Abstract
Objective
miR-146a-5p was found to be significantly upregulated in cartilage tissue of patients with osteoarthritis (OA). NUMB was shown to be involved in the autophagy regulation process of cells. We aimed to learn whether NUMB was involved in the apoptosis or autophagy process of chondrocytes in OA and related with miR-146a-5p.
Methods
QRT-PCR was used to detect miR-146a-5p level in 22 OA cartilage tissues and 22 controls. The targets of miR-146a-5p were analyzed using software and the luciferase reporter experiment. The apoptosis and autophagy, and related proteins were detected in chondrocytes treated with miR-146a-5p mimic/inhibitor or pcDNA3.1-NUMB/si-NUMB and IL-1β, respectively. In vivo experiment, intra-articular injection of miR-146a-5p antagomir/NC was administered at the knee of OA male mice before and after model construction. Chondrocyte apoptosis and the expression of apoptosis and autophagy-related proteins were also detected.
Results
miR-146a-5p was highly expressed in knee cartilage tissue of patients with OA, while NUMB was lowly expressed and negatively regulated by miR-146a-5p. Upregulation of miR-146a-5p can promote cell apoptosis and reduce autophagy of human and mouse chondrocytes by modulating the levels of cleaved caspase-3, cleaved PARP, Bax, Beclin 1, ATG5, p62, LC3-I, and LC3-II. Increasing the low level of NUMB reversed the effects of miR-146a-5p on chondrocyte apoptosis and autophagy. Intra-articular injection of miR-146a-5p antagomir can also reverse the effects of miR-146a-5p on the apoptosis and autophagy of knee joint chondrocytes in OA mice.
Conclusion
Downregulation of miR-146a-5p suppresses the apoptosis and promotes autophagy of chondrocytes by targeting NUMB in vivo and in vitro.
Keywords: osteoarthritis, chondrocyte, miR-146a-5p, NUMB, apoptosis, autophagy
Introduction
Osteoarthritis (OA) is the most common joint disease, characterized by articular cartilage degeneration and bone remodeling.1,2 Chondrocytes are the unique type of cells in articular cartilage. Not only do they synthesize the components of ECM,3,4 but their quantity and activity are also more suitable for the physiological state of cartilage tissue. Chondrocyte apoptosis, 5 autophagy, 6 and reductions in chondrocyte numbers 7 caused by inflammation play an important regulatory role in the pathogenesis of OA.
Noncoding RNA molecules, miRNAs, regulate cell proliferation, apoptosis, inflammation, and autophagy.8,9 Studies have shown that miRNA is abnormally expressed in OA tissues and plays an important role in the pathogenesis of OA. The levels of miR-146a and miR-155 in patients with OA were increased at the progressive stages (grade 3 and 4). 10 MiR-335-5p alleviated inflammation by activating autophagy 11 and miR-128a-induced loss of Atg12 inhibited chondrocyte autophagy, which aggravated the progress of OA. 12 The level of miRNA-140 was significantly reduced in chondrocytes and synovial fluid derived from human OA cartilage, and upregulated miR-140 increased the number of chondrocytes, cartilage thickness, and collagen expression in rat cartilage. 7
We screened out a total of 10 target genes (TRAF6, IRAK1, SEC23IP, PPP1R11, NUMB, RARB, STRBP, ZFYVE1, LRRC15, PRX) for miR-146a-5p through 4 online tools, including TargetScan 7.2 (http://www.targetscan.org/vert_72/), MicroT-CDS (http://www.microrna.gr/microT-CDS), RNA22 full sets of predictions (https://cm.jefferson.edu/rna22/Precomputed/), PicTar (http://www.pictar.org/), miRDB (http://mirdb.org/), and all 10 target genes were conserved among human beings, mice and rats. Then, what we found from previous study was that miR-146a-5p is significantly upregulated (>2-fold change) in the cartilage tissue of patients with OA. 13 MiR-146a-5p has also been found to significantly promote the apoptosis of prostate cancer cells, 14 rat cardiomyocytes 15 indicating that it plays an important role in the process of cell apoptosis. Another study has shown that miR-146a-5p as a major microRNA in CRCSCs targets NUMB to regulate the Wnt-β-catenin pathway, which is reported to be involved in chondrocyte apoptosis in OA.16-18 Some studies have shown that a novel role of NUMB as a regulator of pro-inflammatory cytokine production 19 and NUMB was involved in autophagy regulation.20,21 Therefore, we hypothesize that NUMB may be involved in the apoptosis or autophagy process of chondrocytes in OA.
The inflammatory cytokine interleukin-1β (IL-1β) is not only highly expressed in OA tissues 22 but is also related to the occurrence and progression of OA. 23 In this study, we investigated the expression of miR-146a-5p and candidate target gene (NUMB) in OA tissues, and verified the relationship between them. We also explored the role of miR-146a-5p and target genes in apoptosis and autophagy of IL-1β-induced human and mouse chondrocytes in vitro. On this basis, we further examined the effect of intra-articular injection of miR-146a-5p antagomir on chondrocyte apoptosis and autophagy in the OA tissues of rat knees. Our results demonstrated that high-level miR-146a-5p increases OA chondrocyte apoptosis and reduces cell autophagy by reducing NUMB expression level.
Materials and Methods
Samples
OA cartilage specimens were collected from 22 patients (aged 62.3 [SD = 5.2] years) with OA of the knee requiring total knee arthroplasty and normal cartilage specimens were collected from 22 patients (age 55.8 (SD = 4.6] years) underwent amputation at our hospital. This study was approved by the Ethics Committee of our institution, and written informed consent was obtained from each patient. Patients with primary knee OA diagnosed according to the American College of Rheumatology (ACR) knee OA criteria, 24 and the classification is based on the Kellgren- Lawrence scoring system. 25 The Kellgren-Lawrence score ranged from 0 to 4, where 0 = normal radiographs; 1 = suspected pathology; 2= small osteophytes, possibly stenosis, cysts, and sclerosis; 3 = moderate, defined osteophytes with moderate joint space narrowing; and 4 = severe, with large osteophytes and definite joint space narrowing. In our study, the number of patients in each grade is 22 (grade 0), 3 (grade 1), 7 (grade 2), 7 (grade 3), and 5 (grade 4).
Chondrocytes Isolation and Culture
Chondrocytes were isolated from normal fresh cartilage specimens of human knee or mouse knee using 0.25% trypsin and type II collagenase. 11 Cells were cultured in high–Dulbecco’s modified Eagle medium (high-DMEM) medium containing 10% fetal bovine serum and transfected with the miR-146a-5p mimics (100 nM) or inhibitor (100 nM) using lipofectamine 3000. Cells were treated with IL-1β (10 ng/mL) for 24 hours to establish OA cell model 48 hours after transfection. Furthermore, chondrocytes were co-transfected with miRNA-146a-5p mimics (100 nM) or inhibitor (100 nM) and pcDNA3.1-NUMB or siNUMB for 48 hours to detect the expression of apoptosis or autophagy related proteins. miRNA-146a-5p mimics, inhibitor, plasmid pcDNA3.1-NUMB, and siNUMB were synthesized by Shanghai GenePharma Co., Ltd. (Shanghai, China).
Luciferase Reporter Assay
The wild-type sequence and mutation sequence of NUMB 3′UTR containing the miR-146a-5p binding site were cloned into pmirGLO vector (Promega, Madison, WI, USA), respectively. Luciferase assay was performed in HEK-293T cells (24-well plate) co-transfected with luciferase reporter constructs (200 ng) and 400 ng miR-146a-5p mimics or NC mimics, miR-146a-5p inhibitor or NC inhibitor. Luciferase activity was detected using the Dual Luciferase Reporter Assay kit (Promega) at 24 hours after transfection.
Immunofluorescence (IF)
Chondrocytes were fixed with 100% methanol, permeabilized with 0.1% Triton X-100, and blocked with 1% bovine serum albumin (BSA)/10% normal goat serum/0.3 M glycine in 0.1% phosphate buffered saline (PBS)-Tween (Sigma Aldrich, Germany) in PBS. Then cells were incubated with LC3 antibody (1:1000, Abcam) overnight at 4 °C and subsequently incubated with goat secondary antibody to rabbit IgG (Alexa Fluor 488) (1:500, Abcam) at room temperature for 1 hour and stained with DAPI. The images were collected by a Nikon Eclipse 50i electron microscope.
Quantitative Real-Time Reverse Transcription–Polymerase Chain Reaction (qRT-PCR)
Total RNA was extracted from cartilage specimens or chondrocytes using TRIzol reagent. One microgramme total RNA was reversely transcribed into cDNAs by PrimeScript RT reagent kit (Takara, China), and cDNA was amplified with TB Green Premix Ex Taq using a qTOWER3G detection system (Analytikjena, Jena, Germany). Target genes expression levels were normalized with U6 RNA and GAPDH mRNA. Gene expression levels were calculated using the 2−ΔΔCt method.
OA Model
OA model of rat knee was constructed using anterior cruciate ligament transection (ACLT) method as previously described. 12 All rats were randomly divided into 4 groups with 24 rats in each group. Sham group: only exposed the cartilage surface of the knee joint, and then sutured. OA group: rats underwent ACLT to induce the formation of OA. OA/NC group: rats underwent ACLT and intra-articular injection of miR-146a-5p negative control (NC) (50 nmol/L) at 6 hours before surgery and 12 weeks after surgery (once a week). OA/antagomir group: rats underwent ACLT and intra-articular injection of miR-146a-5p antagomir (50 nmol/L) at 6 hours before surgery and 12 weeks after surgery (once a week). Six random rats in each group were sacrificed every 4 weeks.
Western Blotting
Total protein was extracted with RIPA lysis buffer containing protease inhibitor (Beyotime). Fifty micrograms protein was conducted on a 10% gel using sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) and transferred to a polyvinylidene difluoride membrane (Millipore, Burlington, MA, USA). After sealing in 5% skimmed milk, the membrane was incubated with primary antibodies for cleaved caspase 3 (1:1000, Abcam), cleaved PARP (1:1000, Abcam), Bax (1:1000, Abcam), Bcl-2 (1:500, Abcam), Beclin 1 (1:1000, Abcam), NUMB (1:1000, Abcam), ATG5 (1:5000, Abcam), p62 (1:1000, Abcam), LC3 (1:2000, Abcam), GAPDH (1:5000, Abcam) overnight at 4 °C. Then membrane was incubated with goat anti-rabbit IgG H&L (HRP) (1:50000, Abcam). Finally, an enhanced chemiluminescence (ECL) kit was used to detect the signal.
Immunohistochemistry (IHC)
Cartilage specimens from rat knee were fixed in 10% formalin and decalcified in 10% ethylenediaminetetraacetic acid before embedding in paraffin. IHC was carried out using EnVision Ⅲ Detection System/Mo&Rb (Dako, Glostrup, Denmark). Sections (7 µm) were obtained to perform the deparaffinage, rehydration, and blocking the endogenous peroxidases with 3% hydrogen peroxide in methanol (Sigma-Aldrich). The sections were incubated with NUMB (1:1000, Abcam) or LC3-II (1:200, Abcam) antibody at 4 °C overnight and subsequently incubated with secondary antibody at 37 °C for 30 minutes. Then sections were stained with diaminobenzidine chromogen and counterstained with hematoxylin (ZYMED Laboratories/Invitrogen). Photomicrographs were captured with a Nikon Eclipse 50i microscope (Nikon, Milville, NY, USA) and Nikon DS-FiI digital camera.
Assessment of Cell Apoptosis
Cell apoptosis of chondrocytes were confirmed using flow cytometry (BD FACSCanto II, San Jose, CA, USA) and TUNEL staining. Cell apoptosis of OA specimens from rat were confirmed using TUNEL staining. Flow cytometry was performed using Annexin V-FITC/PI Apoptosis Detection Kit (Elabscience, China) and TUNEL staining was performed using One Step TUNEL Apoptosis Assay Kit (Beyotime, China) according to the manufacturer’s instructions.
Statistical Analysis
All statistical analysis was performed using GraphPad Prism 8 (GraphPad Software, San Jose, USA). First, we used three methods (D ‘Agootino-Pearson method, Kolmogrov-Smirnov method and Shapiro-Wilk method) provided by GraphPad Prism 8 to analyze whether the data were in accordance with normal distribution. And then, the correlation between the expression levels of miR-146a-5p and NUMB mRNA was analyzed using Pearson correlation analysis. All quantitative data were expressed as mean ± standard deviation (SD). Differences between different groups were analyzed using Student t test or one-way analysis of variance. When P < 0.05, the difference was considered statistically significant.
Results
Aberrant Expression of miR-146a-5p and NUMB mRNA in OA Cartilage
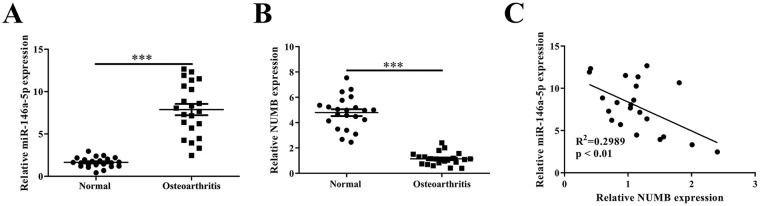
According to qRT-PCR, we verified that miR-146a-5p was upregulated in OA cartilage, compared with normal cartilage (P < 0.001) ( Fig. 1A ); while the level of NUMB mRNA was decreased significantly in OA cartilage compared with normal cartilage (P < 0.001) ( Fig. 1B ). Furthermore, Pearson correlation analysis showed that the miR-146a-5p expression was negatively related to the expression of NUMB mRNA in human OA cartilage (R2 = 0.2989) ( Fig. 1C ).
Figure 1.
Expression levels of miR-146a-5p and NUMB in cartilage tissues from osteoarthritis and nonosteoarthritis patients. (A) QRT-PCR showed the relative miR-146a-5p levels in clinical specimens. (B) QRT-PCR showed the relative NUMB mRNA expression. (C) The expression of miR-146a-5p was negatively correlated with the NUMB expression in the osteoarthritis cartilage.
MiR-146a-5p Targeted NUMB to Inhibit Its Expression
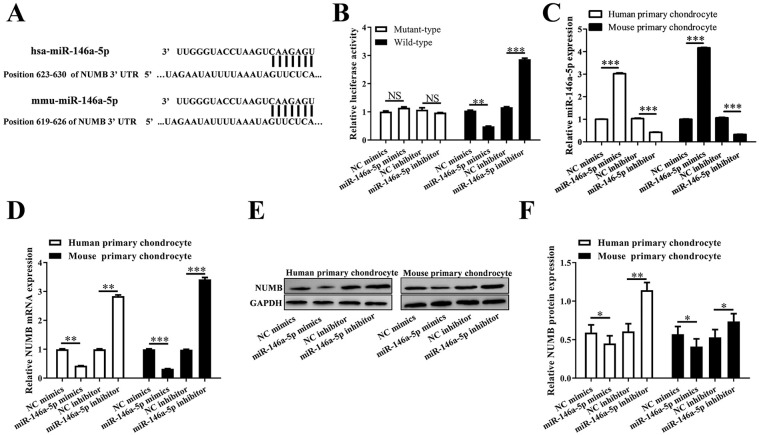
The binding site of has-miR-146a-5p or mmu-miR-146a-5p within NUMB 3′UTR was predicted using 5online tools ( Fig. 2A ). And the relative luciferase activity in NUMB 3′UTR wild-type + miR-146a-5p mimics group was reduced remarkably compared with that in NUMB 3′UTR wild-type + NC mimics group (P < 0.01). While the relative luciferase activity in NUMB 3′UTR wild-type + miR-146a-5p inhibitor group was increased notably than that in NUMB 3′UTR wild-type + NC inhibitor group (P < 0.001) ( Fig. 2B ). The level of miR-146a-5p in human chondrocytes was decreased significantly (P < 0.001) or increased significantly (P < 0.001) when transfecting with miR-146a-5p mimics or miR-146a-5p inhibitor, respectively, as same as that in mouse chondrocytes ( Fig. 2C ). Furthermore, NUMB mRNA level and protein level were also declined notably in miR-146a-5p mimics treated human or mouse chondrocytes (P < 0.05), while promoted notably in miR-146a-5p inhibitor treated human or mouse chondrocytes (P < 0.01) ( Fig. 2D-F ).
Figure 2.
MiR-146a-5p negatively regulated NUMB expression via a direct binding site at 3′UTR of NUMB mRNA. (A) There is a potential miR-146a-5p binding sequence in the 3′UTR of NUMB mRNA. (B) The relative luciferase activity after the co-transfection of miR-146a-5p mimic/inhibitor and 3′UTR of NUMB mRNA. (C, D) QRT-PCR showed the miR-146a-5p and NUMB mRNA levels in the 2 types of cells after transfection with miR-146a-5p mimic or inhibitor. (E, F) Western blotting showed the NUMB protein levels in the 2 types of cells after transfection with miR-146a-5p mimic or inhibitor.
Elevated miR-146a-5p Promoted IL-1β-Induced Chondrocyte Apoptosis
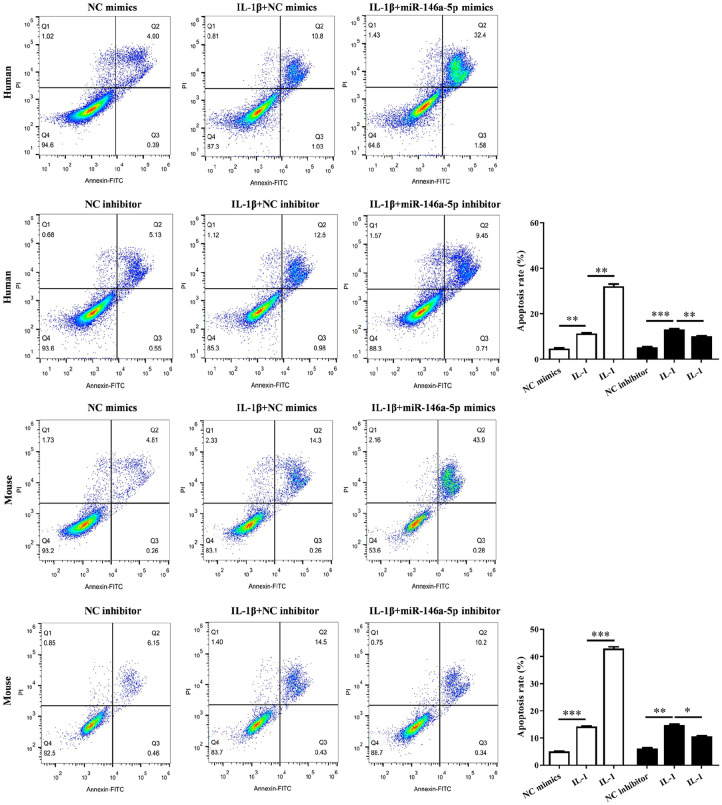
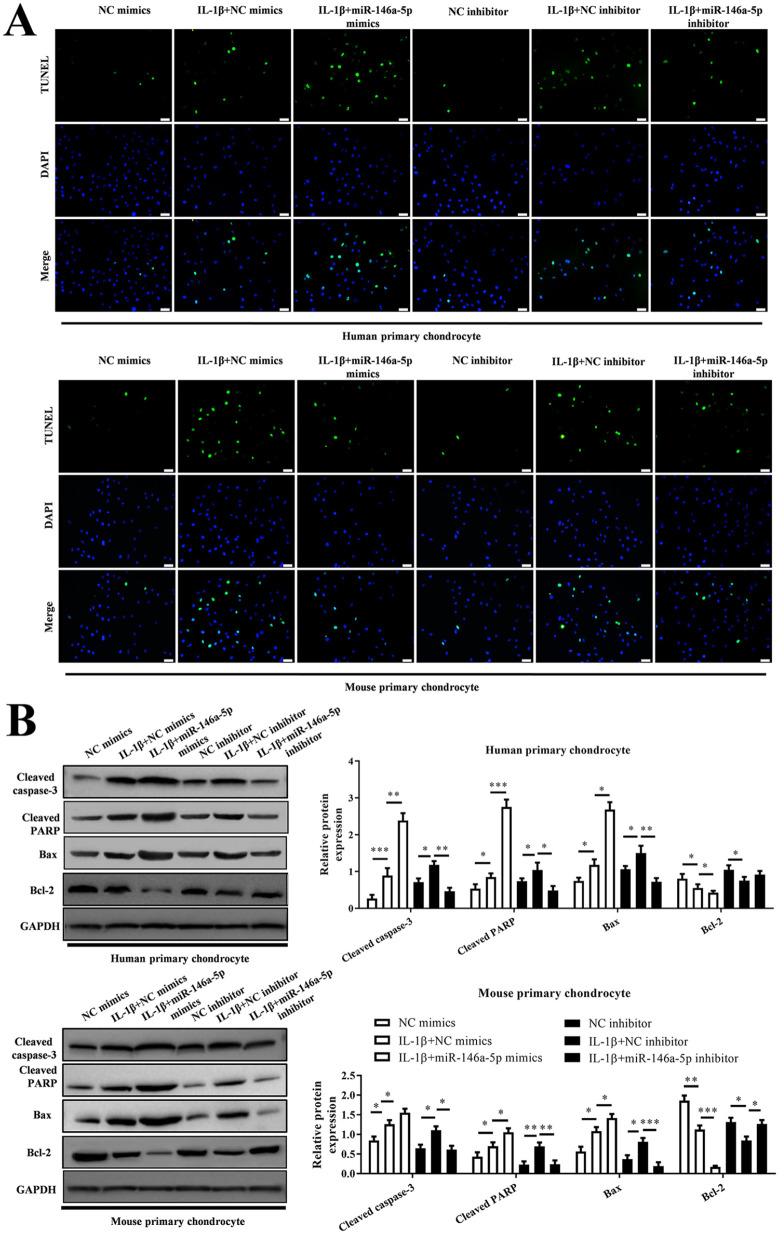
According to flow cytometry, both in human chondrocytes and mouse chondrocytes, we verified the increased apoptosis in IL-1β-induced chondrocytes (P < 0.01), while miR-146a-5p mimics enhanced the cell apoptosis (P < 0.01) but miR-146a-5p inhibitor weakened the cell apoptosis (P < 0.05) ( Fig. 3 ). This was also observed in TUNEL assay ( Fig. 4A ). In addition, the apoptosis-related proteins were also abnormally expressed in these chondrocytes. The levels of cleaved caspase-3 and PARP, Bax were upregulated significantly and Bcl-2 was decreased significantly in IL-1β + miR-146a-5p mimics group as well as those in IL-1β group, compared with those in control group (P < 0.05). But the effect of miR-146a-5p inhibitor on these proteins was opposite to that of miR-146a-5p mimics ( Fig. 4B ).
Figure 3.
Cell apoptosis of the chondrocytes analyzed using flow cytometry (FCM). The histogram showed the results based on 3parallel experiments.
Figure 4.
MiR-146a-5p promotes IL-1β-induced chondrocyte apoptosis in osteoarthritis (OA) progression. (A) TUNEL assays were performed to evaluate apoptosis of chondrocytes. (B) The protein level of cleaved caspase 3, cleaved PRAP, Bax, and Bcl2 measured with Western blotting.
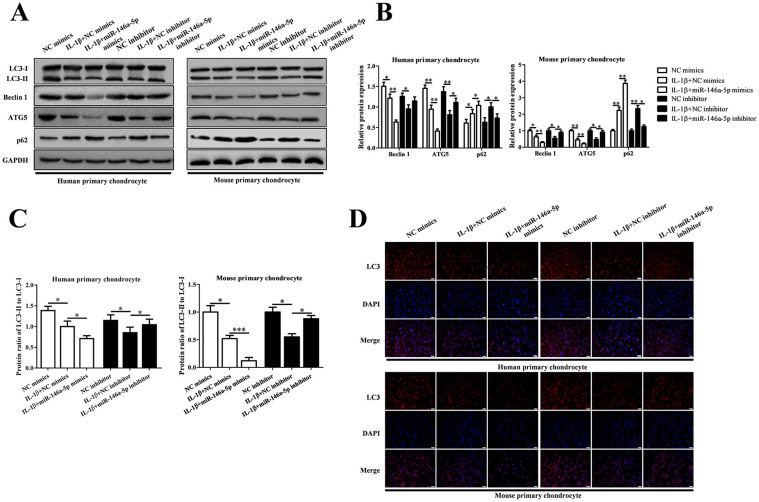
MiR-146a-5p Regulated the Autophagy in IL-1β-Induced Chondrocytes
To investigate the effects of miR-146a-5p on autophagy in IL-1β-induced human and mouse chondrocytes, we detected the autophagy related proteins (Beclin 1, ATG5, p62, LC3-II and LC3-I) in IL-1β group, IL-1β + miR-146a-5p mimics group, IL-1β +miR-146a-5p inhibitor group using Western blotting and IF ( Fig. 5 ). Elevated miR-146a-5p intensified the reduction of Beclin 1, ATG5 and the ratio of LC3-II/ LC3-I, and the increase of p62 in IL-1β-induced chondrocytes. Declined miR-146a-5p reversed the effects of IL-1β on chondrocyte autophagy.
Figure 5.
Knockdown of miR-146a-5p reversed IL-1β-mediated chondrocyte autophagy inhibition in osteoarthritis (OA) progression. (A) The protein level of LC3, Beclin1, ATG5, and p62 measured with Western blotting. (B, C) The histogram showed the relative levels of Beclin1, ATG5, and p62 and the ratio of LC3-II to LC3-I. (D) LC3-II was determined by immunofluorescence staining (magnification 200×).
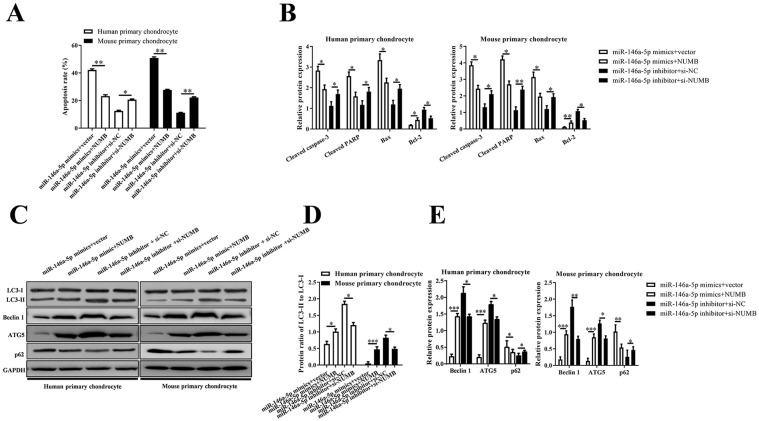
NUMB Mediated the Effects of miR-146a-5p on Primary Chondrocytes
Human or mouse primary chondrocytes were, respectively, co-transfected with miR-146a-5p mimics or miR-146a-5p inhibitor and pcDNA3.1-NUMB or siNUMB to investigate the function of NUMB in the effects of miR-146a-5p on primary chondrocytes. Overexpression of NUMB attenuated the apoptosis of miR-146a-5p mimics treated chondrocytes but improved the apoptosis of miR-146a-5p inhibitor treated chondrocytes (P < 0.05) ( Fig. 6A ). In addition, the levels of cleaved caspase-3, cleaved PARP, and Bax were downregulated significantly in miR-146a-5p mimics + NUMB group, while Bcl-2 was upregulated significantly, compared with those in miR-146a-5p mimics + vector group. Si-NUMB also reversed the effects of miR-146a-5p inhibitor on primary chondrocytes (P < 0.05) ( Fig. 6B ). The autophagy related proteins were also modulated by NUMB in miR-146a-5p mimics or miR-146a-5p inhibitor treated primary chondrocytes ( Fig. 6C-E ).
Figure 6.
Human primary chondrocytes and mouse primary chondrocytes were transfected with miR-146a-5p mimic or inhibitor, and/or NUMB or empty vector, si-NUMB or negative control siRNAs, and then stimulated with IL-1β. (A) The histogram showed the cell apoptosis rate of the chondrocytes. (B) The mRNA levels of cleaved caspase 3, cleaved PRAP, Bax, and Bcl2 measured with qRT-PCR. (C) The protein levels of LC3, Beclin1, ATG5, and p62 measured with Western blotting. (D, E) The histogram showed the relative levels of Beclin1, ATG5, and p62 and the ratio of LC3-II to LC3-I.
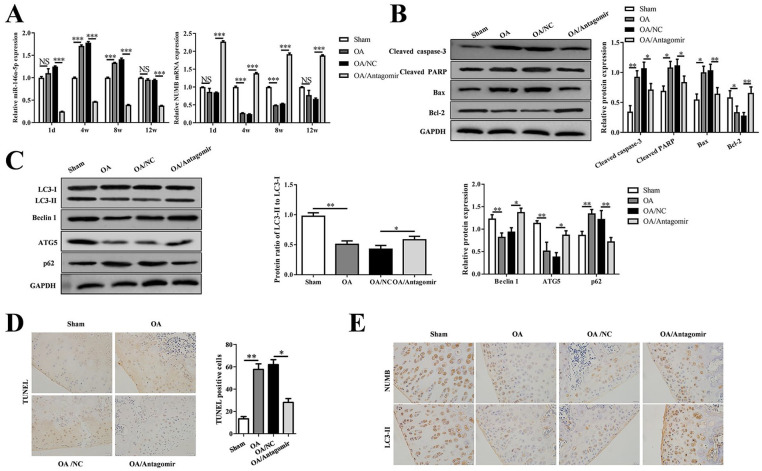
MiR-146a-5p Antagomir Suppressed the Articular Chondrocyte Apoptosis while It Promoted Autophagy In Vivo
The level of miR-146a-5p was upregulated significantly (P < 0.001) in OA cartilage of rat knees at 4 and 8 weeks after ALCT surgery while the level of NUMB mRNA was downregulated significantly (P < 0.001). The continuous injection of antagomir significantly reduced the level of miR-146a-5p (P < 0.001) and increased the level of NUMB mRNA (P < 0.001) ( Fig. 7A ). In addition, the expression of apoptosis-related proteins and autophagy-related proteins in OA cartilage of rat knee were modulated as same as those in miR-146a-5p mimics treated chondrocyte, and reversed by antagomir as well as the effects of miR-146a-5p inhibitor on primary chondrocytes ( Fig. 7B and C). TUNEL assay also showed the decreased apoptosis in OA + antagomir group compared with apoptosis in OA group and OA+ NC group ( Fig. 7D ). Furthermore, the levels of NUMB and LC3-II proteins were also suppressed in the OA and OA + NC groups, but were improved in the OA + antagomir group ( Fig. 7E ).
Figure 7.
miR-146a-5p antagomir suppressed the articular chondrocyte apoptosis while it promoted autophagy in vivo. (A) QRT-PCR showed the miR-146a-5p and NUMB levels in the tibial cartilage on 1 day, 3, 4, or 6 weeks after surgery. (B) Western blotting showed the protein levels of cleaved caspase 3, cleaved PRAP, Bax, and Bcl2, and the corresponding statistical results. (C) Western blotting showed the protein levels of LC3, Beclin1, ATG5, and p62, and the corresponding statistical results. (D) TUNEL staining showed the apoptotic chondrocytes in the cartilage, and the cell counting results of the positive stained were presented in the histogram. (E) Immunohistochemical staining showed the NUMB and LC3-II expression.
Discussion
OA is a chronic arthritis characterized by the gradual degradation of articular cartilage and inflammation of the synovial membrane. 26 Studies have shown that in OA cartilage tissue, there may be an interaction between cell apoptosis and cartilage degradation, 26 and autophagy can help inhibit cartilage cell degeneration. 27 In OA cartilage, cell apoptosis is obvious 28 and autophagy is reduced. 29 This study shows that miR-146a-5p was highly expressed in human OA tissues, and high levels of miR-146a-5p in chondrocytes increased chondrocyte apoptosis and decreased autophagy, which indicates that miR-146a-5p can promote the process of OA. In addition, intra-articular injection of antagomir significantly inhibited the level of miR-146a-5p in the cartilage tissue of the knee joint of OA rats, and inhibited chondrocyte apoptosis and increased autophagy. This further validates the role of miR-146a-5p in the development of OA.
Caspase activation and PARP degradation are molecular markers of apoptosis. 30 Bax is a pro-apoptotic gene that exists in the cytoplasm. It can be expressed in large quantities during cell apoptosis and promotes apoptosis. 31 The anti-apoptotic protein Bcl-2 prevents apoptosis by protecting the integrity of the outer mitochondrial membrane. 32 In this study, upregulated miR-146a-5p promoted caspase-3 activation, PARP degradation, and Bax expression, but suppressed Bcl-2 expression. While downregulated miR-146a-5p inhibited caspase-3 activation, PARP degradation and Bax expression, but increased Bcl-2 expression. This indicates that the pro-apoptotic effect of miR-146a-5p is related to change in activation of caspase-3 and protein levels of PARP, Bax, and Bcl-2.
Beclin 1 pathway activation is related to autophagy activation, 33 and Beclin 1 can be combined with class I phosphoinositide 3-kinase to cause autophagy. 34 The conversion of LC3-I to LC3-II is an initiating step in autophagy.35,36 ATG5 is also an autophagy protein. 37 The autophagy substrate p62 assists the transmission of inflammasomes to autophagosomes and participates in the process of inflammation. 38 In this study, upregulated miR-146a-5p suppressed the expression of Beclin 1 and ATG5, but promoted p62 expression and the conversion of LC3-I to LC3-II. While downregulated miR-146a-5p promoted the expression of Beclin 1 and ATG5, but decreased p62 expression and the conversion of LC3-I to LC3-II. This indicates that miR-146a-5p directly or indirectly inhibits autophagy, as decrease in key regulators of autophagy is accompanied by increase in apoptosis, 39 and the cleavage of Beclin 1 and ATG5 eliminate cytoprotective autophagy and promote apoptosis. 40
We found that NUMB was negatively regulated by miR-146a-5p and was lowly expressed in human OA tissues. NUMB is an evolutionarily conserved cargo-selective endocytic adaptor protein, which plays a key role in cell division, differentiation, migration, and endocytosis.41-43 Overexpression of NUMB reversed the effects of miR-146a-5p on chondrocytes apoptosis and autophagy in this study, indicating that NUMB is the target gene of miR-146a-5p, and participates in the process of miR-146a-5p’s regulating cell apoptosis and autophagy.
MiR-146a-5p modulates the expression of apoptosis- or autophagy-related proteins by targeting NUMB, and affects chondrocyte apoptosis and autophagy. This is of great significance to the dysfunction of chondrocytes, the decrease in chondrocyte number, and the degradation of cartilage in OA tissue. Therefore, detecting the expression level of miR-146a-5p in different stages of OA progression, as well as the expression of miR-146a-5p in the synovium, subchondral bone, and meniscus besides cartilage tissue may be helpful to understand the role of miR-146a-5p in the development of OA.
In summary, this study showed that miRNA-146a-5p is highly expressed in human OA chondrocytes and negatively regulates the expression of NUMB, which is involved in chondrocyte apoptosis and autophagy. Our results confirm that increasing the level of miRNA-146a-5p in human chondrocytes or mouse chondrocytes will reduce the expression level of NUMB, thereby modulating the expression of apoptosis- or autophagy-related proteins, and promoting the increase of chondrocyte apoptosis and the decrease of autophagy. Our results also show that intra-articular injection of miR-146a-5p antagomir can reduce chondrocyte apoptosis and increase autophagy in the knee joint of OA rats. This provides new clues for understanding the complex molecular mechanism of chondrocyte apoptosis and autophagy in OA tissue.
Footnotes
Acknowledgments and Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was supported by Ministry of Science and Technology National Key Research and Development Program (Grant No.: 122300411149), Natural Science Foundation of Henan Province (Grant No.: 182300410349), and the Joint Construction Project of Henan Provincial Health Committee and Ministry of Health (Grant Nos.: 201701017 and B20190316).
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Ethical Approval: This study was approved by the Ethics Committee of our institution (approval no.: ZD20190318).
Informed Consent: Written informed consent was obtained from each patient.
Trial Registration: Not applicable.
ORCID iDs: Jia Zheng  https://orcid.org/0000-0003-2484-6513
https://orcid.org/0000-0003-2484-6513
References
- 1. Glyn-Jones S, Palmer AJ, Agricola R, Price AJ, Vincent TL, Weinans H, et al. Osteoarthritis. Lancet. 2015;386(9991): 376-87. [DOI] [PubMed] [Google Scholar]
- 2. Aicher WK, Rolauffs B. The spatial organisation of joint surface chondrocytes: review of its potential roles in tissue functioning, disease and early, preclinical diagnosis of osteoarthritis. Ann Rheum Dis. 2014;73(4):645-53. [DOI] [PubMed] [Google Scholar]
- 3. Yan S, Wang M, Zhao J, Zhang H, Zhou C, Jin L, et al. MicroRNA-34a affects chondrocyte apoptosis and proliferation by targeting the SIRT1/p53 signaling pathway during the pathogenesis of osteoarthritis. Int J Mol Med. 2016;38(1):201-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Goldring MB. Update on the biology of the chondrocyte and new approaches to treating cartilage diseases. Best Pract Res Clin Rheumatol. 2006;20(5):1003-25. [DOI] [PubMed] [Google Scholar]
- 5. Guilak F, Nims RJ, Dicks A, Wu CL, Meulenbelt I. Osteoarthritis as a disease of the cartilage pericellular matrix. Matrix Biol. 2018;71-72:40-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Lotz MK, Carames B. Autophagy and cartilage homeostasis mechanisms in joint health, aging and OA. Nat Rev Rheumatol. 2011;7(10):579-87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Si HB, Zeng Y, Liu SY, Zhou ZK, Chen YN, Cheng JQ, et al. Intra-articular injection of microRNA-140 (miRNA-140) alleviates osteoarthritis (OA) progression by modulating extracellular matrix (ECM) homeostasis in rats. Osteoarthritis Cartilage. 2017;25:1698-707. [DOI] [PubMed] [Google Scholar]
- 8. Zhao XJ, Yu HW, Yang YZ, Wu WY, Chen TY, Jia KK, et al. Polydatin prevents fructose-induced liver inflammation and lipid deposition through increasing miR-200a to regulate Keap1/Nrf2 pathway. Redox Biol. 2018;18:124-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Mo YY. MicroRNA regulatory networks and human disease. Cell Mol Life Sci. 2012;69(21):3529-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Soyocak A, Kurt H, Ozgen M, Turgut Cosan D, Colak E, Gunes HV. miRNA-146a, miRNA-155 and JNK expression levels in peripheral blood mononuclear cells according to grade of knee osteoarthritis. Gene. 2017;627:207-11. [DOI] [PubMed] [Google Scholar]
- 11. Zhong G, Long H, Ma S, Shunhan Y, Li J, Yao J. miRNA-335-5p relieves chondrocyte inflammation by activating autophagy in osteoarthritis. Life Sci. 2019;226:164-72. [DOI] [PubMed] [Google Scholar]
- 12. Lian WS, Ko JY, Wu RW, Sun YC, Chen YS, Wu SL, et al. MicroRNA-128a represses chondrocyte autophagy and exacerbates knee osteoarthritis by disrupting Atg12. Cell Death Dis. 2018;9(9):919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kopanska M, Szala D, Czech J, Gablo N, Gargasz K, Trzeciak M, et al. MiRNA expression in the cartilage of patients with osteoarthritis. J Orthop Surg Res. 2017;12(1):51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Xu B, Huang Y, Niu X, Tao T, Jiang L, Tong N, et al. Hsa-miR-146a-5p modulates androgen-independent prostate cancer cells apoptosis by targeting ROCK1. Prostate. 2015;75(16):1896-903. [DOI] [PubMed] [Google Scholar]
- 15. Lin G, Huang J, Chen Q, Chen L, Feng D, Zhang S, et al. miR-146a-5p mediates intermittent hypoxia-induced injury in H9c2 cells by targeting XIAP. Oxid Med Cell Longev. 2019;2019:6581217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hwang WL, Yang MH. Numb is involved in the non-random segregation of subcellular vesicles in colorectal cancer stem cells. Cell Cycle. 2016;15(20):2697-703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Sun Y, Wang F, Sun X, Wang X, Zhang L, Li Y. CX3CR1 regulates osteoarthrosis chondrocyte proliferation and apoptosis via Wnt/β-catenin signaling. Biomed Pharmacother. 2017;96:1317-23. [DOI] [PubMed] [Google Scholar]
- 18. Zhong G, Liang R, Yao J, Li J, Jiang T, Liu J, et al. Artemisinin ameliorates osteoarthritis by inhibiting the Wnt/β-catenin signaling pathway. Cell Physiol Biochem. 2018;51(6):2575-90. [DOI] [PubMed] [Google Scholar]
- 19. Kueanjinda P, Roytrakul S, Palaga T. A novel role of numb as a regulator of pro-inflammatory cytokine production in macrophages in response to toll-like receptor 4. Sci Rep. 2015;5:12784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Sun H, Liu Y, Zhang L, Shao X, Liu K, Ding Z, et al. Numb positively regulates autophagic flux via regulating lysosomal function. Biochem Biophys Res Commun. 2017;491(3):780-6. [DOI] [PubMed] [Google Scholar]
- 21. Wu X, Fleming A, Ricketts T, Pavel M, Virgin H, Menzies FM, et al. Autophagy regulates Notch degradation and modulates stem cell development and neurogenesis. Nat Commun. 2016;7:10533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Pelletier JP, Faure MP, DiBattista JA, Wilhelm S, Visco D, Martel-Pelletier J. Coordinate synthesis of stromelysin, interleukin-1, and oncogene proteins in experimental osteoarthritis. An immunohistochemical study. Am J Pathol. 1993;142(1):95-105. [PMC free article] [PubMed] [Google Scholar]
- 23. Kapoor M, Martel-Pelletier J, Lajeunesse D, Pelletier JP, Fahmi H. Role of proinflammatory cytokines in the pathophysiology of osteoarthritis. Nat Rev Rheumatol. 2011;7(1):33-42. [DOI] [PubMed] [Google Scholar]
- 24. Altman R, Asch E, Bloch D, Bole G, Borenstein D, Brandt K, et al. Development of criteria for the classification and reporting of osteoarthritis. Classification of osteoarthritis of the knee. Diagnostic and Therapeutic Criteria Committee of the American Rheumatism Association. Arthritis Rheum. 1986;29(8):1039-49. [DOI] [PubMed] [Google Scholar]
- 25. Kellgren JH, Lawrence JS. Radiological assessment of osteo-arthrosis. Ann Rheum Dis. 1957;16(4):494-502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Hwang HS, Kim HA. Chondrocyte apoptosis in the pathogenesis of osteoarthritis. Int J Mol Sci. 2015;16(11):26035-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Shapiro IM, Layfield R, Lotz M, Settembre C, Whitehouse C. Boning up on autophagy: the role of autophagy in skeletal biology. Autophagy. 2014;10(1):7-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Aigner T, Hemmel M, Neureiter D, Gebhard PM, Zeiler G, Kirchner T, et al. Apoptotic cell death is not a widespread phenomenon in normal aging and osteoarthritis human articular knee cartilage: a study of proliferation, programmed cell death (apoptosis), and viability of chondrocytes in normal and osteoarthritic human knee cartilage. Arthritis Rheum. 2001;44(6):1304-12. [DOI] [PubMed] [Google Scholar]
- 29. Carames B, Hasegawa A, Taniguchi N, Miyaki S, Blanco FJ, Lotz M. Autophagy activation by rapamycin reduces severity of experimental osteoarthritis. Ann Rheum Dis. 2012;71(4):575-81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Sun XM, MacFarlane M, Zhuang J, Wolf BB, Green DR, Cohen GM. Distinct caspase cascades are initiated in receptor-mediated and chemical-induced apoptosis. J Biol Chem. 1999;274(8):5053-60. [DOI] [PubMed] [Google Scholar]
- 31. Oltvai ZN, Milliman CL, Korsmeyer SJ. Bcl-2 heterodimerizes in vivo with a conserved homolog, Bax, that accelerates programmed cell death. Cell. 1993;74(4):609-19. [DOI] [PubMed] [Google Scholar]
- 32. Kang MH, Reynolds CP. Bcl-2 inhibitors: targeting mitochondrial apoptotic pathways in cancer therapy. Clin Cancer Res. 2009;15(4):1126-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Chen G, Ke Z, Xu M, Liao M, Wang X, Qi Y, et al. Autophagy is a protective response to ethanol neurotoxicity. Autophagy. 2012;8(11):1577-89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Su Y, Qu Y, Zhao F, Li H, Mu D, Li X. Regulation of autophagy by the nuclear factor kappaB signaling pathway in the hippocampus of rats with sepsis. J Neuroinflammation. 2015;12:116. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 35. He H, Dang Y, Dai F, Guo Z, Wu J, She X, et al. Post-translational modifications of three members of the human MAP1LC3 family and detection of a novel type of modification for MAP1LC3B. J Biol Chem. 2003;278(31):29278-87. [DOI] [PubMed] [Google Scholar]
- 36. Satoo K, Noda NN, Kumeta H, Fujioka Y, Mizushima N, Ohsumi Y, et al. The structure of Atg4B-LC3 complex reveals the mechanism of LC3 processing and delipidation during autophagy. EMBO J. 2009;28(9):1341-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Almonte-Becerril M, Navarro-Garcia F, Gonzalez-Robles A, Vega-Lopez MA, Lavalle C, Kouri JB. Cell death of chondrocytes is a combination between apoptosis and autophagy during the pathogenesis of osteoarthritis within an experimental model. Apoptosis. 2010;15(5):631-8. [DOI] [PubMed] [Google Scholar]
- 38. Shi CS, Shenderov K, Huang NN, Kabat J, Abu-Asab M, Fitzgerald KA, et al. Activation of autophagy by inflammatory signals limits IL-1β production by targeting ubiquitinated inflammasomes for destruction. Nat Immunol. 2012;13(3):255-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Carames B, Olmer M, Kiosses WB, Lotz MK. The relationship of autophagy defects to cartilage damage during joint aging in a mouse model. Arthritis Rheumatol. 2015;67(6):1568-76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Eisenberg-Lerner A, Bialik S, Simon HU, Kimchi A. Life and death partners: apoptosis, autophagy and the cross-talk between them. Cell Death Differ. 2009;16(7):966-75. [DOI] [PubMed] [Google Scholar]
- 41. Dho SE, French MB, Woods SA, McGlade CJ. Characterization of four mammalian numb protein isoforms. Identification of cytoplasmic and membrane-associated variants of the phosphotyrosine binding domain. J Biol Chem. 1999;274(46):33097-104. [DOI] [PubMed] [Google Scholar]
- 42. Gulino A, Di Marcotullio L, Screpanti I. The multiple functions of numb. Exp Cell Res. 2010;316(6):900-6. [DOI] [PubMed] [Google Scholar]
- 43. Santolini E, Puri C, Salcini AE, Gagliani MC, Pelicci PG, Tacchetti C, et al. Numb is an endocytic protein. J Cell Biol. 2000;151(6):1345-52. [DOI] [PMC free article] [PubMed] [Google Scholar]