Abstract
Objective
To compare the effects of the complex triamcinolone acetonide-hydroxypropyl-β-cyclodextrin (TA-CD) on in vitro inflamed primary human articular chondrocytes in the presence or absence of the mixture hyaluronic acid-Chitlac, a lactose-modified chitosan (HA-CTL).
Design
Changes in cell viability and pro-inflammatory cytokines gene expression were analyzed in human chondrocytes using an in vitro model of macrophage-mediated inflammation. Human monocytes U937 were differentiated to macrophages by phorbol 12-myristate 13-acetate (PMA) and lipopolysaccharides (LPS). The anti-inflammatory effects of the complex TA-CD and HA-CTL mixture were assessed on chondrocytes exposed for 24 hours to U937 conditioned medium (CM), by quantitative polymerase chain reaction analysis.
Results
The TA-CD viability was enhanced by the presence of the HA-CTL mixture in chondrocyte cultures. The exposure of cells to CM significantly increased interleukin-1β and interleukin-6 gene expression, and when the complex TA-CD was added to the inflamed cells, gene transcription of cytokines was restored to near baseline values, both in the presence or in the absence of HA-CTL mixture.
Conclusion
The addition of HA-CTL mixture significantly attenuated cytotoxicity induced by TA and preserved the anti-inflammatory effects, thus confirming the chondroprotective role of the HA-CTL mixture.
Keywords: triamcinolone, hyaluronic acid, osteoarthritis
Osteoarthritis (OA) is an inflammatory condition that leads to articular disability and pain. It is associated with mononuclear cells’ infiltration that cause the release of pro-inflammatory mediators such as interleukin (IL)-1β and IL-6, catabolic enzymes that accelerate the degradation of extracellular matrix (ECM) molecules. 1 The most widely used pharmacological treatments to contain inflammation and to reduce symptoms associated with OA are nonsteroidal and corticosteroidal (CSs) anti-inflammatory drugs, such as hydrocortisone, methylprednisolone, dexamethasone, and triamcinolone. 2 Although discrepancies in their efficacy are reported, adverse effects on cell viability and cartilage loss remained a concern. In particular, some authors found that triamcinolone acetonide (TA), one of the most used CSs, exerts its cytotoxicity action in a dose-dependent manner, 3 whereas others demonstrated their chondral protective effects when administrated in vivo at low doses. 4 To attenuate CS adverse effects, while maintaining their strong anti-inflammatory effects, some authors have proposed a combination of these drugs with some chondroprotective molecules such as hyaluronic acid (HA), whose protective action is well known.5,6 Recently, the mixture of HA with a lactose-modified chitosan (Chitlac; CTL), named ARTY-DUO, was proposed to improve the therapeutic activity of HA injection. 7 The results of this in vivo study demonstrated a significantly increased cartilage regeneration and a reduced synovial inflammation in animals treated with NaCl and with HA. It was hypothesized that this protective effect of the HA-CTL combination might be due not only to the chondroprotective role of HA but also to the ability of CTL to stimulate cartilage growth. Moreover, it was demonstrated that CS bioavailability was improved by their combination with some excipients such cyclodextrin (CD), a cyclic oligomer of glucose, currently used as drug carrier to enhance their solubility. 8
The purpose of the present study was to evaluate the viability and anti-inflammatory effects of TA-CD complex in the presence or absence of the HA-CTL mixture on an inflammatory model of human primary articular chondrocytes treated with the conditioned medium (CM) of U937 macrophage induced by phorbol 12-myristate 13-acetate (PMA) lipopolysaccharides (LPS). We used hydroxypropyl-β-cyclodextrin and triamcinolone-acetonide from Cavitron W7 HP7, (Ashland, OR) and a HA-CTL mixture composed of 1.25 mg/mL sodium HA (molecular weight 1000-1600 kDa) and different concentrations of CTL (molecular weight 800-2500 kDa), pH 7.4.
TA and CD Induce Changes in Chondrocyte Viability That Are Reduced by the Presence of the Mixture HA-CTL
First, we tested the effect of HA-CTL mixture on cell viability cultivating the cells with the combination of HA and CTL in the same ratios (1.7:1) as that used in a previous in vivo study, 7 and we found that the mixture HA 1.25 mg/mL and CTL 0.75 mg/mL did not exert cytotoxic effects (data not shown). Primary articular chondrocytes were isolated from joint cartilage biopsies 9 of OA patients who underwent total knee or hip replacement. The study was approved by the Local Ethical Committee (4744/AO/19) and biopsies were collected after patients signed written informed consent.
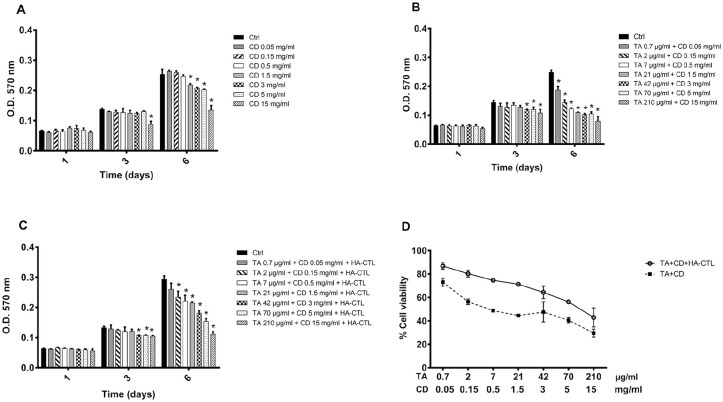
To determine whether the complex TA-CD alone or in combination with HA-CTL mixture affected the viability of chondrocytes (using a modified Denizot 10 method), the cells obtained from joint cartilage biopsies of 3 different donors were separately cultured in the presence or in the absence of different concentrations of CD, of the complex TA-CD and of TA-CD and HA-CTL mixture. These compounds were initially added to cell cultures, without performing medium changes during the observation time. The experiments were performed in triplicate for each cell preparation and the results are reported in Figure 1 . CD at the concentrations up to 0.5 mg/mL did not affect cell proliferation ( Fig. 1A ), whereas a significant reduction of viability was found after 6 days of treatment, in the presence of all the tested TA-CD combined concentrations (CD from 0.05 to 15 mg/mL and TA from 0.7 to 212 µg/mL; P < 0.05; Fig. 1B ). However, when the TA-CD complex was administered together with the HA-CTL mixture, cell viability was enhanced for all the tested TA-CD concentrations and returned to control levels in the presence of 0.05 mg/mL CD and 0.7 µg/mL TA ( Fig. 1C ). In conclusion, the chondrocyte viability increased in the presence of the HA-CTL mixture when compared with that of TA-CD administrated alone to the cell cultures, while remaining significantly lower than the controls ( Fig. 1D ).
Figure 1.
Time-dependent effects of TA and CD, alone or in combination with HA-CTL mixture on human chondrocyte viability. A total of 7000/cm2 cells were seeded in 24-well culture dishes and treated with TA, CD, or their combination, in the presence or absence of HA-CTL (A) CD, (B) CD + TA at different combinations, (C) CD + TA in the presence or absence of HA-CTL mixture. The MTT test was performed at 1, 3, and 6 days after treatment. In (D) the viability of treated cells at 6 days was expressed as percentage of untreated controls. Each experiment was performed in triplicate, using different cell preparations taken from different donors. Data are reported as mean ± SD of the 3 independent experiments. Statistical differences based on unpaired Student’s t test. *P < 0.05 versus untreated cells. TA = triamcinolone acetonide; CD = hydroxypropyl-β-cyclodextrin; HA = hyaluronic acid; CTL = chitosan (Chitlac).
HA-CTL Mixture with TA Did Not Affect the Reduction of Pro-Inflammatory Molecules’ Expression in Chondrocytes
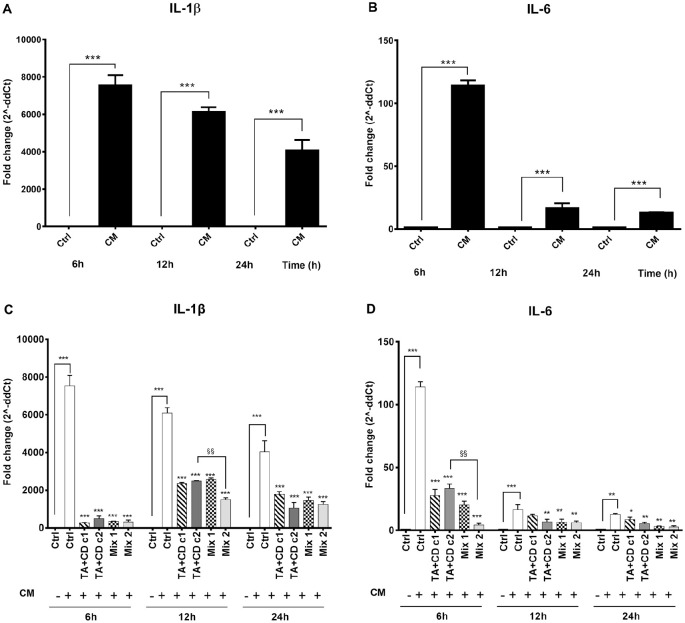
Human monocytes U937 (Thermo Scientific; Wilmington, DE) were differentiated to macrophages by the addition of PMA (Sigma-Aldrich) at a final concentration of 50 ng/mL for 48 hours followed by the exposure to 1 µL/mL LPS (Sigma) for 1 hour. Cells were then washed and cultivated with complete RPMI for 24 hours to produce the inflammatory CM collected. The PMA/LPS activation of U937 was confirmed by a significant (P < 0.0001) upregulation of CD68 (data not shown) and of the pro-inflammatory cytokines, IL-1β ( Fig. 2A ) and IL-6 ( Fig. 2B ). For this purpose, at 6, 12, and 24 hours after treatment, total RNA was extracted by TRIzol (Life Technologies, Carlsbad, CA), reversely transcribed, and analyzed by quantitative polymerase chain reaction (qPCR). Subsequently, the anti-inflammatory effect of the complex TA-CD was tested with selected concentrations of TA and CD (c1, 21 µg/mL TA and 1.5 mg/mL CD; c2, 70 µg/mL TA and 5 mg/mL CD) on chondrocyte treated for 24 hours with the CM of activated U937, in the presence or absence of the HA-CTL mixture, for 24 hours. These CD and TA concentrations were chosen on the basis of preliminary studies on the anti-inflammatory effects of TA on cells. The results showed that the TA-CD complex, in the presence or absence of HA-CTL mixture, induced a downregulation of IL-1β at 6, 12, and 24 hours after treatment. Moreover, the combination of CD+TA c2 with the HA-CTL mixture (mix2) induced a significantly improved reduction of the expression of IL-1β and of IL-6 at 6 hours post-treatment.
Figure 2.
Chondrocyte expression of pro-inflammatory molecules after exposure to TA + CD in the presence or absence of HA-CTL mixture. Human chondrocytes were exposed for 24 hours to CM of activated U937 and then cultured with the studied molecules. RNA transcript levels specific for IL-1β and IL-6 were evaluated by qPCR. Activated U937 cell expression of (A) IL-1β and (B) IL-6. Human chondrocytes expression for (C) IL-1β and (D) IL-6. c1: 1.5 mg/mL CD and 21 µg/mL TA; c2: 5 mg/mL CD and 70 µg/mL TA; mix1: c1 + HA-CTL; mix2: c2 + HA-CTL. Each experiment was performed in triplicate, using different cell preparations taken from different donors. Statistical differences are based on one-way ANOVA test and data are expressed as mean ± SD obtained from the 3 independent experiments. CM = chondrocytes cultures exposed to U937 CM. *P < 0.05, **P < 0.001, and ***P < 0.0001 versus respective CM treated control. §§P < 0.001 versus respective chondrocyte culture treatment. TA = triamcinolone acetonide; CD = hydroxypropyl-β-cyclodextrin; HA = hyaluronic acid; CTL = chitosan (Chitlac).
Taken together, the results of the present study demonstrated that the presence of the mixture HA-CTL in primary human articular chondrocyte cultures partially counteracted the cytotoxic effect of TA while maintaining the well-known anti-inflammatory activity of this compound. 11 In fact, when the complex TA-CD was added to cells exposed to the CM of activated U937 human monocytes, gene transcription for IL-1β and IL-6 were restored to near baseline values, both in the presence or in the absence of HA-CTL mixture. Changes in cell viability induced by the complex TA-CD are reduced by the presence of HA-CTL mixture, thus suggesting that, combining corticosteroids with chondroprotective polysaccharides, such as HA, may be effective in maintaining viability of normal chondrocytes and in restoring survival of cells while providing a steroidal anti-inflammatory ingredient.6,12 Our results also validate the findings of the recent in vivo study performed in a rat OA model demonstrating that HA in combination with CTL counteract the cartilage degradation better than HA alone. HA injections have been widely used as viscosupplementation since this molecule has a central role in maintaining synovial fluid viscosity. 13 However, residence time of HA in the joint cavity is short, and it was demonstrated that its combination with other molecules such as chitosan 14 increased its biological half-life providing cartilage protection. It is also well known that CTL is a molecule able to bind to HA, 15 and a recent unpublished work performed by our laboratory has found that CTL is also able to exert anti-inflammatory activities (data not shown). In conclusion, the results of our study confirmed the efficacy of the TA as an anti-inflammatory molecule and validate the use of HA-CTL mixture as a chondroprotective mixture. Further in vitro and in vivo investigations should be performed to confirm the promising therapeutic applications of the TA-CD complex with HA-CTL mixture.
Footnotes
Acknowledgments and Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by Jointherapeutics s.r.l. (Padua, Italy). Jointherapeutics did not take part in the study design, data analysis and interpretation, and in the writing of the manuscript.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Ethical Approval: The study was approved by the Local Ethical Committee of University of Padova (4744/AO/19).
Informed Consent: All patients signed written informed consent.
ORCID iDs: Pietro Ruggieri  https://orcid.org/0000-0001-9617-9882
https://orcid.org/0000-0001-9617-9882
Paola Brun  https://orcid.org/0000-0002-4204-3549
https://orcid.org/0000-0002-4204-3549
References
- 1. Glyn-Jones S, Palmer AJ, Agricola R, Price AJ, Vincent TL, Weinans H, et al. Osteoarthritis. Lancet. 2015;386:376-87. doi: 10.1016/S0140-6736(14)60802 [DOI] [PubMed] [Google Scholar]
- 2. Evans CH, Kraus VB, Settn LA. Progress in intra-articular therapy. Nat Rev Rheumatol. 2014;10:11-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Syed HM, Green L, Bianski B, Jobe CM, Wongworawat MD. Bupivacaine and triamcinolone may be toxic to human chondrocytes: a pilot study. Clin Orthop Relat Res. 2011;469:2941-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Williams JM, Brandt KD. Triamcinolone hexacetonide protects against fibrillation and osteophyte formation following chemically induced articular cartilage damage. Arthritis Rheum. 1985;28:1267-74. [DOI] [PubMed] [Google Scholar]
- 5. Di Paola R, Fusco R, Impellizzeri D, Cordaro M, Britti D, Morittu VM, et al. Adelmidrol, in combination with hyaluronic acid, displays increased anti-inflammatory and analgesic effects against monosodium iodoacetate-induced osteoarthritis in rats. Arthritis Res Ther. 2016;18:291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Hangody L, Szody R, Lukasik P, Zgadzaj W, Lénárt E, Dokoupilova E, et al. Intraarticular injection of a cross-linked sodium hyaluronate combined with triamcinolone hexacetonide (Cingal) to provide symptomatic relief of osteoarthritis of the knee: a randomized, double-blind, placebo-controlled multicenter clinical trial. Cartilage. 2018;9:276-83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Salamanna F, Giavaresi G, Parrilli A, Martini L, Aldini NN, Abatangelo G, et al. Effects of intra-articular hyaluronic acid associated to Chitlac (arty-duo®) in a rat knee osteoarthritis model. J Orthop Res. 2019;37:867-76. [DOI] [PubMed] [Google Scholar]
- 8. Uekama K, Hirayama F, Arima H. Recent aspect of cyclodextrin-based drug delivery system. J Incl Phenom. 2006;56:3-8. doi: 10.1007/s10847-006-9052-y [DOI] [Google Scholar]
- 9. Brun P, Abatangelo G, Radice M, Zacchi V, Guidolin D, Daga-Gordini D, et al. Chondrocyte aggregation and reorganization into three-dimensional scaffolds. J Biomed Mater Res. 1999;46:337-46. [DOI] [PubMed] [Google Scholar]
- 10. Denizot F, Lang R. Rapid colorimetric assay for cell growth and survival. Modifications to the tetrazolium dye procedure giving improved sensitivity and reliability. J Immunol Methods. 1986;89:271-7. [DOI] [PubMed] [Google Scholar]
- 11. Siengdee P, Radeerom T, Kuanoon S, Euppayo T, Pradit W, Chomdej S, et al. Effects of corticosteroids and their combinations with hyaluronanon on the biochemical properties of porcine cartilage explants. BMC Vet Res. 2015;11:298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Avenoso A, D’Ascola A, Scuruchi M, Mandraffino G, Calatroni A, Saitta A, et al. Hyaluronan in the experimental injury of the cartilage: biochemical action and protective effects. Inflamm Res. 2018;67:5-20 [DOI] [PubMed] [Google Scholar]
- 13. Axe JM, Snyder-Mackler L, Axe M J. The role of viscosupplementation. Sports Med Arthrosc Rev. 2013;21:18-22. [DOI] [PubMed] [Google Scholar]
- 14. Kaderli S, Boulocher C, Pillet E, Watrelot-Virieux D, Rougemont AL, Roger T, et al. A novel biocompatible hyaluronic acid–chitosan hybrid hydrogel for osteoarthrosis therapy. Int J Pharm. 2015;483:158-68. [DOI] [PubMed] [Google Scholar]
- 15. Donati I, Stredanska S, Silvestrini G, Vetere A, Marcon P, Marsich E, et al. The aggregation of pig articular chondrocyte and synthesis of extracellular matrix by a lactose-modified chitosan. Biomaterials. 2005;26:987-9. [DOI] [PubMed] [Google Scholar]