Abstract
Objective
Alteration of the cellular microenvironment may influence the intra- and intercellular communication and contribute to cartilage injury and repair. The purpose of this study was to investigate how matrix elasticity/stiffness affects chondrogenic activities, including cell survival, phenotypic expression, and the release of both pro- and anti-inflammatory cytokines.
Design
Human articular chondrocytes (HACs) cultured on traditional 2-dimensional (2D) plastic surfaces were compared with those cultured within 3D hydrogel matrices of varying stiffness. Chondrogenic proliferation, differentiation, and the expression of pro- and anti-inflammatory cytokines were evaluated. Both interleukin-1-beta (IL-1β) and human synovial fluid–derived cells (hSFCs) were introduced to study the effects of matrix stiffness on chondrocyte response.
Results
Cells demonstrated the most robust chondrogenic differentiation and secreted the least pro-inflammatory cytokines when the matrix stiffness was close to their native microenvironment. The IL-1β effects were attenuated when HACs were co-cultured with hSFCs.
Conclusion
Modifying the matrix stiffness to mimic the native cartilage microenvironment not only optimized chondrogenic expression but also was essential for the regulation of physiological homeostasis. This study proposed a new toolkit to study cell-molecule, cell-cell, and cell-matrix influence on cartilage physiology.
Keywords: chondrogenic differentiation, microenvironment, extracellular matrix, material compliance, inflammation, chondrocyte expression
Introduction
Cartilage injuries are recognized as a significant source of morbidity and pain in the patients. 1 The self-healing capacity of articular cartilage is minimal because of the lack of endogenous cell sources, vasculature for nutrient supply, 2 and scar tissue formation. 3 Osteoarthritis (OA) develops after damage to the articular cartilage and the inability to achieve full-thickness hyaline cartilage repair. 4 Cell-based tissue engineering such as autologous chondrocyte implantation (ACI) and matrix autologous chondrocyte implantation (MACI) has demonstrated promising clinical results.5-7 However, ACI treatment frequently induces fibrocartilage with compromised biological functions, commonly requiring further surgical procedures.4,8 ACI and MACI therapy includes steps such as cartilage tissue harvesting, isolation, cell expansion, and transplantation into patients. The expansion of human articular chondrocytes (HACs) onto 2-dimensional (2D) surfaces introduces functional changes in the cells that dramatically affects their therapeutic utility. 9 Cellular expansion of HACs in vitro represents a formidable challenge, given that cells in their original niche tend to de-differentiate progressively.10,11
Although 3D cell culture models can retain the chondrogenic potential of HACs better than 2D cell culture systems, further optimization of chondrogenic phenotypes by adjusting the matrix compliance to mimic the native tissue environment is warranted.12,13 A growing body of research shows that the matrix plays an important role in influencing cell growth and differentiation.14,15 When cells were seeded in substrates mimicking the compliance of brain, muscle, and bone tissues, differentiation markers corresponding to these cells were expressed at maximum levels.13,16 Controlling the material compliance to mimic the elasticity of the native proteoglycan matrix of cartilage (E ~ 25 kPa)17,18 can maximize chondrogenic potential and yield more optimized chondrocyte phenotypes.
Matrix compliance and dimensionality are determined by a multitude of factors, including pore size, porosity, nutrient diffusion rate, pH, oxygen tension, and interstitial pressure. Matrix characteristics dictate the mean cell responses, ranging from cell spreading, controlling cell shape, and molecular organization.19-21 Cells need an optimal microenvironment to support their biological function. Physically, a soft material cannot be assembled into a 3D structure that can support cell survival throughout a long-term study, while a rigid material limits cell survival and restricts variation in substrate rigidity. 22
Transglutaminase cross-linked gelatin (Col-Tgel) can be fine-tuned to mimic the native extracellular matrix (ECM) stiffness and has been studied as a hypoxic platform without compromising critical nutrient and gas exchange that supports cellular activities.21,23 Using this gel allows for research into complex multicellular processes and endogenous matrix turnover which are vital for the study of cartilage repair. 24 Col-Tgel is not only suitable for in vitro experiments, but it can also be used for in vivo delivery and repair. Previous studies have successfully used Col-Tgel to investigate cell-matrix interactions and optimization of myogenic and osteogenic differentiation in vitro and in vivo.21,23,25
This study formulated the matrix compliance of Col-Tgel into three different levels of rigidity to investigate chondrogenic activities, including cell proliferation, morphology, differentiation, and the release of autocrine pro-/anti-inflammatory cytokines. Secondary objectives examined chondrocyte response to paracrine stimulation (i.e., interleukin-1-beta [IL-1β]) 26 under different matrix conditions. Additionally, human synovial fluid–derived cells (hSFCs) were added to measure their effect on chondrogenesis.27,28 The present study provides new insights into chondrocyte physiology and activity while establishing a model for chondrocyte and cartilage regeneration.
Methods
All chemicals and reagents were purchased from Sigma-Aldrich (St. Louis, MO, USA). Details on the preparation of microbial transglutaminase (TG) and gelatin gel are described previously.21,29,30 Briefly, gelatin type A 300 Bloom (Sigma Aldrich, MO) was dissolved and made into final concentrations of 3%, 6%, and 9%. TG from Streptomyces mobaraense was obtained from Ajinomoto (Tokyo, Japan), and was further purified with SP Sepharose Fast Flow beads (Sigma Aldrich, MO). The activity of TG was titrated by the o-phthaldialdehyde (OPA) assay using casein as a substrate, and the protein concentration was tested by the Bradford method (Bio-Rad, Hercules, CA) utilizing bovine serum albumin (BSA) as a standard.
The final elasticity of gels was determined through conventional unconfined compression tests. 21 Three different rigidities of scaffolds were investigated: 0.9 ± 0.1 kPa (Soft Col-Tgel), 25 ± 5 kPa (Med Col-Tgel), and 40 ± 10 kPa (Stiff Col-Tgel). Med Col-Tgel was tuned to mimic the elasticity of the native proteoglycan matrix between the collagen fibers of cartilage (E ~ 25 kPa).17,18
In Vitro Cell Isolation and Expansion
Isolation of HACs and hSFCs
Discarded cartilage tissue and synovial fluid were obtained from a healthy, de-identified donor. Cartilage was minced and digested with 0.2% collagenase (Worthington Biochemical, Lakewood, NJ, USA) to isolate HACs. hSFCs were extracted through centrifugation of synovial fluid (180 × g). The digested HACs and the extracted hSFCs were filtered and washed twice with DMEM (Dulbecco’s modified Eagle medium)/F-12 (Mediatech, Manassas, VA, USA) supplemented with 10% (v/v) fetal bovine serum III (FBS; Thermo Fisher Scientific, Waltham, MA, USA) and 1% (v/v) penicillin-streptomycin (PS; Mediatech).
Primary Cell Culture of HACs and hSFCs
Cells were cultured in DMEM/F-12 (Mediatech) supplemented with 10% (v/v) FBS and 1% (v/v) PS in a humidified atmosphere of 95% O2/5% CO2 at 37°C. The medium was changed every 2 to 3 days followed cells reached 70% confluence, they were passaged by digestion with trypsin/EDTA solution.
Three-Dimensional HAC Monoculture
The in vitro 3D Col-Tgel-Cell constructs were created as described previously. 23 Briefly, 100 µL of the gel solution was evenly mixed with 2 × 105 HACs (passage 4) and 5 µL of TG to create Col-Tgel cell mixtures. Aliquots (20 μL) of the Col-Tgel mixture were seeded into 48-well plates. Once the gel had solidified, 0.5 mL of growth medium was added. Conventional 2D cell culture was used as a control, where 4 × 104 cells per well were seeded into 48-well tissue culture plates. Samples were collected on days 5, 7, 14, and 28 for analysis. Data obtained from cells embedded in the Col-Tgels were normalized to those on the 2D surface.
External Stimulation with HAC and hSFCs
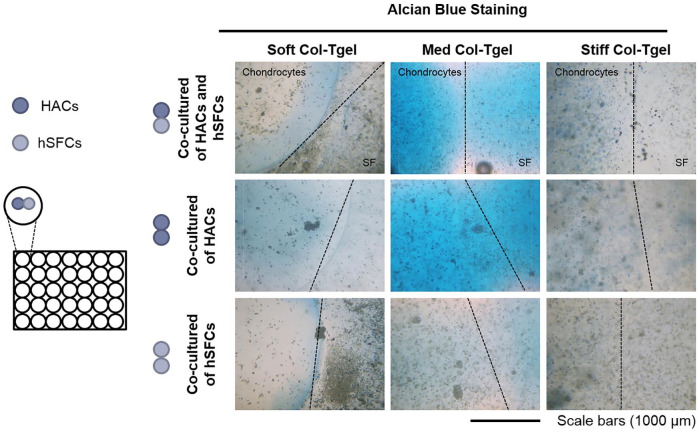
To study the crosstalk between HACs and hSFCs, 3 different co-culture conditions were created by seeding Col-Tgels side-by-side ( Fig. 5 ): HACs with HACs, HACs with hSFCs, and hSFCs with hSFCs. Briefly, 100 µL of the gel solution was evenly mixed with 2 × 105 HACs or hSFCs (passage 4) and 5 µL of TG to create Col-Tgel cell mixtures. Aliquots (10 μL) of the Col-Tgel mixture containing embedded cells were seeded into 48-well suspension plates. After the first gel droplet solidified, 10 μL of the second gel-cell mixture was seeded next to the first gel-cell mixture. Five hundred microliters of DMEM/F-12 (Mediatech) supplemented with 10% (v/v) FBS and 1% (v/v) PS was added per well following gel solidification.
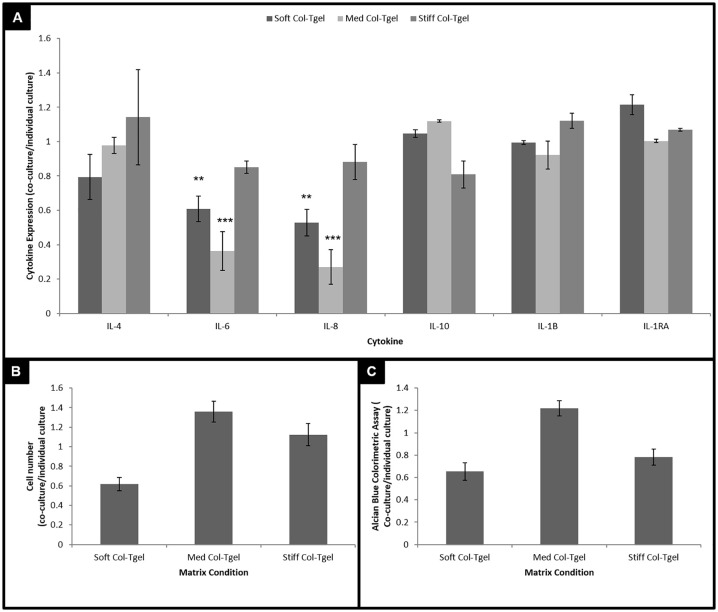
Figure 5.
The response of co-cultured cells to interleukin-1-beta (IL-1β). Human articular chondrocytes (HACs) and human synovial fluid–derived cells (hSFCs) were cultured under different matrix compliances (i.e., Soft, Med and Stiff Col-Tgel) side-by-side. Construct were treated with or without IL-1β on day 3, and the medium was refreshed on day 5. On day 7, samples were harvested for analysis and experimental outcomes were compared with predicted data. (A) Inflammatory cytokine profiles. Co-cultured cells especially those in the Med Col-Tgel expressed significantly less IL-6 and IL-8 than expected. (B) Cell counting. (C) Alcian blue. Cells embedded in the Med Col-Tgel contained the highest number of viable cells and deposited the most proteoglycans than expected. Data are presented as mean ± standard deviation, from 6 samples, statistical differences (*P < 0.05; **P < 0.01; ***P < 0.001).
Stimulation of IL-1β
The growth medium of 2D and 3D cell cultures was supplemented with 10 ng/mL IL-1β (R&D Systems, Minneapolis, MN, USA) on day 3 to evaluate the cellular activity in response to external stimuli (i.e., paracrine effects). The medium was refreshed on day 5 and samples were collected on day 7. Cytokine up/down regulation was determined by comparing the mean expression levels of cytokines between the treated cultures to their untreated counterparts.
Cellular Response Analysis
Cell Morphology Analysis
Chondrocyte cell morphology and cytoskeleton organization were visualized using an F-actin staining kit (Invitrogen, Carlsbad, CA, USA). The assays were performed according to the manufacturer’s recommendations. Briefly, the Col-Tgel-cell construct was rinsed with Tris buffer with 0.05% Tween 20 (TBST, pH 7.4), fixed in 10% neutral formalin solution for 10 min, incubated in blocking buffer (5% BSA in TBST) for 30 minutes, and then incubated with the prepared working solution. After a 2-hour incubation at 4°C, the Col-Tgel-cell construct was counterstained with 4′,6-diamidino-2-phenylindole (DAPI; Biotium, Hayward, CA, USA) for 5 minutes and visualized under an EVOS fluorescence microscope (Advanced Microscopy Group, Bothell, WA, USA).
Chondrogenic Expression Analysis
Proteoglycan deposition was visualized and quantified using Alcian blue (AB) and toluidine blue (TB) staining and colorimetric assays as described previously.31,32 Briefly, after fixation with paraformaldehyde (PFA) for 15 minutes, construct was stained with AB (0.05% Alcian blue 8GX in 75% ethanol) or (0.1% toluidine blue, 0.4% NaCl, and 20 mM HCl) overnight. After 5 washes with ddH2O to remove unbounded dye, images were captured to analyze the unique characteristics of HACs. For AB colorimetric assay, the construct was incubated overnight in 500 μL 6M guanidine hydrochloride. The supernatant was collected, and absorbance measured using a microplate reader (Molecular Devices, Sunnyvale, CA).
In situ immunohistochemical staining was performed at room temperature to characterize chondrogenic differentiation. Briefly, the Col-Tgel-Cell construct was fixed in 10% neutral formalin solution for 10 minutes, incubated in peroxidase suppressor solution (Thermo Scientific, Newington, NH) for 30 minutes, and then in the blocking buffer (5% BSA in the mixture of Tris-buffered saline and Tween-20) for 30 minutes. Next, the sample was incubated overnight in the primary antibody Col II (1: 200, R&D System) or Sox-9 (1:400, Santa Cruz) the signals were amplified either with biotin-conjugated goat anti-rabbit antibody (1:800, Sigma-Aldrich, MO) and visualized with DAB-peroxidase substrate kit (ThermoFisher Scientific, NY). For fluorescence images, signals were detected by Alexa 555 conjugated anti-rabbit secondary antibodies (1:800, ThermoFisher Scientific, NY). Cell grafts images were obtained by a fluorescence microscope (EVOS, ThermoFisher Scientific, NY).
Cell Survival and Cytotoxicity Assay
For cell viability, MTT (3-(4,5-dimethylthiazol-2-yl)-2, 5-diphenyltetrazolium bromide, Sigma-Aldrich, MO, 100 µL/well, 5 mg/mL) was added in each well for the last 4 hours of incubation. The absorbance of DMSO-dissolved-solution was recorded by plate-reader at OD 570 to 650 nm. Cell proliferation rate was compared with the nontreated control.
Cell counting was performed to determine the number of viable cells in Col-Tgels.21,23 Cells were released from Col-Tgel-cell constructs using 20 units of bacterial collagenase and incubated in at 37°C for 30 minutes prior to cell counting.
Pro-/Anti-Inflammatory Mediators, Immunoanalysis
The pro-/anti-inflammatory mediators known involved in joint inflammation/pathology were assayed, including IL-6, IL-8, IL-1β, IL-1 receptor antagonist (IL-1RA), IL-4, and IL-10.33-37. Col-Tgel-cell constructs were refreshed with the conditioned medium on day 5. After 48 hours, the conditioned medium was collected for enzyme-linked immunosorbent assay (ELISA), following the manufacturer’s protocol (Peprotech, London, UK). Since the concentration of secreted cytokines depended on the number of viable cells, the data were normalized. To better elucidate the interplay relationship of co-cultures, the difference between the experimental HAC-hSFC co-cultures and individual HAC or hSFC monoculture was assessed in response to IL-1β ( Figure 5 ). The co-cultured cell number, glycosaminoglycan (GAG) expression, and cytokine expression were expressed as a relative ratio to their individual culture.
Statistical Analysis
Statistical differences were determined using analysis of variance and the Tukey-Kramer honest significant difference (HSD) test for pairwise comparisons (SAS, Cary, NC, USA). Values of P < 0.05 were considered to indicate statistically significant differences.
Results
Re-differentiation of HACs in a 3D Controllable System
Studies have shown that substrates mimicking the compliance of a specific tissue maximized differentiation markers corresponding to that specific tissue. Therefore, we hypothesized that HACs de-differentiated due to in vitro 2D cell expansion could regain their original structure and function when HACs were seeded in the matrix of compliance that mimicked the elasticity of the native matrix of cartilage (E ~ 25 kPa).17,18 To test this hypothesis, HACs (passages 4-6) were encapsulated in Soft (0.9 ± 0.1 kPa), Med (25 ± 5 kPa), and Stiff (40 ± 10 kPa) Col-Tgels and compared with HACs cultured on a 2D surface. Cell morphology, cytoskeletal organization, differentiation, and proliferation were analyzed on day 28 to demonstrate the long-term effects of Col-Tgel on the expression of chondrogenic phenotypes.
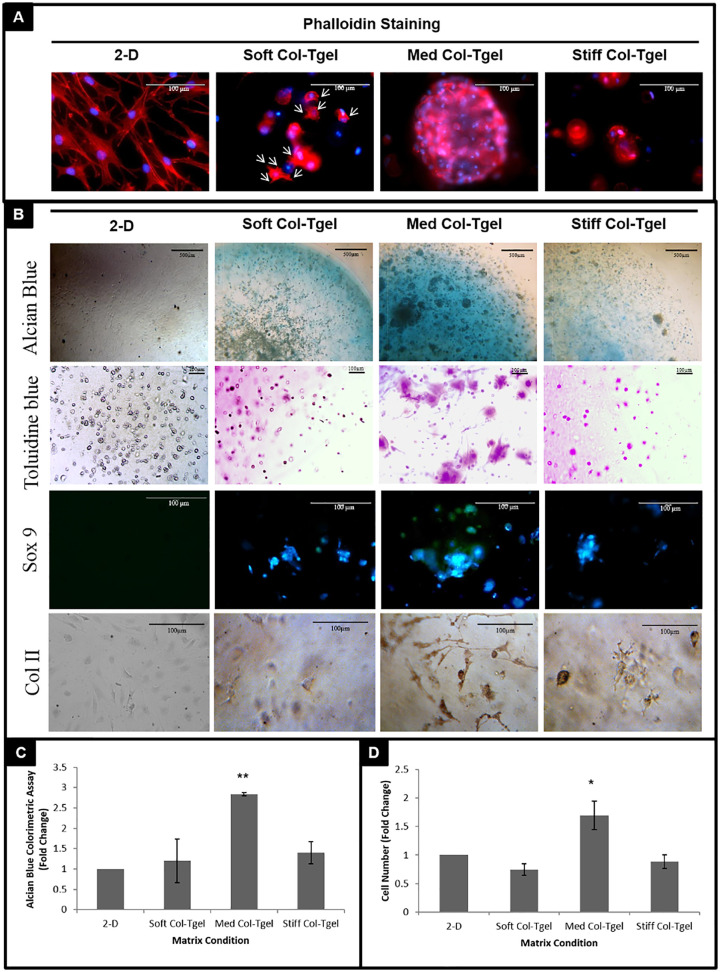
Phalloidin immunofluorescence staining was performed to visualize the organization of HAC actin filaments on 2D and 3D cultures ( Fig. 1A ). Two-dimensional cultured HACs exhibited an elongated fibrotic morphology, with abundant F-actin filaments. In contrast, HACs embedded in the 3D matrices were generally more spherical in shape. While extended filopodia was observed in the Soft Col-Tgel, dot-like actin-microfilaments and filopodia were found in the HACs that were embedded in the Med and Stiff Col-Tgels (white arrows in Fig. 1A ). Notably, cell clusters were observed in the Med Col-Tgel.
Figure 1.
The effect of matrix compliance on chondrogenic phenotype. Human articular chondrocytes (HACs) were cultured under different conditions (i.e., on 2D surface or encapsulated in Soft, Med, and Stiff Col-Tgels). (A) Phalloidin staining. (B) Alcian blue, Toluidine blue histochemical staining and Sox-9 and Col II IHC staining. (C) Alcian blue colorimetric assay. (D) Cell number. Data are given in mean ± standard deviation, n = 6, statistical differences (*P < 0.05; **P < 0.01; ***P < 0.001).
Chondrogenic marker expressions were characterized by histochemical and immunohistochemical staining from different culture conditions ( Fig. 1B ). HACs cultured on 2D surfaces lost their capacity to make proteoglycans, whereas HACs cultured in the 3D Col-Tgels displayed robust GAGs staining (AB, TB staining). Sox9, a master regulator for chondrogenesis 38 stained positive in 3D embedded HACs. Type II collagen IHC staining confirmed chondrogenic differentiation induced by 3D. While all 3 experimental gel conditions exhibited greater staining of GAG, Sox-9 and Col II than the 2D cultured cells, the increase was most obvious for HACs encapsulated in the Med Col-Tgel matrices. AB colorimetric assay was used to evaluate the production of GAG ( Fig. 1C ). Consistent with staining results, HACs embedded in Med Col-Tgel matrices exhibited the most significant increase of AB compared with 2 other gel conditions and 2D (P < 0.01).
Cell counting was performed ( Fig. 1D ) to determine the difference in cell proliferative capacities. There were significantly more HACs in the Med Col-Tgel than in the Soft and Stiff Col-Tgels (P < 0.05). The number of HACs embedded in the Soft and Stiff Col-Tgels was not significantly different from the 2D cell cultures. The Med Col-Tgel had approximately 1.5 times more cells compared with the other culture conditions.
Matrix-Dependent Autocrine Cytokine Secretion of HACs
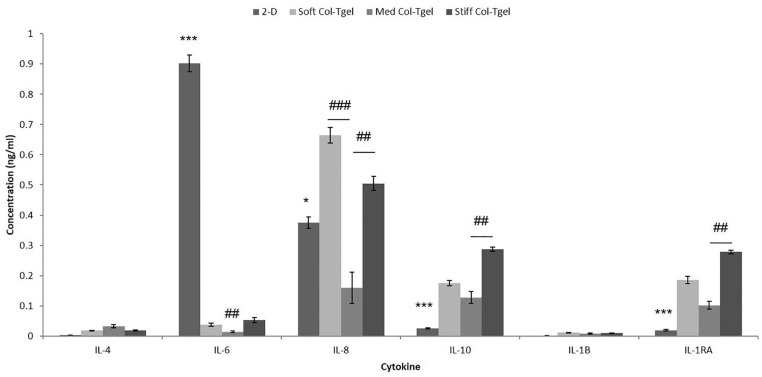
Excessive pro-inflammatory mediators present in the joint have been shown to not only contribute to cartilage degeneration, but also hinder the progression of cartilage repair and recovery.39,40 The effect of matrix compliance on cytokine expression was investigated on day 7 using a sandwich ELISA immuno assay ( Fig. 2 ). HACs cultured on 2D surfaces expressed abundant pro-inflammatory cytokines (IL-6) and minimal anti-inflammatory cytokines (IL-10, IL-1RA, and IL-4). In contrast, HACs embedded in 3D matrices expressed approximately 15 to 70 fold less pro-inflammatory cytokines (i.e., IL-6, P < 0.001) and approximately 5 to 10 fold more anti-inflammatory cytokines (i.e., IL-1RA, IL-4, and IL-10, P < 0.05). The exception to this general trend was IL-8, which was more than 2-fold higher in the Soft and Stiff Col-Tgels as compared with the 2D cell culture (P < 0.05). However, IL-8 remained minimally expressed by HACs encapsulated in the Med Col-Tgel (P < 0.05). HACs embedded in the Med Col-Tgel generally minimally expressed all the evaluated cytokines.
Figure 2.
The impact of matrix compliance on inflammatory secretions. Human articular chondrocytes (HACs) were cultured under different conditions (i.e., on 2D surface or encapsulated in Soft, Med and Stiff Col-Tgels). Constructs were harvested on day 7 for inflammatory cytokine expression analysis. The data were normalized to the number of viable cells in each condition. HACs encapsulated in 3D Col-Tgels generally expressed less pro- and more anti-inflammatory cytokines than HACs culture on 2D surface. Comparing the 3 gel conditions, HACs embedded in the Med Col-Tgel generally had minimal cytokine secretion. Data are presented as mean ± standard deviation, from 6 samples, statistical differences of 3-D compared with 2-D cell culture (*P < 0.05; **P < 0.01; ***P < 0.001) and comparison between different Col-Tgel (#P < 0.05; ##P < 0.01; ###P < 0.001).
Matrix Compliance Modulated HACs in Response to Exogenous Pro-Inflammatory Signaling
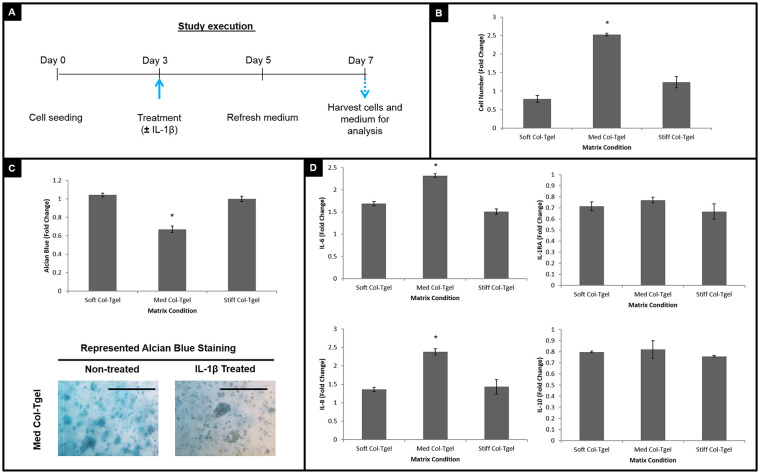
To mimic OA-like changes in vitro,49,50 human recombinant IL-1β was added on day 3 ( Fig. 3A ). On day 7, the effects of IL-1β and matrix compliance for chondrogenic activities of HACs were assessed by examining cell proliferation, GAG expression, and cytokine excretion ( Fig. 3 ). Cell numbers, proteoglycan deposition, and cytokine expression in the medium with IL-1β was normalized against the medium without IL-1β. The addition of IL-1β significantly increased cell proliferation of HACs encapsulated in Med Col-Tgel (P < 0.05). There were no demonstrable changes in cell numbers of HACs encapsulated in the Soft and Stiff Col-Tgels in both treated and untreated with IL-1β ( Fig. 3B ).
Figure 3.
The effect of interleukin-1-beta (IL-1β) treatment. Human articular chondrocytes (HACs) treated with or without IL-1β were cultured under different matrix conditions (i.e., Soft, Med, and Stiff Col-Tgels) for 7 days. (A) Study execution. (B) Cell counting. (C) Alcian blue staining and colorimetric assay. (D) Inflammatory cytokine expression. Cells were treated with IL-1β on day 3 and the medium was refreshed on day 5. On day 7, samples were harvested for analysis. In general, HACs encapsulated in the Med Col-Tgel that had been treated with IL-1β exhibited significantly more cell numbers and less proteoglycan expression as compared with nontreated HACs embedded in the same matrix condition. The downregulation of proteoglycan deposition was accompanied by an upregulation of pro-I (i.e., IL-6 and IL-8) and downregulation of anti- (i.e., IL-1RA and IL-10) inflammatory cytokine expression. Data are presented as mean ± standard deviation, from 6 samples, statistical differences (*P < 0.05; **P < 0.01; ***P < 0.001).
In contrast to cell proliferation, IL-1β significantly decreased proteoglycan deposition of HACs embedded in Med-Col-Tgel, which had the most chondrogenic inductive effect among three gel conditions (P < 0.05) ( Fig. 3C ). HACs encapsulated in the Soft and Stiff Col-Tgels did not exhibit significant changes in GAG staining after exposure to IL-1β due to the initially low GAG expression levels under those conditions. Figure 3D shows that HACs cultured in all three gel conditions expressed more pro-inflammatory (i.e., IL-6 and IL-8) and less anti-inflammatory (i.e., IL-1RA and IL-10) cytokines after being stimulated with IL-1β. Among the 3 gel conditions, the differential secretion of pro-inflammatory cytokines by HACs in the Med Col-Tgel was most pronounced following IL-1β addition (P < 0.05).
Crosstalk between HACs and hSFCs
hSFCs were chosen because of their interaction with the articular cartilage surface, as well as their ease of accessibility and their chondrogenic potential.28-31 Two gel droplets of the same matrix compliance that encapsulated either HACs or hSFCs were seeded side-by-side ( Fig. 4 ). Each well contained either homogeneous or heterogeneous cell types (i.e., HACs-HACs, HACs-hSFCs, or hSFCs-hSFCs). Regardless of cell type, elevated proteoglycan deposition was observed in the Med Col-Tgel as compared with other gel conditions. The hSCFs of HACs-hSFCs co-cultures expressed greater proteoglycan deposition when cultured in the Med Col-Tgel when compared with hSFC monocultures.
Figure 4.
Crosstalk of human articular chondrocytes (HACs), human synovial fluid–derived cells (hSFCs), and matrix compliance. HACs and hSFCs were cultured under different matrix compliances (i.e., Soft, Med, and Stiff Col-Tgel) side-by-side, as shown in the figure. Constructs were harvested on day 7 for Alcian blue staining. hSFCs expressed more proteoglycan deposition when co-culture with chondrocytes in Med Col-Tgel.
Crosstalk between HACs, hSFCs, and IL-1β
HAC-hSFC co-cultures in different matrix compliances were supplemented with IL-1β ( Fig. 5 ). The HAC-hSFC co-cultures expressed significantly less pro-inflammatory cytokines (i.e., IL-6 and IL-8) than individual culture ( Fig. 5A , P < 0.01). There were no observational differences between co-cultures and individual cultures on the expression of anti-inflammatory cytokines. When comparing gel conditions, experimental HAC-hSFC co-cultures normalized with individual HAC + hSFC after stimulation with IL-1β. Cells embedded in the Med Col-Tgel contained the highest number of viable cells, deposited the most proteoglycans, and secreted the least pro-inflammatory cytokines (i.e., IL-6 and IL-8).
Discussion
The ECM is an important regulator of cartilage development, homeostasis, pathology, remodeling, and regeneration. The matrix compliances of most currently available scaffolds are not optimized for chondrogenic activities. Matrix compliance affects the secretion of pro- and anti-inflammatory cytokines involved in cartilage destruction and repair. This study examined the cellular responses to various matrix compliances and demonstrated that there were significant differences between chondrogenic expression and pro-/anti-inflammatory profiles when cultured on 2D surfaces compared with encapsulation in 3D Col-Tgels. These findings were the most significant for HACs encapsulated in the Med Col-Tgel (i.e., the condition that was tuned to mimic cartilage proteoglycan ECM stiffness). This Med Col-Tgel condition exhibited greater proteoglycan deposition and generally produced less pro-inflammatory and less anti-inflammatory cytokines. These data showed that HACs embedded in the Med Col-Tgel demonstrated a physiological sensitivity in response to IL-1β, and when cocultured with hSFCs in the Med Col-Tgel; the HACs were able to differentiate into chondrocyte-like cells and provided chondroprotective effects against catabolic stress.
This study used passage 4-6 of HACs, which has been shown to exhibit irreversible phenotypic alterations.41,42 Similar to previous studies, HACs de-differentiated on 2D surfaces and exhibited extremely high levels of pro-inflammatory (especially IL-6) and minimal levels of anti-inflammatory cytokines. The drawbacks of 2D in vitro studies were overcome by using the 3D scaffold (i.e., Col-Tgel). The elongated, de-differentiated HACs in the 2D system regained their physiological phenotypic expression and sensitivity in the 3D scaffold. Moreover, the 3D system did not compromise the proliferative capability of HACs, suggesting that the 3D in vitro system could provide a more accurate physiological microenvironment for HACs than a 2-D system and a superior platform for in vitro cell expansion.
Matrix stiffness has a profound effect on chondrogenic phenotypic expression.14-16 Substrates with a low stiffness (E ~ 4 kPa) can maintain their chondrogenic phenotype due to the round morphology of chondrocytes. 43 However, in the soft gel, HAC cells do not have enough binding sites or a matrix strong enough to support cell suspension. 44 Therefore, as shown in Figure 1 , extended filopodia was only observed in the Soft Col-Tgel group instead of cell clusters. This study showed that chondrogenic proliferation and expression levels were enhanced and maintained in the matrix compliance (i.e., the Med Col-Tgel, E = 25 ± 5 kPa) that mimicked the native elasticity and stiffness of physiological ECM of cartilage (E ~ 25 kPa).17,18 Spherical cell morphology, decreased actin cytoskeletal organization, and cell clusters that facilitate the outcome of cartilage repair26,45 improved chondrogenesis due to the increase in intercellular contacts.46,47 Suboptimal chondrogenic expression levels were found when cells were subjected to conditions that deviate from the native-like cellular microenvironment (i.e., the Soft and Stiff Col-Tgels, 0.9 ± 0.1 and 40 ± 10 kPa, respectively).
The discrepancy between this study and previous scaffold studies 43 may be secondary to the specific hydrogel 3D microenvironment. Other material properties such as porosity, pore size, and cell-adhesion site contribute to discrepancies. Two methods of proteoglycan staining were used in this study, Alcian blue (AB) and toluidine blue (TB). AB was first used in the gels but was discovered that it did not stain hard or soft gels properly. Even after extensive de-staining, there was still left a high background staining due to the 3D material trapping dye. TB was used after AB to confirm the results and demonstrated strong positive staining of cell clusters proteoglycan staining in the Med gel was the most positive in AB and TB staining. The toluidine blue demonstrated better staining characteristics than AB staining and demonstrated clear positive staining around the cell clusters in the 3D culture. Proteoglycan in cartilage can reach molecular weights between 1 and 4 MDa depending on the amount of glycosylation. 48 Differences in cross-linking densities affect the diffusivity of hydrogels and could cause issues with proteoglycan staining. 49 A better understanding of the dynamic mechanisms involved in cell interaction within the matrix microenvironment could result in predictive control of cell fate and function, enabling rational design strategies for tissue scaffold engineering.
Both the matrix as well as soluble mediators can influence the behavior of cells in a very distinct and cooperative way. 18 Cells encapsulated in the Med Col-Tgel maintained a relatively low secretion of pro- and anti-inflammatory cytokines. The balance between pro- and anti-inflammatory cytokines were better maintained in a 3D scaffold when compared with a 2D cell culture ( Fig. 2 ). For example, the upregulation of pro-inflammatory cytokine expression was accompanied by the upregulation of anti-inflammatory regulators in the Soft and Stiff Col-Tgels, suggesting a positive feedback mechanism of cell signaling in a 3D microenvironment. Mimicking the cartilage microenvironment was demonstrated to be an important strategy for regulating cellular expression and homeostasis in this study.
Although matrix compliance can optimize chondrogenic activities, the matrix itself may not be sufficient to provoke chondrogenic differentiation under pathological conditions. As demonstrated in this study, HACs embedded in the chondrogenic scaffold (i.e., Med Col-Tgel) were more sensitive to IL-1β as evidenced by the significant downregulation of proteoglycan expression in response to the addition of IL-1β. Similar to previous studies,50,51 the matrix degradation was accompanied by the upregulation of cell proliferation and inflammatory responses, especially IL-6 and IL-8. As HACs regained their sensitivity in response to IL-1β, HACs co-delivered with scaffold may fail to repair cartilage injury with the substantial pro-inflammatory presence in the injured joint. The notion that specific, nonnative culture conditions lead to cellular changes that reduce the responsiveness of chondrocytes may be attributable to cell abnormalities or de-differentiation, which was supported by previous studies demonstrating reduced responsiveness to IL-1β stimulation in osteoarthritic chondrocytes. 27 This study showed that soluble mediators and the matrix were important and need to be considered when designing an effective treatment for cartilage defects.
A number of studies have shown that homeostasis of the ECM of articular cartilage under normal and pathological conditions is dependent on the responses of HACs to paracrine anabolic and catabolic pathways.24,25 hSFCs were selected for this study because of their multipotency and availability. A number of studies report that SFCs increase after injury or in degenerated joints.52,53 This study reported that hSFCs coupled with a finetuned scaffold may beneficially influence cartilage repair treatments. The chondrogenic differentiation potential of hSFCs was enhanced in the Med Col-Tgel and the Med Col-Tgel HAC-hSFC co-cultures were the most “resistant” to IL-1β-induced stress, and the complex chronic inflammatory pathways contributing to cartilage injury. Crosstalk between joint tissues within the innate immune inflammatory network of the joint play an important role in cartilage development, disease progression, and remodeling. 54 Previous studies have shown that IL-1β is capable of stimulating cartilage breakdown in living HACs monocultures, but not in the presence of living synoviocytes. 55 The apparent resistance of co-cultures observed in this study is consistent with these findings. While further optimization of the matrix by co-culturing hSFCs and HACs in different matrix compliances might be needed, this study showed that crosstalk between HACs and hSFCs in the chondrogenic-like microenvironment may provide chondro protective effects.
Scaffold and cells are highly coupled in the regulation of chondrogenic expression, function, homeostasis, and sensitivity in response to exogenous stress. The 3D cell culture system allows cell-cell, cell-matrix, and cell-molecule interactions that make it an ideal “toolkit”/model for the study of cartilage development, progression, and repair. Manipulating the matrix compliance allows for the study of cellular responses under pathophysiological conditions that could result in differently designed scaffolds for tissue engineering. While further studies are necessary to determine the applicability of these findings to clinical disease, these results provide preliminary support for studies aimed at understanding and treating cartilage pathology.
Limitations
The tissue samples used in this experiment, discarded cartilage tissue and synovial fluid, were collected from a single healthy, de-identified donor. However, there are varied cartilage proteins and cytokines expressed between individuals.56,57 Additionally there are concerns that the chondrocytes in humans with OA have an altered pattern of gene expression compared to healthy patients. 58 Cartilage tissue from OA patients may be limited in their capacity to regain a cartilage matrix phenotype. A wider range of donors, including healthy patients and patients with confirmed knee OA, are needed to address the issues on donor variability.
Additional compression loading tests are warranted to test hydrogel stiffness and intactness after culture. Hydrogels degrading after culture could explain the lowered cell numbers and proteoglycan deposition of the soft hydrogel group.
Footnotes
Author Contributions: Conception and design: B. Han. Development of methodology and data acquisition: S.J. Tan. Analysis and interpretation data: S.J. Tan, W. Fang, C.T. Vangsness Jr., B. Han. Writing, review, and/or revision of the manuscript: S.J. Tan, W. Fang, C.T. Vangsness Jr., and B. Han. All authors reviewed the manuscript before final submission.
Acknowledgments and Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This project is partially supported by National Institutes of Health (NIH) Grant R21AG053746 (B. Han) and Nimni Cordoba Fund (B. Han)
Declaration of Conflicting Interests: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: C.T. Vangsness Jr. reports nonfinancial support from KeraLink International, CarthroniX Inc, Align-Med Inc, Parcus Medical LLC, and Replenish Inc, outside the submitted work. The other authors report no potential competing interests.
Ethical Approval: Not applicable.
Informed Consent: Not applicable.
Trial Registration: Not applicable.
ORCID iDs: William Fang  https://orcid.org/0000-0003-1703-6969
https://orcid.org/0000-0003-1703-6969
C. Thomas Vangsness Jr.  https://orcid.org/0000-0002-0143-0155
https://orcid.org/0000-0002-0143-0155
References
- 1. Moyad TF. Cartilage injuries in the adult knee: evaluation and management. Cartilage. 2011;2(3):226-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Minas T. A primer in cartilage repair and joint preservation of the knee (e-book: expert consult). Elsevier Health Sciences; 2011. [Google Scholar]
- 3. Silver FH, Glasgold AI. Cartilage wound healing. An overview. Otolaryngol Clin North Am. 1995;28(5):847-64. [PubMed] [Google Scholar]
- 4. Bohaček I, Bojanić I, Gajović S, Josipović M, Jelić M. Articular cartilage repair techniques exploiting intrinsic healing capacity—which one is the best? Period Biol. 2015;117(1):125-33. [Google Scholar]
- 5. Nesic D, Whiteside R, Brittberg M, Wendt D, Martin I, Mainil-Varlet P. Cartilage tissue engineering for degenerative joint disease. Adv Drug Deliv Rev. 2006;58(2_suppl):300-22. [DOI] [PubMed] [Google Scholar]
- 6. Mandelbaum B, Browne JE, Fu F, Micheli LJ, Moseley JB, Jr, Erggelet C, et al. Treatment outcomes of autologous chondrocyte implantation for full-thickness articular cartilage defects of the trochlea. Am J Sports Med. 2007;35(6):915-21. [DOI] [PubMed] [Google Scholar]
- 7. Brittberg M. Autologous chondrocyte implantation—technique and long-term follow-up. Injury. 2008;39(Suppl 1):S40-S49. [DOI] [PubMed] [Google Scholar]
- 8. Peterson L, Brittberg M, Kiviranta I, Åkerlund EL, Lindahl A. Autologous chondrocyte transplantation: biomechanics and long-term durability. Am J Sports Med. 2002;30(1):2-12. [DOI] [PubMed] [Google Scholar]
- 9. Caron MM, Emans PJ, Coolsen MM, Voss L, Surtel DAM, Cremers A, et al. Redifferentiation of dedifferentiated human articular chondrocytes: comparison of 2D and 3D cultures. Osteoarthritis Cartilage. 2012;20(10):1170-8. [DOI] [PubMed] [Google Scholar]
- 10. von der Mark K, Gauss V, von der Mark H, Müller P. Relationship between cell shape and type of collagen synthesised as chondrocytes lose their cartilage phenotype in culture. Nature. 1977;267(5611):531-2. [DOI] [PubMed] [Google Scholar]
- 11. Benya PD, Shaffer JD. Dedifferentiated chondrocytes reexpress the differentiated collagen phenotype when cultured in agarose gels. Cell. 1982;30(1):215-24. [DOI] [PubMed] [Google Scholar]
- 12. Discher DE, Janmey P, Wang YL. Tissue cells feel and respond to the stiffness of their substrate. Science. 2005;310(5751):1139-43. [DOI] [PubMed] [Google Scholar]
- 13. Engler AJ, Sen S, Sweeney HL, Discher DE. Matrix elasticity directs stem cell lineage specification. Cell. 2006;126(4):677-89. [DOI] [PubMed] [Google Scholar]
- 14. Gao Y, Liu SY, Huang JX, Guo W, Chen J, Zhang L, et al. The ECM-cell interaction of cartilage extracellular matrix on chondrocytes. Biomed Res Int. 2014;2014:648459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lai JH, Rogan H, Kajiyama G, Goodman SB, Smith RL, Maloney W, et al. Interaction between osteoarthritic chondrocytes and adipose-derived stem cells is dependent on cell distribution in three-dimension and transforming growth factor-β3 induction. Tissue Eng Part A. 2015;21(5-6):992-1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hu X, Park SH, Gil ES, Xia XX, Weiss AS, Kaplan DL. The influence of elasticity and surface roughness on myogenic and osteogenic-differentiation of cells on silk-elastin biomaterials. Biomaterials. 2011;32(34):8979-89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Stolz M, Raiteri R, Daniels AU, VanLandingham MR, Baschong W, Aebi U. Dynamic elastic modulus of porcine articular cartilage determined at two different levels of tissue organization by indentation-type atomic force microscopy. Biophys J. 2004;86(5):3269-83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Buxboim A, Ivanovska IL, Discher DE. Matrix elasticity, cytoskeletal forces and physics of the nucleus: how deeply do cells “feel”—outside and in? J Cell Sci. 2010;123(pt 3):297-308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Engler A, Bacakova L, Newman C, Hategan A, Griffin M, Discher D. Substrate compliance versus ligand density in cell on gel responses. Biophys J. 2004;86(1 pt 1):617-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kong HJ, Polte TR, Alsberg E, Mooney DJ. FRET measurements of cell-traction forces and nano-scale clustering of adhesion ligands varied by substrate stiffness. Proc Natl Acad Sci U S A. 2005;102(12):4300-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Tan S, Fang JY, Yang Z, Nimni ME, Han B. The synergetic effect of hydrogel stiffness and growth factor on osteogenic differentiation. Biomaterials. 2014;35(20):5294-306. [DOI] [PubMed] [Google Scholar]
- 22. Ochsner M, Textor M, Vogel V, Smith ML. Dimensionality controls cytoskeleton assembly and metabolism of fibroblast cells in response to rigidity and shape. PLoS One. 2010;5(3):e9445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Tan S, Fang J, Wu Y, Yang Z, Liang G, Han B. Muscle tissue engineering and regeneration through epigenetic reprogramming and scaffold manipulation. Sci Rep. 2015;5:16333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Patel S, Kurpinski K, Quigley R, Gao H, Hsiao BS, Poo MM, et al. Bioactive nanofibers: synergistic effects of nanotopography and chemical signaling on cell guidance. Nano Lett. 2007;7(7):2122-8. [DOI] [PubMed] [Google Scholar]
- 25. Fang J, Yang Z, Tan S, Tayag C, Nimni ME, Urata M, et al. Injectable gel graft for bone defect repair. Regen Med. 2014;9(1):41-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Acharya C, Adesida A, Zajac P, Mumme M, Riesle J, Martin I, et al. Enhanced chondrocyte proliferation and mesenchymal stromal cells chondrogenesis in coculture pellets mediate improved cartilage formation. J Cell Physiol. 2012;227(1):88-97. [DOI] [PubMed] [Google Scholar]
- 27. Jones EA, Crawford A, English A, Henshaw K, Mundy J, Corscadden D, et al. Synovial fluid mesenchymal stem cells in health and early osteoarthritis: detection and functional evaluation at the single-cell level. Arthritis Rheum. 2008;58(6):1731-40. [DOI] [PubMed] [Google Scholar]
- 28. de Sousa EB, Casado PL, Neto VM, Duarte MEL, Aguiar DP. Synovial fluid and synovial membrane mesenchymal stem cells: latest discoveries and therapeutic perspectives. Stem Cell Res Ther. 2014;5(5):112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kuwahara K, Fang JY, Yang Z, Han B. Enzymatic crosslinking and degradation of gelatin as a switch for bone morphogenetic protein-2 activity. Tissue Eng Part A. 2011;17(23-24):2955-64. [DOI] [PubMed] [Google Scholar]
- 30. Kuwahara K, Yang Z, Slack GC, Nimni ME, Han B. Cell delivery using an injectable and adhesive transglutaminase-gelatin gel. Tissue Eng Part C Methods. 2010;16(4):609-18. [DOI] [PubMed] [Google Scholar]
- 31. Delorme B, Charbord P. Culture and characterization of human bone marrow mesenchymal stem cells. Methods Mol Med. 2007;140:67-81. [DOI] [PubMed] [Google Scholar]
- 32. Kawaguchi J, Mee PJ, Smith AG. Osteogenic and chondrogenic differentiation of embryonic stem cells in response to specific growth factors. Bone. 2005;36(5):758-69. [DOI] [PubMed] [Google Scholar]
- 33. Hart P, Ahern M, Smith M, Finlay-Jones J. Comparison of the suppressive effects of interleukin-10 and interleukin-4 on synovial fluid macrophages and blood monocytes from patients with inflammatory arthritis. Immunology. 1995;84(4):536-42. [PMC free article] [PubMed] [Google Scholar]
- 34. Kapoor M, Martel-Pelletier J, Lajeunesse D, Pelletier JP, Fahmi H. Role of proinflammatory cytokines in the pathophysiology of osteoarthritis. Nat Rev Rheumatol. 2011;7(1):33-42. [DOI] [PubMed] [Google Scholar]
- 35. Mishima Y, Lotz M. Chemotaxis of human articular chondrocytes and mesenchymal stem cells. J Orthop Res. 2008;26(10):1407-12. [DOI] [PubMed] [Google Scholar]
- 36. Sadouk MB, Pelletier JP, Tardif G, Kiansa K, Cloutier JM, Martel-Pelletier J. Human synovial fibroblasts coexpress IL-1 receptor type I and type II mRNA. The increased level of the IL-1 receptor in osteoarthritic cells is related to an increased level of the type I receptor. Laboratory investigation; a journal of technical methods and pathology. 1995;73(3):347-55. [PubMed] [Google Scholar]
- 37. Ashraf S, Cha BH, Kim JS, Ahn J, Han I, Park H, et al. Regulation of senescence associated signaling mechanisms in chondrocytes for cartilage tissue regeneration. Osteoarthritis Cartilage. 2016;24(2_suppl):196-205. [DOI] [PubMed] [Google Scholar]
- 38. Akiyama H. Control of chondrogenesis by the transcription factor Sox9. Mod Rheumatol. 2008;18(3):213-9. [DOI] [PubMed] [Google Scholar]
- 39. Yang KGA, Saris DBF, Verbout AJ, Creemers LB, Dhert WJA. The effect of synovial fluid from injured knee joints on in vitro chondrogenesis. Tissue Eng. 2006;12(10):2957-64. [DOI] [PubMed] [Google Scholar]
- 40. Rodrigo J, Steadman J, Syftestad G, Benton H, Silliman J. Effects of human knee synovial fluid on chondrogenesis in vitro. Am J Knee Surg. 1994;8(4):124-9. [PubMed] [Google Scholar]
- 41. Benya PD, Padilla SR, Nimni ME. Independent regulation of collagen types by chondrocytes during the loss of differentiated function in culture. Cell. 1978;15(4):1313-21. [DOI] [PubMed] [Google Scholar]
- 42. Kang SW, Yoo SP, Kim BS. Effect of chondrocyte passage number on histological aspects of tissue-engineered cartilage. Biomed Mater Eng. 2007;17(5):269-76. [PubMed] [Google Scholar]
- 43. Schuh E, Kramer J, Rohwedel J, Notbohm H, Müller R, Gutsmann T, et al. Effect of matrix elasticity on the maintenance of the chondrogenic phenotype. Tissue Eng Part A. 2010;16(4):1281-90. [DOI] [PubMed] [Google Scholar]
- 44. Levental I, Georges PC, Janmey PA. Soft biological materials and their impact on cell function. Soft Matter. 2007;3(3):299-306. [DOI] [PubMed] [Google Scholar]
- 45. Chung C, Burdick JA. Influence of three-dimensional hyaluronic acid microenvironments on mesenchymal stem cell chondrogenesis. Tissue Eng Part A. 2008;15(2_suppl):243-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Fang J, Yong Q, Zhang K, Sun W, Yan S, Cui L, et al. Novel injectable porous poly (γ-benzyl-l-glutamate) microspheres for cartilage tissue engineering: preparation and evaluation. J Mater Chem B. 2015;3(6):1020-31. [DOI] [PubMed] [Google Scholar]
- 47. Zhang K, Wang L, Han Q, Heng BC, Yang Z, Ge Z. Relationship between cell function and initial cell seeding density of primary porcine chondrocytes in vitro. Biomed Eng Appl Basis Commun. 2013;25(5):1340001. [Google Scholar]
- 48. Hardingham TE, Fosang AJ. Proteoglycans: many forms and many functions. FASEB J. 1992;6(3):861-70. [PubMed] [Google Scholar]
- 49. Nicodemus GD, Skaalure SC, Bryant SJ. Gel structure has an impact on pericellular and extracellular matrix deposition, which subsequently alters metabolic activities in chondrocyte-laden PEG hydrogels. Acta Biomater. 2011;7(2_suppl):492-504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Vincenti MP, Brinckerhoff CE. Early response genes induced in chondrocytes stimulated with the inflammatory cytokine interleukin-1beta. Arthritis Res. 2001;3(6):381-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Röhner E, Matziolis G, Perka C, Füchtmeier B, Gaber T, Burmester GR, et al. Inflammatory synovial fluid microenvironment drives primary human chondrocytes to actively take part in inflammatory joint diseases. Immunol Res. 2012;52(3):169-75. [DOI] [PubMed] [Google Scholar]
- 52. Morito T, Muneta T, Hara K, Ju YJ, Mochizuki T, Makino H, et al. Synovial fluid-derived mesenchymal stem cells increase after intra-articular ligament injury in humans. Rheumatology (Oxford). 2008;47(8):1137-43. [DOI] [PubMed] [Google Scholar]
- 53. Sekiya I, Ojima M, Suzuki S, Yamaga M, Horie M, Koga H, et al. Human mesenchymal stem cells in synovial fluid increase in the knee with degenerated cartilage and osteoarthritis. J Orthop Res. 2012;30(6):943-9. [DOI] [PubMed] [Google Scholar]
- 54. Liu-Bryan R. Synovium and the innate inflammatory network in osteoarthritis progression. Curr Rheumatol Rep. 2013;15(5):323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Steenvoorden M, Bank R, Ronday H, Toes R, Huizinga T, DeGroot J. Fibroblast-like synoviocyte-chondrocyte interaction in cartilage degradation. Clin Exp Rheumatol. 2007;25(2_suppl):239-45. [PubMed] [Google Scholar]
- 56. Wu J, Liu W, Bemis A, Wang E, Qiu Y, Morris EA, et al. Comparative proteomic characterization of articular cartilage tissue from normal donors and patients with osteoarthritis. Arthritis Rheum. 2007;56(11):3675-84. [DOI] [PubMed] [Google Scholar]
- 57. Tsuchida AI, Beekhuizen M, C’t Hart M, Radstake TRDJ, Dhert WJA, Saris DBF, et al. Cytokine profiles in the joint depend on pathology, but are different between synovial fluid, cartilage tissue and cultured chondrocytes. Arthritis Res Ther. 2014;16(5):441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Brew C, Andrew J, Boot-Handford R, Hardingham T. Increased collagen I expression in osteoarthritic cartilage suggests some loss of normal chondrocyte phenotype. Poster presented at: 50th Annual Meeting of the Orthopaedic Research Society, March 7-10, 2004; San Francisco, CA. Poster No. 0940. [Google Scholar]