Abstract
Objective
Advanced glycation end-product (AGE) accumulation is implicated in osteoarthritis (OA) pathogenesis in aging and diabetic populations. Here, we develop a representative nonenzymatic glycation-induced OA cartilage explant culture model and investigate the effectiveness of resveratrol, curcumin, and eugenol in inhibiting AGEs and the structural and biological hallmarks of cartilage degeneration.
Design
Bovine cartilage explants were treated with AGE–bovine serum albumin, threose, and ribose to determine the optimal conditions that induce physiological levels of AGEs while maintaining chondrocyte viability. AGE crosslinks, tissue stiffness, cell viability, metabolism and senescence, nitrite release and loss of glycosaminoglycans were assessed. Explants were cotreated with resveratrol, curcumin, or eugenol to evaluate their anti-AGE properties. Blind docking analysis was conducted to estimate binding energies of drugs with collagen II.
Results
Treatment with 100 mM ribose significantly increased AGE crosslink formation and tissue stiffness, resulting in reduced chondrocyte metabolism and enhanced senescence. Blind docking analysis revealed stronger binding energies of both resveratrol and curcumin than ribose, with glycation sites along a human collagen II fragment, indicating their increased likelihood of competitively inhibiting ribose activity. Resveratrol and curcumin, but not eugenol, successfully inhibited AGE crosslink formation and its associated downstream biological response.
Conclusions
We establish a cartilage explant model of OA that recapitulates several aspects of aged human cartilage. We find that resveratrol and curcumin are effective anti-AGE therapeutics with the potential to decelerate age-related and diabetes-induced OA. This in vitro nonenzymatic glycation-induced model provides a tool for screening OA drugs, to simultaneously evaluate AGE-induced biological and mechanical changes.
Keywords: osteoarthritis, advanced glycation end-product (AGE), resveratrol, curcumin, eugenol, cartilage
Introduction
Osteoarthritis (OA) is a degenerative joint disease, characterized by the breakdown of articular cartilage and subchondral bone, whose incidence increases with age.1,2 Diabetes is also a major risk factor for OA,3,4 possibly due to the perpetual state of serum hyperglycemia and accumulation of advanced glycation end-products (AGEs) in tissues throughout the body. 5 Driven by the Maillard reaction, AGEs accumulate on low-turnover proteins as a result of nonenzymatic glycation as reducing sugars like glucose, ribose, and other aldoses react with lysine and arginine residues in type II collagen of cartilage to form AGE crosslinks like pentosidine and N-(ε)-carboxymethyl-lysine (CML). 6 Due to the low-turnover rate of collagen, AGE accumulation on the protein increases with age,7,8 stiffening the cartilage tissue; therefore, making it more brittle and susceptible to fracture.1,2,9 AGE accumulation has also been correlated with elevated senescent activity leading to reduced chondrocyte metabolism and biosynthesis rates, increasing the likelihood of cartilage breakdown and OA progression.2,10,11 AGEs, through binding with and activation of the receptor for AGEs (RAGE), have further been shown to upregulate several catabolic and inflammatory markers, including interleukins IL-1, IL-6, IL-8, matrix metalloproteinase (MMP)-13, COX-2, PGE2, and NO,12 -14 which can degrade the cartilage matrix resulting in loss of glycosaminoglycans (GAGs) and collagen. Therefore, inhibiting AGE formation is a plausible therapeutic strategy to slow down aging-induced OA, especially in the diabetic population.
Natural compounds resveratrol, curcumin, and eugenol have been shown to possess anti-AGE properties due to the presence of para-hydroxyl groups in their structure that have strong binding affinity for arginine and lysine residues, which can competitively inhibit AGE formation.15,16 Resveratrol was shown to suppress the IL-1-induced inflammatory response of AGE-treated porcine chondrocytes. 17 Similarly, AGE-induced inflammation was reduced in rabbit chondrocytes treated with curcumin. 18 Eugenol was recently shown to reduce glycation of serum albumin 15 but has not yet been investigated in cartilage. Most of the OA-focused studies evaluating biological response to AGEs and their inhibitors have utilized cells and not cartilage explants; therefore, the role of these drugs in suppressing AGE accumulation in the cartilage matrix to restore tissue biomechanical properties and attenuate the resulting downstream matrix and cellular dysfunction remains unexplored. In vitro studies using chondrocytes treated with AGE–bovine serum albumin (BSA) have shown that activation of RAGE triggers an inflammatory response, however these models are unable to evaluate the glycating effects on the tissue.12,14 Treatment of cartilage explants with high concentrations of threose and ribose has been used to mimic the aging process of AGE crosslink accumulation and altered tissue mechanical properties.1,9,10,19,20 However, use of high sugar concentrations results in complete chondrocyte death making it difficult to study both the biochemical and cellular effects of AGE crosslinks within the same explant model.
Here, we optimize sugar concentration to create a live cartilage explant culture model of AGE-induced OA. This model exhibits increased tissue matrix stiffness while maintaining chondrocyte viability but reduced metabolic activity that accurately mimics the properties of OA tissue in vivo. We use this model, with addition of inflammatory cytokine IL-1, to study the anti-AGE properties of resveratrol, curcumin, and eugenol in inhibiting AGE-induced nonenzymatic mechanical crosslinking and the resulting downstream biological response associated with upregulation of catabolic and inflammatory markers at matrix and cellular levels.
Methods
Materials
Dulbecco’s modified Eagle medium (DMEM) was purchased from Cellgro (Masassas, VA). Media supplements including nonessential amino acids (NEAA), HEPES buffer, and insulin-transferrin-selenium (ITS) were from Gibco (Carlsbad, CA). l-Proline and ascorbic acid were from Fisher Bioreagents (Pittsburgh, PA). AGE-BSA, threose, and ribose and additional reagents not explicitly mentioned were from Sigma-Aldrich (St. Louis, MO). Human recombinant IL-1α was purchased from PeproTech (Rocky Hill, NJ). Resveratrol and eugenol were from Alfa Aesar (Tewksbury, MA) whereas curcumin was from Aeros Organics (Geel, Belgium).
Harvest of Cartilage Tissue Explants and Articular Chondrocytes
Cylindrical cartilage plugs were harvested from the femoropatellar groove of 2- to 3-week-old bovine knee joints (Research 87, Boylston, MA) using a 3-mm dermal punch. Cartilage plugs were sliced to obtain a 1-mm thick disk inclusive of the superficial zone.21,22 Prior to any treatment, explants were equilibrated in serum-free culture media containing low-glucose DMEM for 2 days at 37°C and 5% CO2 as previously described. 21 Cartilage slices were obtained from the medial and lateral femoral condyles and then digested using pronase and collagenase enzymes using previously published methods.23,24 The articular bovine chondrocytes were then frozen in liquid nitrogen until further use for senescence experiments.
Treatment of Cartilage Explants with Glycating Agents AGE-BSA, Threose, and Ribose
To induce glycation, cartilage explants were incubated with a wide dose range of AGE-modified bovine serum albumin (AGE-BSA, 300-900 μg/mL), threose (10 mM), or ribose (10, 30, 100, and 300 mM) for 8 days at 37°C and 5% CO2 with media replenished every 2 days. Following treatment, explants were evaluated for AGE crosslinks, equilibrium modulus, cell viability, metabolism, nitrite release, and GAG loss. The concentration range of glycating agents are based on prior literature evaluating biological effectiveness in cell culture or change in tissue mechanical properties using dead cartilage explants.12,20
Blind Docking
In order to anticipate the competitive binding efficacy of natural, anti-aging drugs to the glycation sites of type II collagen—arginine and lysine residues—blind docking simulations were conducted. Interactions between the molecular structures of ligands (ribose, resveratrol, curcumin, and eugenol obtained from DrugBank) and a 38-residue, human type II collagen fragment (RCSB Protein Data Bank: 6HG7) were simulated using the Achilles Blind Docking Server. A weighted binding energy (WBE) score for each binding site was estimated by multiplying the BE by the number of conformations at that site and dividing by the total number of conformations. The WBEs for each protein-ligand interaction were plotted along the horizontal length of collagen. Ligand-residue interactions were visualized for each ligand at the site of highest BE. Further details can be found in the supplemental information.
Treatment of Cartilage Explants with Potential AGE-Inhibitors
Frozen-dead cartilage explants were incubated in phosphate buffered saline (PBS) with 100 mM ribose and 500 μM resveratrol, 500 μM curcumin, or 20 mM eugenol for 6 days at 37°C. High drug doses were used for this study to clearly establish presence or absence of anti-AGE properties and to produce measurable changes in mechanical properties using the methods described. Following the 6-day culture treatment, explants were evaluated for AGE crosslinks, equilibrium modulus, and permeability.
An 8-day follow-up experiment was then designed using live, 100 mM ribose-treated cartilage explants that were administered therapeutic doses of resveratrol (50 μM), curcumin (12 μM), and eugenol (200 μM). Resveratrol and curcumin doses were chosen based on prior published work 25 ; however, 50 μM curcumin was found to be cytotoxic (Supplemental Fig. S1a), which is consistent with the results of Clutterbuck et al. 26 Thus, a reduced dose of 12 μM curcumin was used for biological studies. In order to induce an inflammatory response similar to one caused by AGEs activating RAGE, 27 explants were cotreated with exogenous IL-1α. Following treatment, explants were evaluated for AGE crosslinks, equilibrium modulus, cell viability, metabolism, nitrite release, and GAG loss. Drug doses were chosen based on prior literature either showing therapeutic effects in AGE-treated chondrocyte culture or no cytotoxicity.17,18,25,26,28
Mechanical Properties of Cartilage Explants and Quantification of AGE Crosslinks
Following culture, explants were subjected to uniaxial, unconfined stress-relaxation testing using a dynamic mechanical analyzer (ElectroForce 5500, TA Instruments) with 3 step-strain intervals of 4%, 6%, and 8%, with strains applied at 0.0001 mm/s and held for 600 seconds (Supplemental Fig. S2a). WinTest software was used to collect stress response data as a function of time which was then fitted to the standard linear viscoelastic (SLV) model (Supplemental Fig. S2b). The model allowed for the determination of equilibrium modulus (E) from the ratio of equilibrium residual stress in tissue ( ) and applied strain (ε) as well as the characteristic time constant (τ) over which 63.2% of the stress in the tissue relaxes as a result of fluid flow, using which, tissue permeability (K) was estimated as described previously.29,30 To measure AGE crosslinks in cartilage, explants were digested using proteinase K and fluorescence readings were obtained at 370 nm excitation and 440 nm emission. 31
Chondrocyte Viability and Metabolism
At the end of culture, 100- to 200-μm thick cross-sections of cartilage explants were stained with fluorescein diacetate and propidium iodide and imaged using a Nikon fluorescent microscope at 4× objective. 21 Percentage of viable cells was quantified using ImageJ and is reported in Supplemental Figure S3. Note that viability images for curcumin treatment were obtained from chondrocyte cultures instead of cartilage tissues as the potent yellow color of curcumin brightly stained the tissue matrix, hindering imaging under fluorescent light. Chondrocyte staining is shown in Supplemental Figure S1. The Alamar Blue assay was used to assess cell metabolism at the end of culture as previously described, with fluorescence measurements obtained on a microplate reader (Biotek). 21
Nitrite Release and GAG Loss from Cartilage Explants
Nitrite released into the media from cartilage explants was determined using the Griess Assay with sodium nitrite as a standard. 21 At the end of culture, digested cartilage explants and culture media were assessed for total GAG content using the dimethyl-methylene blue (DMMB) assay. 32
Chondrocyte Senescence
Primary bovine chondrocytes were seeded at 200,000 cells per well in 48-well plates in culture media containing high-glucose DMEM, supplemented with 10% fetal bovine serum (FBS), and GlutaMAX and antibiotic-antimycotic at 1% (V/V) each. Cells were allowed to adhere for 24 hours prior to any treatment. Cells were cultured in media supplemented with 100 mM ribose and then treated with or without IL-1α (2 ng/mL). Cultures were also administered 50 μM resveratrol, 12 μM curcumin, or 200 μM eugenol and incubated for 8 days with media being replenished every 3 days. At the end of culture, chondrocytes were evaluated for senescence using a Senescence Detection Kit (BioVision, Milpitas, CA), per manufacturer’s recommendations and imaged using a Nikon microscope at 200× magnification.
Statistical Analysis
For in vitro culture experiments, general linear mixed effects model was used with animal as a random variable. Tukey’s honestly significant difference (HSD) was used for comparing data across treatment conditions. No statistical differences were found between animals; thus, the data were pooled together. Seven bovine joints were used (3 independent repeats) with n = 12 to 18 explants (or wells) per treatment condition. All biochemical assays were performed in triplicate. Data are presented as mean ± 95% confidence interval. P < 0.05 was considered statistically significant.
Results
Ribose Treatment Induces AGEs while Keeping Chondrocytes Viable Simulating In Vitro Model of Aged OA Cartilage Explant
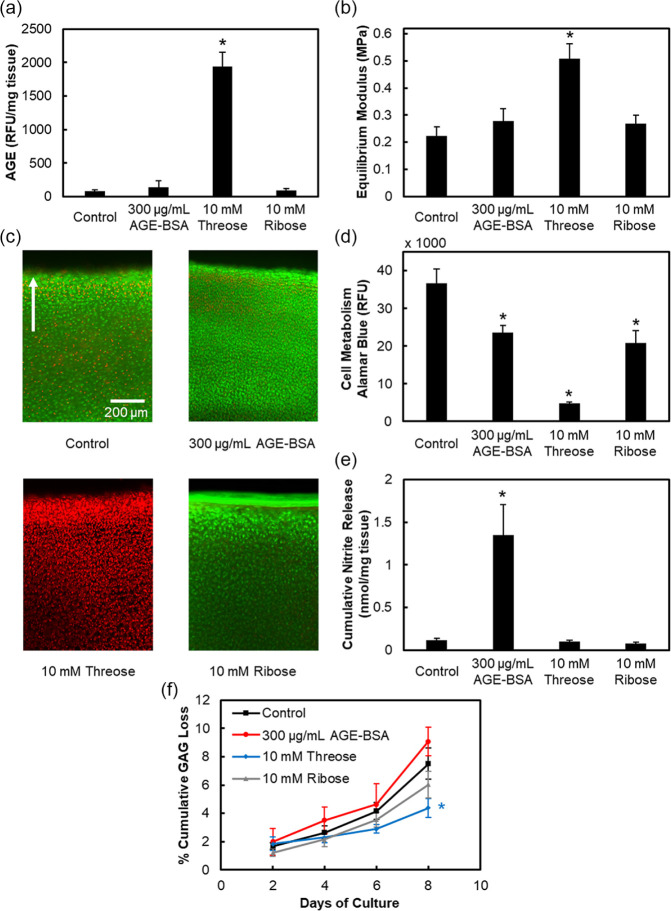
Our data shows that treatment with 300 to 900 μg/mL AGE-BSA (data shown only for lowest concentration) did not increase AGE crosslinks or cartilage stiffness due to the inability of a preglycated form of BSA to crosslink collagen ( Fig. 1a and b ). Cells remained mostly viable similar to control ( Fig. 1c , Supplemental Fig. S3a), but the cell metabolism dropped by 1.5× (P < 0.0001, Fig. 1d ) releasing 12× higher levels of nitrites compared to control ( Fig. 1e ). On the other hand, 10 mM threose led to 24× higher levels of AGEs making cartilage 2.2 times stiffer than control (P < 0.0001, Fig. 1a and d ). These crosslinks also prevented release of GAGs from the tissue as shown by significantly lower GAG loss compared with control by day 8 (P = 0.0005 at day 8, Fig. 1f ). However, complete cell death and loss of metabolism was observed which precluded its further use in developing a live AGE-induced OA cartilage model ( Fig. 1c and d ).
Figure 1.
Evaluating efficacy of various glycating agents. Bovine cartilage explants cultured for 8 days and treated with either 300 μg/mL advanced glycation end-product–bovine serum albumin (AGE-BSA), 10 mM threose or 10 mM ribose. Quantification of (a) AGE crosslinks and (b) equilibrium modulus. Chondrocyte viability images in (c) with green and red colors used to show viable and nonviable cells, respectively. Arrow points toward superficial layer of cartilage. Refer to Supplemental Figure S3a for quantification of viability. Cell metabolism in (d), cumulative nitrite release in (e), and cumulative GAG loss in (f). Data are presented as mean ± 95% confidence interval. *Versus control (P < 0.05). Statistical markers are colored with respect to the data.
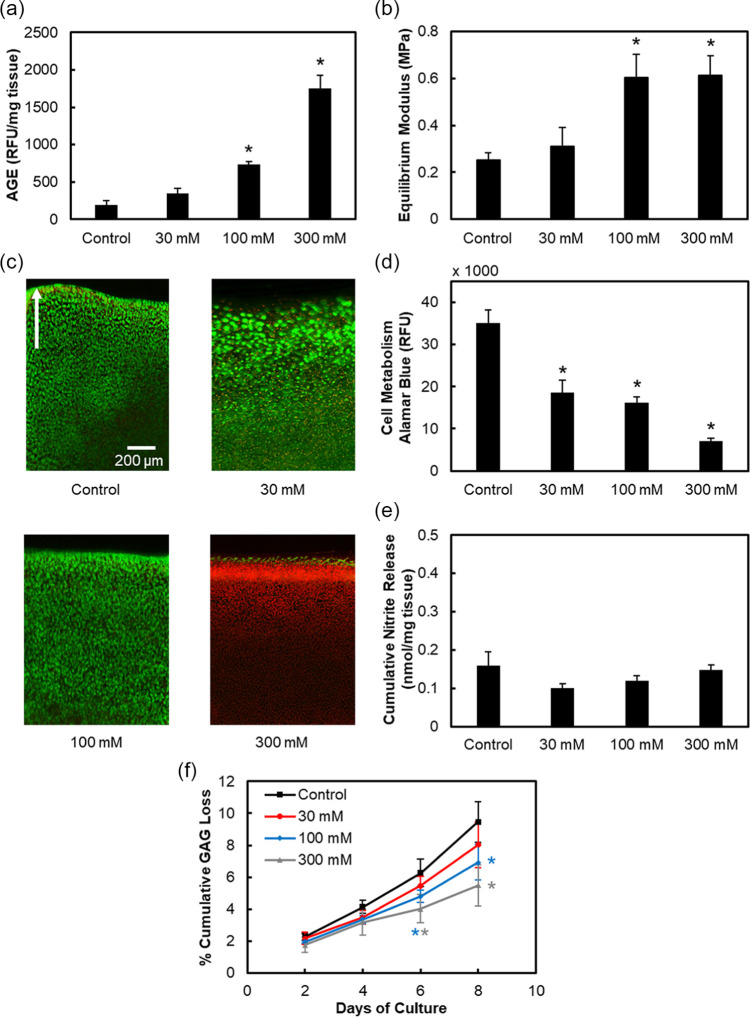
Ten micromolar (10 mM) ribose treatment showed maintenance of cell viability while reducing metabolism; however, despite trending upward, it was unable to significantly increase the AGE content or cartilage stiffness. Thus, an 8-day dose optimization study was conducted using higher ribose concentrations ( Fig. 2 ). A dose-dependent increase in AGE crosslinks was observed with 30, 100, and 300 mM ribose elevating to 1.8×, 3.8× (P < 0.0001), and 9× (P < 0.0001) of control, respectively ( Fig. 2a ). Both 100 and 300 mM ribose resulted in 2.4× stiffer cartilage than control (P < 0.0001, Fig. 2b ). After 8 days of culture, 30 and 100 mM ribose-treated explants had viable cells, whereas the 300 mM condition resulted in complete cell death ( Fig. 2c , Supplemental Figure S3b). All 3 concentrations significantly lowered chondrocyte metabolism after 8 days in a dose-dependent manner (P < 0.0001), with 300 mM having the most profound impact ( Fig. 2d ). Unlike treatment with AGE-BSA, ribose did not initiate an inflammatory response via nitrite release, despite inducing significant accumulation of AGE crosslinks ( Fig. 2e ). Ribose reduced GAG loss in a dose-dependent manner compared with control from day 4 through day 8 ( Fig. 2f ). This can be attributed to increased AGE crosslinks ( Fig. 2a ) that reduce matrix permeability, preventing escape of GAG fragments from the tissue and to the reduced cell metabolism ( Fig. 2d ) that limits proteoglycan biosynthesis. 33
Figure 2.
Ribose dose response. Bovine cartilage explants cultured for 8 days and treated with 30, 100, or 300 mM ribose. Quantification of (a) AGE crosslinks and (b) equilibrium modulus. Chondrocyte viability images in (c) with green and red colors used to show viable and nonviable cells, respectively. Arrow points toward superficial layer of cartilage. Refer to Supplemental Figure S3b for quantification of viability. Cell metabolism in (d), cumulative nitrite release in (e), and cumulative GAG loss in (f). Data are presented as mean ± 95% confidence interval. *Versus control (P < 0.05). Statistical markers are colored with respect to the data.
Lique laborem atem assint.
Resveratrol and Curcumin Bind More Strongly to Collagen II Glycation Sites than Ribose
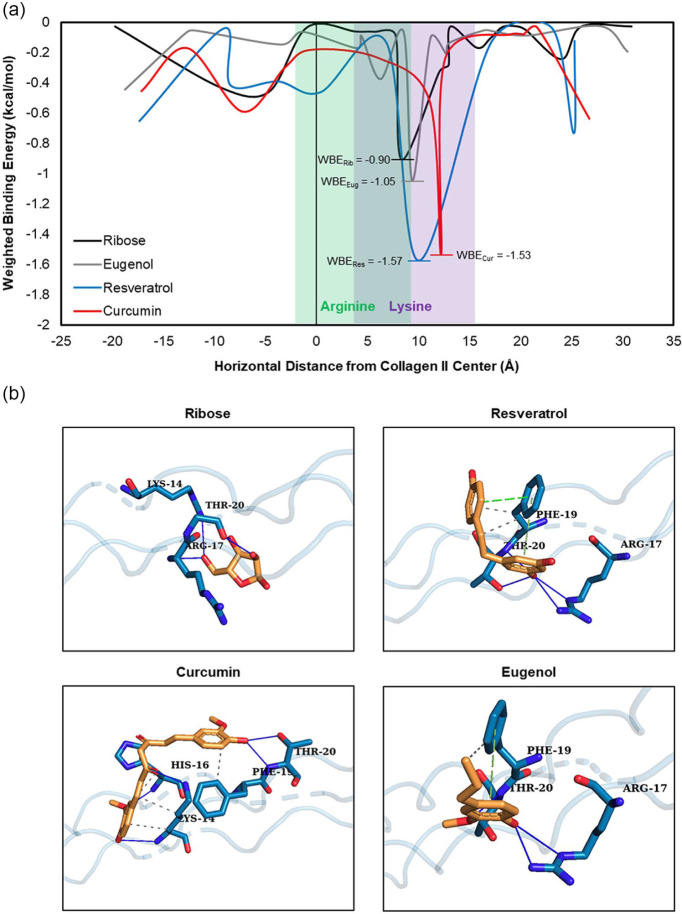
In silico blind docking analysis revealed that ribose, and all 3 experimental therapeutics—resveratrol, curcumin, and eugenol—strongly associated with arginine and lysine residues on the human collagen II fragment in multiple conformations relative to other binding sites on the protein fragment. WBE scores were plotted against the horizontal binding coordinate for each protein-ligand combination to visualize BE across the entire surface of the collagen II fragment ( Fig. 3a ). The strongest WBE for ribose and each experimental drug occurred at the location of arginine and lysine residues, particularly Lys-14 and Arg-17, which correspond to Lys-1130 and Arg-1133 of human collagen II alpha I chain, 34 as visualized by the ligand-residue interactions ( Fig. 3b ). Of the 4 ligands, ribose had the weakest WBE at this site, with a value of −0.9028 kcal/mol. The WBE of eugenol was similar at −1.0472 kcal/mol, while the WBE for curcumin and resveratrol were stronger at −1.5306 and −1.5741 kcal/mol, respectively ( Table 1 ). A complete table of WBEs for each protein-ligand combination can be found in Supplemental Table 1.
Figure 3.
Blind docking analysis of a human collagen II fragment with ribose, resveratrol, curcumin, and eugenol. (a) Weighted binding energy (WBE) of protein-ligand interactions at each binding site along the horizontal length of the collagen II fragment. The location of arginine and lysine residues within the protein structure are shown in green and purple, respectively. The global minimum WBE is marked and displayed for each ligand. (b) Images show each ligand, in orange, binding to specific residues, in teal, at the point of greatest binding energy for each molecule. Hydrogen bonds are represented by solid blue lines, pi stacking is represented by dashed green lines, and hydrophobic interactions are represented by dashed gray lines.
Table 1.
Location of Strongest Weighted Binding Energy (WBE) for Each Protein-Ligand Interaction along Type II Collagen Fragment.
| Ligand | Horizontal Coordinate (Å from Protein Center) | Binding Energy (kcal/mol) | No. of Conformations in Cluster | WBE (kcal/mol) |
|---|---|---|---|---|
| Ribose | 8.35 | −3.9 | 25 | −0.9028 |
| Resveratrol | 10.03 | −5.0 | 34 | −1.5741 |
| Curcumin | 12.23 | −5.7 | 29 | −1.5306 |
| Eugenol | 9.39 | −3.9 | 29 | −1.0472 |
Resveratrol and Curcumin, but Not Eugenol, Inhibit Ribose-Induced AGE Crosslinks
To validate the in silico blind docking results and evaluate the anti-AGE efficacy of potential AGE inhibitors in vitro, 100 mM ribose treatment was used as it effectively increased AGE crosslinks to physiological concentrations that increased matrix stiffness and reduced GAG loss while maintaining cell viability with reduced metabolism. Following 6-day treatment of 500 μM resveratrol, 500 μM curcumin, or 20 mM eugenol on frozen-dead cartilage explants treated with 100 mM ribose, resveratrol and curcumin showed significantly reduced AGE crosslinks, but eugenol did not ( Fig. 4a ). Consistent with these trends, cartilage stiffness reduced, while permeability increased by 3 to 4 times compared to ribose with resveratrol and curcumin treatment, but no change was seen with eugenol ( Fig. 4b and c ).
Figure 4.
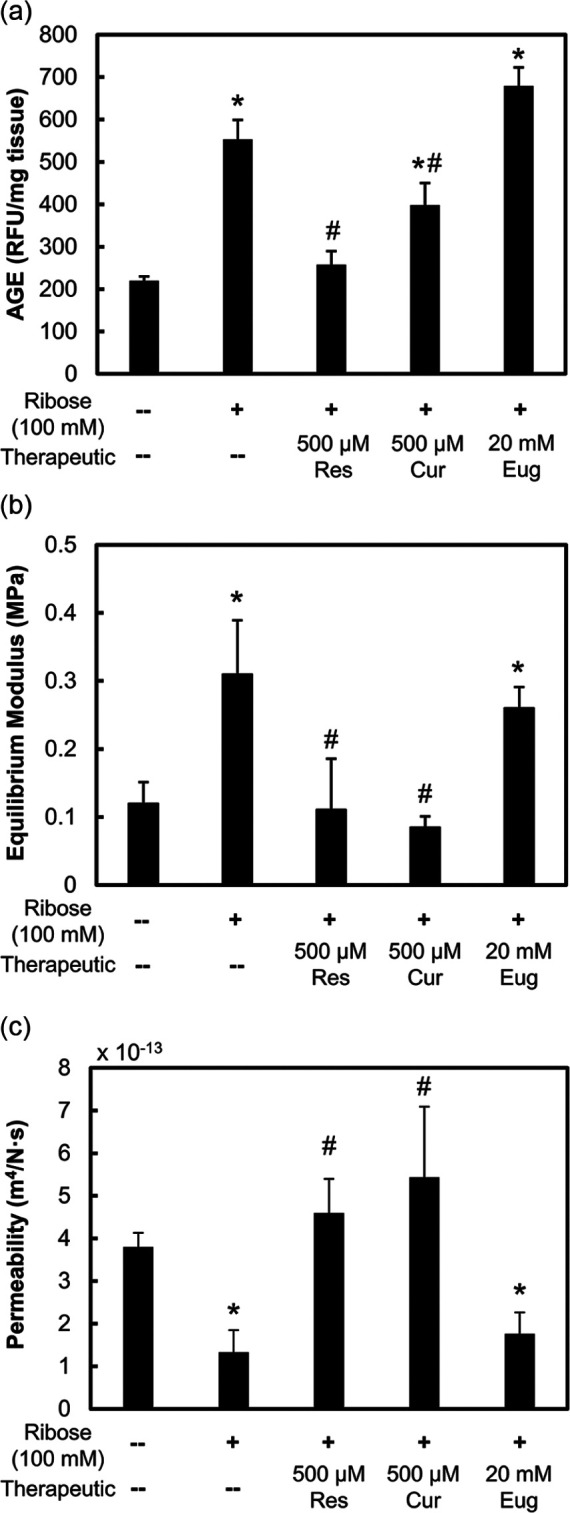
Mechanical response to potential advanced glycation end-product (AGE) inhibitors. Frozen-dead bovine cartilage explants incubated for 6 days and treated with 100 mM ribose and 500 μM resveratrol, 500 μM curcumin, or 20 mM eugenol. Data are presented as mean ± 95% confidence interval for (a) AGE crosslinks, (b) equilibrium modulus, and (c) permeability. *Versus control, #versus 100 mM ribose condition (P < 0.05).
Resveratrol and Curcumin Can Rescue Both Nonenzymatic Glycation and Enzymatic Degradation at Matrix and Cellular Level, but Eugenol Cannot
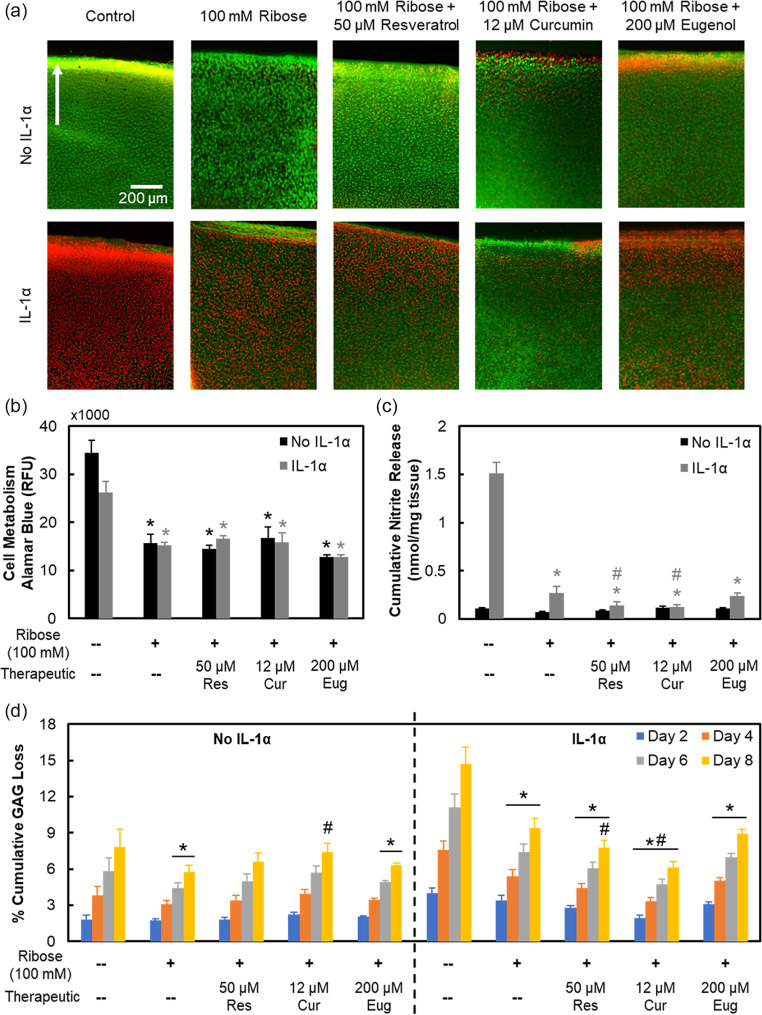
At therapeutic doses (not cytotoxic), resveratrol (50 μM) and curcumin (12 μM) were effective in partially inhibiting ribose-induced AGE crosslinks while reducing cartilage stiffness and increasing its permeability, but eugenol (200 μM) was not ( Fig. 5a-c ). Presence of IL-1 did not alter AGE concentration in ribose or drug treated conditions.
Figure 5.
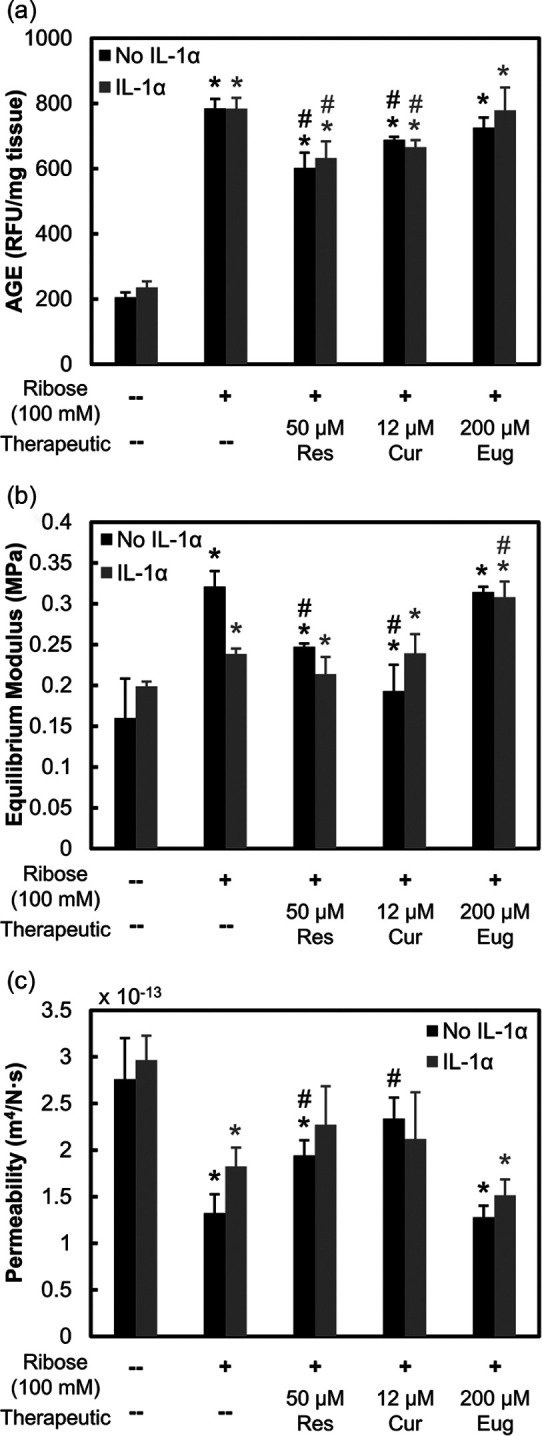
Biomechanical response to potential advanced glycation end-product (AGE) inhibitors. Bovine cartilage explants cultured for 8 days and treated with 100 mM ribose with or without interleukin-1-alpha (IL-1α) (2 ng/mL). Explants were further treated with 50 μM resveratrol, 12 μM curcumin, or 200 μM eugenol. Data are presented as mean ± 95% confidence interval for (a) AGE crosslinks, (b) equilibrium modulus, and (c) permeability. *Versus respective control, #versus respective 100 mM ribose condition (P < 0.05). Statistical markers are colored with respect to the bars.
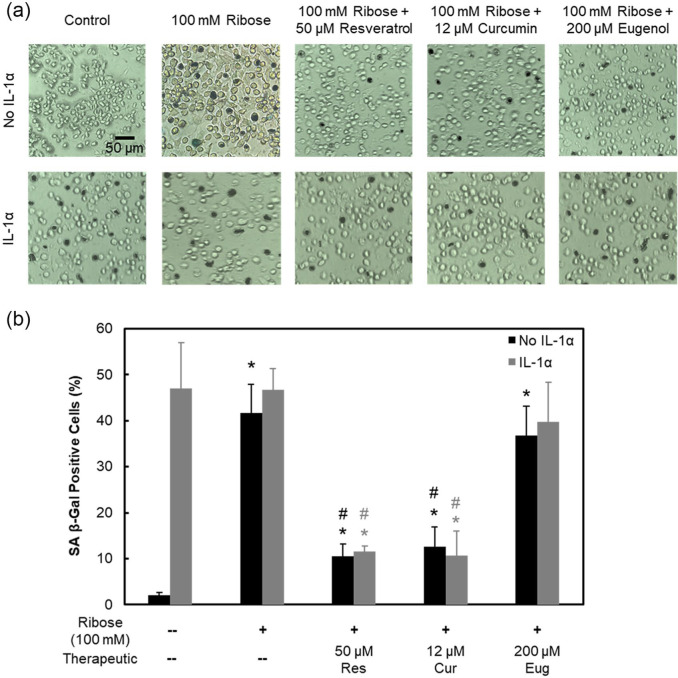
As ribose treatment alone did not produce a measurable inflammatory response within the short 8-day culture ( Fig. 2e ), IL-1, a downstream inflammatory marker of AGEs, was also administered to live cartilage explants for biological studies. IL-1 treatment resulted in complete cell death by 8 days of culture in both control and 100 mM Ribose conditions ( Fig. 6a , Supplemental Fig. S3c); however, no further drop in cellular metabolism than with ribose treatment was observed. Resveratrol and curcumin effectively rescued cell death induced by IL-1 but did not restore cellular metabolism levels ( Fig. 6b ). IL-1 treatment significantly enhanced nitrite release, which was suppressed in the presence of ribose. Since ribose is an integral component of ATP (adenosine triphosphate), its supplementation can enhance ATP availability as a cellular fuel source, thereby limiting the inflammatory effects of IL-1. Treatment with resveratrol and curcumin further suppressed this nitrite release ( Fig. 6c ). By day 8, both resveratrol and curcumin were able to bring back GAG loss levels that were suppressed by ribose to control level ( Fig. 6d ); both drugs are thus effective at inhibiting ribose-induced AGE crosslinks, thereby increasing tissue matrix permeability, which allows for easier release of GAGs, which were otherwise trapped within the dense AGE crosslinked collagen network of tissue. IL-1, as expected, upregulated GAG loss compared with untreated control, losing 1.9× more GAGs by day 8 (P < 0.0001), but in the presence of ribose-induced AGE crosslinks, GAG loss significantly dropped from day 4 onward (P = 0.0016) compared with IL-1 only treatment. Consistent with trends observed in the no IL-1 condition, both resveratrol and curcumin increased GAG loss levels by 1.2 times (P = 0.021) and 1.5 times (P < 0.0001) compared with the IL-1 + 100 mM ribose condition by day 8, respectively. Eugenol treatment resulted in statistically similar GAG loss to IL-1 + 100 mM ribose-treated explants. Finally, ribose treatment with or without IL-1 increased chondrocyte senescence by day 8, which was also rescued by both resveratrol and curcumin but not by eugenol ( Fig. 7 ).
Figure 6.
Biological response to potential advanced glycation end-product (AGE) inhibitors. Bovine cartilage explants cultured for 8 days and treated with 100 mM ribose with or without interleukin-1-alpha (IL-1α) (2 ng/mL). Explants were further treated with 50 μM resveratrol, 12 μM curcumin, or 200 μM eugenol. (a) Cartilage tissue viability images with green and red colors used to show viable and nonviable cells, respectively. Arrow points toward superficial layer of cartilage. Refer to Supplemental Figure S3c for quantification of viability. Twelve micromolar curcumin images shown are representative tissue images based on observations from 8-day chondrocyte cultures. Refer to Supplemental Figure S1b for chondrocyte culture images. Data are presented as mean ± 95% confidence interval for (b) cell metabolism, (c) cumulative nitrite release, and (d) cumulative glycosaminoglycan (GAG) loss. *Versus respective control, #versus respective 100 mM ribose condition (P < 0.05). Statistical markers are colored with respect to the bars.
Figure 7.
Senescence staining in response to potential advanced glycation end-product (AGE) inhibitors. Bovine articular chondrocytes cultured for 8 days and treated with 100 mM ribose with or without interleukin-1-alpha (IL-1α) (2 ng/mL). Chondrocytes were further treated with 50 μM resveratrol, 12 μM curcumin, or 200 μM eugenol. (a) Chondrocyte senescence images obtained using senescence-associated β-galactosidase (SA β-Gal) assay. Representative images of each condition showing presence of staining indicative of senescence. (b) Quantification of SA β-Gal positive cells shown as a percentage of total cells. Data are presented as mean ± 95% confidence interval. *Versus respective control, #versus respective 100 mM ribose condition (P < 0.05). Statistical markers are colored with respect to the bars.
Discussion
Excessive levels of AGEs in tissues are pathogenic and result in elevated levels of oxidative stress and inflammation that contributes to aging, diabetic complications, and several degenerative cartilage diseases including OA. 35 In cartilage, AGEs are formed through a nonenzymatic reaction between sugars and primary amines on collagen II and accumulate with age or are accelerated in response to chronic hyperglycemia in diabetes. AGEs have adverse effects on the cartilage extracellular matrix as well as on cellular health. Their accumulation increases collagen crosslinking, which increases cartilage stiffness, making the tissue more brittle and susceptible to fracture.1,2,9 In chondrocytes, AGEs can bind to the receptor RAGE, increasing production of inflammatory cytokines (e.g., IL-1, IL-6, and TNF-α), inflammatory mediators (e.g., nitric oxide), and degradative enzymes (e.g., MMPs), which both degrade and suppress the biosynthesis of GAGs and type II collagen in cartilage.12 -14 Therefore, inhibiting AGEs is a plausible therapeutic strategy to slow down aging-induced OA especially in the diabetic population. Here, we developed a cartilage explant culture model that better represents age-related OA in vivo by increasing AGE crosslinking (3-4 times) and tissue stiffness (2 times) levels comparable to those of 60-year-old human cartilage,8,36 while lowering metabolic activity and enhancing senescence yet minimally affecting cell viability. 2 We used a blind docking approach to predict the anti-AGE capabilities of resveratrol, curcumin, and eugenol before utilizing the developed live explant culture model for evaluating the drugs’ in vitro ability to inhibit nonenzymatic AGE-induced mechanical crosslinking and the resulting downstream biological response in the presence and absence of IL-1α.
Blind docking analysis revealed that resveratrol and curcumin possessed higher binding energies for lysine and arginine residues on the collagen II fragment compared with ribose, thereby indicating that these drugs have a strong likelihood of competitively inhibiting AGE accumulation in vitro, which was confirmed by our explant studies ( Table 1 , Figs. 4 and 5 ). The drugs’ efficacy can be attributed to their plant-based, polyphenolic, antioxidant nature because of the presence of free para-hydroxyl (4′-OH) groups 16 that can contribute to their anti-glycation ability. This was further confirmed by blind docking analysis as strong hydrogen bonding was seen between the 4′-OH groups and collagen II arginine and lysine residues ( Fig. 4b ). The greater number of free hydroxyl groups in resveratrol and curcumin over eugenol may also explain their stronger association to primary glycation sites. It should be noted that lysines present throughout the collagen II structure may be involved in other types of interactions such as glycosylation and intermolecular crosslinking during the formation of the 3-dimensional crystalline structure of the molecule; thus, not all amines may be available for AGE crosslinking. However, our explant studies, which show reduced AGE crosslink formation, confirm the strong preferential binding of resveratrol and curcumin to amines available for glycation.
Validating the blind docking simulation results, we show that both resveratrol and curcumin can effectively inhibit ribose-induced AGE crosslinks in articular cartilage, restoring its mechanical properties even in presence of the degradative effects of IL-1α ( Fig. 5 ). Both of these drugs also rescued IL-1-induced chondrocyte death and suppressed nitrite release ( Fig. 6 ). Our results also show the rescuing of ribose and IL-1-induced cellular senescence with both resveratrol and curcumin. This can be attributed to the ability of these drugs to activate peroxisome proliferator-activated receptor gamma (PPARγ), 37 which regulates glucose metabolism. PPARγ, on activation, has shown the ability to suppress IL-1-induced nitrite release and MMP-13 expression in human chondrocytes. 38 In another study, diabetic mice treated with pioglitazone, a PPARγ agonist, showed lower levels of IL-6 and MMP-13 in addition to a reduction in glucose and AGE levels. 39
AGE accumulation also reduced GAG release from the cartilage matrix ( Figs. 1f , 2f , and 6d ) potentially due to collagen crosslinking that reduced matrix permeability by preventing escape of GAGs, as well as suppressed chondrocyte metabolism that can limit production of new proteoglycans.2,11,33,40 Both resveratrol and curcumin were able to reverse the ribose suppressed GAG release levels to control in presence of IL-1α, which can be attributed to their ability to inhibit AGE crosslinks, as the 2 drugs were unable to restore cellular metabolic activity. Combination therapy of resveratrol or curcumin together or in combination with an anabolic factor may be explored for restoring metabolic activity. For example, curcumin delivered in combination with microparticles encapsulating chondrogenic kartogenin significantly suppressed hypertrophic and inflammatory activity in a rabbit cartilage defect model, leading to enhanced cartilage repair compared to kartogenin microparticle monotherapy. 41 Senolytic agents such as UBX0101, which can selectively target and kill senescing cells, are also of interest for combination therapy. 42 UBX0101 is a senolytic known for inhibiting the intracellular inactivation of p53 by MDM2 (murine double minute 2), thereby allowing for p53 to continue its role in triggering apoptosis of damaged or senescing cells that contribute to inflammatory activity.42,43 Recently, UBX0101 was shown not only to significantly suppress cartilage erosion and MMP-13 and IL-1β expression but also to elevate COL2A1 and ACAN levels, indicating increased cellular metabolic activity and post-traumatic OA attenuation following repetitive intra-articular (IA) injections in ACLT mice. 42 Additionally, several studies have reported that resveratrol and curcumin can suppress RAGE expression in various cell types.44 -46 Thus, while not examined here, the inhibition of AGE-induced downstream biological activity by resveratrol and curcumin may be attributed toward their ability to suppress RAGE in addition to other biological pathways that contribute to their anti-IL-1 properties such as PPARγ. Eugenol, however had no effect on AGE crosslink formation or enzymatic degradation induced by IL-1.
Resveratrol and curcumin can rescue both enzymatic degradation at matrix and cellular levels as well as nonenzymatic glycation-induced structural changes. A few prior reports have evaluated their effectiveness in rescuing enzymatic degradation. For example, oral resveratrol reduced apoptosis and cartilage destruction in experimental OA rabbits. 47 Following 24-hour pretreatment with resveratrol, porcine chondrocytes showed suppression of AGE-induced iNOS, NO, and NF-κB expression as well as reduced proteoglycan degradation. 17 Csaki et al. 25 pretreated human articular chondrocytes with 50 μM each of resveratrol and curcumin before administering IL-1β for 24 h and observed significant suppression of NF-κB, MMP-3, and MMP-9 expression. In cartilage explant models, curcumin reduced IL-1-induced MMP-3, GAG, and PGE2 release.26,48 These studies further aid to the promise of using resveratrol and curcumin as anti-AGE and anti-IL-1 therapeutics; however, our study is the first to report the ability of either of these drugs to suppress both AGE crosslinking and IL-1-induced catabolic activity in a live tissue matrix model. To study nonenzymatic glycation, past work has either crosslinked the collagen network of dead cartilage explants using high sugar concentrations to study changes in tissue mechanical properties or separately evaluated the downstream cellular response of AGE accumulation using chondrocyte culture. As a result, it had not been possible to study the downstream biological effects of AGE crosslinks on cell health. This is thus the first time that anti-AGE drugs, resveratrol, curcumin, and eugenol, have been evaluated for inhibiting AGE crosslink formation in a live cartilage matrix to simultaneously restore tissue biomechanical properties and the resulting downstream matrix and cellular dysfunction. Our results show that resveratrol and curcumin have the potential to treat aging-induced OA especially in the diabetic population who are at a higher risk of early OA onset due to rapid AGE accumulation in their tissue matrix. The in vitro age-related, nonenzymatic, glycation-induced OA explant culture model established in this study provides a tool for screening anti-glycating drugs by concurrently evaluating changes in tissue mechanical properties and the resulting downstream biological effects on cell and matrix health.
While desirable, it is nontrivial to determine the ideal time of intervention, dosing, patient sub-type and the duration of clinical trials evaluating preventive OA therapies. As such, resveratrol and curcumin have only been tested clinically for treating older patients suffering from OA-associated pain and/or stiffness. Marouf et al. 49 reported that 500 mg/d oral resveratrol alleviated knee pain severity in a time-dependent manner and reduced serum concentrations of inflammatory markers after 90 days treatment in combination with meloxicam. 49 A dose of 1500 mg/d oral curcumin led to similar improvements in WOMAC (Western Ontario and McMaster Universities Osteoarthritis Index) scores through 4 weeks as ibuprofen, however, with significantly fewer gastrointestinal issues. 50 A clinical trial investigating oral 1000 mg/d curcuminoid capsules (Curamin) showed improvements in WOMAC scores and physical performance tests but only after 3 months of treatment. 51 These trials, however, did not quantify serum or synovial fluid AGE concentrations, thus the anticrosslinking effect of these drugs on cartilage tissue in human has yet to be confirmed.
Local IA administration of these drugs can elicit a greater therapeutic benefit compared with oral administration by targeting the avascular cartilage tissue and the residing chondrocytes. In a proof-of-concept study, Elmali et al. 52 reported that daily IA injections of resveratrol into lipopolysaccharide (LPS)-treated rabbit knees decreased cartilage destruction and preserved matrix proteoglycan content at 3 weeks’ time, an effect also shown in response to a rabbit traumatic knee injury model utilized by Wang et al. 47 However, repetitive injections are clinically infeasible due to concerns surrounding toxicity, injection site infection risk and patient discomfort. 21 IA-administered small molecule drugs like resveratrol suffer from very short intra-joint residence time due to the rapid turnover of synovial fluid and an inability to penetrate the dense cartilage matrix packed with highly negatively charged aggrecans. 53 This can be overcome by delivering drugs using optimally charged cationic carriers that utilize electrostatic interactions to enhance their targeting, uptake and retention within the negatively charged cartilage.53 -58 AGE accumulation within cartilage reduces GAG release levels ( Figs. 1f , 2f , and 6d ) and is associated with the loss of primary amines on collagen II, which can increase the tissue’s net negative fixed charge density (FCD). This high negative FCD can be further utilized for enhancing intra-cartilage targeting53,57 -59 and residence time of such drugs following their IA administration by making them positively charged.54,56,60
It is important to note that this study used ribose to induce AGE crosslinking, whereas in the body, there are several sugars, including ribose, which contribute to the formation of several different AGEs. 20 Thus, quantification of general AGE levels instead of specific AGEs allows for a more accurate comparison between in vitro and in vivo crosslinking. Furthermore, it should be noted that we used tissues from young bovine joints of tightly controlled age to develop an AGE-induced OA model of aging, in order to minimize animal-to-animal variation. Similar glycating effects and biological response should be expected with adult cartilage. Finally, since AGEs stimulate the mitogen-activated protein kinase (MAPK) and NF-κB pathways, both of which activate the iNOS gene resulting in enhanced nitrite release from chondrocytes, 27 nitrite release with ribose was expected, but not observed. It is possible that an 8-day culture was too short to produce detectable levels of nitrite by the Griess assay, and that its inflammatory response remained upstream at transcriptional genetic level, which was not studied here. However, to address this limitation, IL-1, an inflammatory marker naturally upregulated in response to AGEs, was added to the system.
In conclusion, we have developed a live cartilage explant culture model of ribose-treated AGE-induced OA that better represents aged cartilage in vivo and provides a tool for evaluating the effectiveness of drugs in inhibiting AGE crosslink formation. Our results suggest that resveratrol and curcumin are promising compounds to treat both age-related and diabetes-induced OA, as they are natural compounds with strong antioxidant and anti-glycation properties. Their local delivery into the joint space can be enabled by using cartilage targeting cationic nanocarriers that are especially relevant as cartilage tissue with a high AGE concentration possesses a greater net negative FCD which represents a promising area of future work.29,56,61
Supplemental Material
Supplemental material, sj-docx-1-car-10.1177_1947603520988768 for Resveratrol and Curcumin Attenuate Ex Vivo Sugar-Induced Cartilage Glycation, Stiffening, Senescence, and Degeneration by Shikhar Mehta, Cameron C. Young, Matthew R. Warren, Sumayyah Akhtar, Sandra J. Shefelbine, Justin D. Crane and Ambika G. Bajpayee in CARTILAGE
Supplemental material, sj-docx-2-car-10.1177_1947603520988768 for Resveratrol and Curcumin Attenuate Ex Vivo Sugar-Induced Cartilage Glycation, Stiffening, Senescence, and Degeneration by Shikhar Mehta, Cameron C. Young, Matthew R. Warren, Sumayyah Akhtar, Sandra J. Shefelbine, Justin D. Crane and Ambika G. Bajpayee in CARTILAGE
Supplemental material, sj-tex-1-car-10.1177_1947603520988768 for Resveratrol and Curcumin Attenuate Ex Vivo Sugar-Induced Cartilage Glycation, Stiffening, Senescence, and Degeneration by Shikhar Mehta, Cameron C. Young, Matthew R. Warren, Sumayyah Akhtar, Sandra J. Shefelbine, Justin D. Crane and Ambika G. Bajpayee in CARTILAGE
Supplemental material, sj-tex-2-car-10.1177_1947603520988768 for Resveratrol and Curcumin Attenuate Ex Vivo Sugar-Induced Cartilage Glycation, Stiffening, Senescence, and Degeneration by Shikhar Mehta, Cameron C. Young, Matthew R. Warren, Sumayyah Akhtar, Sandra J. Shefelbine, Justin D. Crane and Ambika G. Bajpayee in CARTILAGE
Supplemental material, sj-tex-3-car-10.1177_1947603520988768 for Resveratrol and Curcumin Attenuate Ex Vivo Sugar-Induced Cartilage Glycation, Stiffening, Senescence, and Degeneration by Shikhar Mehta, Cameron C. Young, Matthew R. Warren, Sumayyah Akhtar, Sandra J. Shefelbine, Justin D. Crane and Ambika G. Bajpayee in CARTILAGE
Footnotes
Acknowledgments and Funding: We would like to thank our funding sources. The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: The Northeastern University Global Resilience Institute, Northeastern University Office of Undergraduate Research and Fellowships, United States Department of Defense through the Congressionally Directed Medical Research Programs (CDMRP) under W81XWH-17-1-0085 and National Institutes of Health (1R01AR075121-01A1). These funding sources were not involved in the writing of this manuscript, study design, or the collection, analysis and interpretation of any data.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Ethical Approval: Ethical approval was not sought for the present study because this research did not require Ethics Board approval because it does not involve human or animal subjects.
ORCID iD: Shikhar Mehta  https://orcid.org/0000-0002-9818-8560
https://orcid.org/0000-0002-9818-8560
Supplementary material for this article is available on the Cartilage website at https://journals.sagepub.com/home/car.
References
- 1. Bank RA, Bayliss MT, Lafeber FP, Maroudas A, Tekoppele JM. Ageing and zonal variation in post-translational modification of collagen in normal human articular cartilage. The age-related increase in non-enzymatic glycation affects biomechanical properties of cartilage. Biochem J. 1998;330(Pt 1):345-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Lotz M, Loeser RF. Effects of aging on articular cartilage homeostasis. Bone. 2012;51:241-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Eymard F, Parsons C, Edwards MH, Petit-Dop F, Reginster JY, Bruyere O, et al. Diabetes is a risk factor for knee osteoarthritis progression. Osteoarthritis Cartilage. 2015;23:851-9. [DOI] [PubMed] [Google Scholar]
- 4. Schett G, Kleyer A, Perricone C, Sahinbegovic E, Iagnocco A, Zwerina J, et al. Diabetes is an independent predictor for severe osteoarthritis: results from a longitudinal cohort study. Diabetes Care. 2013;36:403-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Berenbaum F. Diabetes-induced osteoarthritis: from a new paradigm to a new phenotype. Ann Rheum Dis. 2011;70:1354-6. [DOI] [PubMed] [Google Scholar]
- 6. Gautieri A, Redaelli A, Buehler MJ, Vesentini S. Age- and diabetes-related nonenzymatic crosslinks in collagen fibrils: candidate amino acids involved in advanced glycation end-products. Matrix Biol. 2014;34:89-95. [DOI] [PubMed] [Google Scholar]
- 7. DeGroot J, Verzijl N, Wenting-van Wijk MJ, Jacobs KM, Van El B, Van Roermund PM, et al. Accumulation of advanced glycation end products as a molecular mechanism for aging as a risk factor in osteoarthritis. Arthritis Rheum. 2004;50:1207-15. [DOI] [PubMed] [Google Scholar]
- 8. Verzijl N, DeGroot J, Oldehinkel E, Bank RA, Thorpe SR, Baynes JW, et al. Age-related accumulation of Maillard reaction products in human articular cartilage collagen. Biochem J. 2000;350(Pt 2):381-7. [PMC free article] [PubMed] [Google Scholar]
- 9. Chen AC, Temple MM, Ng DM, Verzijl N, DeGroot J, TeKoppele JM, et al. Induction of advanced glycation end products and alterations of the tensile properties of articular cartilage. Arthritis Rheum. 2002;46:3212-7. [DOI] [PubMed] [Google Scholar]
- 10. DeGroot J, Verzijl N, Jacobs KM, Budde M, Bank RA, Bijlsma JW, et al. Accumulation of advanced glycation endproducts reduces chondrocyte-mediated extracellular matrix turnover in human articular cartilage. Osteoarthritis Cartilage. 2001;9:720-6. [DOI] [PubMed] [Google Scholar]
- 11. Loeser RF. Aging and osteoarthritis: the role of chondrocyte senescence and aging changes in the cartilage matrix. Osteoarthritis Cartilage. 2009;17:971-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Nah SS, Choi IY, Lee CK, Oh JS, Kim YG, Moon HB, et al. Effects of advanced glycation end products on the expression of COX-2, PGE2 and NO in human osteoarthritic chondrocytes. Rheumatology (Oxford). 2008;47:425-31. [DOI] [PubMed] [Google Scholar]
- 13. Rasheed Z, Akhtar N, Haqqi TM. Advanced glycation end products induce the expression of interleukin-6 and interleukin-8 by receptor for advanced glycation end product-mediated activation of mitogen-activated protein kinases and nuclear factor-κB in human osteoarthritis chondrocytes. Rheumatology (Oxford). 2011;50:838-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Yammani RR, Carlson CS, Bresnick AR, Loeser RF. Increase in production of matrix metalloproteinase 13 by human articular chondrocytes due to stimulation with S100A4: role of the receptor for advanced glycation end products. Arthritis Rheum. 2006;54:2901-11. [DOI] [PubMed] [Google Scholar]
- 15. Singh P, Jayaramaiah RH, Agawane SB, Vannuruswamy G, Korwar AM, Anand A, et al. Potential dual role of eugenol in inhibiting advanced glycation end products in diabetes: proteomic and mechanistic insights. Sci Rep. 2016;6:18798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Bors W, Michel C. Chemistry of the antioxidant effect of polyphenols. Ann N Y Acad Sci. 2002;957:57-69. [DOI] [PubMed] [Google Scholar]
- 17. Liu FC, Hung LF, Wu WL, Chang DM, Huang CY, Lai JH, et al. Chondroprotective effects and mechanisms of resveratrol in advanced glycation end products-stimulated chondrocytes. Arthritis Res Ther. 2010;12:R167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Yang Q, Wu S, Mao X, Wang W, Tai H. Inhibition effect of curcumin on TNF-α and MMP-13 expression induced by advanced glycation end products in chondrocytes. Pharmacology. 2013;91:77-85. [DOI] [PubMed] [Google Scholar]
- 19. Fick JM, Huttu MR, Lammi MJ, Korhonen RK. In vitro glycation of articular cartilage alters the biomechanical response of chondrocytes in a depth-dependent manner. Osteoarthritis Cartilage. 2014;22:1410-8. [DOI] [PubMed] [Google Scholar]
- 20. Verzijl N, DeGroot J, Ben ZC, Brau-Benjamin O, Maroudas A, Bank RA, et al. Crosslinking by advanced glycation end products increases the stiffness of the collagen network in human articular cartilage: a possible mechanism through which age is a risk factor for osteoarthritis. Arthritis Rheum. 2002;46:114-23. [DOI] [PubMed] [Google Scholar]
- 21. Mehta S, Akhtar S, Porter RM, Önnerfjord P, Bajpayee AG. Interleukin-1 receptor antagonist (IL-1Ra) is more effective in suppressing cytokine-induced catabolism in cartilage-synovium co-culture than in cartilage monoculture. Arthritis Res Ther. 2019;21:228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Pouran B, Arbabi V, Bajpayee AG, van Tiel J, Töyräs J, Jurvelin JS, et al. Multi-scale imaging techniques to investigate solute transport across articular cartilage. J Biomech. 2018;78:10-20. [DOI] [PubMed] [Google Scholar]
- 23. He T, Li B, Colombani T, Joshi-Navare K, Mehta S, Kisiday J, et al. Hyaluronic acid-based shape-memory cryogel scaffolds for focal cartilage defect repair. Tissue Eng Part A. Published online October 27, 2020. doi: 10.1089/ten.TEA.2020.0264 [DOI] [PubMed] [Google Scholar]
- 24. Kisiday JD, Kurz B, DiMicco MA, Grodzinsky AJ. Evaluation of medium supplemented with insulin–transferrin–selenium for culture of primary bovine calf chondrocytes in three-dimensional hydrogel scaffolds. Tissue Eng. 2005;11:141-51. [DOI] [PubMed] [Google Scholar]
- 25. Csaki C, Mobasheri A, Shakibaei M. Synergistic chondroprotective effects of curcumin and resveratrol in human articular chondrocytes: inhibition of IL-1β-induced NF-κB-mediated inflammation and apoptosis. Arthritis Res Ther. 2009;11:R165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Clutterbuck AL, Allaway D, Harris P, Mobasheri A. Curcumin reduces prostaglandin E2, matrix metalloproteinase-3 and proteoglycan release in the secretome of interleukin 1β-treated articular cartilage. F1000Res. 2013;2:147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Loeser RF, Yammani RR, Carlson CS, Chen H, Cole A, Im HJ, et al. Articular chondrocytes express the receptor for advanced glycation end products: potential role in osteoarthritis. Arthritis Rheum. 2005;52:2376-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Ho YC, Huang FM, Chang YC. Mechanisms of cytotoxicity of eugenol in human osteoblastic cells in vitro. Int Endod J. 2006;39:389-93. [DOI] [PubMed] [Google Scholar]
- 29. Bajpayee AG, Rodolfo E, Scheu M, Varady NH, Yannatos IA, Brown LA, et al. Sustained intra-cartilage delivery of low dose dexamethasone using a cationic carrier for treatment of post traumatic osteoarthritis. Eur Cell Mater. 2017; 34:341-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Sah RL, Trippel SB, Grodzinsky AJ. Differential effects of serum, insulin-like growth factor-I, and fibroblast growth factor-2 on the maintenance of cartilage physical properties during long-term culture. J Orthop Res. 1996;14:44-52. [DOI] [PubMed] [Google Scholar]
- 31. Monnier VM, Kohn RR, Cerami A. Accelerated age-related browning of human collagen in diabetes mellitus. Proc Natl Acad Sci U S A. 1984;81:583-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Farndale RW, Buttle DJ, Barrett AJ. Improved quantitation and discrimination of sulphated glycosaminoglycans by use of dimethylmethylene blue. Biochim Biophys Acta. 1986;883:173-7. [DOI] [PubMed] [Google Scholar]
- 33. DeGroot J, Verzijl N, Bank RA, Lafeber FP, Bijlsma JW, TeKoppele JM. Age-related decrease in proteoglycan synthesis of human articular chondrocytes: the role of nonenzymatic glycation. Arthritis Rheum. 1999;42:1003-9. [DOI] [PubMed] [Google Scholar]
- 34. UniProt. UniProtKB—P02458 (CO2A1_HUMAN). Published April 10, 2018. Accessed July 28, 2020. https://www.uniprot.org/uniprot/P02458
- 35. Verzijl N, Bank RA, TeKoppele JM, DeGroot J. AGEing and osteoarthritis: a different perspective. Curr Opin Rheumatol. 2003;15:616-22. [DOI] [PubMed] [Google Scholar]
- 36. Verzijl N, DeGroot J, Thorpe SR, Bank RA, Shaw JN, Lyons TJ, et al. Effect of collagen turnover on the accumulation of advanced glycation end products. J Biol Chem. 2000;275:39027-31. [DOI] [PubMed] [Google Scholar]
- 37. Shen X, Wang M, Bi X, Zhang J, Wen S, Fu G, et al. Resveratrol prevents endothelial progenitor cells from senescence and reduces the oxidative reaction via PPAR‑γ/HO‑1 pathways. Mol Med Rep. 2016;14:5528-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Fahmi H, Di Battista JA, Pelletier JP, Mineau F, Ranger P, Martel-Pelletier J. Peroxisome proliferator–activated receptor γ activators inhibit interleukin-1β–induced nitric oxide and matrix metalloproteinase 13 production in human chondrocytes. Arthritis Rheum. 2001;44:595-607. [DOI] [PubMed] [Google Scholar]
- 39. Chen YJ, Chan DC, Lan KC, Wang CC, Chen CM, Chao SC, et al. PPARγ is involved in the hyperglycemia-induced inflammatory responses and collagen degradation in human chondrocytes and diabetic mouse cartilages. J Orthop Res. 2015;33:373-81. [DOI] [PubMed] [Google Scholar]
- 40. Vos P, Mastbergen S, Huisman A, de Boer T, DeGroot J, Polak A, et al. In end stage osteoarthritis, cartilage tissue pentosidine levels are inversely related to parameters of cartilage damage. Osteoarthritis Cartilage. 2012;20:233-40. [DOI] [PubMed] [Google Scholar]
- 41. Asgari N, Bagheri F, Eslaminejad MB, Ghanian MH, Sayahpour FA, Ghafari AM. Dual functional construct containing kartogenin releasing microtissues and curcumin for cartilage regeneration. Stem Cell Res Ther. 2020;11:289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Jeon OH, Kim C, Laberge RM, Demaria M, Rathod S, Vasserot AP, et al. Local clearance of senescent cells attenuates the development of post-traumatic osteoarthritis and creates a pro-regenerative environment. Nat Med. 2017;23:775-81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Nag S, Qin J, Srivenugopal KS, Wang M, Zhang R. The MDM2-p53 pathway revisited. J Biomed Res. 2013;27:254-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Khazaei M, Karimi J, Sheikh N, Goodarzi MT, Saidijam M, Khodadadi I, et al. Effects of resveratrol on receptor for advanced glycation end products (RAGE) expression and oxidative stress in the liver of rats with type 2 diabetes. Phytother Res. 2016;30:66-71. [DOI] [PubMed] [Google Scholar]
- 45. Lin J, Tang Y, Kang Q, Feng Y, Chen A. Curcumin inhibits gene expression of receptor for advanced glycation end-products (RAGE) in hepatic stellate cells in vitro by elevating PPARγ activity and attenuating oxidative stress. Br J Pharmacol. 2012;166:2212-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Moridi H, Karimi J, Sheikh N, Goodarzi MT, Saidijam M, Yadegarazari R, et al. Resveratrol-dependent down-regulation of receptor for advanced glycation end-products and oxidative stress in kidney of rats with diabetes. Int J Endocrinol Metab. 2015;13:e23542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Wang J, Gao JS, Chen JW, Li F, Tian J. Effect of resveratrol on cartilage protection and apoptosis inhibition in experimental osteoarthritis of rabbit. Rheumatol Int. 2012;32:1541-8. [DOI] [PubMed] [Google Scholar]
- 48. Clutterbuck AL, Mobasheri A, Shakibaei M, Allaway D, Harris P. Interleukin-1β–induced extracellular matrix degradation and glycosaminoglycan release is inhibited by curcumin in an explant model of cartilage inflammation. Ann N Y Acad Sci. 2009;1171:428-35. [DOI] [PubMed] [Google Scholar]
- 49. Marouf BH, Hussain SA, Ali ZS, Ahmmad RS. Resveratrol supplementation reduces pain and inflammation in knee osteoarthritis patients treated with meloxicam: a randomized placebo-controlled study. J Med Food. 2018;21:1253-9. [DOI] [PubMed] [Google Scholar]
- 50. Kuptniratsaikul V, Dajpratham P, Taechaarpornkul W, Buntragulpoontawee M, Lukkanapichonchut P, Chootip C, et al. Efficacy and safety of Curcuma domestica extracts compared with ibuprofen in patients with knee osteoarthritis: a multicenter study. Clin Interv Aging. 2014;9:451-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Haroyan A, Mukuchyan V, Mkrtchyan N, Minasyan N, Gasparyan S, Sargsyan A, et al. Efficacy and safety of curcumin and its combination with boswellic acid in osteoarthritis: a comparative, randomized, double-blind, placebo-controlled study. BMC Complement Altern Med. 2018;18:260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Elmali N, Baysal O, Harma A, Esenkaya I, Mizrak B. Effects of resveratrol in inflammatory arthritis. Inflammation. 2007; 30:1-6. [DOI] [PubMed] [Google Scholar]
- 53. Bajpayee AG, Grodzinsky AJ. Cartilage-targeting drug delivery: can electrostatic interactions help? Nat Rev Rheumatol. 2017;13:183-93. [DOI] [PubMed] [Google Scholar]
- 54. He T, Zhang C, Vedadghavami A, Mehta S, Clark HA, Porter RM, et al. Multi-arm Avidin nano-construct for intra-cartilage delivery of small molecule drugs. J Control Release. 2019; 318:109-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Mehta S, He T, Bajpayee AG. Recent advances in targeted drug delivery for treatment of osteoarthritis. Curr Opin Rheumatol. 2020;33:94-109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Vedadghavami A, Wagner EK, Mehta S, He T, Zhang C, Bajpayee AG. Cartilage penetrating cationic peptide carriers for applications in drug delivery to avascular negatively charged tissues. Acta Biomater. 2019;93:258-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Vedadghavami A, Zhang C, Bajpayee AG. Overcoming negatively charged tissue barriers: drug delivery using cationic peptides and proteins. Nano Today. 2020;34:100898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Young CC, Vedadghavami A, Bajpayee AG. Bioelectricity for drug delivery: the promise of cationic therapeutics. Bioelectricity. 2020;2(2_suppl):68-81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Vedadghavami A, Mehta S, Bajpayee AG. Characterization of intra-cartilage transport properties of cationic peptide carriers. J Vis Exp. 2020;(162). doi: 10.3791/61340 [DOI] [PubMed] [Google Scholar]
- 60. Wagner EK, Vedadghavami A, Jacobsen TD, Goel SA, Chahine NO, Bajpayee AG. Avidin grafted dextran nanostructure enables a month-long intra-discal retention. Sci Rep. 2020;10:12017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Bajpayee AG, Quadir MA, Hammond PT, Grodzinsky AJ. Charge based intra-cartilage delivery of single dose dexamethasone using avidin nano-carriers suppresses cytokine-induced catabolism long term. Osteoarthritis Cartilage. 2016;24:71-81. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-car-10.1177_1947603520988768 for Resveratrol and Curcumin Attenuate Ex Vivo Sugar-Induced Cartilage Glycation, Stiffening, Senescence, and Degeneration by Shikhar Mehta, Cameron C. Young, Matthew R. Warren, Sumayyah Akhtar, Sandra J. Shefelbine, Justin D. Crane and Ambika G. Bajpayee in CARTILAGE
Supplemental material, sj-docx-2-car-10.1177_1947603520988768 for Resveratrol and Curcumin Attenuate Ex Vivo Sugar-Induced Cartilage Glycation, Stiffening, Senescence, and Degeneration by Shikhar Mehta, Cameron C. Young, Matthew R. Warren, Sumayyah Akhtar, Sandra J. Shefelbine, Justin D. Crane and Ambika G. Bajpayee in CARTILAGE
Supplemental material, sj-tex-1-car-10.1177_1947603520988768 for Resveratrol and Curcumin Attenuate Ex Vivo Sugar-Induced Cartilage Glycation, Stiffening, Senescence, and Degeneration by Shikhar Mehta, Cameron C. Young, Matthew R. Warren, Sumayyah Akhtar, Sandra J. Shefelbine, Justin D. Crane and Ambika G. Bajpayee in CARTILAGE
Supplemental material, sj-tex-2-car-10.1177_1947603520988768 for Resveratrol and Curcumin Attenuate Ex Vivo Sugar-Induced Cartilage Glycation, Stiffening, Senescence, and Degeneration by Shikhar Mehta, Cameron C. Young, Matthew R. Warren, Sumayyah Akhtar, Sandra J. Shefelbine, Justin D. Crane and Ambika G. Bajpayee in CARTILAGE
Supplemental material, sj-tex-3-car-10.1177_1947603520988768 for Resveratrol and Curcumin Attenuate Ex Vivo Sugar-Induced Cartilage Glycation, Stiffening, Senescence, and Degeneration by Shikhar Mehta, Cameron C. Young, Matthew R. Warren, Sumayyah Akhtar, Sandra J. Shefelbine, Justin D. Crane and Ambika G. Bajpayee in CARTILAGE