Abstract
Objective
The search for an effective and long-lasting strategy to treat osteochondral defects (OCD) is a great challenge. Regenerative medicine launched a new era of research in orthopaedics for restoring normal tissue functions. The aim of this study was to test the healing potential of Rigenera micrografting technology in a rat model of OCD by investigating 2 cartilage donor sites.
Methods
Full-thickness OCD was bilaterally created in the knee joints of rats. Animals were randomly divided into 2 groups based on the anatomical site used for micrograft collection: articular (TO) and xiphoid (XA). Micrograft was injected into the knee via an intra-articular approach. The contralateral joint served as the control. Euthanasia was performed 2 months after the set-up of OCD. Histological evaluations foresaw hematoxylin/eosin and safranin-O/fast green staining, the modified O’Driscoll score, and collagen 1A1 and 2A1 immunostaining. Kruskal-Wallis and the post hoc Dunn test were performed to evaluate differences among groups.
Results
Histological results showed defect filling in both autologous micrografts. The TO group displayed tissue repair with more hyaline-like characteristics than its control (P < 0.01). A fibrocartilaginous aspect was instead noticed in the XA group. Immunohistochemical assessments on type 2A1 and type 1 collagens confirmed the best histological results in the TO group.
Conclusions
TO and XA groups contributed to a different extent to fill the OCD lesions. TO group provided the best histological and immunohistochemical results; therefore, it could be a promising method to treat OCD after the validation in a larger animal model.
Keywords: osteochondral defects, autologous micrograft, osteochondral repair, immunohistopathology, rat model
Introduction
OCD of the knee is among the most frequent musculoskeletal disorders that impair the patients’ quality of life with a great burden on the health care system. 1 To date, there are several approaches to repair osteochondral injuries after a traumatic event. Conventional debridement and various methods of penetrating subchondral bone often lead to fibrocartilage formation, which lacks the typical biochemical and biomechanical properties of hyaline articular cartilage. 2 Several therapeutic strategies, including autologous chondrocyte implantation (ACI) and periosteum, perichondrium or osteochondral grafting, are already used in clinics for many years.3,4
To address some problems about the use of the ACI procedure, a second-generation technique was introduced by exploiting the intrinsic potential of suitable scaffolds to drive matrix synthesis and tissue organization. 5 This approach, based on autologous matrix-induced chondrogenesis (AMIC) 6 or matrix-induced autologous chondrocyte implantation (MACI), was successful to treat large cartilage defects. 7 Nowadays, ACI is in its fourth generation and can be regarded as an effective tissue engineering procedure. 8
Despite the wide range of therapeutic strategies for OCD,1-8 there is a need for identifying novel perspectives for cartilage repair to overcome current limitations and ensure a long-lasting repair. 9 Employment of 1-step techniques is fast rising in the orthopedic field thanks to different point-of-care medical devices. This approach exploits the multipotency and paracrine abilities of progenitor cells from different tissue sources, like bone marrow or adipose tissue, thanks to their direct processing in the surgical room.10-14 Among 1-step strategies, the use of minced cartilage is fast emerging, based on the employment of small graft fragments to promote tissue repair by covering tissue lesions.15-17 The surgical technique of slicing viable cartilage into small pieces and direct reimplantation is not novel. Cicero Parker Meek conceived it in 1958 to treat skin burns. 18 Then, micrografting technology was used in different clinical settings, including oral and maxillofacial surgeries, hair loss, 19 several kinds of wounds,20,21 and bone defects.22-25
When referring to OCD, the feasibility of using cartilage fragments as a regenerative biological solution could benefit from a precious source of articular chondrocytes and progenitor cells entrapped in an extracellular matrix. Recently, some authors have demonstrated that primary human chondrocytes cultured with micrografts, obtained with an innovative protocol (Rigeneracons, Human BrainWave Srl, Torino, Italy), 26 maintain their phenotypical characteristics and promote collagen and glycosaminoglycans synthesis. 27 This technology, based on the use of a specific class I (CE) medical device, performs the mechanical microfragmentation of human autologous tissues in micrografts of 80 µm in sterile saline solution by overcoming the use of enzymatic treatments. In the present study, we hypothesized that the use of micrografts, thanks to their biological properties, could have beneficial effects to treat OCD. Therefore, the purpose of this preclinical investigation was to test the efficacy of intra-articular injection of autologous micrografts got from 2 anatomical sites (articular cartilage and xiphoid appendix) in a rat model of OCD. Histological and immunohistochemical analyses were carried out to evaluate tissue architecture and typical cartilaginous markers after the micrografting technology.
Materials and Methods
Animal Experimental Design
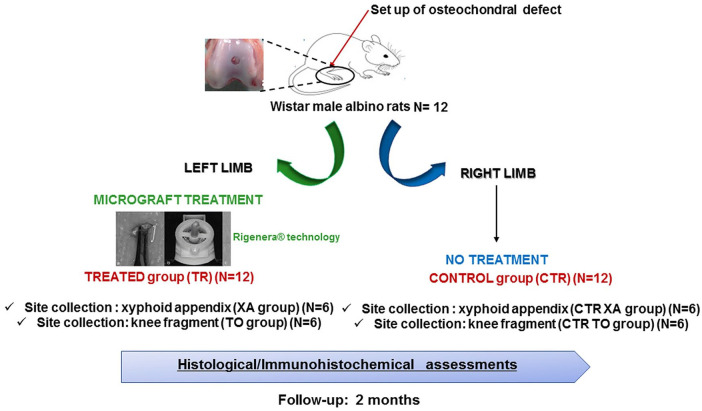
The present study was approved by the Ethics Committee on the Use of Animals, Federal Rural University of Pernambuco (UFRPE) with license 126/2017. The experiments followed international, national, and/or institutional guidelines for animal treatment and complied with relevant legislation. Twelve male adult albino Wistar rats (subspecies: Rattus norvegicus, body weight: 300 ± 50 g) were used ( Fig. 1 ). The animals were kept in suitable cages under controlled illumination and temperature (12-hour light-dark cycle and approximately 23 °C), feed and water ad libitum.
Figure 1.
Graphical scheme of the experimental design. Twelve Wistar male albino rats underwent bilateral osteochondral defects. Animals were divided into 4 groups: (i) group treated with micrograft got from the xiphoid appendix (XA) (n = 6; right knee); (ii) untreated group after collecting the XA, CTR (XA) (n = 6; left knee); (iii) group treated with micrograft got from the knee fragment (TO) (n = 6; right knee); (iv) untreated group after collecting the TO, CTR (TO) (n = 6; left knee). Animals were euthanized at 2 months of follow-up. Histological and immunohistochemical analyses were carried out.
At the time of surgery, rats were anaesthetized using oxygen in a flow of 3 L/min and 3% isoflurane. The preanesthetic action was performed by intramuscular tight administration of meloxicam 0.2% (dose of 2 mg/kg), volume of 0.4 mL; morphine 1% (dose of 5 mg/kg), volume of 0.2 mL. The maintenance was performed in an anesthetic mask diluted in oxygen 3 L/min, at an isoflurane concentration of 2.5%. Full-thickness trochlear osteochondral defects of 2.5 mm diameter and 2.0 mm depth were created bilaterally in all 12 rats (24 articular joints). Briefly, a Bard-Parker type scalpel cable mounted with blade no. 11 was used and an initial puncture of the bone with the aid of punch, in contra-angle and at right angles to the cortical bone involved were performed. Then, enlargement and deepening of the bone were created using a 2.5 mm punch ( Fig. 2A ). Once the knee joint was exposed, the joint capsule was cut for intra-articular access, the patella was displaced, allowing access to the trochlea and the extraction of a small fragment. During the injury, the region was irrigated with saline to avoid necrosis. The animals were randomly divided into 2 groups. In the first group tissue samples for preparing micrografts were collected from the knee following the procedure described above (TO group), while in the second group tissue samples were collected from the xiphoid appendix (XA group).
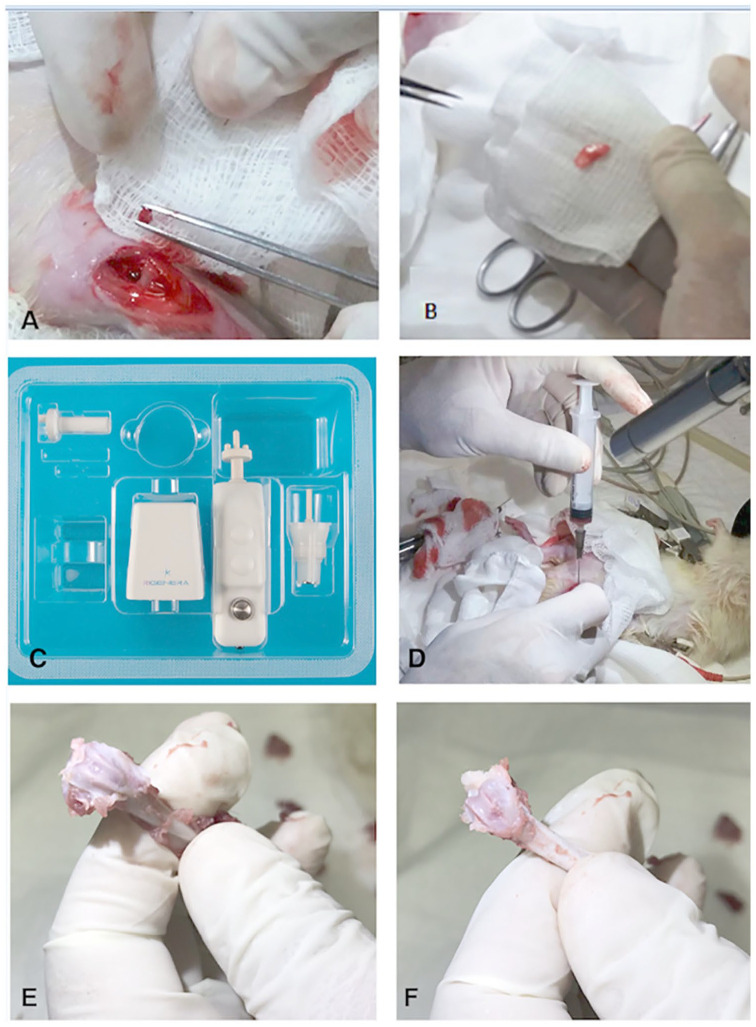
Figure 2.
Schematic representation of surgical and processing procedures for preparing the autologous tissue micrograft in the rat model. (A) The set-up of an osteochondral defect of 2.5 mm in diameter and 2.0 mm depth in the left knee. (B) Fragment extraction from the xiphoid appendix. (C) Image of a medical device called Rigeneracons® (CE certified class I) to provide autologous micrografts from tissue disaggregation. (D) Intra-articular delivery of autologous liquid micrografts suspension in the animal’s left knee. At 2 months of follow-up, femur extraction of left (E) and right (F) limb for each animal and tissue processing for histological assessment.
For the XA group, micrografts were generated from a tissue sample collected from the xiphoid appendix. Animals were positioned in dorsal decubitus to allow surgical access to the sternum and the knee. A Bard-Parker scalpel cable mounted with blade no. 11. Once the musculature of the area was exposed, the fascia was sectioned in the same direction as the cutaneous incision. Using a pair of scissors, a fragment of the xiphoid appendix (approximately 1 cm) was extracted ( Fig. 2B ). In both control groups CTR (TO) and CTR (XA), the process of the articular defect in the right knee was described above then treated only with saline solution.
After the surgical procedure, the animals were sutured with absorbable wires and kept in recovery cages until they stabilized and returned to the individual cages where they received the following recovery medication: meloxicam 0.2%: 0.2 mL, (subcutaneously [SC]) one a day, for 3 days; tramadol 5%: 0.08 mL (SC) twice an day, and enrofloxacin 2.5%: 0.08 mL (SC) twice a day, for 5 days all animals were recovered with the medication.
Autologous Micrografts Preparation and Grafting
To generate autologous micrografts from the collected tissues, we followed the method established by Ceccarelli et al. 28 The procedure is based on the use of a class IIa CE medical device named Rigeneracons ( Fig. 2C ), composed of (1) grid with hexagonal holes, each hole is equipped at the edges with microblade and (2) a rotating helix. Through this device, the rotating helix repeatedly pushes the tissue sample on the blades allowing the formation of microfragments particles called “micrografts.” The tissues collected during the surgery (articular cartilage for TO group and xiphoid cartilage for XA group) described above were inserted in the devices along with sterile saline solution. Tissue fragmentation was carried out in 4 minutes at room temperature. After processing, the tissues were completely disaggregated in micrometer-sized particles composed of cell clusters (micrografts) in sterile saline solution. The micrograft suspension was intra-articularly injected into the articular lesions in the treated groups ( Fig. 2D ).
Histological Assessments
Animals were euthanized by using intramuscular anaesthesia with ketamine and xylazine, followed by CO2 in a chamber until death at 2 month follow-up. An authorized veterinarian carried the euthanasia process out following the protocol recommended by the Brazilian Board for Animal Experimentation. After reaching the bone plane and locating the defect, the femur was disarticulated and removed ( Fig. 2E and F ). The soft tissues were removed by dissection and the samples were placed in containers with 10% buffered formalin solution for fixation at room temperature and then bones were decalcified in 12.5% formic acid solution and 20% sodium citrate for 20 days. After the decalcification, tissue samples were first washed in running water and then processed in a gradual series of alcohol and xylene for tissue dehydration and diaphanization up to their embedding with paraffin. Microscopic slides (thickness 7 μm) were stained with Gill III hematoxylin/eosin (Sigma Aldrich, St. Louis, MO) and 0.1% safranin O/0.03% fast green (Sigma Aldrich) to assess tissue morphology and the proteoglycan content in the extracellular matrix.
A modified O’Driscoll (mO’Driscoll) score was used for providing a semiquantification of histological features in the control and experimental groups (TO and XA groups). This score considers 8 parameters: nature of the repair tissue, matrix staining, structural integrity, surface regularity, filling of the defect, bonding to host tissue, degenerative changes of the repair tissue, degenerative changes of the adjacent cartilage. 29 It has a range from 0 (no repair) up to 21 (osteochondral repair). Six microscopic fields, spaced in 5 sections, were assessed for each specimen by 2 blinded investigators with the Eclipse 90i microscope (Nikon, Melville, NY).
Immunohistochemical Assessments
Analyses for type 1 and type 2 collagens were carried out. After deparaffinization, the antigen retrieval was performed incubating the tissue sections with 0.1% proteinase (Sigma Aldrich) and 0.1% hyaluronidase (Sigma Aldrich) at 37 °C for 15 minutes, respectively. After washing steps with phosphate-buffered saline (PBS), sections were blocked with 1% bovine serum albumin (BSA) at room temperature for 20 minutes. Then, the incubation with monoclonal anti-mouse collagen type 2 (2 µg/mL; Abcam, Cambridge, UK) and collagen type 1 (5 µg/mL; Sigma Aldrich) was carried out at room temperature for an hour. Ready-to-use anti-mouse biotinylated secondary antibody and alkaline-labeled streptavidin (Biocare Medical, Pacheco, CA) were used. The histochemical detection was performed with Fast Red Substrate Kit (Biocare Medical). Specific negative controls were carried out by using an isotype-matched control or omitting the primary antibodies. The nuclear component was stained with Gill III hematoxylin. Three microscopic fields (100× magnification) were assessed for each sample by a blinded investigator with a semiquantitative method.
We segmented and selected the neo-formed tissues for each marker by avoiding the surrounding adjacent native tissues. Image acquisition and processing were carried out with the Eclipse 90i microscope (Nikon) and NIS-Elements through the hue/saturation/intensity (HSI) system. We set the threshold value for H was set for positive pixels between 225 and 255. Ranges from 0 to 155 were threshold values for S and I. Positive cells/ area for each marker of neoformed tissue were measured (10× objective lens) and expressed as a percentage of positive cells/ area on a scale from 0 (no protein expression) to 100 (the highest protein expression).
Statistical Analysis
Statistical analysis was performed with GraphPad Prism software. The Kolmogorov-Smirnov test was used to test the data distribution. We used the Kruskal-Wallis followed by the post hoc Dunn’s test to assess differences in the control and treated groups. Data were expressed as the 95% confidence intervals (CI) of the mean ± standard deviation (SD). P ≤ 0.05 was considered statistically significant.
Results
Histological Evaluations
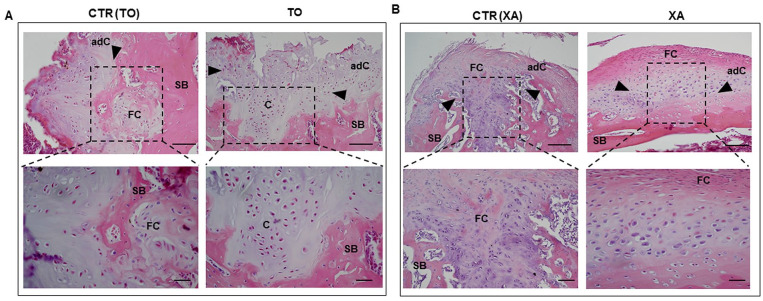
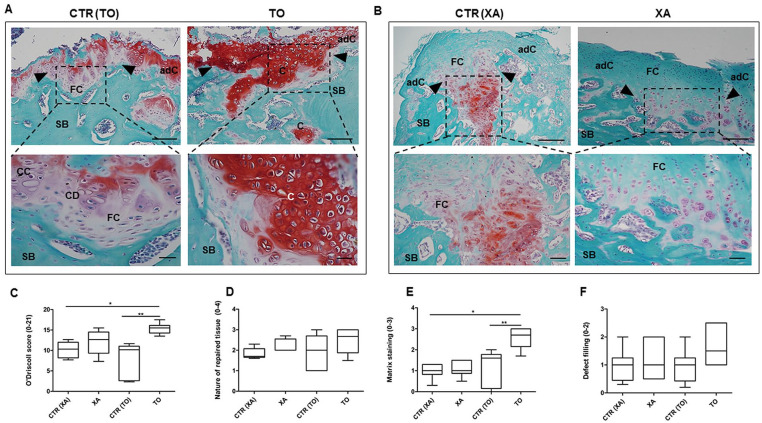
Histological evaluation showed that the treatment with autologous micrografts promoted defect filling to a different extent in both TO and XA groups. In the CTR (TO) group, the defect was filled with fibrocartilage which displays impaired tissue integrity, some blood vessels, empty lacunae and a poor content of proteoglycan. Moderate hypocellularity with cell clusters and little bone remodelling were further histological features observed in the defect area in the CTR (TO) group ( Figs. 3A and 4A ). Conversely, the defect area from the TO group showed a repair tissue with hyaline-like histological features. This neoformed tissue displayed a partial bonding with the host tissue, islets of chondrons dispersed in the extracellular matrix and several processes of osteochondral ossification ( Figs. 3B and 4B ). Good structural integrity and normal cellularity with several round cells surrounded by proteoglycans were further histological features noticed in the TO group ( Figs. 3B and Fig 4B ). The CTR (XA) group showed an abnormal tissue formation with an altered tissue architecture, several fibrillations, low proteoglycan content, and immature bone formation ( Figs. 3B and Fig.4B ). The OCD lesions were filled with fibrocartilage in the XA group, which displayed a slight cellular positivity for safranin O staining in the deep zone. The repaired cartilage tissue was immature with no tidemark and the bone displayed several spaces with osteoprogenitors ( Figs. 3B and Fig. 4B ).
Figure 3.
Histological staining with hematoxylin and eosin. Representative images of hematoxylin/eosin staining in the TO (knee fragment), XA (xiphoid appendix), CTR (TO), and CTR (XA) groups (A and B). Dashed rectangular represents the repaired tissues in the different groups with indications of the adjacent cartilage (adC), fibrocartilage (FB), and bone areas (SB). The upper panel shows representative micrographs with low magnification (scale bar: 100 μm) whereas the lower panel has high magnification (scale bar: 50 μm).
Figure 4.
Histological staining with safranin O/fast green. Representative images of safranin O/fast green staining (red staining, proteoglycan content; green staining, collagen content) in the TO (knee fragment), XA (xiphoid appendix), CTR (TO), and CTR (XA) groups. (A and B) Dashed rectangular represents the repaired tissues in the different groups with indications of adjacent cartilage (adC), fibrocartilage (FB), cartilaginous areas (C), and bone areas (SB). The upper panel shows representative micrographs with low magnification (scale bar: 100 μm), whereas the lower panel has high magnification (scale bar: 50 μm). (C) Graphical representation of modified O’Driscoll score in the TO, CTR (TO), XA, and CTR (XA) groups; **P < 0.01: TO versus CTR (TO) group; *P < 0.05: TO versus CTR (XA) group. (D) Graphical representation of the repaired tissue, a subparameter of mO’Driscoll score, in all control and experimental groups. (E) Graphical representation of the matrix staining, a subparameter of mO’Driscoll score, in all groups. Data are expressed as 95% confidence intervals (CIs) of the mean ± standard deviation (SD), **P < 0.01: TO versus CTR (TO) group; *P < 0.05: TO versus CTR (XA) group. (F) Graphical representation of the defect filling, a subparameter of the score, in all groups.
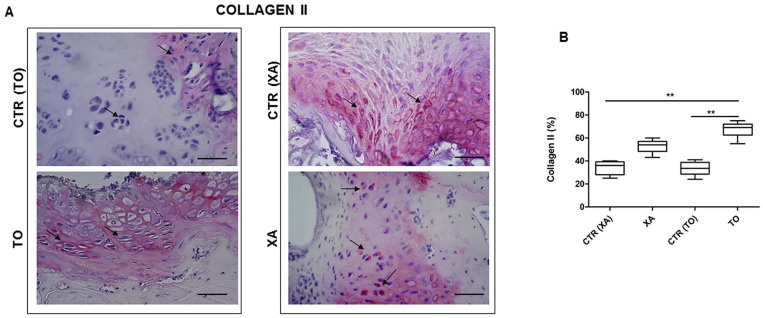
The semiquantification of histological features was performed with the mO’Driscoll score. The TO group showed a higher mO’Driscoll score (15.36 ± 0.5) than the XA group (12.07 ± 1.3) ( Fig. 4C ). In particular, the TO group (15.36 ± 0.5) displayed a higher score than its control, CTR (TO) (7.9 ± 1.7) (**P < 0.01) and CTR (XA) (10.14 ± 0.89) (*P < 0.05). The XA group showed a higher mO’Driscoll score than its control, but with no statistical evidence ( Fig. 4C ). Among the histological parameters within the mO’Driscoll score, the TO group (2.6 ± 0.2) showed a higher matrix staining than CTR (TO) (1.2 ± 0.3) (P < 0.01) and CTR (XA) (0.98 ± 0.1) (P < 0.05) groups ( Fig. 4E ).
Immunohistochemical Assessments
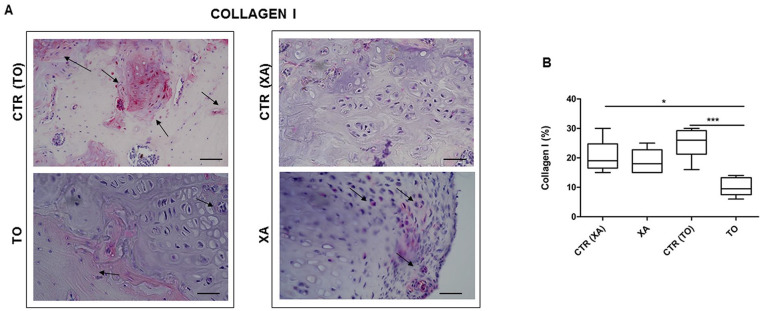
To further evaluate the quality of the neoformed tissues in the control and experimental groups, we performed immunostaining for type 2A1 and 1 collagens. Both TO and XA groups showed strong positivity for type 2A1 collagen at the cellular and extracellular levels ( Fig. 5A ). They displayed a higher protein expression for type 2A1 than their respective controls. In particular, the TO group showed a higher significant value for type 2A1 than the CTR (TO) (P < 0.01) and CTR (XA) (P < 0.05) groups ( Fig. 5B ). Regarding type 1 collagen, both groups treated with the micrografts showed lower protein values at a cellular level than their respective controls ( Fig. 6A ). The TO group showed a lower protein expression for type 1 collagen than CTR (TO) (P < 0.01) and CTR (XA) (P < 0.05) ( Fig. 6B ).
Figure 5.
Immunohistochemical analysis: type 2A1 collagen. (A) Representative images for the immunohistochemical analysis for type 2A1 collagen in the TO (knee fragment), XA (xiphoid appendix), CTR (TO), and CTR (XA) groups. (B) Graphical representation of protein expression for type 2A1 collagen with hue/saturation/intensity (HSI) system with software NIS-Elements in all groups. Data are expressed as 95% CI of the mean ± SD. **P < 0.01: TO versus CTR (TO) group; *P < 0.05: TO versus CTR (XA) group.
Figure 6.
Immunohistochemical analysis: type 1 collagen. (A) Representative images for the immunohistochemical analysis for type 1 collagen in the TO (knee fragment), XA (xiphoid appendix), CTR (TO), CTR (XA). (B) Graphical representation of protein expression for type 1 collagen after image analysis with hue/saturation/intensity (HSI) system with software NIS-Elements in all groups. Data are expressed as 95% CI of the mean ± SD. **P < 0.01: TO versus CTR (TO) group; *P < 0.05: TO versus CTR (XA) group.
Discussion
The main finding of this preclinical in vivo study is that the intra-articular delivery of autologous cartilage micrograft can be a promising mini-invasive 1-step approach to treat OCD. Employing autologous micrograft as a biological solution can offer significant benefits, allowing to overcome current limitations of therapeutic approaches, like invasiveness, donor site morbidity, cell death and allogeneic response. Albrecht 30 introduced the concept of cartilage chips in 1983. Early preclinical and clinical studies provided promising results on the use of various minced cartilage techniques through different shaver medical devices, delivery systems and the enrichment with bioactive molecules for chondrocyte activation.31-35 In particular, Lind and Larsen 36 provided preclinical evidence on the equal repair capacity of 1-step minced cartilage under collagen scaffold to standard ACI technique in sheep. Mostly, studies on cartilage micrograft investigated its combination with bioactive molecules and/or biomimetic scaffold and implantation into the defect site by arthroscopy.31-35 To date, full knowledge of the role of micrografts in musculoskeletal tissue regeneration, and especially for the repair of OCD, is still scarce.15,16,37
The main aim of this study is to raise the level of knowledge of autologous micrograft to treat OCD in a rat model via an intra-articular approach by testing its effectiveness after tissue fragmentation with a novel medical device (CE-certified class I), named Rigeneracons. This system produces small fragments of 80 µm, capable of being incorporated into the native tissue to promote chondrocyte activation and tissue engraftment. In-depth investigation clarified the influence of two harvesting sites, articular cartilage and xiphoid appendix, to promote tissue repair. Preliminary in vitro studies showed that this technology does not affect cell viability in articular cartilage and the chondrogenic potential. 27 Herein, we investigated the efficacy of micrograft and some critical characteristics conditioning tissue repair. Our study showed that the autologous micrografts from articular cartilage show attractive properties to treat OCD with important perspectives in clinics. The micrograft from xiphoid appendix displayed some repair processes with immature fibrous features. The different biological responses from the two tissue harvesting sites could depend on the different anatomical architectures, 38 which do not guarantee proper biomechanics. Immunohistochemical analyses showed that the TO group displayed various islets of chondrocytes embedded in the extracellular matrix in the defect area. These cell islets showed a high positivity for type 2 collagen and low levels of type 1 collagen, promoting a repair tissue with typical hyaline-like features. Regarding the bone component, this group exhibited tissue restoration, which is a critical factor during osteochondral defect healing as it ensures adequate nutrient and growth factor supply to cartilage tissue. 39 The potential of this technology could derive from the ability to obtain ready-to-use micrografts containing viable chondrons, the morpho-functional units of articular cartilage. The feasibility of implanting instructive “cell niche” could enhance endogenous tissue regeneration by recruiting progenitor cells from the surrounding tissues in the lesion site. Indeed, using the native extracellular matrix scaffold can restore typical spatial organization of the articular cartilage at the treatment site, thus ensuring suitable mechanical tissue properties and cellular functions. Besides the chondrocyte population, micrograft contains progenitor cells, which may further promote tissue repair thanks to their biological properties. 26 Progenitor cells could act as “growth factors secreting factories” and exert a therapeutic effect through the release of soluble mediators.26,40-42 This study provides the first preliminary interesting concepts on the role of micrografts to treat OCD via an intra-articular approach. However, it presents some limitations due to (1) the use of a small animal model, (2) the small sample number, and (3) short-term follow-up. In conclusion, our data show that this micrografting technology could be a potential low-cost strategy to treat OCD since it promotes a repair tissue with typical hyaline-like features. Further studies are, however, needed to validate the potential biological relevance of this micrograft in a larger animal model before considering its clinical application.
Footnotes
Acknowledgments and Funding: The author(s) disclosed receipt of the following financial support for research, authorship and/or publication of the article: This study was financed in part by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior–Brasil (CAPES)–Finance Code 001.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Ethical Approval: The study was carried out after being approved by the Ethics Committee on the Use of Animals, Federal Rural University of Pernambuco (UFRPE) with License 126/2017.
ORCID iDs: Giovanna Desando  https://orcid.org/0000-0003-3338-5747
https://orcid.org/0000-0003-3338-5747
Brunella Grigolo  https://orcid.org/0000-0002-3990-6745
https://orcid.org/0000-0002-3990-6745
References
- 1. Gorbachova T, Melenevsky Y, Cohen M, Cerniglia BW. Osteochondral lesions of the knee: differentiating the most common entities at MRI. Radiographics. 2018;38:1478-95. doi: 10.1148/rg.2018180044 [DOI] [PubMed] [Google Scholar]
- 2. Armiento AR, Alini M, Stoddart MJ. Articular fibrocartilage—why does hyaline cartilage fail to repair? Adv Drug Deliv Rev. 2019;146:289-305. doi: 10.1016/j.addr.2018.12.015 [DOI] [PubMed] [Google Scholar]
- 3. Brittberg M. Autologous chondrocyte implantation—technique and long-term follow-up. Injury. 2008;39(Suppl 1):S40-S49. doi: 10.1016/j.injury.2008.01.040 [DOI] [PubMed] [Google Scholar]
- 4. Bugbee WD, Convery FR. Osteochondral allograft transplantation. Clin Sports Med. 1999;18(1):67-75. doi: 10.1016/s0278-5919(05)70130-7 [DOI] [PubMed] [Google Scholar]
- 5. Niemeyer P, Salzmann G, Feucht M, Pestka J, Porichis S, Ogon P, et al. First-generation versus second-generation autologous chondrocyte implantation for treatment of cartilage defects of the knee: a matched-pair analysis on long-term clinical outcome. Int Orthop. 2014;38(10):2065-70. doi: 10.1007/s00264-014-2368-0 [DOI] [PubMed] [Google Scholar]
- 6. Gao L, Orth P, Cucchiarini M, Madry H. Autologous matrix-induced chondrogenesis: a systematic review of the clinical evidence. Am J Sports Med. 2019;47(1):222-31. doi: 10.1177/0363546517740575 [DOI] [PubMed] [Google Scholar]
- 7. Schenker H, Wild M, Rath B, Tingart M, Driessen A, Quack V, et al. Current overview of cartilage regeneration procedures [in German]. Orthopade. 2017;46(11):907-13. doi: 10.1007/s00132-017-3474-7 [DOI] [PubMed] [Google Scholar]
- 8. Kessler MW, Ackerman G, Dines JS, Grande D. Emerging technologies and fourth generation issues in cartilage repair. Sports Med Arthrosc Rev. 2008;16(4):246-54. doi: 10.1097/JSA.0b013e31818d56b3 [DOI] [PubMed] [Google Scholar]
- 9. Mendes LF, Bosmans K, Van Hoven I, Viseu SR, Maréchal M, Luyten FP. Developmental engineering of living implants for deep osteochondral joint surface defects. Bone. 2020;139:115520. doi: 10.1016/j.bone.2020.115520 [DOI] [PubMed] [Google Scholar]
- 10. Buda R, Vannini F, Cavallo M, Baldassarri M, Luciani D, Mazzotti A, et al. One-step arthroscopic technique for the treatment of osteochondral lesions of the knee with bone marrow-derived cells: three years results. Musculoskelet Surg. 2013;97(2_suppl):145-51. doi: 10.1007/s12306-013-0242-7 [DOI] [PubMed] [Google Scholar]
- 11. Li Q, Zhao F, Li Z, Duan X, Cheng J, Zhang J, et al. Autologous fractionated adipose tissue as a natural biomaterial and novel one-step stem cell therapy for repairing articular cartilage defects. Front Cell Dev Biol. 2020;8:694. doi: 10.3389/fcell.2020.00694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Caforio M, Nobile C. Intra articular administration of autologous purified adipose tissue associated with arthroscopy ameliorates knee osteoarthritis symptoms. J Clin Med. 2021;10(10):2053. doi: 10.3390/jcm10102053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Van Genechten W, Vuylsteke K, Martinez PR, Swinnen L, Sas K, Verdonk P. Autologous micro-fragmented adipose tissue (MFAT) to treat symptomatic knee osteoarthritis: early outcomes of a consecutive case series. J Clin Med. 2021;10:2231. doi: 10.3390/jcm10112231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Chahla J, Cinque ME, Schon JM, Liechti DJ, Matheny LM, LaPrade RF, et al. Bone marrow aspirate concentrate for the treatment of osteochondral lesions of the talus: a systematic review of outcomes. J Exp Orthop. 2016;3(1):33. doi: 10.1186/s40634-016-0069-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Salzmann GM, Ossendorff R, Gilat R, Cole BJ. Autologous minced cartilage implantation for treatment of chondral and osteochondral lesions in the knee joint: an overview. Cartilage. Epub 2020 July 25. doi: 10.1177/1947603520942952 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Astarita C, Arora CL, Trovato L. Tissue regeneration: an overview from stem cells to micrografts. J Int Med Res. 2020;48(6):0300060520914794. doi: 10.1177/0300060520914794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Marcarelli M, Zappia M, Rissolio L, Baroni C, Astarita C, Trovato L, et al. Cartilage micrografts as a novel non-invasive and non-arthroscopic autograft procedure for knee chondropathy: three-year follow-up study. J Clin Med. 2021;10(2_suppl):322. doi: 10.3390/jcm10020322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Meek CP. Successful microdermagrafting using the Meek-Wall microdermatome. Am J Surg. 1958;96(4):557-8. doi: 10.1016/0002-9610(58)90975-9 [DOI] [PubMed] [Google Scholar]
- 19. Ruiz JC, Ruiz RG, Rosell JMC, Ceccarelli G, De Sio C, De Angelis GC, et al. Progenitor-cell-enriched micrografts as a novel option for the management of androgenetic alopecia. J Cell Physiol. 2020;235(5):4587-93. doi: 10.1002/jcp.29335 [DOI] [PubMed] [Google Scholar]
- 20. Andreone A, den Hollander D. A retrospective study on the use of dermis micrografts in platelet-rich fibrin for the resurfacing of massive and chronic full-thickness burns. Stem Cells Int. 2019;2019:8636079. doi: 10.1155/2019/8636079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Riccio M, Marchesini A, Zingaretti N, Carella S, Senesi L, Onesti MG, et al. A multicentre study: the use of micrografts in the reconstruction of full-thickness posttraumatic skin defects of the limbs—a whole innovative concept in regenerative surgery. Stem Cells Int. 2019:2019:5043518. doi: 10.1155/2019/5043518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Araújo CRG, Astarita C, D’Aquino R, Pelegrine AA. Evaluation of bone regeneration in rat calvaria using bone autologous micrografts and xenografts: histological and histomorphometric analysis. Materials (Basel). 2020;13(19):4284. doi: 10.3390/ma13194284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Lupi SM, Baena ARY, Todaro C, Ceccarelli G, Rodriguez Y, Baena R. Maxillary sinus lift using autologous periosteal micrografts: a new regenerative approach and a case report of a 3-year follow-up. Case Rep Dent. 2018;2018:3023096. doi: 10.1155/2018/3023096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ferrarotti F, Romano F, Gamba MN, Quirico A, Giraudi M, Audagna M, et al. Human intrabony defect regeneration with micrografts containing dental pulp stem cells: a randomized controlled clinical trial. J Clin Periodontol. 2018;45(7):841-50. doi: 10.1111/jcpe.12931 [DOI] [PubMed] [Google Scholar]
- 25. Dorta Fernandez A, Baroni Luengo A. Biostimulation of knee cartilage using autologous micro-grafts: a preliminary study of the Rigenera protocol in osteochondral lesions of the knee. Rehabil Sci. 2018;3(1):8-12. doi: 10.11648/j.rs.20180301.12 [DOI] [Google Scholar]
- 26. Trovato L, Monti M, Del Fante C, Cervio M, Lampinen M, Ambrosio L, et al. A new medical device rigeneracons allows to obtain viable micro-grafts from mechanical disaggregation of human tissues. J Cell Physiol. 2015;230:2299-303. doi: 10.1002/jcp.24973 [DOI] [PubMed] [Google Scholar]
- 27. Viganò M, Tessaro I, Trovato L, Colombini A, Scala M, Magi A, et al. Rationale and pre-clinical evidence for the use of autologous cartilage micrografts in cartilage repair. J Orthop Surg Res. 2018;13(1):279. doi: 10.1186/s13018-018-0983-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Ceccarelli G, Gentile P, Marcarelli M, Balli M, Ronzoni FL, Benedetti L, et al. In vitro and in vivo studies of alar-nasal cartilage using autologous micro-grafts: the use of the Rigenera® protocol in the treatment of an osteochondral lesion of the nose. Pharmaceuticals (Basel). 2017;10(2_suppl):53.doi: 10.3390/ph10020053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Shimizu M, Matsumoto T, Kikuta S, Ohtaki M, Kano K, Taniguchi H, et al. Transplantation of dedifferentiated fat cell-derived micromass pellets contributed to cartilage repair in the rat osteochondral defect model. J Orthop Sci. 2018;23(4):688-96. doi: 10.1016/j.jos.2018.03.001 [DOI] [PubMed] [Google Scholar]
- 30. Albrecht FH. Closure of joint cartilage defects using cartilage fragments and fibrin glue. Fortschr Med. 1983;101(37):1650-2. [PubMed] [Google Scholar]
- 31. Lu Y, Dhanaraj S, Wang Z, Bradley DM, Bowmann SM, Cole BJ, et al. Minced cartilage without cell culture serves as an effective intraoperative cell source for cartilage repair. J Orthop Res. 2006; 24(6):1261-70. doi: 10.1002/jor.20135 [DOI] [PubMed] [Google Scholar]
- 32. Salzmann GM, Calek AK, Preiss S. Second-generation autologous minced cartilage repair technique. Arthrosc Tech. 2017;6(1):e127-e131. doi: 10.1016/j.eats.2016.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Levinson C, Cavalli E, Sindi DM, Kessel B, Zenobi-Wong M, Preiss S, et al. Chondrocytes from device-minced articular cartilage show potent outgrowth into fibrin and collagen hydrogels. Orthop J Sports Med. 2019;7:2325967119867618. doi: 10.1177/2325967119867618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Cugat R, Alentorn-Geli E, Navarro J, Cuscó X, Steinbacher G, Seijas R, et al. A novel autologous-made matrix using hyaline cartilage chips and platelet-rich growth factors for the treatment of full-thickness cartilage or osteochondral defects: preliminary results. J Orthop Surg (Hong Kong). 2020;28(1):2309499019887547. doi: 10.1177/2309499019887547 [DOI] [PubMed] [Google Scholar]
- 35. Christensen BB, Olesen ML, Hede KTC, Bergholt NL, Foldager CB, Lind M. Particulated cartilage for chondral and osteochondral repair: a review. Cartilage. Epub 2020 February 13. doi: 10.1177/1947603520904757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Lind M, Larsen A. Equal cartilage repair response between autologous chondrocytes in a collagen scaffold and minced cartilage under a collagen scaffold: an in vivo study in goats. Connect Tissue Res. 2008;49:437-42. doi: 10.1080/03008200802325037 [DOI] [PubMed] [Google Scholar]
- 37. Gentile P, Scioli MG, Bielli A, Orlandi A, Cervelli V. Reconstruction of alar nasal cartilage defects using a tissue engineering technique based on a combined use of autologous chondrocyte micrografts and platelet-rich plasma: preliminary clinical and instrumental evaluation. Plast Reconstr Surg Glob Open. 2016;4(10):e1027. doi: 10.1097/GOX.0000000000001027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Anderson BW, Holme MR, Burns B. Anatomy, thorax, xiphoid process. StatPearls; 2021. [PubMed] [Google Scholar]
- 39. Lepage SIM, Robson N, Gilmore H, Davis O, Hooper A, St John S, et al. Beyond cartilage repair: the role of the osteochondral unit in joint health and disease. Tissue Eng Part B Rev. 2019;25(2_suppl):114-25.doi: 10.1089/ten [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Monti M, Graziano A, Rizzo S, Perotti C, Del Fante C, d’’Aquino R, et al. In vitro and in vivo differentiation of progenitor stem cells obtained after mechanical digestion of human dental. Pulp J Cell Physiol. 2017;232(3):548-55. doi: 10.1002/jcp.25452 [DOI] [PubMed] [Google Scholar]
- 41. Cosenza S, Ruiz M, Toupet K, Jorgensen C, Noël D. Mesenchymal stem cells derived exosomes and microparticles protect cartilage and bone from degradation in osteoarthritis. Sci Rep. 2017;7(1):16214. doi: 10.1038/s41598-017-15376-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Cosenza S, Ruiz M, Maumus M, Jorgensen C, Noël D. Pathogenic or therapeutic extracellular vesicles in rheumatic diseases: role of mesenchymal stem cell-derived vesicles. Int J Mol Sci. 2017;18(4):889. doi: 10.3390/ijms18040889 [DOI] [PMC free article] [PubMed] [Google Scholar]