Abstract
Objective
To investigate meniscal regeneration and prevent cartilage degeneration using wrapping treatment for meniscal horizontal tears that have been difficult to repair in rabbits.
Design
Thirty knees from 15 Japanese white rabbits were divided into the horizontal (horizontal tears) or wrapping (horizontal tears with wrapping treatment) groups. Horizontal tears were created and wrapped with a sheet scaffold containing polyglycolic acid, polylactic acid, and polycaprolactone. The meniscus was stained with Safranin-O/Fast Green and evaluated with modified Pauli scores at 8, 12, and 16 weeks after implantation (n = 5). Cell morphology was determined with hematoxylin and eosin staining. Mature collagen was confirmed with Picrosirius Red staining. Furthermore, immunohistochemical analysis of inducible nitric oxide synthase (iNOS) for inflammation, Ki-67 for proliferation, and type II collagen for regeneration was performed. Medial femoral cartilage was stained with Safranin-O/Fast Green and evaluated with the Osteoarthritis Research Society International score at 8 and 16 weeks.
Results
The wrapping group had significantly better regeneration than the horizontal group, especially at 16 weeks (P < 0.05). Wrapping treatment induced fibrochondrocyte-like cells at 16 weeks. After wrapping treatment, iNOS was overexpressed at 8 weeks, Ki-67 at 8 and 12 weeks, and type II collagen at 16 weeks. Cartilage degeneration in the wrapping group did not progress significantly compared with that in the horizontal group at 16 weeks (P < 0.05).
Conclusions
Wrapping treatment for meniscal horizontal tears induced meniscal regeneration as the sheet scaffold might induce intrinsic and extrinsic repair. Regaining the meniscal function by the wrapping treatment prevented cartilage degeneration.
Keywords: wrapping treatment, horizontal tear, rabbit model, meniscus, repair
Introduction
The meniscus plays a key role in knee homeostasis for load distribution, shock absorption, joint stability, lubrication, and proprioception.1-4 Meniscectomy is still widely accepted for meniscal tears, and it has been recognized as one of the risk factors for knee osteoarthritis because of the loss of meniscal function.5,6 Although good results of meniscal repair have been reported,7-9 the reoperation rate of meniscal repair is 5 times higher than partial meniscectomy. 10 One of the reasons for meniscal reoperation depends on the extent of vascular richness at the injured area. 11 The longitudinal tears are an adaptation of meniscal repairs because the sites of meniscal tear are at the vascular area, 9 whereas the treatment of horizontal tears is challenging because most of the lesions are located at an avascular area, 12 even though various operative techniques have been performed for the horizontal tear.13,14
Wrapping treatment for meniscal tears was first reported in 2003. 15 Recently, a wrapping treatment for meniscal complex tears was reported to have good clinical and arthroscopic outcomes. 16 Although the concept of the wrapping treatment is to improve the biomechanical and biological environment of the meniscal tear, histological assessment for the wrapping treatment for horizontal tears has not yet been reported, while the histological assessments of the wrapping treatment for meniscal longitudinal and radial tears have been reported.17,18
In this study, we focused on the wrapping treatment using a sheet scaffold for horizontal tears in rabbit and investigated meniscal regeneration and cartilage preservation. The purpose of this study was the histological evaluation of the wrapping treatment for horizontal tears, and the hypothesis of this study was that the wrapping treatment for meniscal horizontal tear supported not only meniscal regeneration but also prevented cartilage degeneration.
Methods
Materials
Sheet meniscal scaffold, which has already been clinically used as a dural substitute for synthetic bioabsorbable polymers (SEAMDURA; Gunze, Kyoto, Japan), was selected. This sheet consists of polyglycolic acid (PGA) and poly-L-lactic acid and caprolactone (P(LA/CL)). The manufacturing methods and characteristics of the sheet scaffold has been described previously. 19 Briefly, granules of P(LA/CL) (50% L-lactic acid, 50% caprolactone) copolymer were compressed and cooled to form a 100-µm-thick film. A sheet of nonwoven PGA fabric was sandwiched between 2 copolymer films and compressed ( Fig. 1 ). The composite sheet thus obtained was used as a sheet scaffold after sterilization with ethylene oxide gas. The thickness of the scaffold was 0.2 mm.
Figure 1.
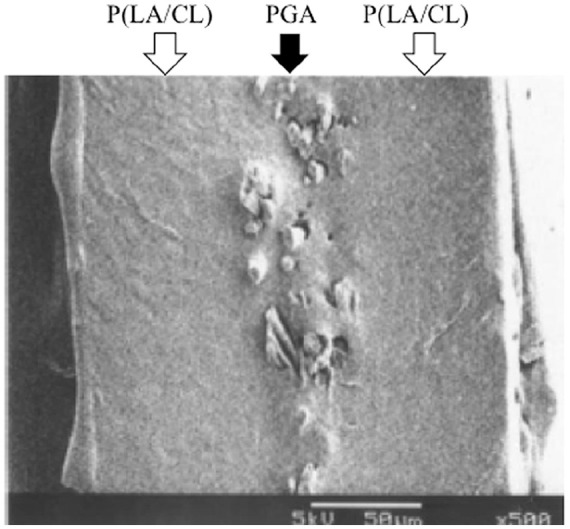
Scanning electron micrograph of the sheet scaffold. Black arrow indicates polyglycolic acid (PGA) fabric fibers sandwiched between poly-L-lactic acid and caprolactone (P(LA/CL)) copolymer films, indicated by the white arrow. Bar = 50 µm.
Animal Experiments
The Animal Research Committee of Osaka Medical College approved all experiments (No. 26101). Thirty knees of 15 skeletally mature Japanese white rabbits (6 months old; mean weight 3.2 kg; range 3.0-3.4 kg) without degenerative change were obtained from Oriental BioService, Inc. (Kyoto, Japan).
Surgical Procedures
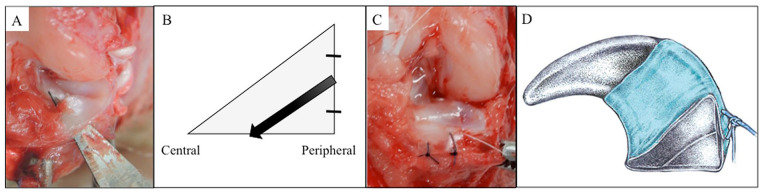
Rabbits were anesthetized with an intravenous injection of pentobarbital sodium (30 mg/kg; Somnopentyl; Kyoritsu Seiyaku, Tokyo, Japan) and a subcutaneous injection of 1% lidocaine hydrochloride (Xylocaine; AstraZeneca, Osaka, Japan). Knee surgery was performed under sterile conditions as described previously.20,21 Briefly, a medial parapatellar incision was made, and the medial meniscus was dislocated by pulling it out anteriorly when the tibia was externally rotated after partial dissection of the medial collateral ligament. The anterior medial meniscus was exposed and left from the anterior joint capsule to check the peripheral meniscus wall. Horizontal tear was created using a surgical blade from the outside of the meniscus to the tibial surface at the anterior medial meniscus ( Fig. 2A and B ). The tear was 4-mm wide and started halfway from the peripheral meniscal wall. Each knee (n = 30) was randomly divided into 2 groups: horizontal group (n = 15), in which the menisci of the rabbits had horizontal tear without treatment, and the wrapping group (n = 15), in which rabbits had horizontal tears with wrapping treatment. The horizontal tear was wrapped and sutured on the outside of the meniscus wall with the sheet scaffold fabricated to 4-mm wide at 2 points via 6-0 monofilament nylon sutures (Akiyama Medical Co, Ltd, Tokyo, Japan) ( Fig. 2C and D ). After the reduction of the medial meniscus, the medial collateral ligament was tightly sutured with 4-0 nylon sutures, the anterior joint capsule was repaired with 4-0 nylon sutures, and the skin was closed with 6-0 nylon sutures. The rabbits were returned to the cage and allowed to engage in full weight-bearing activities after surgery. Samples of this study were harvested at 8, 12, and 16 weeks after implantation in both groups.
Figure 2.
Horizontal tear model in rabbit. (A) Horizontal tear created using a surgical blade. (B) The starting position of the horizontal tear was half of the peripheral meniscal wall. Black arrow indicates the direction of the horizontal tear making. (C and D) The horizontal tear was wrapped by PGA-combined P(LA/CL) sheet scaffold.
Morphological Analysis
To evaluate inflammation and to observe the appearance of scaffold, macrophotographs were obtained using the COOLPIX310 camera (Nikon, Tokyo, Japan).
Histological Analysis of the Meniscus
Radial sections were obtained at the medial anterior menisci. They were fixed in 10% neutral buffered formalin. Paraffin-embedded samples were sectioned at 5 µm, deparaffinized in xylene after passing through ethanol, and stained with Safranin-O/Fast Green. The sheet scaffold was removed through decalcification process. Menisci were stained with Safranin-O/Fast Green and evaluated with modified Pauli’s histological tissue quality score (range 0-12; higher score indicated better meniscal regeneration and lower score indicated more degenerative change).22,23 Hematoxylin and eosin (H&E) staining was performed to investigate the morphology of the cells around the meniscal tear. Harris’ H&E staining protocol was used, in which the nuclei stained blue and the cytoplasm red. 24 Cell count was determined at the meniscal tear site at 8, 12, and 16 weeks, and average number of cells was calculated after counting twice at different meniscal tear sites. Picrosirius Red staining was performed to investigate the morphology of the meniscus regenerative collagen fibers at 16 weeks with BZ-X710 fluorescence microscope (KEYENCE, Osaka, Japan).
Immunohistochemical Analysis
Anti-inducible nitric oxide synthase (iNOS) and Ki-67 were investigated for the inflammatory tissue of the meniscus and cell proliferation from peripheral to central meniscus tear and evaluated at 8, 12, and 16 weeks. Type II collagen was investigated for the structure of the regenerated tissue at the meniscal avascular area and evaluated at 8 and 16 weeks. Before immunohistochemical staining, we performed heat-induced epitope retrieval for antigen retrieval by pressure cooking as reported previously. 25 Briefly, a water bath was preheated until 95°C to 100°C. The staining dish containing ethylenediaminetetraacetic acid (EDTA) buffer was immersed in the water bath and pressurized in a pressure cooker for 3 minutes. They were removed from the water bath and cooled to room temperature for 15 minutes and soaked in cold water for 30 minutes before the staining procedure. The sections were washed with phosphate-buffered saline (PBS) and blocked with skim milk at room temperature for 10 minutes. Primary antibodies were diluted at 1:200 of iNOS, which was a rabbit polyclonal antibody (Merck Millipore, Darmstadt, Germany), and at 1:300 of Ki-67, which was a rabbit polyclonal antibody (Novus Biologicals, Centennial, CO). Primary antibody was diluted at 1:20 of type II collagen, which was a mouse polyclonal antibody (Developmental Studies Hybridoma Bank, Iowa City, Iowa). The sections were incubated overnight at 4°C. After washing with PBS, they were treated with biotinylated anti-rabbit immunoglobulin G (Vector Laboratories, Burlingame, CA) for iNOS and Ki-67, and with biotinylated anti-mouse immunoglobulin G (Vector Laboratories, Burlingame, CA) for type II collagen as a secondary antibody for 60 minutes at room temperature. After washing with PBS, they were treated with diaminobenzene (DAB) chromogen/substrate kit (ScyTek Laboratories, West Logan, UT) for 5 minutes. They were washed with PBS and then counterstained with hematoxylin.
Histological Analysis of the Cartilage
The distal femur was fixed in 10% neutral buffered formalin for 48 hours, decalcified with 10% EDTA for 28 days, and embedded in paraffin. Paraffin-embedded samples, as well as the menisci, were sectioned at 5 µm, deparaffinized in xylene after passing through ethanol, and stained with Safranin-O/Fast Green. 21 The sections were stained with Safranin-O/Fast Green and evaluated with Osteoarthritis Research Society International (OARSI) histological score for articular cartilage of the medial distal femur (range 0-24; higher score indicated higher degenerative change of articular cartilage). 26
Statistical Analysis
Comparisons between the groups were performed by the Wilcoxon rank sum test using JMP Pro version 13.0.0 (SAS 196 Institute Japan, Tokyo, Japan). The results were reported as means ± standard deviation, and P < 0.05 was considered significant. The intraclass correlation coefficients (ICCs) of intra- and interobserver reliabilities for histological scores of the meniscus and the articular cartilage were assessed by 2 independent blinded observers using SPSS version 22 (IBM Corporation, Armonk, NY).
Results
Morphological Analysis
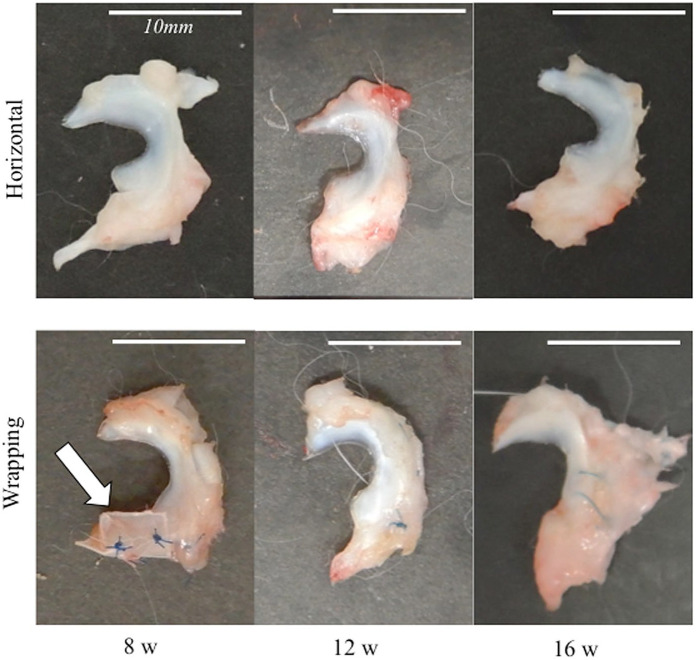
There were no macroscopic inflammations around the meniscus surface at 8, 12, and 16 weeks after the wrapping treatment. The scaffold was not dislocated at 8 weeks and was not detected at 12 and 16 weeks ( Fig. 3 ).
Figure 3.
Macroscopic appearance of the meniscus. (A) Horizontal group at 8, 12, and 16 weeks. (B) Wrapping group at 8, 12, and 16 weeks. White arrow indicates the sheet scaffold. The bar indicates 10 mm.
Histological Analysis
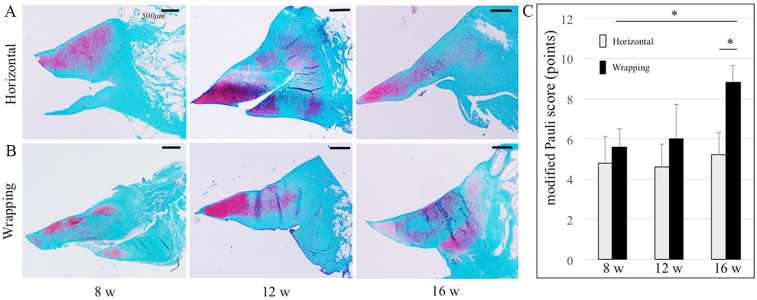
In Safranin-O/Fast Green staining, the menisci in both horizontal and wrapping groups showed healing at the peripheral part, but not at the central part at 12 weeks ( Fig. 4A, B ). The meniscus in the wrapping group was gradually restored from 8 to 12 weeks but not in the horizontal group. The meniscus in the wrapping group was almost healed and intensely stained at 16 weeks. In the horizontal group, the modified Pauli score at 16 weeks (5.2 ± 1.1) did not improve compared to that at 8 and 12 weeks (4.8 ± 1.3 and 4.6 ± 1.1; Fig. 4C ) (P = 0.83, 0.58). In the wrapping group, the score at 16 weeks (8.8 ± 0.8) had improved significantly compared to that at 8 and 12 weeks (5.6 ± 0.9 and 6.0 ± 1.7) (P < 0.05). At 16 weeks, the score in the wrapping group was significantly higher than that in the horizontal group (P < 0.05). The ICCs of intra- and interobserver reliabilities of the score were 0.83 and 0.88, respectively.
Figure 4.
Comparison of meniscal healing between the horizontal and wrapping groups by Safranin-O/Fast Green staining. (A) Horizontal group at 8, 12, and 16 weeks. (B) Wrapping group at 8, 12, and 16 weeks. The bar indicates 500 µm. (C) Modified Pauli score is shown: range 0-12, higher score indicates better regeneration. *P < 0.05.
Cell Morphology Analysis
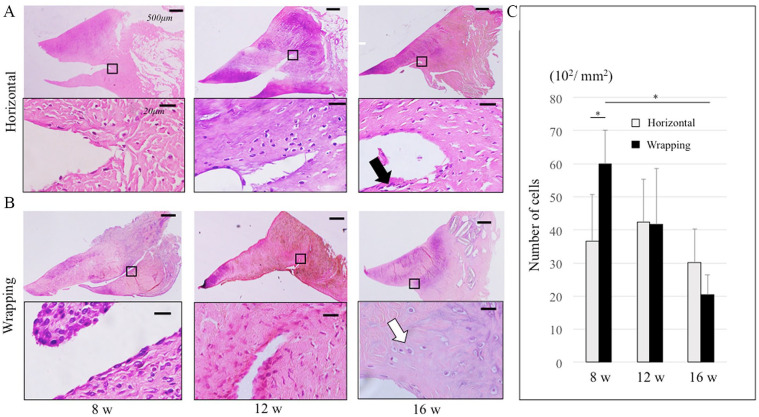
There were few cells around the meniscal tear in the horizontal group at 8 weeks, while there were many cells around not only the peripheral but also the central meniscal tear in the wrapping group ( Fig. 5A, B ). There were fewer cells at 12 weeks than at 8 weeks in the wrapping group. Cell morphology was unclear at 8 and 12 weeks in both groups. However, at 16 weeks, there were round cells in the wrapping group, while there were spindle cells in the horizontal group at the avascular area. Macrophage and foreign body multinucleated giant cells (FBMGCs) were not confirmed in both groups. This result indicated that there was no strong inflammatory reaction. There were significantly more cells at the meniscus tear site at 8 weeks in the wrapping group (6000/mm2) than in the horizontal group (3660/mm2) (P < 0.05) ( Fig. 5C ). In the wrapping group, there were significantly more cells at 8 weeks than at 16 weeks (2040/mm2) (P < 0.05). There was no significant difference in the number of the cells between the 2 groups at 12 and 16 weeks.
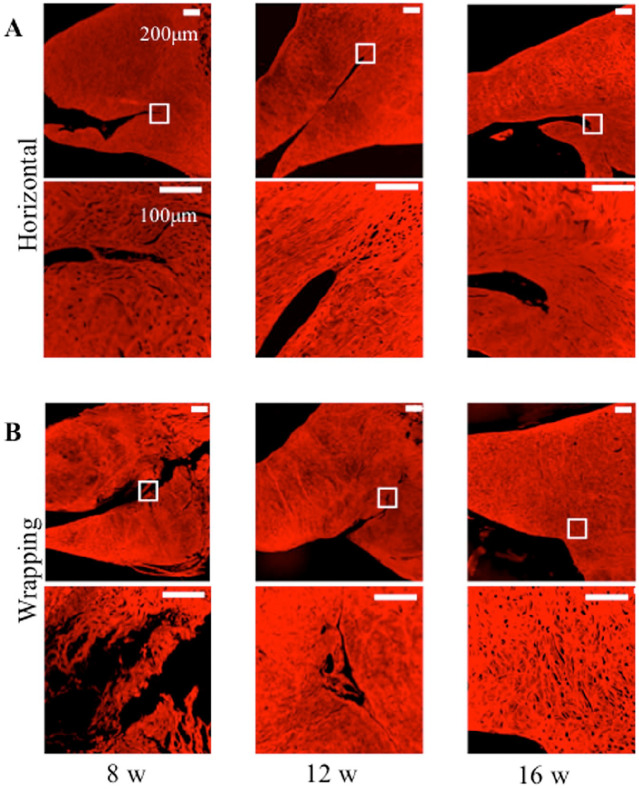
Figure 5.
Cell morphology with hematoxylin and eosin staining of the meniscus. Black square in the upper figure shows the peripheral meniscal tear in the lower figure. (A) Horizontal group at 8, 12, and 16 weeks. Black arrow indicates fibroblast-like cell at 16weeks. (B) Wrapping group at 8, 12, and 16 weeks. White arrow indicates fibrochondrocyte-like cell at 16 weeks. In the upper figures, the bar indicates 500 µm. In the lower figures, the bar indicates 20 µm. (C) This graph indicated the average number of cells at the meniscal tear site at 8, 12, and 16 weeks in both groups. *P < 0.05.
In Picrosirius red staining, the wrapping group had detected more collagen fiber formations compared to the horizontal group at 16 weeks, while both groups at 8 and 12 weeks had unmatured collagen fiber formations ( Fig. 6A and B ).
Figure 6.
Picrosirius red staining for collagen fibers of meniscus tear. White square in the upper figure shows the peripheral meniscal tear in the lower figure. (A) Horizontal group at 8, 12, and 16 weeks. (B) Wrapping group at 8, 12, and 16 weeks. In the upper figures, the bar indicates 200 µm. In the lower figures, the bar indicates 100 µm.
Immunohistochemical Analysis
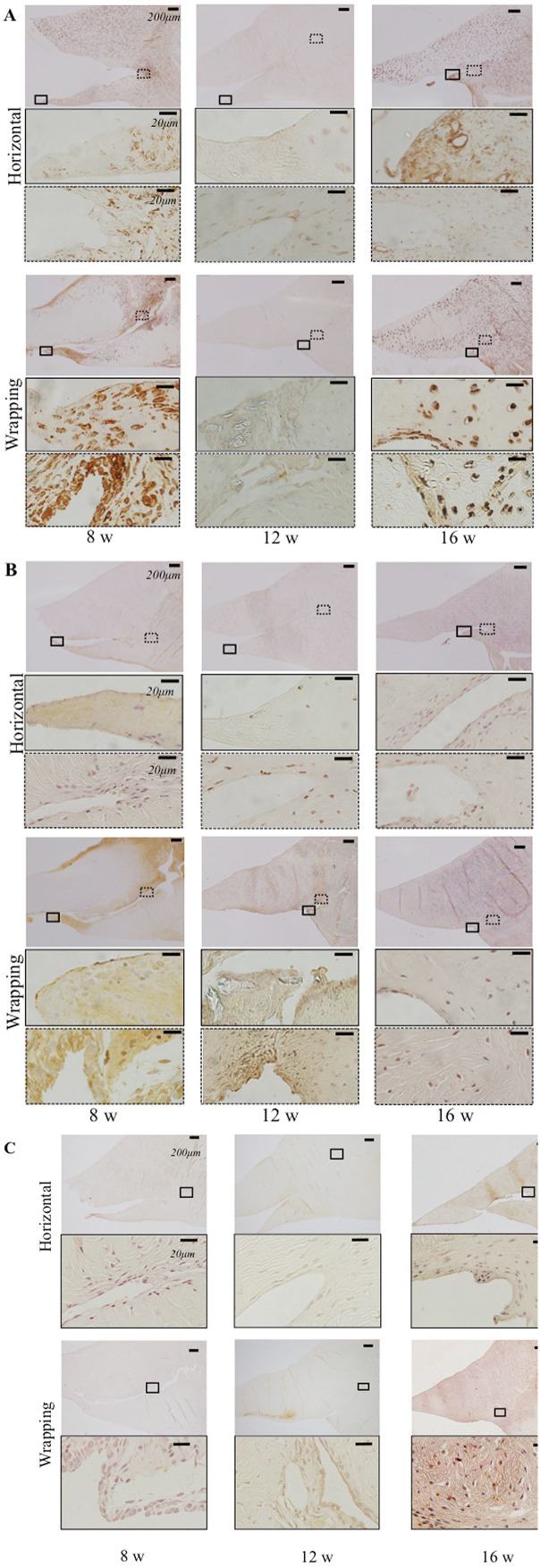
iNOS was overexpressed at the central part of meniscal tear and peripheral meniscal tear in the wrapping group at 8 weeks and at the meniscal tear in the horizontal group at 16 weeks ( Fig. 7A ). The result indicated inflammation in the wrapping group at an early phase compared to the horizontal group.
Figure 7.
Immunohistochemistry for the meniscus in horizontal and wrapping group at 8, 12, and 16 weeks. Black square in the upper shows the central part of the meniscal tear in the middle figure. Dotted square shows the most peripheral meniscal tear in the lower figure. (A) Immunohistochemistry of iNOS. (B) Immunohistochemistry of Ki67. (C) Immunohistochemistry of type II collagen. In the upper figures, the bar indicates 200 µm. In the middle and lower figures, the bar indicates 20 µm.
Ki-67 was overexpressed at the central part of meniscal tear and peripheral meniscal tear in the wrapping group at 8 and 12 weeks rather than at 16 weeks and in the horizontal tear group ( Fig. 7B ). This result indicated that cell proliferation occurred in the wrapping group at an early phase.
Type II collagen was overexpressed at the meniscal central part in the wrapping group at 16 weeks rather than in the wrapping group at 8 and 12 weeks and in the horizontal group ( Fig. 7C ). The result indicated that the central meniscus tear had regenerative tissue which had chondrocyte characteristics at a late phase in the wrapping group.
Histological Analysis for Femoral Cartilage
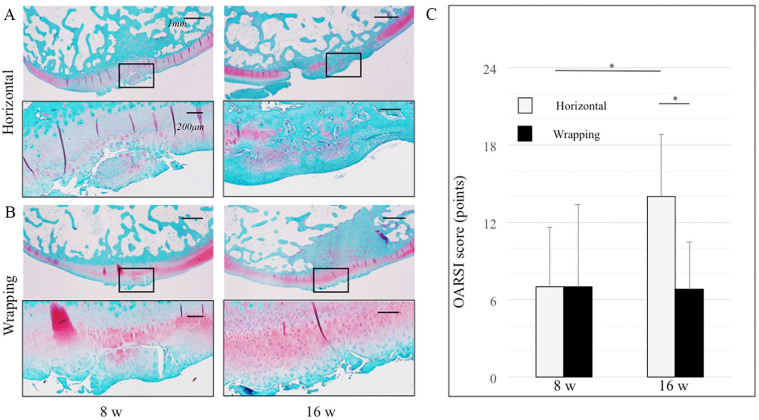
As for the cartilage degeneration, the articular cartilage exhibited more degenerative change at 16 weeks than at 8 weeks in the horizontal group; however, cartilage degeneration did not appear to progress from 8 to 16 weeks in the wrapping group ( Fig. 8A, B ). The OARSI score at 16 weeks (6.8 ± 3.7) was lower than that at 8 weeks in the wrapping group (7.0 ± 6.4), though not significantly (P = 0.60); however, the score at 16 weeks (14.0 ± 4.8) was significantly higher than that at 8 weeks in the horizontal group (7.0 ± 4.6; Fig. 8C ) (P < 0.05). The ICCs of intra- and interobserver reliabilities of the score were 0.94 and 0.95, respectively.
Figure 8.
Articular cartilage stained with Safranin-O/Fast Green stain. Black square in the upper figure shows the cartilage degeneration area in the lower figure. (A) Horizontal group at 8 and 16 weeks. (B) Wrapping group at 8 and 16 weeks. In the upper figures, the bar indicates 1 mm. In the lower figures, the bar indicates 200 µm. (C) OARSI score is shown: range 0-24. A higher score was indicative of increased degenerative changes in the articular cartilage. *P < 0.05.
Discussion
This study demonstrated that the wrapping treatment with PGA combined with P(LA/CL) sheet scaffold for meniscal horizontal tear induced meniscal regeneration and prevented cartilage degeneration. To the best of our knowledge, this study is the first to investigate the histological evaluation of meniscus and cartilage after the wrapping treatment for horizontal tear model in rabbits.
Meniscal healing process was described as having intrinsic and extrinsic pathways.27,28 The extrinsic pathway is based on meniscal progenitor-like cells from the peripheral vascular area, although the intrinsic pathway has a self-repair capacity. In this study, both pathways might be accelerated by wrapping because of cell proliferation at the central part of the meniscal tear and peripheral meniscal tear. Inflammation is the first step in the healing process, and the second to final processes are proliferation and maturity. 29 The meniscus with wrapping treatment at 8 weeks had inflammation in the peripheral part of the meniscal tear and the bottom of the meniscal tear, although the meniscus without treatment did not have inflammation, and the central part of the meniscus at 16 weeks after treatment had fibrochondrocyte-like cells, collagen maturity, and overexpression of type II collagen. In the normal meniscus, the central matrix, synthesized by fibrochondrocytes, has a small amount of type II collagen. 30 Our finding suggested that the wrapping treatment accelerated the healing process from the partial inflammation at an early phase to the maturity of cell and extracellular matrix at the second to final processes. Horizontal tears often included irreparable and degenerative tears and difficult to repair. The wrapping technique might be a new effective treatment for irreparable horizontal or complex tears in which suture treatment is not feasible.
Macrophage and FBMGCs were reported to induce immune response after scaffold implantation. 31 This study showed a partial inflammation from the overexpression of iNOS without macrophage and FBMGC at 8 weeks after the wrapping treatment. Horizontal and complex tears were highly associated with the severity of cartilage degeneration. 32 No significant difference in cartilage degeneration was found between the wrapping group and the horizontal group at 8 weeks, but cartilage degeneration was preserved by wrapping treatment after 16 weeks. This indicated that cartilage degeneration was preserved not to induce macrophage and FBMGC with wrapping treatment. The prevention of cartilage degeneration indicated that wrapping treatment also induced meniscal function.
The horizontal tear model covers the avascular inner menisci at the tibial surface in rabbits. The pathology of horizontal tears has been investigated because only a few studies have focused on the histological analysis of horizontal tears using animal models. Narita had first reported a horizontal tear model in a rabbit, in which the tears were made from the peripheral rim to the central part of the meniscus. 33 As an advantage, this animal model evaluated the length of the meniscus tear; however, this model was an incomplete tear and might not be induced by instability with shear stress. In contrast, the current horizontal tear model might be presented more precisely by the clinical status.
We selected synthetic bioabsorbable polymers (PGA-combined P(LA/CL)) for the wrapping treatment. The sheet scaffold has been mostly used in combination surgery with autologous chondrocyte implantation,34,35 but there are a few reports about the meniscus.17,18 A cell-free scaffold has the advantage of reducing cost, time to treatment, and infection risk compared to a cell-based strategy. The PGA-combined P(LA/CL) sheet has already been used clinically as an artificial dura, and the reported degradation time of the sheet was 24 weeks 19 ; therefore, it is safe and feasible. This sheet might be a good candidate material for meniscal wrapping treatment. In this study, the wrapping treatment gave stability to the meniscal tear site and induced cell proliferation from the peripheral soft tissue to the central meniscus; when the PGA-combined P(LA/CL) sheet was used, the cells might have enough time to migrate and reached the meniscal tear site. This study has some limitations. First, our horizontal tear was an acute model. Second, our horizontal tears reached the vascular area. The rabbits were too small to create the horizontal tear at the avascular area alone. In future study, research with pure avascular tear model might be required using bigger animals. Third, this study did not elucidate the biomechanical property. Although PGA-combined P(LA/CL) sheet showed good healing with wrapping treatment, suitable materials for the meniscal wrapping treatment should be researched in the future.
In conclusion, the wrapping treatment with PGA-combined P(LA/CL) sheet scaffold for meniscal horizontal tear induced meniscal regeneration and prevented cartilage degeneration, and the wrapping treatment might have a potential for treating irreparable horizontal tear.
Footnotes
Acknowledgments and Funding: We thank Yoshie Seki and Noriko Kinoshita for their expert technical assistance. The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: We acknowledge the grant support from KAKENHI 15K10498 and Terumo Life Science.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Ethical Approval: Ethical approval for this study was obtained from Animal Research Committee of Osaka Medical College (Approval Number: 26101).
Animal Welfare: The present study followed international, national, and/or institutional guidelines for humane animal treatment and complied with relevant legislation.
ORCID iD: Kosuke Nakagawa  https://orcid.org/0000-0003-4081-0000
https://orcid.org/0000-0003-4081-0000
References
- 1. Walker PS, Erkman MJ. The role of the menisci in force transmission across the knee. Clin Orthop Relat Res. 1975;(109):184-92. [DOI] [PubMed] [Google Scholar]
- 2. Voloshin AS, Wosk J. Shock absorption of meniscectomized and painful knees: a comparative in vivo study. J Biomed Eng. 1983;5:157-61. [DOI] [PubMed] [Google Scholar]
- 3. Renstrom P, John son R. Anatomy and biomechanics of the menisci. Clin Sports Med. 1990;9:523-38. [PubMed] [Google Scholar]
- 4. Karahan M, Kocaoglu B, Cabukoglu C, Akgun U, Nuran R. Effect of partial medial meniscectomy on the proprioceptive function of the knee. Arch Orthop Trauma Surg. 2010;130:427-31. [DOI] [PubMed] [Google Scholar]
- 5. Andersson-Molina H, Karlsson H, Rockborn P. Arthroscopic partial and total meniscectomy: a long-term follow-up study with matched controls. Arthroscopy. 2002;18:183-89. [DOI] [PubMed] [Google Scholar]
- 6. Verdonk R, Madry H, Shabshin N, Dirisamer F, Peretti GM, Pujol N, et al. The role of meniscal tissue in joint protection in early osteoarthritis. Knee Surg Sports Traumatol Arthrosc. 2016;24:1763-74. [DOI] [PubMed] [Google Scholar]
- 7. Kurzweil PR, Lynch NM, Coleman S, Kearney B. Repair of horizontal meniscus tears: a systematic review. Arthroscopy. 2014;30:1513-19. [DOI] [PubMed] [Google Scholar]
- 8. Barber-Westin SD, Noyes FR. Clinical healing rates of meniscus repairs of tears in the central-third (red-white) zone. Arthroscopy. 2014;30:134-46. [DOI] [PubMed] [Google Scholar]
- 9. Noyes FR, Chen RC, Barber-Westin SD, Potter HG. Greater than 10-year results of red-white longitudinal meniscal repairs in patients 20 years of age or younger. Am J Sports Med. 2011;39:1008-17. [DOI] [PubMed] [Google Scholar]
- 10. Paxton ES, Stock MV, Brophy RH. Meniscal repair versus partial meniscectomy: a systematic review comparing reoperation rates and clinical outcomes. Arthroscopy. 2012;27:1275-88. [DOI] [PubMed] [Google Scholar]
- 11. Henning CE, Lynch MA, Clark JR. Vascularity for healing of meniscal repairs. Arthroscopy. 1987;3:13-8. [DOI] [PubMed] [Google Scholar]
- 12. Englund M, Roos EM, Roos HP, Lohmander LS. Patient-relevant outcomes fourteen years after meniscectomy: influence of type of meniscal tear and size of resection. Rheumatology (Oxford). 2001;40:631-39. [DOI] [PubMed] [Google Scholar]
- 13. Kamimura T, Kimura M. Meniscal repair of degenerative horizontal cleavage tears using fibrin clots: clinical and arthroscopic outcomes in 10 cases. Orthop J Sports Med. 2014:2:2325967114555678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ahn JH, Kwon OJ, Nam TS. Arthroscopic repair of horizontal meniscal cleavage tears with marrow-stimulating technique. Arthroscopy. 2015;31:92-8. [DOI] [PubMed] [Google Scholar]
- 15. Jacob M, Jakob RP. Meniscal repair: enhancement of healing process. In: Beaufils P, Verdonk R, editors. The Meniscus. Berlin: Springer; 2010. p. 129-35. [Google Scholar]
- 16. Piontek T, Ciemniewska-Gorzela K, Naczk J, Jakob R, Szulc A, Grygorowicz M, et al. Complex meniscus tears treated with collagen matrix wrapping and bone marrow blood injection: a 2-year clinical follow-up. Cartilage. 2016;7:123-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Jülke H, Mainil-Varlet P, Jakob RP, Brehm W, Schäfer B, Nesic D. The role of cells in meniscal guided tissue regeneration: a proof of concept study in a goat model. Cartilage. 2015;6:20-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Shimomura K, Rothrauff BB, Hart DA, Hamamoto S, Kobayashi M, Yoshikawa H, et al. Enhanced repair of meniscal hoop structure injuries using an aligned electrospun nanofibrous scaffold combined with a mesenchymal stem cell derived tissue engineered construct. Biomaterials. 2019;192:346-54. [DOI] [PubMed] [Google Scholar]
- 19. Yamada K, Miyamoto S, Nagata I, Kikuchi H, Ikada Y, Iwata H, et al. Development of dural substitute from synthetic bioabsorbable polymers. J Neurosurg. 1997;86:1012-17. [DOI] [PubMed] [Google Scholar]
- 20. Oda S, Otsuki S, Kurokawa Y, Hoshiyama Y, Nakajima M, Neo M. A new method for meniscus repair using type I collagen scaffold and infrapatellar fat pad. J Biomater Appl. 2015;29:1439-48. [DOI] [PubMed] [Google Scholar]
- 21. Murakami T, Otsuki S, Nakagawa K, Okamoto Y, Inoue T, Sakamoto Y, et al. Establishment of novel meniscal scaffold structures using polyglycolic and poly-l-lactic acids. J Biomater Appl. 2017;32:150-61. [DOI] [PubMed] [Google Scholar]
- 22. Pauli C, Grogan SP, Patil S, Otsuki S, Hasegawa A, Koziol J, et al. Macroscopic and histopathologic analysis of human knee menisci in aging and osteoarthritis. Osteoarthritis Cartilage. 2011;19:1132-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Toratani T, Nakase J, Numata H, Oshima T, Takata Y, Nakayama K, et al. Scaffold-free tissue-engineered allogenic adipose-derived stem cells promote meniscus healing. Arthroscopy. 2017;33:346-54. [DOI] [PubMed] [Google Scholar]
- 24. Llewellyn BD. Nuclear staining with alum hematoxylin. Biotech Histochem. 2009;84:159-77. [DOI] [PubMed] [Google Scholar]
- 25. Norton AJ, Jordan S, Yeomans P. Brief, high-temperature heat denaturation (pressure cooking): a simple and effective method of antigen retrieval for routinely processed tissues. J Pathol. 1994;173:371-9. [DOI] [PubMed] [Google Scholar]
- 26. Laverty S, Girard CA, Williams JM, Hunziker EB, Prizker KP. The OARSI histopathology initiative—recommendations for histological assessments of osteoarthritis in the rabbit. Osteoarthritis Cartilage. 2010;18(Suppl 3):S53-S65. [DOI] [PubMed] [Google Scholar]
- 27. Ishida K, Kuroda R, Miwa M, Tabata Y, Hokugo A, Kawamoto T, et al. The regenerative effects of platelet-rich plasma on meniscal cells in vitro and in vivo application with biodegradable gelatin hydrogel. Tissue Eng. 2007;13:1103-12. [DOI] [PubMed] [Google Scholar]
- 28. De Albornoz PM, Forriol F. The meniscal healing process. Muscles Ligaments Tendons J. 2012;17;2:10-8. [PMC free article] [PubMed] [Google Scholar]
- 29. Wong ME, Hollinger JO, Pinero GJ. Integrated process responsible for soft tissue healing. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1996;82:475-92. [DOI] [PubMed] [Google Scholar]
- 30. Verdonk P. Histology-ultrastructure-biology. In: Beaufils P, Verdonk R, editors. The Meniscus. Berlin: Springer; 2010. p. 19-27. [Google Scholar]
- 31. Kon E, Filardo G, Tschon M, Fini M, Giavaresi G, Marchesini Reggiani L, et al. Tissue engineering for total meniscal substitution: animal study in sheep model results at 12 months. Tissue Eng Part A. 2012;18:1573-82. [DOI] [PubMed] [Google Scholar]
- 32. Christoforakis J, Pradhan R, Sanchez-Ballester J, Hunt N, Strachan RK. Is there an association between articular cartilage changes and degenerative meniscus tears? Arthroscopy. 2005;21:1366-9. [DOI] [PubMed] [Google Scholar]
- 33. Narita A, Takahara M, Sato D, Ogino T, Fukushima S, Kimura Y, et al. Biodegradable gelatin hydrogels incorporating fibroblast growth factor 2 promote healing of horizontal tears in rabbit meniscus. Arthroscopy. 2012;28:255-63. [DOI] [PubMed] [Google Scholar]
- 34. Iwasa J, Engebretsen L, Shima Y, Ochi M. Clinical application of scaffolds for cartilage tissue engineering. Knee Surg Sports Traumatol Arthrosc. 2009;17:561-77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Willers C, Chen J, Wood D, Xu J, Zheng MH. Autologous chondrocyte implantation with collagen bioscaffold for the treatment of osteochondral defects in rabbits. Tissue Eng. 2005;11:1065-76. [DOI] [PubMed] [Google Scholar]