Abstract
Background
Angiopoietin-like protein 2 (ANGPTL2) is a secreted molecule with numerous physiologic and pathologic functions, for example, in angiogenesis, hematopoiesis, and tumorigenesis. Although recent studies implicated ANGPTL2 in chronic inflammation in mouse peritoneal macrophages, human ligamentum flavum fibroblasts, and human retinal microvascular endothelial cells, the mechanism underlying ANGPTL2-associated inflammation in chondrocytes remains unclear. Therefore, it was investigated whether ANGPTL2 is expressed in or functions in chondrocytes.
Methods
Expression of ANGPTL2 and its receptor, integrin α5β1 were examined over time in ATDC5 cells using real-time RT-PCR (reverse transcription–polymerase chain reaction) analysis. ATDC5 cells were then incubated with or without ANGPTL2 for 3 hours, and expression of the IL-1β, TNF-α, COX-2, aggrecanase (ADAMTS)-5, matrix metalloproteinase (MMP)-3, and MMP-13 genes were examined using real-time RT-PCR. Additionally, phosphorylation of ERK, JNK, p38, Akt, and NF-κB was examined by western blotting. Furthermore, it was also investigated for the effect of anti-integrin α5β1 antibody on the expression of inflammatory markers and intracellular signaling pathways.
Results
ANGPTL2 induced the phosphorylation of all 3 MAPKs, Akt, and NF-κB and dramatically upregulated the expression of inflammation-related factor genes. Inhibiting the activation of integrin α5β1 suppressed these reactions.
Conclusion
ANGPTL2 may induce inflammatory factors by stimulating the integrin α5β1/MAPKs, Akt, and NF-κB signaling pathway.
Keywords: ANGPTL2, ATDC5, chondrocyte, inflammation, integrin α5β1
Introduction
Osteoarthritis (OA) is one of the most common joint chronic conditions. 1 It occurs often in the knees, fingers, and temporomandibular joints. 2 OA causes joint disability due to articular cartilage and bone degeneration, resulting in severe pain and disfunctions such as limited movements. 3 Articular cartilage is composed of chondrocytes and extracellular matrix (ECM). ECM contains collagen fibers and proteoglycans and functions as a metabolically active tissue. 4 An optimal level of mechanical loading is required for ECM maintenance in cartilage, but excessive mechanical stress is thought to play an important role in cartilage destruction. For example, excessive loading induces the expression of inflammatory cytokines, leading to excessive secretion of protease such as matrix metalloproteinases (MMPs) and ADAMTSs in chondrocytes.5,6
The angiopoietin-like proteins (ANGPTL) family include 7 secreted glycoproteins that are structurally similar to angiopoietin (ANGPT). In contrast to ANGPT, ANGPTLs do not bind to either receptor tyrosine kinase of the Tunica internal endothelial cell kinase family (Tie2 or Tie1).7,8 ANGPTLs contain a N-terminal coiled-coil domain that functions in oligomerization, which is necessary for maximum ANGPTL activity and a C-terminal fibrinogen-like domain that serves as a receptor binding site.9-11 Fibrinogen acts as a ligand of the receptors for integrins such as αvβ3, α5β1, and αMβ2,12,13 which are heterodimeric transmembrane glycoproteins that mediate cell-ECM and cell-cell adhesion. 14 Recent study reported that ANGPTL3 is binding to integrin αvβ3 and induces endothelial cell adhesion and migration, 15 and ANGPTL2 acted on endothelial cells through integrin α5β1 and influenced monocytes/macrophages through integrin α4 or β2. 16 ANGPTLs have been implicated as playing roles in a diverse array of physiologic processes, including lipid metabolism, angiogenesis, and inflammation. 17 Of the 7 members of the ANGPTL family, the expression of ANGPTL2 in particular was reported to be elevated in inflammatory diseases.16,18,19 ANGPTL2 is expressed in a variety of cells and tissues, including the heart, 20 lung, 21 kidney, 22 skeletal muscle, 19 and adipose tissue, 23 most of which are target organs for lifestyle-related metabolic disorders and play important roles in lipid metabolism, 16 angiogenesis, 9 hematopoiesis, 10 and inflammation.24-26 Researchers have also reported that ANGPTL2 induces the expression of inflammation-related genes in mouse peritoneal macrophages, 27 human ligamentum flavum fibroblasts, 28 and human retinal microvascular endothelial cells. 29
Recent studies indicated that expression of ANGPTL2, a mediator of chronic inflammation, is induced under a variety of pathologic conditions, including mechanical stretching stress, 30 hypoxia, undernutrition, and endoplasmic reticulum stress. 16 Other studies showed that ANGPTL2 signaling via integrins activates inflammatory cascades in endothelial cells 10 and induces monocyte/macrophage chemotaxis. 16 With regard to chondrocytes, ANGPTL2 is expressed in both resting and proliferating cells, and ANGPTL2 secreted by chondrocytes accumulates in the surrounding ECM. 31 Integrins are reportedly involved in the activation of intracellular signaling pathways for inflammatory reactions involving chondrocytes. 32 The function of ANGPTL2 in chondrocytes during inflammation have not been elucidated. In this study, therefore, we examined the relationship between integrin α5β1 and the expression of inflammatory factors in the presence of ANGPTL2 in chondrocytes using an integrin α5β1 blocker.
Materials and Methods
Cell Culture
The mouse ATDC5 cell line, a model of multistep in vitro chondrogenic differentiation, was obtained from the RIKEN Cell Bank, Tsukuba, Japan (Cat#RCB0565, RRID:CVCL3894). ATDC5 cells were seeded at a density of 6.0 × 104 cells/mL on 35-mm plates (FALCON, Franklin Lakes, NJ, USA) and cultured in 2 mL of Dulbecco’s modified Eagle’s medium/nutrient F-12 Ham’s (DMEM/F12; Sigma, St Louis, MO, USA) containing 5% fetal bovine serum (FBS; Bio Whittaker, Verviers, Belgium), 10 mg/mL human transferrin (Sigma), and 3 × 10−8 M sodium selenite (Sigma) in a humidified incubator with 5% CO2 at 37°C. After cells reached approximately 50% confluence, the medium was changed to DMEM/Ham’s F12 hybrid medium containing 10 µg/mL of bovine insulin (Sigma) and 37.5 µg/mL of ascorbate-2-phosphatase (Wako Pure Chemicals, Osaka, Japan), and cells were cultured for an additional 14 days to induce differentiation.
Real-Time RT-PCR Analysis
Total RNA was extracted from cultured cells using TRIzol reagent (Invitrogen Life Technologies Inc., Gaithersburg, MD, USA) in accordance with the manufacturer’s protocol. Reverse transcription from total RNA to cDNA was performed using ReverTra Ace (Toyobo, Osaka, Japan). Target gene expression was quantified by real-time reverse transcription–polymerase chain reaction (RT-PCR) using SYBR Green PCR Master Mix (Toyobo) and a LightCycler system (Roche Diagnostics, Mannheim, Germany). Expression of S29 was monitored as reference gene for comparison. Normalized cycle threshold (Ct) values were compared relative to those of the controls. Data were calculated as relative expression according to the 2-ΔCt method, in which the cycle threshold is the beginning of logarithmic amplification and ΔCt is the difference between the target gene Ct and reference gene Ct. Each sample was analyzed in triplicate to ensure accuracy. The primer sequences are shown in Table 1 .
Table 1.
The Primer Sequences for Real-Time Reverse Transcription–Polymerase Chain Reaction.
| Gene | Sequence of Primers (5′-3′) | |
|---|---|---|
| ANGPTL2 | Forward | GGAGGTTGGACTGTCATCCAGAG |
| Reverse | GCCTTGGTTCGTCAGCCAGTA | |
| Adamts5 | Forward | GGCATCATTCATGTGACACC |
| Reverse | CGAGTACTCAGGCCCAAATG | |
| IL-1β | Forward | TGTGCAAGTGTCTGAAGCAGCTATG |
| Reverse | ACACAGGCTCTCTTTGAACAGAATG | |
| TNF-α | Forward | CTCCCTCCAGAAAAGACACCATGA |
| Reverse | CTGACCACTCTCCCTTTGCAGAAC | |
| COX2 | Forward | CAGTTTCTCTACAACAACTCCATC |
| Reverse | TTCATCTCTCTGCTCTGGTC | |
| MMP3 | Forward | CTCAAGGGTGGATGCTGTC |
| Reverse | TGCCATAGCACATGCTGAAC | |
| MMP13 | Forward | CCAAAAGAGGTGAAGAGACTGA |
| Reverse | CGGGGATAATCTTTGTCCATA | |
| COL2A1 | Forward | GTCCTGAAGGTGCTCAAGGTTCTC |
| Reverse | AGGAATACCATCAGTCCCTGGGTTA | |
| COL10A1 | Forward | CTTCATCCCATACGCCATAAAGA |
| Reverse | GCTGATATTCCTGGTGGTCCTG | |
| Aggrecan | Forward | GGCAACCTCCTGGGTGTAAG |
| Reverse | TGGGGTTCGGTGGGCTCACAA | |
| Itgα5 | Forward | CAAGGTGACAGGACTCAGCA |
| Reverse | TGGTGTGGAGAGGTCTCTGG | |
| Itgβ1 | Forward | GGATTCTCCAGAAGGTGGCT |
| Reverse | GGATTCTCCAGAAGGTGGCT | |
| S29 | Forward | GGAGTCACCCACCGGGAAGTTCGG |
| Reverse | GGAAGCACTGGCGGCACATG |
Immunohistochemical (IHC) Analysis
After allowing 14 days for differentiation induction, ATDC5 cells were fixed with 4% paraformaldehyde in phosphate buffered saline (PBS) after rinsing with PBS for 15 minutes. To block nonspecific binding, the cells were incubated with blocking buffer (1× PBS/0.3% Triton-X 100/5% normal goat serum) for 60 minutes at room temperature. Next, a 1:100 dilution of anti-ANGPTL2 (R&D Systems, Cat#AF1444, Minneapolis, MN, USA) and integrin α5β1 (Merck Millipore, Cat#BMB5, Darmstadt, Germany) antibodies in 1% bovine serum albumin (BSA) were added, and the plate was incubated at 4°C overnight. After washing with PBS, the cells were incubated with secondary Alexa Fluor 594–labeled anti-goat antibody and Alexa Fluor 488–labeled anti-rat antibody (Life Technologies) diluted with 1% BSA for 30 minutes at room temperature. After removing the secondary antibody solution and washing with PBS, nuclei were counterstained with 4′,6′-diamidino-2-phenylindole (DAPI). All images were captured under a microscope using image analysis software (Biozero, Keyence, Osaka, Japan).
Stimulation with Recombinant ANGPTL2
After incubating cells for 14 days in a 35-mm multiwell plate (FALCON, Franklin Lakes, NJ, USA) to allow for induction of differentiation, the medium was changed to DMEM/F12 without FBS. The cells were incubated for an additional 24 hours, and then recombinant human ANGPTL2 protein (Ray Biotech, Cat#230-00716, Norcross, GA, USA) was added at concentrations of 0.125, 0.25, 0.5, or 1.0 µg/mL and cells were incubated for 3 hours.
Treatment of ATDC5 Cells with Anti-Integrin α5β1 Antibody
After incubation of ATDC5 cells for 14 days to allow for differentiation induction, cells were maintained for 24 hours in DMEM/F12 containing 0% FBS. The cells were treated with anti-α5β1 integrin antibody (0.2, 2, or 20 µg/mL; Merck Millipore) for 12 hours before treatment with recombinant ANGPTL2 (1.0 µg/mL). Rat IgG (1 µg/mL; Merck Millipore) was used as a control for the anti-α5β1 integrin antibody.
Western Blot Analysis
Whole-cell lysate was prepared using Triton buffer (50 mM Tris, 250 mM NaCl, 0.1% Triton X-100, 1 mM EDTA, 50 mM NaF), and the protein concentration was determined using a Bradford protein assay (Bio-Rad). Equal amounts (20 μg) of protein were resolved in each lane of a tris-glycine SDS-PAGE gel (ATTO, Tokyo, Japan). After electrophoresis, the proteins were transferred onto polyvinylidene difluoride membranes using an i-Blot system (Invitrogen). After blocking with 1% skim milk (Cell Signaling Technology), the membranes were probed with antibody (1:1000 dilution) overnight at 4°C. Anti–phosphorylated p-38 (Cell Signaling Technology [CST], Danvers, MA, USA, Cat# 9211), anti–total p-38 (CST, Cat# 8690), anti–phosphorylated ERK1/2 (CST, Cat# 4370), anti–total ERK1/2 (CST, Cat# 4695), anti–phosphorylated JNK (CST, Cat# 4668S), anti–total JNK (CST, Cat# 9252), anti–phosphorylated Akt (CST, Cat# 4051S), anti–total Akt (CST, Cat# 9272S), anti–phosphorylated NF-κB p65 (CST, Cat# 3033S), anti–total NF-κB p65 (CST, Cat# 6956S), and anti-β-actin (CST, Cat# 4970) were used as primary antibodies. The membranes were then incubated with horseradish peroxidase–conjugated anti-mouse antibody or anti-rabbit antibody (1:20000 dilution; GE Healthcare Life Sciences, Tokyo, Japan) was used as the secondary antibody. Signals were detected using an Odyssey Blot Analyzer (M&S Techno Systems, Osaka, Japan). The protein bands were scanned and qualified as a ratio to β-actin using Image Studio software (LI-COR, Lincoln, NE, USA).
Statistical Analysis
Each experiment was repeated at least 3 times. Data were analyzed for significant differences using the Student t test or one-way analysis of variance and subsequently via Fisher least significant difference post hoc comparisons (StatView for Windows; SAS Institute, Cary, NC, USA) when necessary. A P value of <0.05 was regarded as indicative of a statistically significant difference, whereas a P value of <0.01 was regarded as indicative of a highly significant difference.
Results
Expression of ANGPTL2, Integrin α5, and Integrin β1 Genes in ATDC5 Cells
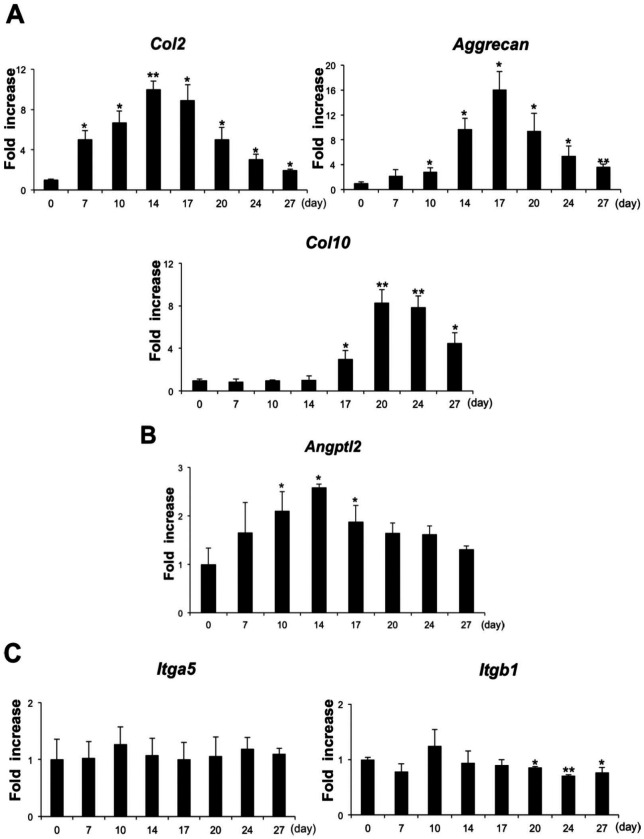
The expression pattern of ANGPTL2 was evaluated in comparison with various chondrocyte differentiation markers in ATDC5 cells. Expression of the gene encoding Col2, a chondrocyte proliferation marker, peaked 14 days after the start of differentiation. Expression of the gene encoding Aggrecan peaked 17 days after the start of differentiation, and that of Col10, a marker of hypertrophic differentiated chondrocytes, peaked 20 days after the start of differentiation ( Fig. 1A ). The level of ANGPTL2 expression peaked at day 14 and then declined significantly during the hypertrophic chondral phase ( Fig. 1B ).
Figure 1.
Transition gene expression pattern in ATDC5 cells at days 0, 7, 10, 14, 17, 20, 24, and 27 of chondrogenic cell differentiation. (A) Gene expression levels of Col2, Col10 and Aggrecan at each time point was determined by real-time reverse transcription–polymerase chain reaction (RT-PCR) analysis. (B) Angptl2 mRNA expression during chondrogenic differentiation of ATDC5 cells. Transcript levels were normalized to S29. (C) Integrin α5 and integrin β1 transcript levels in ATDC5 cells. Data are expressed as mean ± SD, n = 3. *P < 0.05, **P < 0.01 compared with controls at each time point (A-C).
The expression of integrin α5 and integrin β1 was investigated in differentiated ATDC5 cells. Levels of integrin α5 transcripts were fairly uniform in ATDC5 cells over the entire chondrocyte differentiation period. By contrast, levels of integrin β1 transcripts were significantly lower during the hypertrophic chondral phase ([ Fig. 1C ]).
IHC staining was used to assess the expression of ANGPTL2 and integrin α5β1 protein in cultured ATDC5 chondrocytes during the proliferative stage of chondrogenic differentiation. ANGPTL2 staining was observed in the cytoplasm but not the nucleus ( Fig. 2A ), whereas integrin α5β1 staining was observed on the cell surface but not in the nucleus ( Fig. 2B ).
Figure 2.
Immunohistochemistry for ANGPTL2 and Integrin α5β1 in ATDC5 cells. (A) Immunofluorescence staining for ANGPTL2. Nuclei were stained with DAPI (4′,6′-diamidino-2-phenylindole). (B) Immunofluorescence staining for Integrin α5β1. Nuclei were stained with DAPI. Scale bar represents 100 µm in each panel.
ANGPTL2 Induces the Expression of Inflammation-Related Genes
The expression pattern of inflammation-related genes was evaluated in ANGPTL2-treated ATDC5 cells by monitoring transcript levels. Real-time RT-PCR analysis revealed increased expression of the inflammation-related genes IL-1β, TNF-α, COX-2, ADAMTS-5, MMP-3, and MMP-13 at 3 hours after ANGPTL2 stimulation, compared with control cells ( Fig. 3A ). Furthermore, levels of integrin α5 and integrin β1 transcripts were also elevated ( Fig. 3B ).
Figure 3.
Effect of Angptl2 on gene expression of inflammatory mediators and its receptor. (A) ATDC5 treated with various concentrations of ANGPTL2 were subjected for 3 hours. Gene expression of IL-1β, TNF-α, COX-2, Adamts-5, MMP-3, and MMP-13 was measured by real-time reverse transcription–polymerase chain reaction (RT-PCR). (B) Integrin α5 and integrin β1 transcript levels in ANGPTL2-treated ATDC5 cells. Data are expressed as mean ± SD, n = 3. *P < 0.05, ** P < 0.01 compared with group under ANGPTL2 and without.
Effect of Anti-Integrin α5β1 Antibody on Expression of Inflammation Markers
Anti-integrin α5β1 antibody was used to investigate whether ANGPTL2 signaling via integrin α5β1 mediates the expression of inflammation markers. Real-time RT-PCR analysis and western blotting showed that the anti-integrin α5β1 antibody suppressed the mRNA and protein expression of IL-1β, TNF-α, COX-2, ADAMTS-5, MMP-3, and MMP-13 ( Fig. 4A-C ).
Figure 4.
Integrin α5β1 is a candidate receptor for ANGPTL2 in chondrocytes. (A) Gene expression of IL-1β, TNF-α, COX-2, Adamts-5, MMP-3, and MMP-13 in ATDC5 cells at day 14 postdifferentiation was measured by real-time reverse transcription–polymerase chain reaction (RT-PCR). Anti-integrin α5β1 antibody was added to cultures with various concentrations before recombinant ANGPTL2 treatment. RNA was harvested 3 hours after recombinant ANGPTL2 administration. Transcript levels were normalized to S29 and expressed as fold-increase relative to control expression levels (n = 3). Data are expressed as mean ± SD, n = 3. *P < 0.05, ** P < 0.01. (B) ATDC5 cells were treated with anti-integrin α5β1 antibody (20 µg/mL) before recombinant ANGPTL2 treatment. Protein was harvested 12 hours after recombinant ANGPTL2 administration and sample from each condition was subjected to Western immunoblotting analysis. (C) Relative protein expression was quantified using Image Studio software. The data presented are the means ± SD of the 3 independent experiments. **P < 0.01 compared with the control.
Effect of ANGPTL2 on Activation of Phosphorylated ERK, JNK, p38, Akt, and NF-κB
The release of pro-inflammatory cytokines, such as IL-1β and TNF-α, is mediated by the activation of signaling pathways involving 3 major MAP kinase (MAPK) groups (ERK, JNK, and p38) as well as Akt and NF-κB.33,34 The transcription factor NF-κB plays a particularly important role in regulating the expression of MMPs and aggrecanases in many types of cells. 35 We found that ANGPTL2 induced the phosphorylation of all 3 MAPKs within 60 minutes and phosphorylation of Akt within 5 minutes. In addition, phosphorylation of NF-κB was observed within 20 minutes and continued for 120 minutes ( Fig. 5A and B ).
Figure 5.
Effect of ANGPTL2 on activation of MAPKs, NF-κB, and Akt phosphorylation. (A) Whole cell lysis was subjected to Western blot analysis. β-actin was used as a loading control. (B) Relative protein expression was quantified using Image Studio software. The data presented are the means ± SD of the 3 independent experiments. *P < 0.05, **P < 0.01.
To evaluate the role of intracellular signaling pathways in ANGPTL2-induced integrin signaling in chondrocytes, we performed Western blotting of phosphorylated MAPKs, Akt, and NF-κB after treatment with neutralizing integrin α5β1 antibody. The levels of MAPKs, p-Akt, and p-NF-κB decreased markedly in antibody-treated cells. The total levels of MAPKs, Akt, and NF-κB protein remained unchanged under all conditions tested ( Fig. 6A and B ).
Figure 6.
Changes in cell signaling activities in ATDC5. (A) Cells were harvested after 20 and 60 minutes of treatment with 1 µg/mL recombinant ANGPTL2 after 12 hours of treatment with 20 µg/mL anti-integrin α5β1 antibody. Each cell lysate was subjected to Western immunoblotting analysis, followed by the detection of MAPKs, NF-κB, and Akt and their phosphorylated forms. (B) Relative protein expression was quantified using Image Studio software. The data presented are the means ± SD of the 3 independent experiments. *P < 0.05, **P < 0.01.
Discussion
ANGPTL2 is one of the biologically active substances attracting the attention of researchers, however, little has been reported on the expression and the roles of ANGPTL2 on chondrocytes. Tanoue et al. 31 reported ANGPTL2 is expressed mainly in resting and proliferative chondrocytes, and ANGPTL2 functions in chondrocyte differentiation and subsequent endochondral ossification. It was demonstrated that ANGPTL2 is also expressed in ATDC5 cells and that its expression peaks 14 days after initiation of chondrogenic differentiation. Integrins are cell surface mechanoreceptors that mediate adhesion between cells and the ECM and introduce signaling pathways to regulate cell physiology. 36 The integrin α5β1 receptor reportedly functions as an ANGPTL2 receptor in many types of cells. 26 In this study, it was found that integrin α5 is stably expressed during each stage of differentiation in ATDC5 cells; however, the level of integrin β1 expression decreased significantly in the latter stages of chondrogenic differentiation. This is similar to the results obtained from a previous study. 31
ANGPTL2 reportedly plays an important role as a mediator of inflammation in mouse peritoneal macrophages, 27 human ligamentum flavum fibroblasts, 28 and human retinal microvascular endothelial cells. 29 Constitutive activation of ANGPTL2 in skin epithelial cells of transgenic mice was shown to induce inflammation, including vasculature inflammation characterized by attachment of numerous leukocytes to the vessel walls and increased vascular permeability. 37 In dermatomyositis pathology, ANGPTL2 induces inflammation by increasing expression of IL-1β and IL-6, through NF-κB activation in an autocrine or paracrine manner. 19 Here, we demonstrated that ANGPTL2 dramatically upregulates the expression of various inflammation-related genes, such as IL-1β, TNF-α, COX-2, ADAMTS-5, MMP-3, and MMP-13, in mouse chondrogenic cells. The results of our study indicate that ANGPTL2 could play a key role in promoting inflammation in chondrocytes via activation of inflammatory cytokines. Furthermore, the expression of ANGPTL2 mRNA and protein is regulated by the administration of the recombinant ANGPTL2 suggesting an autocrine signaling mechanism for ANGPTL2 ( Fig. 4A-C ). MMP-3 and MMP-13 are known to be secondarily activated by proinflammatory cytokines such as IL-1β.38-40 MMPs have the important role of ECM remodeling, which occurs in processes as diverse as tissue morphogenesis, which is precisely regulated at the transcriptional level during normal tissue remodeling. On the other hand, MMP dysregulation occurs in various pathological conditions, such as rheumatoid arthritis, atherosclerosis, and tumor growth, invasion and metastasis, resulting in destructive tissue remodeling. 41 In this study, the upregulation of MMP-3 and MMP-13 expression after treatment with ANGPTL2 occurred relatively quickly. Recent study reported that in bone tissue, CXCL12-activated CXCR4 signaling promoted by ANGPTL2 accelerated osteolysis and bone engraftment most likely by increasing MMP-13 activity. 42 Therefore, it was hypothesized that MMPs upregulation is done more quickly by CXCL12-activated CXCR4 signaling than by proinflammatory cytokines. In addition, ANGPTL2 enhanced the expression of integrin α5 and β1 transcripts, which may promote inflammation synergistically. Taken together, we speculate that perturbed or constitutive ANGPTL2 expression promotes detrimental tissue remodeling through MMP and chronic inflammation.
Previous reports suggested that ANGPTL2-mediated activation of several intracellular signaling factors, including MAPKs, plays a role in the progression of a number of diseases.22,43 IL-1β upregulates the expression of inflammation-related genes in chondrocytes via activation of the JNK or Akt and NF-κB signaling pathways.44,45 Moreover, upregulation of MMP-3 and MMP-13 expression by IL-1β in articular chondrocytes reportedly involves MAPK pathways as well as NF-κB. 39 Shan et al. 46 reported that ANGPTL2 promotes the expression of IL-1β through p-38 and NF-κB in human primary chondrocytes. On the other hand, we especially focused on the reaction of chondrocytes in hypertrophic period to ANGPTL2. We investigated the effect of ANGPTL2 on these signaling pathways under inflammation using Western blotting. We found that the integrin α5β1 blocker markedly inhibited the phosphorylation of MAPKs, NF-κB, and Akt. These data suggest that the MAPKs, NF-κB, and Akt signaling pathways are activated primarily via integrin α5β1.
Osteoarthritis is characterized by degeneration of articular cartilage and subchondral bone. 47 Recent studies on OA have provided new insights into how chronic lower grade activation of proinflammatory pathways contributes to OA pathogenesis.48,49 The current study found that ANGPTL2 is abundantly expressed and secreted in the RA synovium and suggests that ANGPTL2 plays role in inflammation of the rheumatoid arthritis (RA) synovium via inflammatory vascular remodeling and recruitment of macrophages into RA joints. 18 This study showed that ANGPTL2 regulates MMPs expression, proving that ANGPTL2 might be involved in the progression of OA. In addition, recent reports suggest a possible role of other member of the ANGPTL family, such as ANGPTL450,51 and ANGPTL5 11 in pathogenesis of arthritis. Further investigation to identify the roles of ANGPTL family in inflammation of chondrocytes might therefore open the door to the development of new therapeutic approaches for cartilage lesions.
Conclusions
The results of our study demonstrate that ANGPTL2 induces the expression of various inflammation-related factors in chondrocytes via integrin α5β1.
Footnotes
Acknowledgments and Funding: We would like to thank all members of the Department of Orthodontics and Craniofacial Developmental Biology, Hiroshima University Graduate School of Biomedical & Health Sciences for their support of the present study. The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was funded by JSPS KAKENHI Grant Number 18K098330 from the Ministry of Education, Culture, Sports, Science and Technology of Japan.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
ORCID iD: Mami Takano  https://orcid.org/0000-0001-7928-0735
https://orcid.org/0000-0001-7928-0735
References
- 1. Guccione AA, Felson DT, Anderson JJ, Anthony JM, Zhang Y, Wilson PW, et al. The effects of specific medical conditions on the functional limitations of elders in the Framingham study. Am J Public Health. 1994;84:351-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Robinson WH, Lepus CM, Wang Q, Raghu H, Mao R, Lindstrom TM, et al. Low-grade inflammation as a key mediator of the pathogenesis of osteoarthritis. Nat Rev Rheumatol. 2016;12:580-92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Sellam J, Berenbaum F. The role of synovitis in pathophysiology and clinical symptoms of osteoarthritis. Nat Rev Rheumatol. 2010;6:625-35. [DOI] [PubMed] [Google Scholar]
- 4. Mow VC, Wang CC, Hung CT. The extracellular matrix, interstitial fluid and ions as a mechanical signal transducer in articular cartilage. Osteoarthritis Cartilage. 1999;7:41-58. [DOI] [PubMed] [Google Scholar]
- 5. Su SC, Tanimoto K, Tanne Y, Kunimatsu R, Hirose N, Mitsuyoshi T, et al. Celecoxib exerts protective effects on extracellular matrix metabolism of mandibular condylar chondrocytes under excessive mechanical stress. Osteoarthritis Cartilage. 2014;22:845-51. [DOI] [PubMed] [Google Scholar]
- 6. Asakawa-Tanne Y, Su S, Kunimatsu R, Hirose N, Mitsuyoshi T, Okamoto Y, et al. Effects of enzymatic degradation after loading in temporomandibular joint. J Dent Res. 2015;94:337-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Cursiefen C, Chen L, Borges LP, Jackson D, Cao J, Radziejewski C, et al. VEGF-A stimulates lymphangiogenesis and hemangiogenesis in inflammatory neovascularization via macrophage recruitment. J Clin Invest. 2004;113:1040-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kim I, Moon SO, Koh KN, Kim H, Uhm CS, Kwak HJ, et al. Molecular cloning, expression, and characterization of angiopoietin-related protein. Angiopoietin-related protein induces endothelial cell sprouting. J Biol Chem. 1999;274:26523-8. [DOI] [PubMed] [Google Scholar]
- 9. Oike Y, Yasunaga K, Suda T. Angiopoietin-related/angiopoietin-like proteins regulate angiogenesis. Int J Hematol. 2004;80:21-8. [DOI] [PubMed] [Google Scholar]
- 10. Kubota Y, Oike Y, Satoh S, Tabata Y, Niikura Y, Morisada T, et al. Cooperative interaction of angiopoietin-like proteins 1 and 2 in zebrafish vascular development. Proc Natl Acad Sci U S A. 2005;102:13502-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Hato T, Tabata M, Oike Y. The role of angiopoietin-like proteins in angiogenesis and metabolism. Trends Cardiovasc Med. 2008;18:6-14. [DOI] [PubMed] [Google Scholar]
- 12. Herrick S, Blanc-Brude O, Gray A, Laurent G. Fibrinogen. Int J Biochem Cell Biol. 1999;31:741-6. [DOI] [PubMed] [Google Scholar]
- 13. Mosesson MW. Fibrinogen and fibrin structure and functions. J Thromb Haemost. 2005;3:1894-904. [DOI] [PubMed] [Google Scholar]
- 14. Hynes RO, Lively JC, McCarty JH, Taverna D, Francis SE, Hodivala-Dilke K, et al. The diverse roles of integrins and their ligands in angiogenesis. Cold Spring Harb Symp Quant Biol. 2002;67:143-53. [DOI] [PubMed] [Google Scholar]
- 15. Camenisch G, Pisabarro MT, Sherman D, Kowalski J, Nagel M, Hass P, et al. ANGPTL3 stimulates endothelial cell adhesion and migration via integrin αvβ3 and induces blood vessel formation in vivo. J Biol Chem. 2002;277:17281-90. [DOI] [PubMed] [Google Scholar]
- 16. Tabata M, Kadomatsu T, Fukuhara S, Miyata K, Ito Y, Endo M, et al. Angiopoietin-like protein 2 promotes chronic adipose tissue inflammation and obesity-related systemic insulin resistance. Cell Metab. 2009;10:178-88. [DOI] [PubMed] [Google Scholar]
- 17. Nakao S, Zandi S, Hata Y, Kawahara S, Arita R, Schering A, et al. Blood vessel endothelial VEGFR-2 delays lymphangiogenesis: an endogenous trapping mechanism links lymph- and angiogenesis. Blood. 2011;117:1081-90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Okada T, Tsukano H, Endo M, Tabata M, Miyata K, Kadomatsu T, et al. Synoviocyte-derived angiopoietin-like protein 2 contributes to synovial chronic inflammation in rheumatoid arthritis. Am J Pathol. 2010;176:2309-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ogata A, Endo M, Aoi J, Takahashi O, Kadomatsu T, Miyata K, et al. The role of angiopoietin-like protein 2 in pathogenesis of dermatomyositis. Biochem Biophys Res Commun. 2012;418:494-9. [DOI] [PubMed] [Google Scholar]
- 20. Oike Y, Tian Z, Miyata K, Morinaga J, Endo M, Kadomatsu T. ANGPTL2—a new causal player in accelerating heart disease development in the aging. Circ J. 2017;81:1379-85. [DOI] [PubMed] [Google Scholar]
- 21. Yang W, Liu W, Yu W, Fei D, Meng X, Yang S, et al. Angptl2 deficiency attenuates paraquat (PQ)-induced lung injury in mice by altering inflammation, oxidative stress and fibrosis through NF-κB pathway. Biochem Biophys Res Commun. 2018;503:94-101. [DOI] [PubMed] [Google Scholar]
- 22. Morinaga J, Kadomatsu T, Miyata K, Endo M, Terada K, Tian Z, et al. Angiopoietin-like protein 2 increases renal fibrosis by accelerating transforming growth factor-β signaling in chronic kidney disease. Kidney Int. 2016;89:327-41. [DOI] [PubMed] [Google Scholar]
- 23. Sasaki Y, Ohta M, Desai D, Figueiredo JL, Whelan MC, Sugano T, et al. Angiopoietin like protein 2 (ANGPTL2) promotes adipose tissue macrophage and T lymphocyte accumulation and leads to insulin resistance. PLoS One. 2015;10:e0131176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Aoi J, Endo M, Kadomatsu T, Miyata K, Ogata A, Horiguchi H, et al. Angiopoietin-like protein 2 accelerates carcinogenesis by activating chronic inflammation and oxidative stress. Mol Cancer Res. 2014;12:239-49. [DOI] [PubMed] [Google Scholar]
- 25. Thorin-Trescases N, Thorin E. Angiopoietin-like-2: a multifaceted protein with physiological and pathophysiological properties. Expert Rev Mol Med. 2014;16:e17. [DOI] [PubMed] [Google Scholar]
- 26. Kadomatsu T, Endo M, Miyata K, Oike Y. Diverse roles of ANGPTL2 in physiology and pathophysiology. Trends Endocrinol Metab. 2014;25:245-54. [DOI] [PubMed] [Google Scholar]
- 27. Tazume H, Miyata K, Tian Z, Endo M, Horiguchi H, Takahashi O, et al. Macrophage-derived angiopoietin-like protein 2 accelerates development of abdominal aortic aneurysm. Arterioscler Thromb Vasc Biol. 2012;32:1400-9. [DOI] [PubMed] [Google Scholar]
- 28. Nakamura T, Okada T, Endo M, Nakamura T, Oike Y, Mizuta H. Angiopoietin-like protein 2 promotes inflammatory conditions in the ligamentum flavum in the pathogenesis of lumbar spinal canal stenosis by activating interleukin-6 expression. Eur Spine J. 2015;24:2001-9. [DOI] [PubMed] [Google Scholar]
- 29. Kanda A, Noda K, Oike Y, Ishida S. Angiopoietin-like protein 2 mediates endotoxin-induced acute inflammation in the eye. Lab Invest. 2012;92:1553-63. [DOI] [PubMed] [Google Scholar]
- 30. Nakamura T, Okada T, Endo M, Kadomatsu T, Taniwaki T, Sei A, et al. Angiopoietin-like protein 2 induced by mechanical stress accelerates degeneration and hypertrophy of the ligamentum flavum in lumbar spinal canal stenosis. PLoS One. 2014;9:e85542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Tanoue H, Morinaga J, Yoshizawa T, Yugami M, Itoh H, Nakamura T, et al. Angiopoietin-like protein 2 promotes chondrogenic differentiation during bone growth as a cartilage matrix factor. Osteoarthritis Cartilage. 2018;26:108-17. [DOI] [PubMed] [Google Scholar]
- 32. Askari JA, Buckley PA, Mould AP, Humphries MJ. Linking integrin conformation to function. J Cell Sci. 2009;122(Pt 2):165-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Rao KM. MAP kinase activation in macrophages. J Leukoc Biol. 2001;69:3-10. [PubMed] [Google Scholar]
- 34. Krappmann D, Wegener E, Sunami Y, Esen M, Thiel A, Mordmuller B, et al. The IkappaB kinase complex and NF-kappaB act as master regulators of lipopolysaccharide-induced gene expression and control subordinate activation of AP-1. Mol Cell Biol. 2004;24:6488-500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Vincenti MP, Brinckerhoff CE. Transcriptional regulation of collagenase (MMP-1, MMP-13) genes in arthritis: integration of complex signaling pathways for the recruitment of gene-specific transcription factors. Arthritis Res. 2002;4:157-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Zhu HF, Liu YJ, Chu LX, Feng W. Effects of mechanical stimulation on expression of integrin subunits in chondrocyte [in Chinese]. Zhongguo Gu Shang. 2011;24:266-8. [PubMed] [Google Scholar]
- 37. Aoi J, Endo M, Kadomatsu T, Miyata K, Nakano M, Horiguchi H, et al. Angiopoietin-like protein 2 is an important facilitator of inflammatory carcinogenesis and metastasis. Cancer Res. 2011;71:7502-12. [DOI] [PubMed] [Google Scholar]
- 38. Joosten LA, Helsen MM, Saxne T, van De Loo FA, Heinegard D, van Den Berg WB. IL-1 alpha beta blockade prevents cartilage and bone destruction in murine type II collagen-induced arthritis, whereas TNF-alpha blockade only ameliorates joint inflammation. J Immunol. 1999;163:5049-55. [PubMed] [Google Scholar]
- 39. Liacini A, Sylvester J, Li WQ, Zafarullah M. Inhibition of interleukin-1-stimulated MAP kinases, activating protein-1 (AP-1) and nuclear factor kappa B (NF-κB) transcription factors down-regulates matrix metalloproteinase gene expression in articular chondrocytes. Matrix Biol. 2002;21:251-62. [DOI] [PubMed] [Google Scholar]
- 40. Kapoor M, Martel-Pelletier J, Lajeunesse D, Pelletier JP, Fahmi H. Role of proinflammatory cytokines in the pathophysiology of osteoarthritis. Nat Rev Rheumatol. 2011;7:33-42. [DOI] [PubMed] [Google Scholar]
- 41. Hijova E. Matrix metalloproteinases: their biological functions and clinical implications. Bratisl Leke Listy. 2005;106:127-32. [PubMed] [Google Scholar]
- 42. Masuda T, Endo M, Yamamoto Y, Odagiri H, Kadomatsu T, Nakamura T, et al. ANGPTL2 increases bone metastasis of breast cancer cells through enhancing CXCR4 signaling. Sci Rep. 2015;5:9170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Odagiri H, Kadomatsu T, Endo M, Masuda T, Morioka MS, Fukuhara S, et al. The secreted protein ANGPTL2 promotes metastasis of osteosarcoma cells through integrin α5β1, p38 MAPK, and matrix metalloproteinases. Sci Signal. 2014;7:ra7. [DOI] [PubMed] [Google Scholar]
- 44. Akhtar N, Haqqi TM. Epigallocatechin-3-gallate suppresses the global interleukin-1beta-induced inflammatory response in human chondrocytes. Arthritis Res Ther. 2011;13:R93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Montaseri A, Busch F, Mobasheri A, Buhrmann C, Aldinger C, Rad JS, et al. IGF-1 and PDGF-bb suppress IL-1β-induced cartilage degradation through down-regulation of NF-κB signaling: involvement of Src/PI-3K/AKT pathway. PLoS One. 2011;6:e28663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Shan W, Cheng C, Huang W, Ding Z, Luo S, Cui G, et al. Angiopoietin-like 2 upregulation promotes human chondrocyte injury via NF-κB and p38/MAPK signaling pathway. J Bone Miner Metab. Epub 2019 June 18. [DOI] [PubMed] [Google Scholar]
- 47. Goldring MB, Goldring SR. Osteoarthritis. J Cell Physiol. 2007;213:626-34. [DOI] [PubMed] [Google Scholar]
- 48. Guilak F, Fermor B, Keefe FJ, Kraus VB, Olson SA, Pisetsky DS, et al. The role of biomechanics and inflammation in cartilage injury and repair. Clin Orthop Relat Res. 2004;(423):17-26. [DOI] [PubMed] [Google Scholar]
- 49. Hamerman D. The biology of osteoarthritis. N Engl J Med. 1989;320:1322-30. [DOI] [PubMed] [Google Scholar]
- 50. Hermann LM, Pinkerton M, Jennings K, Yang L, Grom A, Sowders D, et al. Angiopoietin-like-4 is a potential angiogenic mediator in arthritis. Clin Immunol. 2005;115:93-101. [DOI] [PubMed] [Google Scholar]
- 51. Murata M, Yudo K, Nakamura H, Chiba J, Okamoto K, Suematsu N, et al. Hypoxia upregulates the expression of angiopoietin-like-4 in human articular chondrocytes: role of angiopoietin-like-4 in the expression of matrix metalloproteinases and cartilage degradation. J Orthop Res. 2009;27:50-7. [DOI] [PubMed] [Google Scholar]