Abstract
Objective
Shockwave application is a potential treatment for osteoarthritis (OA), but the underlying mechanism remains unknown. Oxidative stress and a counterbalancing antioxidant system might be the key to understanding this mechanism. We hypothesized that reactive oxygen species (ROS) and the transcription factor nuclear factor erythroid 2-related factor 2 (Nrf2),which is an important regulator of cellular redox homeostasis, are plausible elements.
Design
Porcine chondrocytes were cultured in a 3-dimensional pellet model and subjected to shockwaves. The effects of shockwaves with various energy-flux densities on optimal extracellular matrix (ECM) synthesis were assessed. ROS, mitogen-activated protein kinase (MAPK) signaling, and the redox activity of Nrf2 were measured. To investigate the signaling mechanism involved in the shockwave treatment in chondrocytes, specific inhibitors of ROS, MAPK signaling, and Nrf2 activity were targeted.
Results
Shockwaves increased ECM synthesis without affecting cell viability or proliferation. Furthermore, they induced transient ROS production mainly through xanthine oxidase. The phosphorylation of ERK1/2 and p38 and the nuclear translocation of Nrf2 were activated by shockwaves. By contrast, suppression of ROS signaling mitigated shockwave-induced MAPK phosphorylation, Nrf2 nuclear translocation, and ECM synthesis. Pretreatment of chondrocytes with the specific inhibitors of MEK1/2 and p38, respectively, mitigated the shockwave-induced nuclear translocation of Nrf2 and ECM synthesis. Nrf2 inhibition by both small hairpin RNA knockdown and brusatol reduced the shockwave-enhanced ECM synthesis.
Conclusions
Shockwaves activated Nrf2 activity through the induction of transient ROS signaling and subsequently enhanced ECM synthesis in chondrocytes. This study provided fundamental evidence confirming the potential of shockwaves for OA management.
Keywords: shockwave, osteoarthritis, Nrf2, chondrocyte, reactive oxygen species
Introduction
Shockwaves are acoustic pulses characterized by a high positive pressure amplitude and an extremely short buildup time. Emerging evidence suggests that shockwaves have potential for the treatment of osteoarthritis (OA).1-5 However, the chondroprotective effect of shockwaves on chondrocytes and the underlying mechanism are still under investigation. 2 Shockwaves have been demonstrated to enhance the production of reactive oxygen species (ROS) in tissues and cells, including mesenchymal stem cells and adipose-derived stem cells.6-8 A low level of ROS can act as an intracellular second messenger to regulate various crucial cellular activities and mediate the expressions of numerous genes; however, an excess ROS can cause cellular damage and apoptosis. Therefore, ROS have been implicated in the initiation of numerous diseases, including OA.9-12 In a normal situation, oxidative stress is counterbalanced by a delicate antioxidant system that ensures the proper response to oxidants in cells. 13 The balance between ROS and intracellular antioxidants is crucial for maintaining cartilage homeostasis and ensuring cell survival. 12
The transcription factor nuclear factor erythroid 2-related factor 2 (Nrf2) controls several antioxidant pathways and is an important regulator of cellular redox homeostasis. 14 Nrf2 regulates the expressions of gene encoding antioxidants, such as superoxide dismutase 2 and NAD(P)H quinone dehydrogenase 1 (Nqo-1) as well as stress response genes (e.g., heme oxygenase 1 [Ho-1]), antioxidant response elements (AREs), which are types of consensus cis-elements. Recent studies have highlighted the pleiotropic roles of Nrf2 in preventing OA pathology, including as a regulator of chondrocyte apoptosis, inflammatory response, and cellular homeostasis as well as a mechanosensitive transcription factor in endothelial cells and bones.14-18
Studies have revealed that the mild oxidative stress induced by pharmacologic agents can lead to the induction of the Nrf2/ARE pathway and thus exert a potent chondroprotective effect. 16 On the basis of these reports, we hypothesized that the chondroprotective effect of shockwaves can be attributed to the Nrf2/AREs corresponding to shockwave-induced ROS signals. Therefore, we investigated the biological effect of shockwaves on chondrocytes and investigated the mechanism through which chondrocytes sense and respond to the mechanical stimulation of shockwaves.
Methods
Isolation and Culture of Chondrocytes
This study was approved by the Institutional Animal Care and Use Committee of Kaohsiung Medical University. The articular chondrocytes used for primary culture were harvested from slices of the distal femur cartilage from 8-week-old pigs (KHAPS Black Pig). Chondrocytes were isolated from cartilage tissue by heating a 0.1% collagenase P solution digestion to 37 °C overnight. After collagenase digestion, the cells were passed through a nylon cell strainer (100-µm porosity) to filter out nondigested cartilage. Then, the solution was rinsed and centrifuged; after discarding the supernatant, the chondrocytes were resuspended in Dulbecco’s modified Eagle’s medium (DMEM, high glucose) supplemented with 10% (v/v) fetal calf serum (FCS), 2 mM l-glutamine, and 1% antibiotics. Then, this mixture was seeded into a culture dish and maintained at 37 °C in humidified 5% CO2 as a monolayer culture. Additionally, cells were cultured as a 3-dimensional (3D) pellet using a method described previously 19 with some modification. Briefly, the pellets were prepared by adding 5 × 105 cells to DMEM-F12 medium containing 10% FBS, and the mixture was transferred to 15-mL round-bottomed PE centrifuge tubes. Then, the cells were pelleted through centrifugation at 400 × g for 5 minutes at room temperature. Finally, the cell pellets were cultured in the same centrifuge tubes, with loosened lids, at 37 °C in 5% humidified CO2.
Shockwave Treatment
Shockwave treatment was performed using an electrohydraulic device (Duolith SD1; Storz Medical AG, Trägerwilen, Switzerland). The cell pellets were cultured for 5 days before the shockwave was applied to allow the matrix to deposit around the chondrocytes after pellet formation. The pellets were placed in a 15-mL centrifuge tube, which was completely filled with growth medium to prevent air from interfering with impulse transmission. Then, the pellets were exposed to focused-model shockwave applications in a custom-made acrylic thermostatic container with a membrane connecting the shockwave applicator ( Fig. 1B ). The acrylic container was filled with degassed water to avoid cavitation. Shockwaves were focused on the bottom of the centrifuge tube, so that their focal working zone should theoretically impact the chondrocyte pellets. The energy-flux density (EFD) and impulses of shockwaves were chosen based on the result of previous clinical trials and our pilot study. 5
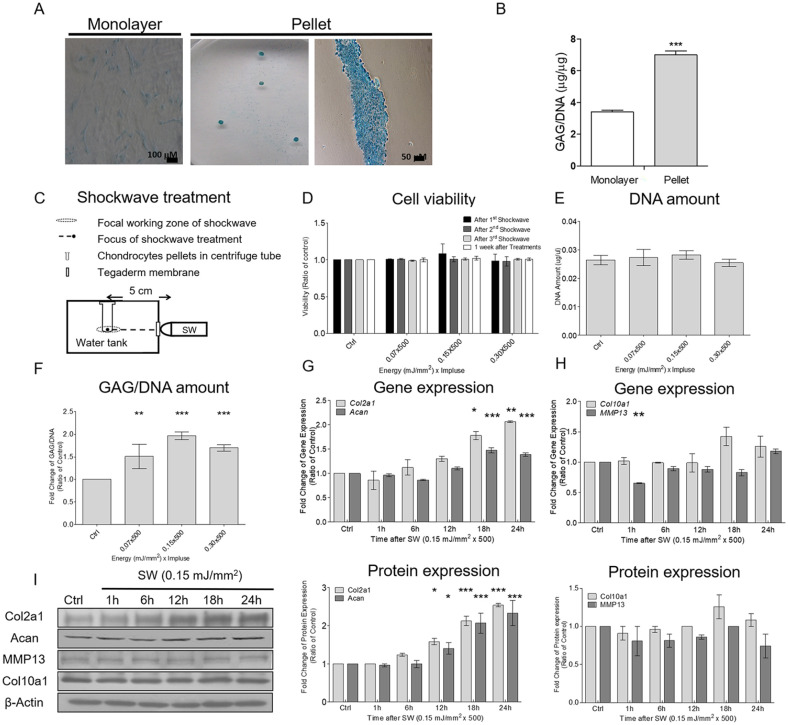
Figure 1.
Shockwaves enhanced extracellular matrix (ECM) production in chondrocytes without affecting cell viability or proliferation. (A) Example of chondrogenic staining with uptake of Alcian blue staining in chondrocytes cultured in a monolayer culture (left panel) and a pellet culture (middle panel); histological sectioning of a pellet cultured (right panel). (B) Comparison of glycosaminoglycan (GAG) content between monolayer and pellet cultures by using DMMB (1,9-dimethylmethylene blue) assays. Data are represented means ± standard deviation (n = 3); ***P < 0.001 compared with the monolayer culture group. (C) Schematic of the shockwave treatment setup. First, cell pellets were cultured for 5 days; then, shockwave treatment was applied every day for 5 days, and the pellets were cultured for an additional 7 days after shockwave application. (D) Cell viability was investigated at different times by using Alamar blue assays. (E) Total DNA content of cell pellets was measured using Hoechst DNA assays 7 days after the final shockwave treatment. (F) Cell pellets and culture supernatants were collected for the measurement of GAG content. The values of GAG content were normalized by the total DNA content of each sample. (G and H) Time-series comparison of the gene expressions of Col2a1, Acan, MMP13, and Col10a1 after a single course of shockwaves (500 impulses at 0.15 mJ/mm2). The threshold cycles (Ct) for each gene were normalized by the value for the GAPDH gene (ΔCt), and the experimental samples were referred to their controls (samples without shockwave treatment) (ΔΔCt). Fold change values were expressed as 2−ΔΔCt. (I) Time-series comparison of the protein expressions of Col2a1, Acan, MMP13, and Col10a1 after a single course of shockwaves (500 impulses at 0.15 mJ/mm2). Each bar represents the mean ± standard deviation, *P < 0.05; **P < 0.01; ***P < 0.001; Ctrl = control group; SW = shockwave treatment group.
alamarBlue Assay for Cell Viability Assessment
For cell viability assessments, chondrocyte pellets were placed in 10% alamarBlue (Bio-Rad AbD Serotec Ltd., Kidlington, UK) with 90% growth medium. After 24 hours, the mixture was collected and analyzed using Tecan GENios with the Magellan 5 software (Tecan, Männedorf, Switzerland) to obtain absorbance readings at 570 and 600 nm for viability analyses. The percentage reduction of alamarBlue, which indicated proliferative activity, was determined from the absorbance readings using the manufacturer’s protocols (Bio-Rad AbD Serotec Ltd.).
Hoechst DNA Assay for DNA Content Assessment and Total Glycosaminoglycan (GAG) Quantification
Total DNA within the pellets was assessed using the Hoechst assay. Cell pellets were digested with proteinase K for 16 hours at 65 °C. Then, 10 μL of the digested sample was combined with 200 μL of Hoechst dye solution (0.7 μg/mL). DNA concentration was assessed against a standard curve of calf thymus DNA after preparation in an assay solution (2 M NaCl, 50 mM sodium monobasic phosphate, pH 7.4). Fluorescence measurements were obtained at an excitation wavelength of 340 nm and an emission wavelength of 465 nm.
Total sulfated GAG content in the pellets and culture supernatant was determined using DMMB (Polysciences, Warrington, PA, USA). Chondroitin sulfate C from shark cartilage was used as a standard for comparison. Briefly, cell pellets were digested with proteinase K solution for 16 hours at 65 °C. The digested sample (100 μL) was then combined with 1 mL of 1,9-dimethylmethylene blue (DMMB) dye solution, and absorbance was immediately measured at 656 nm. GAG content was normalized to the amount of DNA measured per sample and expressed in terms of micrograms of GAG per microgram of DNA.
RNA Isolation and Real-Time Polymerase Chain Reaction (PCR)
Total RNA was extracted from chondrocytes using TRIzol reagent (Thermo Fisher Scientific, Waltham, MA, USA). Then, the extracted total RNA (2 μg) was reverse-transcribed into cDNA using the Maxima First Strand cDNA Synthesis Kit (Thermo Fisher Scientific) according to the manufacturer’s instructions. The solution was incubated at 65 °C for 5 minutes; then, it was mixed with first-strand buffer and dithiothreitol (DTT) for a final volume of 20 μL. This solution was incubated at 42 °C for 60 minutes and then at 70 °C for 15 minutes to prevent reverse transcriptase activity. Real-time PCR was conducted using SYBR Green PCR Master Mix and was processed on a LightCycler PCR and detection system (Roche Diagnostics, Basel, Switzerland). The primers for collagen type II alpha 1 chain (Col2a1), aggrecan (Acan), collagen type X alpha 1 chain (Col10a1), matrix metallopeptidase 13 (MMP13), Ho-1, Nqo-1, and glyceraldehyde 3-phosphate dehydrogenase (GAPDH) are listed in Table 1 . The cycling parameters were as follows: 95 °C for 15 minutes to activate DNA polymerase, followed by 40 cycles of 95 °C for 15 seconds and 60 °C for 1 minute. Melting curves were generated at the end of the reaction. Threshold cycles (Ct) for each tested gene were normalized to the GAPDH gene value (ΔCt), and the experimental samples were referred to their controls (samples without shockwave treatment) (ΔΔCt). Fold change values were expressed as 2−ΔΔCt.
Table 1.
Primer Sequences Used for Real-Time Polymerase Chain Reaction.
| Genes | Forward | Reverse |
|---|---|---|
| Col2a1 | ACTCCTGGCACGGATGGTC | CTTTCTCACCAACATCGCCC |
| Acan | CCCAACCAGCCTGACAACTT | CCTTCTCGTGCCAGATCATCA |
| Col10a1 | TGAACTTGGTTCATGGAGTGTTTTA | TGCCTTGGTGTTGGATGGT |
| MMP13 | ACCCAGGAGCCCTCATGTTTCC | CAGGGTTTCTCCTCGGAGACTG |
| Ho-1 | GGTCCTCGAAGAAGCCAAGACC | GACCGTTGCCACCAGAAAGCTG |
| Nqo-1 | CTGCCATGTATGACAAGGGACC | AGTGTGCCCAATGCTGTACGTC |
| GAPDH | TCACGACCATGGAGAAGGCT | CAGGAGGCATTGCTGATGATC |
Col2a1 = type 2 collagen; Acan = Aggrecan; Col10a1 = type 10 collagen; MMP13 = matrix metallopeptidase 13; Ho-1 = hemeoxygenase-1; Nqo-1 = NAD(P)H:quinone oxidoreductase; GAPDH = glyceraldehyde3-phosphate dehydrogenase.
Western Blot Analysis
The pellets were washed 3 times with cold phosphate-buffered saline (PBS) and cell lysates were extracted on ice. The total protein concentrations of various lysates were determined according to the Bradford Protein Assays (Bio-Rad) using bovine serum albumin (BSA) as a standard. Cell lysates (40 μg of protein for each) or nuclear extracts were prepared from vehicle- or shockwave-treated cells. To perform Western blotting, an analytical 10% gel was first prepared for sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE). After completing SDS-PAGE, proteins were transferred to a polyvinylidene difluoride membrane using a semidry apparatus. We employed the following primary antibodies: porcine Col2a1; Acan; Nrf2; p38; phospho-p38; β-actin (Proteintech, Manchester, UK); phospho-JNK; JNK1/2/3 (MyBioSource.com); ERK1/2; ERK2 and phospho-ERK 1/2 (Cell Signaling Technology, Danvers, MA, USA). Immunoblot analysis was conducted with mouse or rabbit immunoglobulin G (IgG) antibody coupled with horseradish peroxidase, and results were recorded using an enhanced chemiluminescence kit. We used β-actin expression as an internal control. Immunoblot density was determined using an image analysis system that was installed with the BIO-ID software (Vilber Lourmat, Marne-la-Vallée, France).
Detection of ROS Production
ROS production was detected as described previously, with slight modifications. 20 Chondrocytes were incubated with 20 μM dichlorofluorescein diacetate (DCFH-DA) (Sigma-Aldrich Co.) in DMEM for 30 minutes at 37 °C in the dark. DCFH, which is nonfluorescent, is oxidized by intracellular ROS to highly fluorescent dichlorofluorescein (DCF). Cells were trypsinized and washed with ice-cold PBS to quantify the fluorescence emitted from the fluorescent DCF using a fluorescence-activated cell sorter (FACScan, BD Biosciences), which was in turn used to quantify the ROS. Diphenyleneiodonium (DPI) (a nicotinamide adenine dinucleotide phosphate [NADPH] oxidase inhibitor), allopurinol (a xanthine oxidase [XO] inhibitor), and rotenone (a mitochondria complex I inhibitor) (Sigma-Aldrich Co.) were used to identify the sources of shockwave-induced ROS. The chondrocyte pellets were pretreated with the indicated agents 30 minutes before the single course of shockwaves (500 impulses at 0.15 mJ/mm2), and ROS production was detected 10 minutes after shockwave treatment.
Cytosolic and Nuclear Extract Preparation
Dished chondrocytes were washed twice with PBS and scraped in 500 μL of PBS. The cells were collected through centrifugation at 12,000 rpm for 10 seconds, resuspended in 400 μL of buffer A [10 mM 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES; pH 7.9), 1.5 mM MgCl2, and 10 mM KCl], and then stored on ice for 10 minutes. Nuclei were precipitated through centrifugation at 12,000 rpm for 10 seconds. The supernatants were extracted from the cytosol and harvested. Then, the pellets were resuspended in 100 μL of buffer C (20 mM HEPES [pH 7.9], 1.5 mM MgCl2, 0.2 mM ethylenediaminetetraacetic acid [EDTA], 420 mM NaCl, and 25% [vol/vol] glycerol) and stored on ice for 20 minutes. The suspension was centrifuged at 12,000 rpm for 2 minutes, and supernatants were collected and stored at −70 °C until use. Buffer A and buffer C contained 0.5 mM DTT, 2 μg/mL leupeptin, 1 mM orthovanadate, 2 μg/mL pepstatin A, and 0.5 mM phenylmethylsulfonyl fluoride.
Immunofluorescence Analysis
Chondrocytes cultured on coverslips or pellet tissues were fixed with 4% paraformaldehyde for 30 minutes and then permeabilized with 1% Triton X-100 for 1 minute. The samples were incubated with 1% BSA for 20 minutes and then with antibodies against Nrf2 overnight at 4 °C. The samples were washed thrice in PBS and then incubated with FITC 488 goat antirabbit IgG or TRITC 543 goat anti-rabbit IgG for 1 hour at room temperature. Then, the samples were washed and mounted using Fluoroshield with 4′,6-diamidino-2-phenylindole (Sigma-Aldrich) and visualized using an Olympus IX81 (Waltham, MA, USA) inverted fluorescence microscope.
Stable Nrf2-Knockdown Chondrocyte Transfectants
To establish stable Nrf2-knockdown cells, the small hairpin RNA (shRNA) of Nrf2 (pLKO.1-Nrf2-shRNA, which targets the Nrf2 gene sequence 5′-GCTCGGATCTACCTTCCAGTA-3′) and a luciferase control (pLKO.1-shluc) plasmid construct were obtained from the National RNAi Core Facility of the Institute of Molecular Biology/Genomic Research Center, Academia Sinica, Taipei, Taiwan. Then, 8 × 106 293T cells were incubated on 10-cm Petri dishes coated with poly-l-lysine for 24 hours to package the lentiviral vectors. TransIT-LT1 reagent in 750 μL of Opti-MEM was mixed with 6.75 μg of the packaging vector pCMV-ΔR8.91, 0.75 μg of the envelope vector pMD.G, and 7.5 μg of the transfer vector pLKO.1-Nrf2-shRNA or pLKO.1-shluc. After incubation at room temperature for 20 minutes, the plasmid-containing mixture was transferred to the 293T cells. Then, 2 days after transfection, the supernatant of the culture cells was harvested. This medium, which contained the lentiviral vectors, was centrifuged at 2500 rpm for 10 minutes, and the viral supernatants were passed through 0.45-μm filters. Primary chondrocytes (P2) were cultured on 6-cm Petri dishes with 4 mL of medium and then infected with 1 mL of the harvested lentiviral supernatants and 8 μg/mL Polybrene for 24 hours. The infected cells were incubated in a medium containing 2 μg/mL puromycin for the selection of stable Nrf2-knockdown chondrocyte transfectants.
Statistics
All analyses were performed using Statistical Package for Social Sciences (Version 19.0, SPSS Inc., Chicago, IL). Significant differences between the shockwave treatment and control groups were determined using 1-way ANOVA, followed by post hoc analysis using Bonferroni’s test. Representative results were obtained from at least three independent experiments. All values were displayed as the mean ± standard deviation of three determinations (*P < 0.05; **P < 0.01; ***P < 0.001 compared with the vehicle).
Results
Extracellular Matrix (ECM) Synthesis of Chondrocytes Increased After Shockwave Application
The expansion of isolated chondrocytes in vitro through monolayer passaging is known to cause the loss of the chondrogenic phenotype and lead to cell dedifferentiation. To mimic the nature the cartilage environment, we used the 3D pellet cultures to perform experiments. Figure 1A and B compare the ECM depositions of chondrocytes between the monolayer and pellet cultures by using Alcian blue staining and measuring the accumulation of GAG after 5 days. The Alcian blue staining analysis also revealed deep blue staining in the pellet culture. These results were consistent with those of previous studies.21,22After 5 days of culture, shockwaves (500 impulses at EFDs of 0.07, 0.15, or 0.3 mJ/mm2) were applied to the chondrocyte pellets every day for 5 days. Cell viability was measured using the alamarBlue test after the first, second, and third shockwave applications and 7 days after the final application. All chondrocyte pellets remained at >95% viability relative to untreated controls throughout the experimental period ( Fig. 1D ).
GAG synthesis and total DNA were quantified 7 days after the final shockwave treatment. No significant difference was noted in the total DNA per pellet between the control and shockwave groups ( Fig. 1E ). Cell pellets and culture supernatants were collected for the measurement of GAG content. The total GAG/DNA was significantly higher in the shockwave groups than in the control group ( Fig. 1F ). The EFD of 0.15 mJ/mm2 was adopted for the following studies because it was associated with the highest GAG production.
The gene expressions of Col2a1 and Acan significantly increased after a single course of shockwaves (500 impulses at 0.15 mJ/mm2) and peaked after 24 hours ( Fig. 1G ). A time-series Western blot study revealed that the protein expressions of Col2a1 and Acan also significantly increased and peaked 24 hours after shockwave treatment ( Fig. 1I ). By contrast, the gene and protein expressions of Col10a1 and MMP13, markers of hypertrophic chondrocytes, did not significantly increase after shockwave treatment ( Fig. 1H and 1I ).
ROS Production Increased Transiently in Chondrocytes After Shockwave Treatment
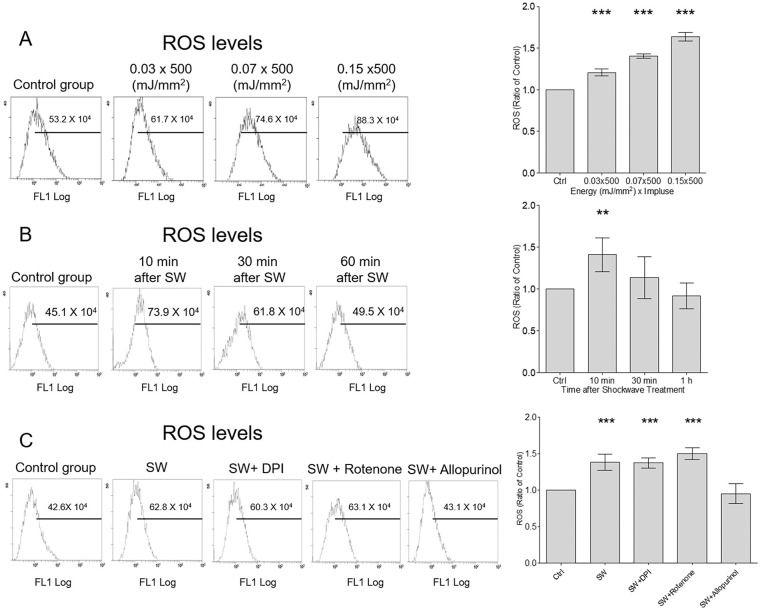
Shockwave treatment (500 impulses at EFDs of 0.03, 0.07, or 0.15 mJ/mm2) was applied to individual chondrocyte pellets; after 10 minutes, the maximum ROS generation was observed in the group with an EFD of 0.15 mJ/mm2 ( Fig. 2A ). ROS increased significantly 10 minutes after shock wave treatment and returned to the baseline after 30 minutes ( Fig. 2B ). To determine whether shockwave-induced ROS were produced by mitochondria, NADPH oxidase, or XO, we examined the effects of rotenone (an inhibitor of mitochondria complex I), DPI (an NADPH oxidase inhibitor), and allopurinol (an XO inhibitor) on shockwave-induced ROS production. Shockwave-induced ROS were significantly attenuated by allopurinol but not by the other inhibitors ( Fig. 2C ).
Figure 2.
Shockwave treatment induced transient reactive oxygen species (ROS) production in chondrocytes in a dose-dependent manner. (A) Chondrocytes were treated using shockwaves with 500 impulses at energy-flux density (EFD) values of 0, 0.03, 0.07, and 0.15 mJ/mm2 and a frequency of 4 Hz. ROS production in cells was detected 10 minutes after shockwave treatment. (B) Time-series of ROS production after a single course of shockwaves (500 impulses at 0.15 mJ/mm2). (C) Chondrocyte pellets were pretreated with diphenyleneiodonium (DPI; an NADPH oxidase inhibitor), rotenone (a mitochondrial complex I inhibitor), or allopurinol (a xanthine oxidase [XO] inhibitor) and then subjected to a single course of shockwaves (500 impulses at 0.15 mJ/mm2). The ROS level was measured 10 minutes after shockwave application. Each bar represents mean ± standard deviation, *P < 0.05; **P < 0.01; ***P < 0.001; Ctrl = control group; SW = shockwave treatment group).
Phosphorylation of p38 MAPKs and ERK1/2 and Nuclear Translocation of Nrf2 in Chondrocytes Increased After Shockwave Treatment
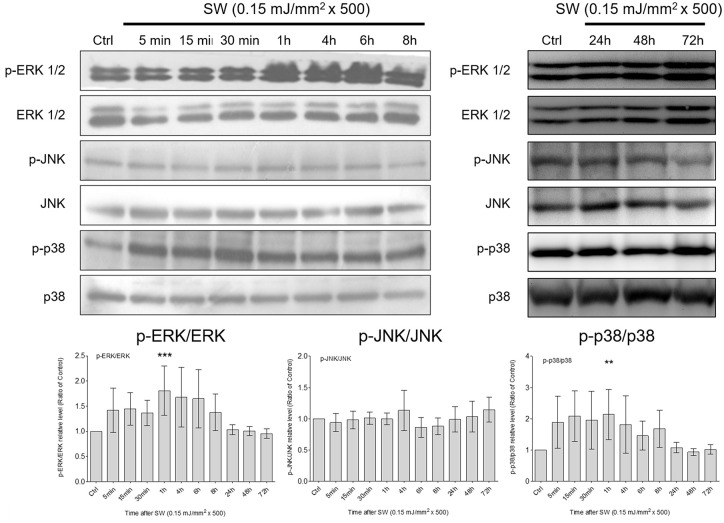
MAPK is a family of protein kinases that mediate signal transduction from extracellular stimulation to the nucleus. 23 Previous studies have suggested that shockwaves produce mechanical stimulation through pressure change, thus initiating a serial cellular function by triggering various intracellular signaling events that involve MAPK signaling.6,24 Therefore, we examined whether the shockwave-induced chondrogenic effect was induced through the activation of MAPKs by analyzing the phosphorylation levels of ERK, JNK, and p38. Phosphorylation of p38 MAPK and ERK1/2 significantly increased 1 hour after shockwave treatment ( Fig. 3 ). By contrast, no significant increase in JNK phosphorylation was observed after shockwave treatment.
Figure 3.
Shockwave treatment activated MAPK signaling in chondrocytes. Time-series comparison of the phosphorylation of MAPKs (ERK, p38, and JNK) aftera single course of shockwaves (500 impulses at 0.15 mJ/mm2). The ratio of phosphorylated MAPK to total MAPK was calculated through densitometry in each sample, with a value of 1 assigned to the control samples. Each bar represents mean ± standard deviation, *P < 0.05; **P < 0.01; ***P < 0.001; Ctrl = control group; SW = shockwave treatment group)
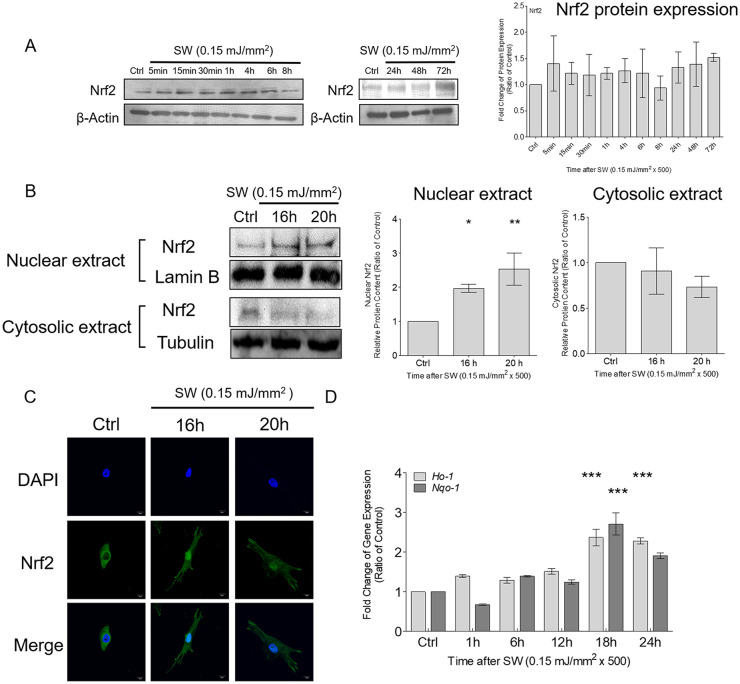
Because shockwaves yielded a transient low-level ROS signal, we examined the adaptive response of redox activity in chondrocytes after shockwave treatment. No significant increase in total Nrf2 was observed within 72 hours of shockwave treatment ( Fig. 4A ). Nuclear translocation of Nrf2 was measured using Western blotting and immunofluorescence. Nuclear and cytoplasmic fractions of chondrocytes were purified after shockwave treatment. Then, tubulin and lamin B were used as loading controls and to distinguish nuclear and cytoplasmic expression, respectively. Nrf2 significantly accumulated in the nucleus after 16 hours and remained for up to 20 hours after shockwave treatment ( Fig. 4B ). Moreover, the immunofluorescence study confirmed the increased nuclear translocation of Nrf2 at 16 hours after shockwave treatment ( Fig. 4C ). Additionally, the downstream gene expression of Nrf2 effectors, including Ho-1 and Nqo-1, was significantly increased at 18 hours after shockwave treatment ( Fig. 4D ).
Figure 4.
Shockwave treatment increased the nuclear translocation of Nrf2 and the expression of Nrf2-dependent genes in chondrocytes. Chondrocyte pellets were treated with a single course of shockwaves (500 impulses at 0.15 mJ/mm2). (A) Times-series of the protein expression of Nrf2. (B) Nuclear translocation of Nrf2 was measured by using Western blotting. Tubulin and lamin B were used as loading controls and to specify nuclear and cytoplasmic expression, respectively. (C) The porcine articular chondrocytes were pelleted first and treated with a single course of shockwaves (500 impulses at 0.15 mJ/mm2), following which the pellets were trypsinized into separated cells and cultured in the 12-well dish for additional 16 and 20 hours. Then the immunofluorescence experiments were subsequently performed. Nuclear translocation of Nrf2 was investigated through immunofluorescence by using a specific antibody against Nrf2 and 4′,6-diamidino-2-phenylindolestaining for nuclei. (D) Time-series of the gene expressions of Ho-1 and Nqo-1after shockwave treatment. Each bar represents mean ± standard deviation, *P < 0.05; **P < 0.01; ***P < 0.001; Ctrl = control group; SW = shockwave treatment group.
Reducing ROS, MAPK Signaling, or the Redox Activity of Nrf2 Suppressed Shockwave-Induced ECM Synthesis
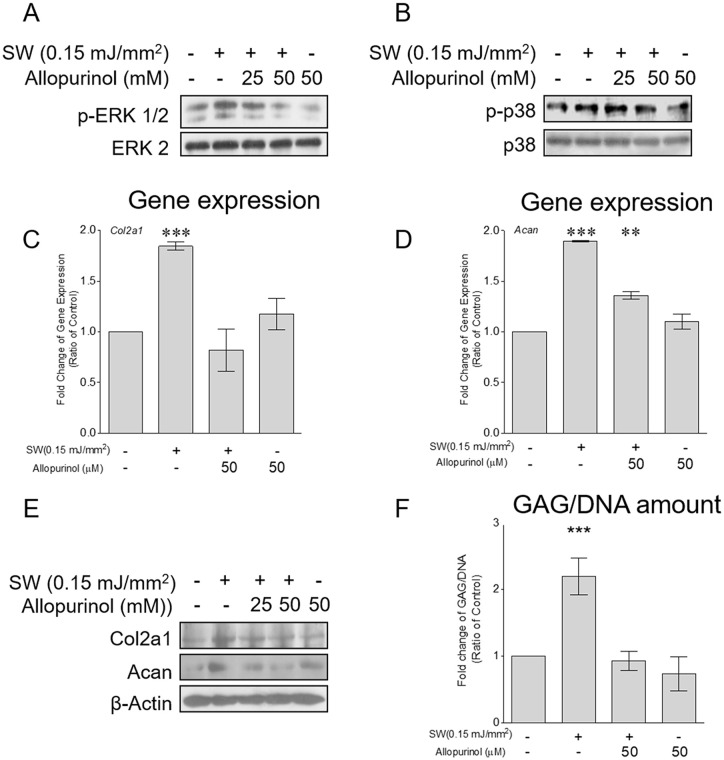
Studies have revealed that MAPK signaling could be activated by ROS in various cell types.16,23 To test whether the shockwave-induced phosphorylation of ERK1/2 and p38 depended on ROS production, chondrocyte pellets were pretreated with allopurinol before shockwave treatment. Shockwave-activated phosphorylation of ERK1/2 and p38 ( Fig. 5A and B ), the gene and protein expressions of Col2a1 and Acan ( Fig. 5C-E ), and GAG production ( Fig. 5F ) were diminished after ROS production was inhibited by allopurinol.
Figure 5.
Shockwave-induced reactive oxygen species (ROS) production activated mitogen-activated protein kinase (MAPK) signaling and extracellular matrix (ECM) synthesis in chondrocytes. Chondrocyte pellets were pretreated with allopurinol (25 or 50 µM) for 1 hour and then subjected to a single course of shockwaves (500 impulses at 0.15 mJ/mm2). (A and B) Phosphorylation of ERK1/2 and p38 measured 1 hour after shockwave application. (C and D) Gene expressions of Col2a1 and Acan measured 24 hours after shockwave application. (E) Protein expressions of Col2a1 and Acan measured 24 hours after shockwave application. (F) Cell pellets were first cultured for 5 days; then, shockwave treatment (500 impulses at 0.15 mJ/mm2) was applied every day for 5 days, and the cell pellets were cultured for an additional 7 days. The cell pellets were pretreated with allopurinol (50 µM) for 1 hour before each shockwave treatment. The cell pellets and the culture supernatants were collected for the measurement of GAG content. The GAG values were normalized by the total DNA content of each sample. Each bar represents mean ± standard deviation, *P < 0.05; **P < 0.01; ***P < 0.001; Ctrl = control group; SW = shockwave treatment group.
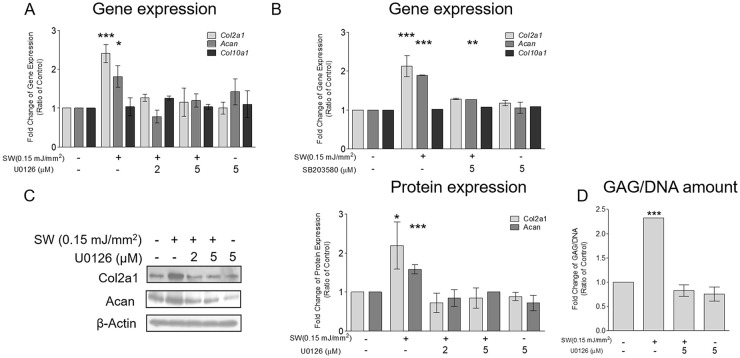
Pretreatment with U0126 and SB203580, the specific inhibitors of MEK1/2 and p38, respectively, suppressed the shockwave-induced gene expressions of Col2a1 and Acan ( Fig. 6A and B ). Moreover, the shockwave-induced protein expressions of Col2a1, Acan, and GAG synthesis decreased after pretreatment with U0126 ( Fig. 6C and D ). Shockwave treatment had no effect on the gene expression of Col10a1 with or without these inhibitors. Taken together, these results indicated that shockwave-induced transient ROS production was mainly from XO. Moreover, it was crucial for the subsequent signal transduction of MAPK and increased the production of ECM in chondrocytes.
Figure 6.
Shockwave treatment upregulated Nrf2 activity through reactive oxygen species (ROS) and mitogen-activated protein kinase (MAPK) signaling. Chondrocyte pellets were pretreated with U0126 (2 or 5 μM) or SB203580 (5 μM) for 1 hour and then subjected to a single course of shockwaves (500 impulses at 0.15 mJ/mm2). (A and B) Gene expressions of Col2a1, Acan, and Col10a1 measured 24 hours after shockwave application. (C) Protein expressions of Col2a1 and Acan measured 24 hours after shockwave application. (D) First, chondrocyte pellets were cultured for 5 days; then, shockwave treatment (500 impulses at 0.15 mJ/mm2) was applied every day for 5 days, and the pellets were cultured for an additional 7 days. The pellets were pretreated with U0126 (5 µM) for 1 hour before each shockwave treatment. The cell pellets and the culture supernatants were collected for the measurement of glycosaminoglycan (GAG) content. GAG values were normalized by the total DNA content of each sample. Each bar represents mean ± standard deviation, *P < 0.05; **P < 0.01; ***P < 0.001; Ctrl = control group; SW = shockwave treatment group.
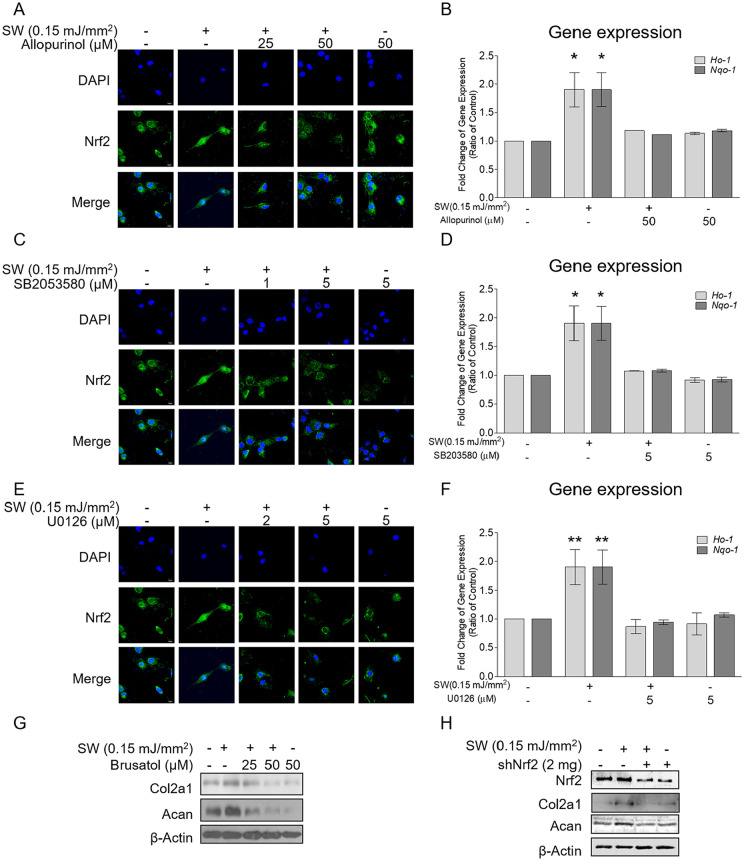
Pretreatment with allopurinol mitigated the shockwave-induced nuclear translocation of Nrf2 and the gene expressions of Ho-1 and Nqo-1 in chondrocytes ( Fig. 7A and B ). Furthermore, the immunofluorescence study revealed that U0126and SB203580 reduced the shockwave-induced nuclear translocation of Nrf2 ( Fig. 7C and E ). The shockwave-enhanced gene expressions of Ho-1 and Nqo-1 returned to the baseline after MAPK signaling was blocked with either U0126 or SB203580 ( Fig. 7D and F ). These results suggested that ROS and MAPK signaling were critical for the shockwave-induced redox activity of Nrf2.
Figure 7.
Shockwave-induced reactive oxygen species (ROS) and mitogen-activated protein kinase (MAPK) signaling affected the nuclear translocation of Nrf2 and the gene expressions of Nrf2-dependent antioxidants and chondrogenics. Chondrocyte pellets were pretreated with allopurinol (25 or 50 μM), SB203580 (1 or 5 μM), or U0126 (2 or 5 μM) for 1 hour and then subjected to a single course of shockwaves (500 impulses at 0.15 mJ/mm2). (A, C, and E) Immunofluorescence results for nuclear translocation of Nrf2 16 hours after shockwave application. (B, D, and F) Gene expressions of Ho-1 and Nqo-1 measured 18 hours after shockwave application. (G) Chondrocyte pellets were pretreated with brusatol (25 or 50 μM) for 1 hour and then subjected to a single course of shockwaves (500 impulses at 0.15 mJ/mm2). The protein expressions of Col2a1 and Acan were measured 24 hours after shockwave application. (H) Nrf2 was knocked down by using an Nrf2-specific shNrf2-expressing lentiviral plasmid. The protein expressions of Nrf2, Col2a1, and Acan were measured 24 hours after a single course of shockwaves (500 impulses at 0.15 mJ/mm2). Each bar represents mean ± standard deviation, *P < 0.05; **P < 0.01; ***P < 0.001; Ctrl = control group; SW = shockwave treatment group).
To further substantiate the role of Nrf2 in shockwave-induced ECM synthesis, we reduced Nrf2 activity using brusatol and knocked down Nrf2 using an Nrf2-specific shNrf2-expressing lentiviral plasmid. 25 The shockwave-induced protein expressions of Col2a1 and Acan were reduced following pretreatment with brusatol ( Fig. 7G ). Furthermore, Nrf2 knockdown in porcine chondrocytes had no significant effect on the protein production of Col2a1 and Acan after shockwave treatment ( Fig. 7H ).
Discussion
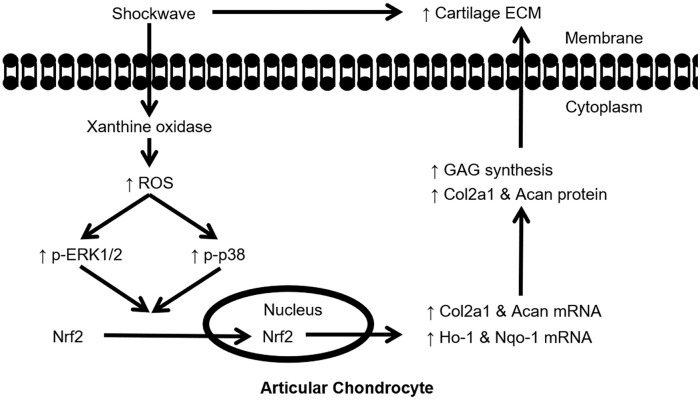
Recent studies have demonstrated the potential of shockwaves for treating OA; however, the efficacy and mechanism have not been fully elucidated.1,5,26 The present study aimed to fill this knowledge gap by exploring the function and mechanism of shockwaves in chondrocytes. By using a 3D culture model, we observed that shockwaves stimulated the transient production of ROS, which activated the MAPK/Nrf2 signaling pathway and led to increased ECM synthesis in chondrocytes ( Fig. 8 ). The present results supported the clinical application of shockwaves for the treatment of OA, with an optimal EFD of 0.15 mJ/mm2.
Figure 8.
Schematic of the proposed mechanism for the shockwave-induced increase in extracellular matrix (ECM) synthesis in chondrocytes. Shockwaves induced reactive oxygen species (ROS) production mainly through xanthine oxidase (XO) in porcine articular chondrocytes. Transient ROS signaling activated the phosphorylation of ERK1/2 and p38, which increased the nuclear translocation of Nrf2 and the downstream gene expressions of Ho-1 and Nqo-1. Activation of Nrf2 function increased ECM production in porcine articular chondrocytes.
Some studies have indicated that shockwaves are potentially cytotoxic, and that the level of cytotoxicity depends on the EFD and number of impulses; an EFD of >0.06 mJ/mm2 or an excessive number of impulses might endanger chondrocyte viability.1,27,28 However, these results were obtained using 2D chondrocyte culture models. In animal studies, limited gross pathological changes were observed in joints after shockwave treatment with EFD and impulse number within the commonly used ranges for musculoskeletal diseases.29,30 Clinical trials have indicated that most patients can tolerate shockwaves for therapeutic purposes for up to 4000 impulses at an EFD of <0.4 mJ/mm2 without serious side effects.3,4,26 We confirmed the safety of shockwave treatment for chondrocytes cultured in a 3D model at certain energy levels (500 impulses at 0.3 mJ/mm2). Taken together, these results supported the safety of the clinical use of shockwaves for OA treatment.
ECM is composed of water, collagen, and Acan, and it is secreted and maintained by chondrocytes. When exposed to mechanical stress, reduced ECM content not only predisposes the cartilage to strain but also induces a series of downstream activities, including ECM remodeling and MMP13 induction.31-33 Our resultsrevealedthat shockwave treatment at specific EFD values enhanced ECM synthesis, possibly preventing further degeneration and improving cartilage health. These findings are in agreement with those of previous rat studies, which have demonstrated that shockwave treatment improved safranin-O staining in the proximal medial tibia of OA knees.34-36 Wang et al.34,35,37,38 proposed that shockwaves elicited these chondroprotective effects through subchondral bone turnover. By contrast, we found that chondrocytes independently sensed the physical signals of shockwaves and then shifted the homeostatic balance toward ECM synthesis. These findings indicated that shockwave treatment should be focused on the proper working zone. For example, to increased ECM synthesis in chondrocyte, shockwaves should be focused on the articular cartilage.
ROS may play a dual role in the regulation of cartilage homeostasis; low physiological levels of ROS might serve as an important cellular messenger to regulate physiological processes and maintain cartilage health; however, excessive ROS may inhibit chondrocyte proliferation and modulate the initiation of hypertrophic changes.8-12,39,40 We found that shockwave-induced ROS production enhanced the ECM synthesis in chondrocytes without affecting cell viability or inducing hypertrophic differentiation. Moreover, shockwave-induced ROS production was transient; this contrasted with the pharmacologic induction of oxidative stress in experimental settings.41,42 The early detection of ROS elevation (usually within 30 minutes after shockwave treatment) was consistent with previous findings according to which ROS is a crucial upstream signal initiating shockwave-induced biological responses.6-8 Wang et al. 8 reported that NADPH oxidase was the main source of shockwave-induced ROS production in osteoblasts. However, we found that XO was mainly responsible for shockwave-induced ROS production in chondrocytes. Moreover, studies have revealed increased XO activity in the synovial membranes of individuals with acute joint injury and metabolic arthritis, but little is known regarding the role of XO activation in chondrocytes.43-45 By contrast, ROS induced by low-concentration XO or another exogenous oxidants frequently stimulated the proteoglycan synthesis of chondrocytes, but a higher XO concentration inhibited these effects.39,46,47 Moreover, H2O2 generated by XO is freely diffusible and important for intra- and intercellular signal transduction.45,48 Additional studies are required to identify whether H2O2 is the key mediator that transfers the mechanical signal of shockwaves from the focal working zone to the surrounding area through the autocrine and paracrine effects in the articular cartilage.
Nrf2—a master regulator of antioxidant responses—is emerging as a therapeutic target for OA.14-16,49 Under normal physiological conditions, Nrf2 is stabilized and bound by Kelch-like ECH-associated protein 1 (Keap1) in the cytoplasm. 13 However, oxidative stress can disrupt the Nrf2-Keap1 complex, leading to the nuclear translocation of Nrf2, which subsequently activates the expressions of genes encoding various antioxidant enzymes. 13 Recent studies have demonstrated that the pharmacological activation or genetic overexpression of Nrf2 and Nrf2-dependent genes can reduce inflammation, matrix degradation, and the oxidative stress caused by interleukin-1β.15,16,50 The present study revealed that the nonpharmacologic activation of Nrf2, Ho-1, and Nqo-1 by shockwaves increased ECM synthesis in chondrocytes. Furthermore, both upregulation and downregulation of Nrf2 yielded abnormal chondrogenesis; an appropriate Nrf2 level is necessary for cartilage homeostasis.51,52 The expressions of Nrf2 and Nrf2-dependent genes, such as Ho-1 and Nqo-1, was significantly higher in OA joints and the damaged zones of human cartilage than in normal cartilage. 15 Nrf2 and Ho-1 expressions in the OA cartilage of patients with type 2 diabetes mellitus (T2DM) were lower than in controls without T2DM. 53 The impairment of the Nrf2-related antioxidant system may increase the inflammatory reaction of the OA cartilage in patients with T2DM. Whether the level of Nrf2/ARE activity before shockwave treatment affects clinical outcomes requires further research; candidate patients should be carefully selected to improve the outcomes of shockwave treatment.
Some limitations of this study should be noted. First, we used healthy porcine chondrocytes rather than human OA chondrocytes to investigate the signal transduction pathway. In the most common clinical scenario, shockwave treatment would be administered in patients with early-stage OA; thus, some chondrocytes may remain sufficiently healthy to respond to the shockwave stimulation. Further research is required to determine whether shockwaves can activate Nrf2/ARE in these relatively healthy chondrocytes and thus improve the homeostasis of the whole articular cartilage. Second, the upstream signaling cascades of shockwave-induced ROS production require further investigation. The present identification of the phosphorylation of MAPK as responsible for shockwave-induced ROS signaling in various cell types is consistent with the literature.6-8,42 We found that the shockwave activated the phosphorylation of ERK and p38 MAPK in chondrocytes through ROS production. Interestingly, other studies have reported that shockwave treatment activated ERK and p38 signaling through cellular ATP release and P2 receptor stimulation in osteoblasts, mesenchymal stem cells, and T cells.24,54,55 The connection between shockwave-induced ROS production and cellular ATP release in signaling cascades requires further investigation.
In conclusion, the present results revealed that shockwaves increase ECM synthesis in chondrocytes without affecting cell viability or proliferation. We claim that the shockwave-induced anabolic response in chondrocytes is mediated through ROS signaling and the activation of Nrf2/ARE pathways. Shockwaves transiently increased ROS production mainly from XO, subsequently enhancing the phosphorylation of ERK1/2 and p38. The activation of MAPK signaling led to the nuclear translocation of Nrf2, the enhanced downstream gene expression of Ho-1 and Nqo-1, and finally increased ECM synthesis in chondrocytes.
Supplemental Material
Supplemental material, sj-pptx-1-car-10.1177_19476035211012465 for Shockwave Treatment Enhanced Extracellular Matrix Production in Articular Chondrocytes Through Activation of the ROS/MAPK/Nrf2 Signaling Pathway by Po-Chih Shen, Shih-Hsiang Chou, Cheng-Chang Lu, Hsuan-Ti Huang, Song-Hsiung Chien, Peng-Ju Huang, Zi-Miao Liu, Chia-Lung Shih, Shu-Jem Su, Li-Min Chen and Yin-Chun Tien in CARTILAGE
Footnotes
Acknowledgements and Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by research grant from the Ministry of Science and Technology (104-2314-B-037-038-MY3) and from Kaohsiung Medical University Hospital (KMUH104-4R41). This manuscript was edited by Wallace Academic Editing.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Ethical Approval: All experiments were approved by the Institutional Animal Care and Use Committee of Kaohsiung Medical University.
Animal Welfare: The present study followed international, national, and/or institutional guidelines for humane animal treatment and complied with relevant legislation.
ORCID iDs: Po-Chih Shen  https://orcid.org/0000-0001-5185-5584
https://orcid.org/0000-0001-5185-5584
Yin-Chun Tien  https://orcid.org/0000-0001-9069-6387
https://orcid.org/0000-0001-9069-6387
Supplementary material for this article is available on the Cartilage website at http://cart.sagepub.com/supplemental.
References
- 1. Ji Q, Wang P, He C. Extracorporeal shockwave therapy as a novel and potential treatment for degenerative cartilage and bone disease: osteoarthritis. A qualitative analysis of the literature. Prog Biophys Mol Biol. 2016;121(3):255-65. doi: 10.1016/j.pbiomolbio.2016.07.001 [DOI] [PubMed] [Google Scholar]
- 2. Ji Q, He C. Extracorporeal shockwave therapy promotes chondrogenesis in cartilage tissue engineering: a hypothesis based on previous evidence. Med Hypotheses. 2016;91:9-15. doi: 10.1016/j.mehy.2016.03.013 [DOI] [PubMed] [Google Scholar]
- 3. Zhao Z, Jing R, Shi Z, Zhao B, Ai Q, Xing G. Efficacy of extracorporeal shockwave therapy for knee osteoarthritis: a randomized controlled trial. J Surg Res. 2013;185(2_suppl):661-6. doi: 10.1016/j.jss.2013.07.004 [DOI] [PubMed] [Google Scholar]
- 4. Chen TW, Lin CW, Lee CL, Chen CH, Chen YJ, Lin TY, et al. The efficacy of shock wave therapy in patients with knee osteoarthritis and popliteal cyamella. Kaohsiung J Med Sci. 2014;30(7):362-70. doi: 10.1016/j.kjms.2014.03.006 [DOI] [PubMed] [Google Scholar]
- 5. Wang YC, Huang HT, Huang PJ, Liu ZM, Shih CL. Efficacy and safety of extracorporeal shockwave therapy for treatment of knee osteoarthritis: a systematic review and meta-analysis. Pain Med. 2020;21:822-35. doi: 10.1093/pm/pnz262 [DOI] [PubMed] [Google Scholar]
- 6. Catalano MG, Marano F, Rinella L, de Girolamo L, Bosco O, Fortunati N, et al. Extracorporeal shockwaves (ESWs) enhance the osteogenic medium-induced differentiation of adipose-derived stem cells into osteoblast-like cells. J Tissue Eng Regen Med. 2017;11(2_suppl):390-9. doi: 10.1002/term.1922 [DOI] [PubMed] [Google Scholar]
- 7. Wang FS, Wang CJ, Sheen-Chen SM, Kuo YR, Chen RF, Yang KD. Superoxide mediates shock wave induction of ERK-dependent osteogenic transcription factor (CBFA1) and mesenchymal cell differentiation toward osteoprogenitors. J Biol Chem. 2002;277(13):10931-7. doi: 10.1074/jbc.M104587200 [DOI] [PubMed] [Google Scholar]
- 8. Wang FS, Wang CJ, Chen YJ, Chang PR, Huang YT, Sun YC, et al. Ras induction of superoxide activates ERK-dependent angiogenic transcription factor HIF-1α and VEGF-A expression in shock wave-stimulated osteoblasts. J Biol Chem. 2004;279(11):10331-7. doi: 10.1074/jbc.M308013200 [DOI] [PubMed] [Google Scholar]
- 9. Henrotin Y, Kurz B, Aigner T. Oxygen and reactive oxygen species in cartilage degradation: friends or foes? Osteoarthritis Cartilage. 2005;13:643-54. doi: 10.1016/j.joca.2005.04.002 [DOI] [PubMed] [Google Scholar]
- 10. Henrotin Y, Deby-Dupont G, Deby C, De Bruyn M, Lamy M, Franchimont P. Production of active oxygen species by isolated human chondrocytes. Br J Rheumatol. 1993;32(7):562-7. [DOI] [PubMed] [Google Scholar]
- 11. Li J, Dong S. The signaling pathways involved in chondrocyte differentiation and hypertrophic differentiation. Stem Cells Int. 2016;2016:2470351. doi: 10.1155/2016/2470351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Lepetsos P, Papavassiliou AG. ROS/oxidative stress signaling in osteoarthritis. Biochim Biophys Acta. 2016;1862(4):576-91. doi: 10.1016/j.bbadis.2016.01.003 [DOI] [PubMed] [Google Scholar]
- 13. Ma Q. Role of nrf2 in oxidative stress and toxicity. Annu Rev Pharmacol Toxicol. 2013;53:401-26. doi: 10.1146/annurev-pharmtox-011112-140320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Marchev AS, Dimitrova PA, Burns AJ, Kostov RV, Dinkova-Kostova AT, Georgiev MI. Oxidative stress and chronic inflammation in osteoarthritis: can NRF2 counteract these partners in crime? Ann N Y Acad Sci. 2017;1401(1):114-35. doi: 10.1111/nyas.13407 [DOI] [PubMed] [Google Scholar]
- 15. Khan NM, Ahmad I, Haqqi TM. Nrf2/ARE pathway attenuates oxidative and apoptotic response in human osteoarthritis chondrocytes by activating ERK1/2/ELK1-P70S6K-P90RSK signaling axis. Free Radic Biol Med. 2018;116:159-71. doi: 10.1016/j.freeradbiomed.2018.01.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Khan NM, Haseeb A, Ansari MY, Devarapalli P, Haynie S, Haqqi TM. Wogonin, a plant derived small molecule, exerts potent anti-inflammatory and chondroprotective effects through the activation of ROS/ERK/Nrf2 signaling pathways in human osteoarthritis chondrocytes. Free Radic Biol Med. 2017;106:288-301. doi: 10.1016/j.freeradbiomed.2017.02.041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Khan NM, Haqqi TM. Pleiotropic roles of Nrf2 as regulators of chondrocyte apoptosis, oxidative stress, inflammatory response and catabolic and anabolic pathways in osteoarthritis. Free Radical Bio Med. 2017;112:191. doi: 10.1016/j.freeradbiomed.2017.10.300 [DOI] [Google Scholar]
- 18. Sun YX, Li L, Corry KA, Zhang P, Yang Y, Himes E, et al. Deletion of Nrf2 reduces skeletal mechanical properties and decreases load-driven bone formation. Bone. 2015;74:1-9. doi: 10.1016/j.bone.2014.12.066 [DOI] [PubMed] [Google Scholar]
- 19. Wu L, Leijten JC, Georgi N, Post JN, van Blitterswijk CA, Karperien M. Trophic effects of mesenchymal stem cells increase chondrocyte proliferation and matrix formation. Tissue Eng Part A. 2011;17(9-10):1425-36. doi: 10.1089/ten.TEA.2010.0517 [DOI] [PubMed] [Google Scholar]
- 20. Liu L, Zhang K, Sandoval H, Yamamoto S, Jaiswal M, Sanz E, et al. Glial lipid droplets and ROS induced by mitochondrial defects promote neurodegeneration. Cell. 2015;160(1-2):177-90. doi: 10.1016/j.cell.2014.12.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Diaz-Romero J, Kursener S, Kohl S, Nesic D. S100B + A1 CELISA: A novel potency assay and screening tool for redifferentiation stimuli of human articular chondrocytes. J Cell Physiol. 2017;232(6):1559-70. doi: 10.1002/jcp.25682 [DOI] [PubMed] [Google Scholar]
- 22. Caron MM, Emans PJ, Coolsen MM, Voss L, Surtel DA, Cremers A, et al. Redifferentiation of dedifferentiated human articular chondrocytes: comparison of 2D and 3D cultures. Osteoarthritis Cartilage. 2012;20(10):1170-8. doi: 10.1016/j.joca.2012.06.016 [DOI] [PubMed] [Google Scholar]
- 23. Son Y, Cheong YK, Kim NH, Chung HT, Kang DG, Pae HO. Mitogen-activated protein kinases and reactive oxygen species: how can ROS activate MAPK pathways? J Signal Transduct. 2011;2011:792639. doi: 10.1155/2011/792639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Weihs AM, Fuchs C, Teuschl AH, Hartinger J, Slezak P, Mittermayr R, et al. Shock wave treatment enhances cell proliferation and improves wound healing by ATP release-coupled extracellular signal-regulated kinase (ERK) activation. J Biol Chem. 2014;289(39):27090-104. doi: 10.1074/jbc.M114.580936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Vartanian S, Ma TP, Lee J, Haverty PM, Kirkpatrick DS, Yu K, et al. Application of mass spectrometry profiling to establish brusatol as an inhibitor of global protein synthesis. Mol Cell Proteomics. 2016;15(4):1220-31. doi: 10.1074/mcp.M115.055509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Li W, Pan Y, Yang Q, Guo ZG, Yue Q, Meng QG. Extracorporeal shockwave therapy for the treatment of knee osteoarthritis: a retrospective study. Medicine (Baltimore). 2018;97(27):e11418. doi: 10.1097/MD.0000000000011418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Dorotka R, Kubista B, Schatz KD, Trieb K. Effects of extracorporeal shock waves on human articular chondrocytes and ovine bone marrow stromal cells in vitro. Arch Orthop Trauma Surg. 2003;123(7):345-8. doi: 10.1007/s00402-003-0551-7 [DOI] [PubMed] [Google Scholar]
- 28. Byron CR, Benson BM, Stewart AA, Stewart MC. Effects of radial shock waves on membrane permeability and viability of chondrocytes and structure of articular cartilage in equine cartilage explants. Am J Vet Res. 2005;66(10):1757-63. [DOI] [PubMed] [Google Scholar]
- 29. Vaterlein N, Lussenhop S, Hahn M, Delling G, Meiss AL. The effect of extracorporeal shock waves on joint cartilage—an in vivo study in rabbits. Arch Orthop Trauma Surg. 2000;120(7-8):403-6. [DOI] [PubMed] [Google Scholar]
- 30. Mayer-Wagner S, Ernst J, Maier M, Chiquet M, Joos H, Muller PE, et al. The effect of high-energy extracorporeal shock waves on hyaline cartilage of adult rats in vivo. J Orthop Res. 2010;28(8):1050-6. doi: 10.1002/jor.21074 [DOI] [PubMed] [Google Scholar]
- 31. Madry H, Luyten FP, Facchini A. Biological aspects of early osteoarthritis. Knee Surg Sports Traumatol Arthrosc. 2012;20(3):407-22. doi: 10.1007/s00167-011-1705-8 [DOI] [PubMed] [Google Scholar]
- 32. Vogel WF, Abdulhussein R, Ford CE. Sensing extracellular matrix: an update on discoidin domain receptor function. Cell Signal. 2006;18(8):1108-16. doi: 10.1016/j.cellsig.2006.02.012 [DOI] [PubMed] [Google Scholar]
- 33. Maldonado M, Nam J. The role of changes in extracellular matrix of cartilage in the presence of inflammation on the pathology of osteoarthritis. Biomed Res Int. 2013;2013:284873. doi: 10.1155/2013/284873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Wang CJ, Weng LH, Ko JY, Wang JW, Chen JM, Sun YC, et al. Extracorporeal shockwave shows regression of osteoarthritis of the knee in rats. J Surg Res. 2011;171(2_suppl):601-8. doi: 10.1016/j.jss.2010.06.042 [DOI] [PubMed] [Google Scholar]
- 35. Wang CJ, Sun YC, Wong T, Hsu SL, Chou WY, Chang HW. Extracorporeal shockwave therapy shows time-dependent chondroprotective effects in osteoarthritis of the knee in rats. J Surg Res. 2012;178(1):196-205. doi: 10.1016/j.jss.2012.01.010 [DOI] [PubMed] [Google Scholar]
- 36. Wang CJ, Huang CY, Hsu SL, Chen JH, Cheng JH. Extracorporeal shockwave therapy in osteoporotic osteoarthritis of the knee in rats: an experiment in animals. Arthritis Res Ther. 2014;16(4):R139. doi: 10.1186/ar4601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Wang CJ, Weng LH, Ko JY, Sun YC, Yang YJ, Wang FS. Extracorporeal shockwave therapy shows chondroprotective effects in osteoarthritic rat knee. Arch Orthop Trauma Surg. 2011;131(8):1153-8. doi: 10.1007/s00402-011-1289-2 [DOI] [PubMed] [Google Scholar]
- 38. Wang CJ, Sun YC, Siu KK, Wu CT. Extracorporeal shockwave therapy shows site-specific effects in osteoarthritis of the knee in rats. J Surg Res. 2013;183(2_suppl):612-9. doi: 10.1016/j.jss.2013.02.006 [DOI] [PubMed] [Google Scholar]
- 39. Lee RB, Urban JP. Functional replacement of oxygen by other oxidants in articular cartilage. Arthritis Rheum. 2002;46(12):3190-200. doi: 10.1002/art.10686 [DOI] [PubMed] [Google Scholar]
- 40. Morita K, Miyamoto T, Fujita N, Kubota Y, Ito K, Takubo K, et al. Reactive oxygen species induce chondrocyte hypertrophy in endochondral ossification. J Exp Med. 2007;204(7):1613-23. doi: 10.1084/jem.20062525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Yu SM, Kim SJ. Production of reactive oxygen species by withaferin A causes loss of type collagen expression and COX-2 expression through the PI3K/Akt, p38, and JNK pathways in rabbit articular chondrocytes. Exp Cell Res. 2013;319(18):2822-34. doi: 10.1016/j.yexcr.2013.08.026 [DOI] [PubMed] [Google Scholar]
- 42. Collins JA, Wood ST, Nelson KJ, Rowe MA, Carlson CS, Chubinskaya S, et al. Oxidative stress promotes peroxiredoxin hyperoxidation and attenuates pro-survival signaling in aging chondrocytes. J Biol Chem. 2016;291(13):6641-54. doi: 10.1074/jbc.M115.693523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Stabler T, Zura RD, Hsueh MF, Kraus VB. Xanthine oxidase injurious response in acute joint injury. Clin Chim Acta. 2015;451(Pt B):170-4. doi: 10.1016/j.cca.2015.09.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Aibibula Z, Ailixiding M, Iwata M, Piao J, Hara Y, Okawa A, et al. Xanthine oxidoreductase activation is implicated in the onset of metabolic arthritis. Biochem Biophys Res Commun. 2016;472(1):26-32. doi: 10.1016/j.bbrc.2016.02.039 [DOI] [PubMed] [Google Scholar]
- 45. Bolduc JA, Collins JA, Loeser RF. Reactive oxygen species, aging and articular cartilage homeostasis. Free Radic Biol Med. 2019;132:73-82. doi: 10.1016/j.freeradbiomed.2018.08.038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Panasyuk A, Frati E, Ribault D, Mitrovic D. Effect of reactive oxygen species on the biosynthesis and structure of newly synthesized proteoglycans. Free Radic Biol Med. 1994;16(2_suppl):157-67. [DOI] [PubMed] [Google Scholar]
- 47. Ramakrishnan P, Hecht BA, Pedersen DR, Lavery MR, Maynard J, Buckwalter JA, et al. Oxidant conditioning protects cartilage from mechanically induced damage. J Orthop Res. 2010;28(7):914-20. doi: 10.1002/jor.21072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Klomsiri C, Karplus PA, Poole LB. Cysteine-based redox switches in enzymes. Antioxid Redox Signal. 2011;14(6):1065-77. doi: 10.1089/ars.2010.3376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Xue EX, Lin JP, Zhang Y, Sheng SR, Liu HX, Zhou YL, et al. Pterostilbene inhibits inflammation and ROS production in chondrocytes by activating Nrf2 pathway. Oncotarget. 2017;8(26):41988-2000. doi: 10.18632/oncotarget.16716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Park C, Hong SH, Shin SS, Lee DS, Han MH, Cha HJ, et al. Activation of the Nrf2/HO-1 signaling pathway contributes to the protective effects of Sargassum serratifolium extract against oxidative stress-induced DNA damage and apoptosis in SW1353 human chondrocytes. Int J Environ Res Public Health. 2018;15(6):1173. doi: 10.3390/ijerph15061173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Hinoi E, Takarada T, Fujimori S, Wang L, Iemata M, Uno K, et al. Nuclear factor E2 p45-related factor 2 negatively regulates chondrogenesis. Bone. 2007;40(2_suppl):337-44. doi: 10.1016/j.bone.2006.08.016 [DOI] [PubMed] [Google Scholar]
- 52. Solomon LA, Berube NG, Beier F. Transcriptional regulators of chondrocyte hypertrophy. Birth Defects Res C Embryo Today. 2008;84(2_suppl):123-30. doi: 10.1002/bdrc.20124 [DOI] [PubMed] [Google Scholar]
- 53. Vaamonde-Garcia C, Courties A, Pigenet A, Laiguillon MC, Sautet A, Houard X, et al. The nuclear factor-erythroid 2-related factor/heme oxygenase-1 axis is critical for the inflammatory features of type 2 diabetes-associated osteoarthritis. J Biol Chem. 2017;292(35):14505-15. doi: 10.1074/jbc.M117.802157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Sun D, Junger WG, Yuan C, Zhang W, Bao Y, Qin D, et al. Shockwaves induce osteogenic differentiation of human mesenchymal stem cells through ATP release and activation of P2X7 receptors. Stem Cells. 2013;31(6):1170-80. doi: 10.1002/stem.1356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Yu T, Junger WG, Yuan C, Jin A, Zhao Y, Zheng X, et al. Shockwaves increase T-cell proliferation and IL-2 expression through ATP release, P2X7 receptors, and FAK activation. Am J Physiol Cell Physiol. 2010;298(3):C457-64. doi: 10.1152/ajpcell.00342.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-pptx-1-car-10.1177_19476035211012465 for Shockwave Treatment Enhanced Extracellular Matrix Production in Articular Chondrocytes Through Activation of the ROS/MAPK/Nrf2 Signaling Pathway by Po-Chih Shen, Shih-Hsiang Chou, Cheng-Chang Lu, Hsuan-Ti Huang, Song-Hsiung Chien, Peng-Ju Huang, Zi-Miao Liu, Chia-Lung Shih, Shu-Jem Su, Li-Min Chen and Yin-Chun Tien in CARTILAGE