of chondrocytes (e.g., proliferation, apoptosis and migration) and aggravate the development of OA.27,28 IL-1β can activate the downstream signaling pathways such as nuclear transcription factor-κB (NF-κB) pathway and MAPK pathway. Inhibiting the downstream signaling pathways of IL-1β can evidently mitigate the progression of OA. 27 For example, thymoquinone suppresses IL-1β-induced production of inflammatory mediators by inhibiting NF-κB and MAPKs signaling pathways in OA, 29 and curcumin can be used to treat OA by inhibiting NF-κB signaling pathways. 30 In this study, IL-1β was used to establish in-vitro OA models, and it was found that IL-1β can obviously increase the expressions of inflammatory cytokines IL-6, IL-8 and TNF-α in chondrocytes and significantly decrease the viability and migration of chondrocytes, thus effectively simulating the inflammatory response in chondrocytes.
Many recent studies have shown that lncRNAs play an important role in the development of many diseases, including OA. For instance, LncRNA SNHG5 can enhance the proliferation and migration of chondrocytes in OA. 31 Upregulated lncRNA HOTAIR contributed to MMP overexpression and chondrocyte apoptosis in temporomandibular joint in IL-1β-induced OA models. 32 OIP5-AS1, as a newly found lncRNA, has been found to promote cell proliferation, migration, and drug tolerance in multiple tumors.33-35 Our study supported that the OIP5-AS1 expression was obviously downregulated in OA tissues and models. After OIP5-AS1-overexpression plasmids were transfected in IL-1β-induced OA models, it was found that the viability was enhanced, the number of migrated cells was increased, and the number of apoptotic cells was decreased accordingly. The proteins related to inflammatory reaction (including inflammatory cytokines IL-6, IL-8, and TNF-α) were reduced notably, indicating that OIP5-AS1 can protect chondrocytes from damage by regulating inflammatory reaction.
MiRNA, a small noncoding RNA molecule, is extensively involved in the pathological mechanisms of almost all diseases.36,37 Recent studies suggested that miRNAs had unique roles in gene regulation and possibly became a new molecular target for the treatment of OA. MiR-10a-5p, for example, promoted chondrocyte apoptosis by targeting HOXA1. 38 MiR-146a could alleviate OA triggered by aging and trauma via inhibiting the catabolism induced by Notch1, IL-6, and IL-1. 39 ] MiR-29b-3p is a recently found miRNA that plays a critical role in OA and many other diseases.19,40 Our study revealed that the miR-29b-3p expression was obviously increased in OA tissues. By inhibiting the miR-29b-3p expression in IL-1β-induced OA models, the viability and migration abilities of chondrocytes were improved, while the number of apoptotic cells and the expression of inflammatory cytokines were significantly reduced. Thus, the upregulated miR-29b-3p expression could promote the development of OA.
Many studies suggested that LncRNA was considered as a ceRNA by sponging miRNA when regulating the mRNA of the target gene.41-43 lncRNA FOXD 2-AS1 could regulate CCND1 and chondrocyte proliferation in OA by acting as the sponge of miR-206 44 LncRNA DANCR regulated the progression of OA as a ceRNA via miR-577/SphK 2 axis. 45 Intriguingly, it was found in online databases that miR-29b-3p is probably a target of OIP5-AS1. Hence, we further presumed that OIP5-AS1 possibly regulated miR-29b-3p through the pathways mentioned above. The results of luciferase reporter assay supported that OIP5-AS1 could bind to miR-29b-3p, and the miR-29b-3p expression was increased after the OIP5-AS1 expression was downregulated in CHON-001 and ATDC5 cells. In addition, the function of OIP5-AS1 in promoting the proliferation and migration of chondrocytes and inhibiting the apoptosis and inflammatory reaction can be partially suppressed by miR-29b-3p. These results indicated that OIP5-AS1 at least partially regulated the viability and migration of chondrocytes by sponging miR-29b-3p and thus ameliorated the damage of inflammation to chondrocytes.
PGRN, a secreting growth factor, is involved in maintaining and regulating the development, proliferation, regeneration and host defense responses in normal tissues. It has been extensively studied in multiple pathophysiological processes, including inflammatory responses. It has been shown that PGRN can bind to TNF receptors and has a therapeutic effect on inflammatory arthritis in mice. 46 PGRN bound to TNF receptors to form atsttrin, which could prevent early OA in mouse and rat models. 47 PGRN plays an important part in suppressing the progression of OA. This study suggested that the PGRN expression was upregulated in OA tissues and models. PGRN and miR-29b-3p expressions were negatively correlated with each other in OA tissues. The PGRN expression was obviously increased in OA models with decreased miR-29b-3p expressions. Considering the results of luciferase reporter assay, miR-29b-3p may bind to 3’-UTR of PGRN and reduce its expression. PGRN and OIP5-AS1 expressions are positively correlated with each other in OA tissues. The PGRN expression was decreased in OA models following OIP5-AS1 knockdown, indicating that the PGRN expression was upregulated by OIP5-AS1. Therefore, OIP5-AS1 probably regulates the PGRN expression in an indirect manner via sponging miR-29b-3p.
Conclusion
Our study indicated that OIP5-AS1 promoted the viability and migration of chondrocytes and inhibited the apoptosis and inflammation in OA by regulating miR-29b-3p/PGRN axis, thereby ameliorating the damage to chondrocytes in OA. OIP5-AS1/miR-29b-3p/PGRN axis may become a novel therapeutic target in the treatment of OA. Nevertheless, the feasibility still requires further verification by in vivo experiments.
Footnotes
Authors’ Note: The data used to support the findings of this study are available from the corresponding author on request.
Acknowledgments and Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study is supported by Medical Research Project of Xi’an Science and Technology Bureau (J201701007) and China postdoctoral science foundation (2017M623215).
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Ethical Approval: This study was approved by the Ethics Committee of Honghui Hospital and the Ethics Committee of Xi’an Jiaotong University.
Informed Consent: Written informed consent was obtained from all patients.
Trial Registration: Not applicable.
ORCID iD: Jianbing Ma  https://orcid.org/0000-0001-9122-3784
https://orcid.org/0000-0001-9122-3784
References
- 1. Loeser RF, Collins JA, Diekman BO. Ageing and the pathogenesis of osteoarthritis. Nat Rev Rheumatol. 2016;12:412-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Appleton CT. Osteoarthritis year in review 2017: biology. Osteoarthritis Cartilage. 2018;26:296-303. [DOI] [PubMed] [Google Scholar]
- 3. Li J, Li Z, Zheng W, Li X, Wang Z, Cui Y, et al. LncRNA-ATB: an indispensable cancer-related long noncoding RNA. Cell Prolif. 2017;50(6). doi: 10.1111/cpr.12381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Liao K, Xu J, Yang W, You X, Zhong Q, Wang X. The research progress of LncRNA involved in the regulation of inflammatory diseases. Mol Immunol. 2018;101:182-8. [DOI] [PubMed] [Google Scholar]
- 5. Pearson MJ, Philp AM, Heward JA, Roux BT, Walsh DA, Davis ET, et al. Long intergenic noncoding RNAs mediate the human chondrocyte inflammatory response and are differentially expressed in osteoarthritis cartilage. Arthritis Rheumatol. 2016;68:845-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Wang Y, Shi F, Xia Y, Zhao H. LncRNA OIP5-AS1 predicts poor prognosis and regulates cell proliferation and apoptosis in bladder cancer. J Cell Biochem. 2019;120(5):7499-505. [DOI] [PubMed] [Google Scholar]
- 7. Deng J, Deng H, Liu C, Liang Y, Wang S. Long non-coding RNA OIP5-AS1 functions as an oncogene in lung adenocarcinoma through targeting miR-448/Bcl-2. Biomed Pharma-cother. 2018;98:102-10. [DOI] [PubMed] [Google Scholar]
- 8. Zheng D, Wang B, Zhu X, Hu J, Sun J, Xuan J, et al. LncRNA OIP5-AS1 inhibits osteoblast differentiation of valve interstitial cells via miR-137/TWIST11 axis. Biochem Biophys Res. Commun. 2019;511:826-32. [DOI] [PubMed] [Google Scholar]
- 9. Bartel DP. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell. 2004;116:281-97. [DOI] [PubMed] [Google Scholar]
- 10. Li B, Wang X, Choi IY, Wang YC, Liu S, Pham AT, et al. MiR-146a modulates autoreactive Th17 cell differentiation and regulates organ-specific autoimmunity. J Clin Invest. 2017;127:3702-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Pan JL, Yuan DZ, Zhao YB, Nie L, Lei Y, Liu M, et al. Progesterone-induced miR-133a inhibits the proliferation of endometrial epithelial cells. Acta Physiol (Oxf). 2017;219:683-92. [DOI] [PubMed] [Google Scholar]
- 12. Zhang M, Zhang Q, Hu Y, Xu L, Jiang Y, Zhang C, et al. MiR-181a increases FoxO1 acetylation and promotes granulosa cell apoptosis via SIRT1 downregulation. Cell Death Dis. 2017;8:e3088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Aryal B, Singh AK, Rotllan N, Price N, Fernández-Hernando C. MicroRNAs and lipid metabolism. Curr Opin Lipidol. 2017;28:273-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hussain N, Zhu W, Jiang C, Xu J, Wu X, Geng M, et al. Down-regulation of miR-10a-5p in synoviocytes contributes to TBX5-controlled joint inflammation. J Cell Mol Med. 2018;22:241-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Wu W, He C, Liu C, Cao AT, Xue X, Evans-Marin HL, et al. miR-10a inhibits dendritic cell activation and Th1/Th17 cell immune responses in IBD. Gut. 2015;64:1755-64. [DOI] [PubMed] [Google Scholar]
- 16. Mesci A, Huang X, Taeb S, Jahangiri S, Kim Y, Fokas E, et al. Targeting of CCBE1 by miR-330-3p in human breast cancer promotes metastasis. Br J Cancer. 2017;116:1350-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hu J, Wang Z, Pan Y, Ma J, Miao X, Qi X, et al. MiR-26a and miR-26b mediate osteoarthritis progression by targeting FUT4 via NF-κB signaling pathway. Int J Biochem Cell Biol. 2018;94:79-88. [DOI] [PubMed] [Google Scholar]
- 18. Zhang X, Wang C, Zhao J, Xu J, Geng Y, Dai L, et al. MiR-146a facilitates osteoarthritis by regulating cartilage homeostasis via targeting Camk2d and Ppp3r2. Cell Death Dis. 2017;8:e2734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Chen L, Li Q, Wang J, Jin S, Zheng H, Lin J, et al. MiR-29b-3p promotes chondrocyte apoptosis and facilitates the occurrence and development of osteoarthritis by targeting PGRN. J Cell Mol Med. 2017;21:3347-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Tian R, Li Y, Yao X. PGRN suppresses inflammation and promotes autophagy in keratinocytes through the Wnt/β-Catenin signaling pathway. Inflammation. 2016;39:1387-94. [DOI] [PubMed] [Google Scholar]
- 21. Daniel R, He Z, Carmichael KP, Halper J, Bateman A. Cellular localization of gene expression for progranulin. J Histochem Cytochem. 2000;48:999-1009. [DOI] [PubMed] [Google Scholar]
- 22. He Z, Ong CH, Halper J, Bateman A. Progranulin is a mediator of the wound response. Nat Med. 2003;9:225-9. [DOI] [PubMed] [Google Scholar]
- 23. Serrero G, Hawkins DM, Yue B, Ioffe O, Bejarano P, Phillips JT, et al. Progranulin (GP88) tumor tissue expression is associated with increased risk of recurrence in breast cancer patients diagnosed with estrogen receptor positive invasive ductal carcinoma. Breast Cancer Res. 2012;14:R26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Yin F, Dumont M, Banerjee R, Ma Y, Li H, Lin MT, et al. Behavioral deficits and progressive neuropathology in progranulin-deficient mice: a mouse model of frontotemporal dementia. FASEB J. 2010;24:4639-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Wei J, Hettinghouse A, Liu C. The role of progranulin in arthritis. Ann N Y Acad Sci. 2016;1383:5-20. [DOI] [PubMed] [Google Scholar]
- 26. Daheshia M, Yao JQ. The interleukin 1beta pathway in the pathogenesis of osteoarthritis. J Rheumatol. 2008;35:2306-12. [DOI] [PubMed] [Google Scholar]
- 27. Wojdasiewicz P, Poniatowski ŁA, Szukiewicz D. The role of inflammatory and anti-inflammatory cytokines in the pathogenesis of osteoarthritis. Mediators Inflamm. 2014;2014:561459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Laavola M, Leppänen T, Hämäläinen M, Vuolteenaho K, Moilanen T, Nieminen R, et al. IL-6 in osteoarthritis: effects of pine stilbenoids. Molecules. 2018;24:E109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Wang D, Qiao J, Zhao X, Chen T, Guan D. Thymoquinone inhibits IL-1β-induced inflammation in human osteoarthritis chondrocytes by suppressing NF-κB and MAPKs signaling pathway. Inflammation. 2015;38:2235-41. [DOI] [PubMed] [Google Scholar]
- 30. Shakibaei M, John T, Schulze-Tanzil G, Lehmann I, Mobasheri A. Suppression of NF-κB activation by curcumin leads to inhibition of expression of cyclo-oxygenase-2 and matrix metalloproteinase-9 in human articular chondrocytes: implications for the treatment of osteoarthritis. Biochem Pharmacol. 2007;73:1434-45. [DOI] [PubMed] [Google Scholar]
- 31. Shen H, Wang Y, Shi W, Sun G, Hong L, Zhang Y. LncRNA SNHG5/miR-26a/SOX2 signal axis enhances proliferation of chondrocyte in osteoarthritis. Acta Biochim Biophys Sin (Shanghai). 2018;50:191-8. [DOI] [PubMed] [Google Scholar]
- 32. Zhang C, Wang P, Jiang P, Lv Y, Dong C, Dai X, et al. Upregulation of lncRNA HOTAIR contributes to IL-1β-induced MMP overexpression and chondrocytes apoptosis in temporomandibular joint osteoarthritis. Gene. 2016;586:248-53. [DOI] [PubMed] [Google Scholar]
- 33. Luan W, Zhang X, Ruan H, Wang J, Bu X. Long noncoding RNA OIP5-AS1 acts as a competing endogenous RNA to promote glutamine catabolism and malignant melanoma growth by sponging miR-217. J Cell Physiol. Epub 2019 February 18. [DOI] [PubMed] [Google Scholar]
- 34. Bai Y, Li S. Long noncoding RNA OIP5-AS1 aggravates cell proliferation, migration in gastric cancer by epigenetically silencing NLRP6 expression via binding EZH2. J Cell Biochem. 2019;121:353-62. [DOI] [PubMed] [Google Scholar]
- 35. Song L, Zhou Z, Gan Y, Li P, Xu Y, Zhang Z, et al. Long noncoding RNA OIP5-AS1 causes cisplatin resistance in osteosarcoma through inducing the LPAATβ/PI3K/AKT/mTOR signaling pathway by sponging the miR-340-5p. J Cell Biochem. 2019;120:9656-66. [DOI] [PubMed] [Google Scholar]
- 36. Ivey KN, Srivastava D, MicroRNAs as developmental regulators. Cold Spring Harb Perspect Biol. 2015;7:a008144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Siomi H, Siomi MC. Posttranscriptional regulation of micro-RNA biogenesis in animals. Mol Cell. 2010;38:323-32. [DOI] [PubMed] [Google Scholar]
- 38. Ma Y, Wu Y, Chen J, Huang K, Ji B, Chen Z, et al. MiR-10a-5p promotes chondrocyte apoptosis in osteoarthritis by targeting HOXA1. Mol Ther Nucleic Acids. 2019;14:398-409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Guan YJ, Li J, Yang X, Du S, Ding J, Gao Y, et al. Evidence that miR-146a attenuates aging- and trauma-induced osteoarthritis by inhibiting Notch1, IL-6, and IL-1 mediated catabolism. Aging Cell. 2018;17:e12752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Su T, Xiao Y, Xiao Y, Guo Q, Li C, Huang Y, et al. Bone marrow mesenchymal stem cells-derived exosomal MiR-29b-3p regulates aging-associated insulin resistance. ACS Nano. 2019;13:2450-62. [DOI] [PubMed] [Google Scholar]
- 41. Wu XS, Wang F, Li HF, Hu YP, Jiang L, Zhang F, et al. LncRNA-PAGBC acts as a microRNA sponge and promotes gallbladder tumorigenesis. EMBO Rep. 2017;18:1837-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Yu C, Li L, Xie F, Guo S, Liu F, Dong N, et al. LncRNA TUG1 sponges miR-204-5p to promote osteoblast differentiation through upregulating Runx2 in aortic valve calcification. Cardiovasc Res. 2018;114:168-79. [DOI] [PubMed] [Google Scholar]
- 43. Leisegang MS. LET’s sponge: how the lncRNA PFL promotes cardiac fibrosis. Theranostics. 2018;8:874-877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Cao L, Wang Y, Wang Q, Huang J. LncRNA FOXD2-AS1 regulates chondrocyte proliferation in osteoarthritis by acting as a sponge of miR-206 to modulate CCND1 expression. Biomed Pharmacother. 2018;106:1220-6. [DOI] [PubMed] [Google Scholar]
- 45. Fan X, Yuan J, Xie J, Pan Z, Yao X, Sun X, et al. Long non-protein coding RNA DANCR functions as a competing endogenous RNA to regulate osteoarthritis progression via miR-577/SphK2 axis. Biochem Biophys Res Commun. 2018;500:658-64. [DOI] [PubMed] [Google Scholar]
- 46. Tang W, Lu Y, Tian QY, Zhang Y, Guo FJ, Liu GY, et al. The growth factor progranulin binds to TNF receptors and is therapeutic against inflammatory arthritis in mice. Science. 2011;332:478-84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Wei JL, Fu W, Ding YJ, Hettinghouse A, Lendhey M, Schwarzkopf R, et al. Progranulin derivative Atsttrin protects against early osteoarthritis in mouse and rat models. Arthritis Res Ther. 2017;19:280. [DOI] [PMC free article] [PubMed] [Google Scholar]