Abstract
Objective
This study aimed to assess the association between synovial fluid (SF) metabolites and magnetic resonance imaging (MRI) measurements of cartilage biochemical composition to identify potential SF biomarkers for detecting the early onset of cartilage degeneration in a rabbit model.
Methods
Both knees of 12 New Zealand White rabbits were used. The anterior cruciate ligament transection (ACLT) model was performed on right knees, and the sham surgery on left knees. MRI UTE-T2* scanning and SF sample collection were performed on ACLT knees at 4 and 8 weeks postsurgery and on sham surgery knees at 4 weeks postsurgery. Ultra-performance liquid chromatography–mass spectrometry and multivariate statistical analysis were used to distinguish samples in three groups. Pathway and receiver operating characteristic analyses were utilized to identify potential metabolite biomarkers.
Results
There were 12 knees in sham surgery models, 11 in ACLT models at 4 weeks postsurgery, and 10 in ACLT models at 8 weeks postsurgery. UTE-T2* values for the lateral tibia cartilage showed significant decreases over the study period. Levels of 103 identified metabolites in SF were markedly different among three groups. Furthermore, 24 metabolites were inversely correlated with UTE-T2* values of the lateral tibia cartilage, while hippuric acid was positively correlated with UTE-T2* values of the lateral tibia cartilage. Among 25 potential markers, N1-acetylspermidine, 2-amino-1,3,4-octadecanetriol, l-phenylalanine, 5-hydroxy-l-tryptophan, and l-tryptophan were identified as potential biomarkers with high area under the curve values and Pearson correlation coefficients.
Conclusion
Five differential metabolites in SF were found as potential biomarkers for the early detection of cartilage degeneration in the rabbit ACLT model.
Keywords: cartilage, degeneration, metabolomics, magnetic resonance imaging, biomarker
Introduction
Anterior cruciate ligament (ACL) injury is one of the most common forms of severe sports-related injury. The long-term outcomes of ACL injury, whether treated with ACL-reconstructive or nonoperative treatment, include recurrent instability and increased risk of posttraumatic osteoarthritis (OA). 1 Cartilage degeneration, which includes cartilage thinning and compositional alterations, is a significant symptom of the early phase of OA. 2
Currently, magnetic resonance imaging (MRI) is the only noninvasive and effective imaging technique to diagnose cartilage injury. However, routine MRI has limited sensitivity for diagnosing early cartilage degenerative changes when cartilage still is morphologically intact. Quantitative MRI, such as T1rho and T2 mapping, are increasingly used to detect biochemical changes in cartilage matrix. Ultrashort echo time-T2* (UTE-T2*) mapping has been reported to be more sensitive to deep zone cartilage changes and early subsurface degeneration than T2 mapping. 3 Synovial fluid (SF) is the primary source of nutrients for the avascular cartilage and contributes to cartilage homeostasis. 4 It contains metabolites that potentially reflect cartilage metabolism. 4 SF biomarkers have gained recent attention because of their ability to provide a window into the molecular milieu associated with various knee joint pathologies. 5 Metabolomics is a powerful tool for biomarker detection and provides new insights into the disease developmental process, since it can offer information about numerous metabolites derived from the interaction between the microenvironment and intracellular pathways. 6 It has been estimated that metabolites in SF may serve as degenerative joint metabolism biomarkers related to OA pathology.
An anterior cruciate ligament transection (ACLT) model in New Zealand rabbits provides a controlled and well-established experimental model for surgically generated OA and has been widely used in many studies. 7 Because of the high resemblance in terms of anatomy, the disease progression of the rabbit ACL-injured knee shows similar features to that of the human knee, which makes it suitable for experimental studies. 8 In this study, we used MRI and untargeted metabolomics with ultra-performance liquid chromatography–mass spectrometry (UPLC-MS) to (1) analyze differences in the metabolic profiles in SF between control and ACL-injured knees in the rabbit model and (2) discover putative metabolic biomarker candidates that could be used to examine the early course of cartilage degeneration.
Materials and Methods
Animal Selection and ACLT Model Construction
The Animal Care and Ethics Committee of our institution approved all experimental procedures using animals. All experiments comply with the ARRIVE guidelines. We used 12 healthy New Zealand White rabbits (6 months old, male, 3.2 kg in weight on average) for this study. Both knees of each rabbit were shaved, prepared in a sterile environment. To generate ACL injured joints, the ACLT model was performed on the right knees that were similar to those performed in the previous study (Supplementary Materials and Methods). 9 As controls, the sham surgery model was performed on the left knees of all 12 rabbits in which the ACL was visualized but not transected. MRI scanning and SF sample collection were performed on the ACLT knees at 4 (TF group) and 8 (TE group) weeks postsurgery and on the sham surgery knees at 4 weeks postsurgery (N group).
MRI Protocol
ACLT models were scanned using a 3-T MRI scanner (MR750; GE Healthcare) with a rabbit-specific surface coil. The rabbits were placed in the supine position and anesthetized by inhalation of a mixture of isoflurane at a rate of 3.0 L/min while performing MRI. All subjects underwent MRI scanning with the sagittal proton density (PD) sequence and UTE-T2* mapping. The sagittal PD sequence was used to evaluate the morphological appearance of the ACL and cartilage. The parameters were as follows: repetition time (TR), 6273.0 ms; echo time (TE), 40.0 ms; flip angle, 142°; slice thickness, 1.0 mm; matrix, 352 × 192; acquisition time, 2 minutes 12 seconds. An experienced musculoskeletal radiologist who was blinded to the surgical procedure evaluated the morphological images to identify whether the ACLT models were successful. The 2-echo UTE-T2* sequence was acquired sagittally for the quantification of the cartilage UTE-T2* values. The parameters were as follows: TR, 85.0 ms; TE, 0.043 and 6.8 ms; flip angle, 12°; slice thickness, 1.0 mm; matrix, 320 × 320; acquisition time, 6 minutes 59 seconds. All images were transferred to the GE workstation for cartilage measurement and postprocessing. The tibiofemoral cartilage was divided into 4 regions of interest (ROIs), including the medial femoral condyle, medial tibia, lateral femoral condyle, and lateral tibia (TL) compartments. The measurements of the UTE-T2* relaxation times of the 4 ROIs are detailed in the Supplementary Materials and Methods. SF samples were collected immediately following MRI scanning at 4 and 8 weeks after surgery.
SF Collection and Preparation
SF collections were performed similar to the previously described methods.10,11 Knees were shaved and disinfected, and then 1 mL sterile saline was injected through the joint capsule in the middle of the medial articular space. Knees were moved into extension and flexion several times to allow the aspiration of as much SF as possible. SF samples (about 800 μL) were collected along with the saline solution under complete aseptic conditions using the insulin needle and centrifuged at 2000 × g for 10 minutes at 4°C, and the supernatant was directly snap frozen and stored at −80°C until metabolomic analysis was performed. All sample collections were performed by the same orthopedic surgeon (with 10 years of experience in the orthopedic surgery).
The samples were thawed on ice for 30 minutes and vortexed thoroughly. Two hundred microliter aliquots from each sample and the standard compound mixture were transferred into new tubes. Proteins were precipitated using 800 μL of cold methanol solution (prechilled to −20°C) containing 2 internal standards, 2-chloro-l-phenylalanine and lysophosphatidylcholine (12:0) at a final concentration of 2.5 μg/mL. Then, the mixture was thoroughly mixed with a vortex mixer for 10 seconds and incubated for 2 hours at −20°C. Subsequently, the mixture was briefly vortexed and then centrifuged twice at 12,000 rpm for 10 minutes at 4°C. The supernatant (800 μL) was collected, aliquoted into 2 separate vials (400 μL) and dried under a vacuum. For the hydrophilic interaction liquid chromatography (HILIC) and reversed-phase liquid chromatography (RP) analyses, the obtained residue was reconstituted in 100 μL of acetonitrile/water (1:1, v/v) for LC-MS analysis. A mixed quality control sample was prepared by mixing equal volumes (20 μL) of each SF sample. One QC sample and 1 blank sample were analyzed after the analysis of every 10 study samples.
Metabolite Analysis and Data Management
The sample analysis was performed on an Ultimate-3000 UPLC system coupled to a Q Exactive hybrid quadrupole-Orbitrap MS system (Thermo Scientific). The autosampler temperature was maintained at 4°C during sample analysis.
Liquid chromatography for the aqueous extraction was carried out using an Acquity UPLC BEH Amide column (1.7 μm × 2.1 mm × 100 mm, Waters) with an Acquity UPLC BEH Amide VanGuard precolumn (1.7 μm × 2.1 mm × 5 mm, Waters). Metabolites were analyzed by LC-MS analysis as previously described with modifications. 12 The mobile phases used in our study were 20 mM ammonium acetate and 20 mM ammonium hydroxide in water/acetonitrile (95:5, v:v) (A) and acetonitrile (B). The gradient elution was as follows: phase B was held at 85% from 0 to 1.0 minute and then decreased linearly to 65% over the following 11 minutes. Then, phase B was decreased to 40% over 0.1 minute and held at 40% for 2.9 minutes, followed by a fast return to the initial conditions between 15.0 and 15.1 minutes, which were held for 4.9 minutes for column reequilibration. The flow rate, injection volume, and column temperature were set at 0.35 mL/min, 5 μL, and 40°C, respectively.
Liquid chromatography for the organic extraction was carried out using a Hypersil GOLD column (1.9 μm × 2.1 mm × 100 mm, Thermo Scientific). The gradient elution of analytes was performed with 0.1% formic acid in acetonitrile (A) and 0.1% formic acid in water/acetonitrile (95:5, v:v) (B). The gradient elution was performed as follows: phase A was increased linearly to 20% from 0 to 1.5 minutes and then increased to 100% over 8.5 minutes. Then, phase A was maintained at 100% between 10.0 and 13.0 minutes and returned to the initial state for 0.5 minutes, followed by 2.5 minutes of reequilibration. The flow rate, injection volume and column temperature were set at 0.35 mL/min, 5 μL, and 45°C, respectively.
The details of the sample extraction and MS parameters are described in the Supplementary Materials and Methods.
Data Management and Metabolite Identification and Characterization
The raw data information was converted to mzXML files with MSConvert in ProteoWizard (version 3.0.9974). The peak identification, filtration, alignment, and isotope annotation were performed using the XCMS program (version 3.0.0) with CAMERA (version 1.34.0) in the R package (version 3.4.3) to obtain the data matrix containing the mass-to-charge, retention times, integrated peak intensity, and isotope annotation. The method parameters were set as follows: centWave method, ppm = 10, snthresh = 10, bw = 30 (HILIC condition)/10 (RP condition), minfrac = 0.5, mzwid = 0.025. The multivariate statistical analysis was performed with SIMCA-P (Umetrics, Umea, Sweden). The differences in the metabolic signatures, trends or outliers were evaluated using unsupervised principal components analysis (PCA). Then, partial least-squares discriminant analysis was performed to maximize the differences between the 3 groups and to select the variables responsible for the separation between them. The cross-validation was carried out to prevent the overfitting of the statistical model. Moreover, the analysis of variance and Fisher’s LSD (least significant difference) post hoc test were used to determine whether variables were statistically significant between the 2 groups. Metabolites with variable importance of projection (VIP) values >1 and P < 0.05 were identified as significantly different metabolites between the 2 groups. The significant metabolites were identified by MS/MS fragment analysis by using databases such as mzCloud, HMDB (http://hmdb.ca) and lipidmaps (http://www.lipidmaps.org) with a confidence limit of 10 ppm to increase the sensitivity and to filter out irrelevant compounds. Thereafter, the recorded KEGG (http://www.kegg.jp) numbers served as input for the metabolomics pathway. The metabolic pathway analysis was performed and the heatmaps were generated with MetaboAnalyst 4.0, in which any pathway with an impact value >0.10 and a false discovery rate <0.05 was regarded as significant.
Univariate Statistical Analysis
Analysis of variance was conducted to compare the differences in the UTE-T2* values and the metabolite concentrations in SF among the 3 groups. Pearson correlation analysis was used to examine the association between each metabolite concentration and the UTE-T2* values. Receiver operating characteristic (ROC) analysis was used to assess the diagnostic power of the potential biomarkers by calculating the area under the curve (AUC). All univariate statistical analyses were performed using SAS version 9.3 software.
Results
MRI Information
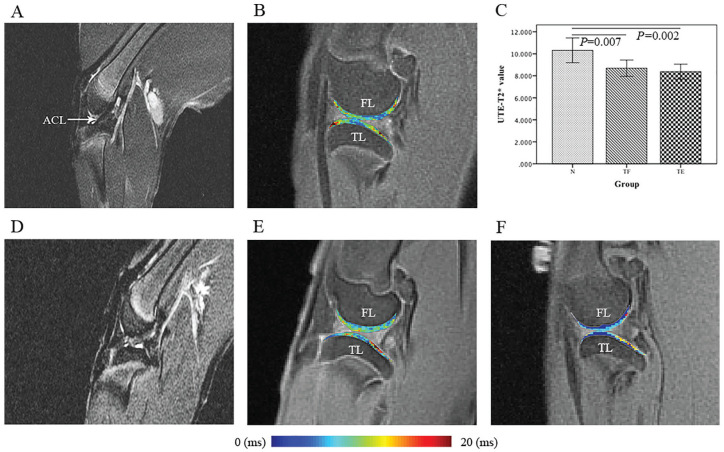
One sample in the ACLT model at 4-week postsurgery and 2 samples in the ACLT model at 8 weeks postsurgery were excluded as they showed swelling in joint capsules and bone marrow edema on PD images. The UTE-T2* values of the tibiofemoral cartilage compartments are shown in Table 1 and Figure 1 . The UTE-T2* values were significantly lower in the injured knees in the TL cartilage compartment compared with those in the controls. Both the medial tibiofemoral and lateral femoral condyle cartilage compartments showed decreased UTE-T2* values without statistical significance.
Table 1.
P Values of the Magnetic Resonance Ultrashort Echo Time-T2* (MR UTE-T2*) Comparisons.
| Results | Compartment | ||||
|---|---|---|---|---|---|
| FM | TM | FL | TL | Average | |
| UTE-T2* relaxation value | |||||
| N | 10.61 ± 1.72 | 9.74 ± 1.68 | 10.46 ± 1.34 | 10.32 ± 1.77 | 10.28 ± 1.17 |
| TF | 10.19 ± 1.73 | 8.77 ± 0.85 | 10.12 ± 2.35 | 8.69 ± 1.10 | 9.44 ± 1.13 |
| TE | 10.59 ± 1.30 | 9.69 ± 1.01 | 11.01 ± 2.56 | 8.38 ± 0.95 | 9.92 ± 0.81 |
| P value | |||||
| Overall | 0.785 | 0.139 | 0.633 | 0.004 | 0.181 |
| TF vs. N | 0.535 | 0.073 | 0.966 | 0.007 | 0.067 |
| TE vs. N | 0.983 | 0.920 | 0.913 | 0.002 | 0.427 |
| TF vs. TE | 0.567 | 0.104 | 0.807 | 0.601 | 0.314 |
FM = medial femoral condyle; TM = medial tibia; FL = lateral femoral condyle; TL = lateral tibia; average = the tibiofemoral cartilage; N = sham surgery model at 4 weeks; TF = anterior cruciate ligament (ACL) transection model at 4 weeks; TE = ACL transection model at 8 weeks.
Figure 1.
Sagittal proton density sequence and ultrashort echo time-T2* (UTE-T2*) color maps of the lateral tibiofemoral compartment. In the UTE-T2* color maps, blue indicates low and red indicates high cartilage UTE-T2* values. (Left) Morphological magnetic resonance images of the anterior cruciate ligament (ACL). (A) Knee before surgery. The white arrow shows a normal ligament. (D) Knee postsurgery with an entire ligament tear. (B, E, F) UTE-T2* color maps of the lateral compartment of the tibiofemoral cartilage in the sham-surgery group (N, B) and 4 (TF, E) and 8 weeks postsurgery (TE, F) in the ACL transection group. In the UTE-T2* color maps, the UTE-T2* values of the lateral tibiofemoral compartment show a decreasing trend according to the corresponding color bar. (C) Boxplots showing the comparison of the TL cartilage compartment UTE-T2* values among the 3 groups.
Significant Metabolism Perturbation in SF in the ACLT Model
All peaks detected by UPLC-MS analysis were subjected to PCA and partial least-squares-discriminant analysis (PLS-DA) to obtain a direct overview of the systemic alterations in metabolites. PCA showed that all QC samples were within 2 standard deviations in the score plots, indicating the acceptable stability and repeatability of the results of the present study (Figure S1 in the Supplementary Material). Meanwhile, as shown in Figure S1 in the Supplementary Material, the metabolic profiles of the samples at 8 weeks postsurgery could be only poorly distinguished from those at 4 weeks, suggesting the occurrence of progressive and overlapping metabolic changes during the cartilage degeneration process. However, the ACL-injured knees were best distinguished from controls in the PCA and PLS-DA models, which showed good fitting and predictive performances, indicating the contribution of altered metabolism to the process associated with cartilage degeneration. The model parameters of the PCA and PLS-DA models as well as the results of the permutation test (200 iterations) are listed in Table S1 in the Supplementary Material.
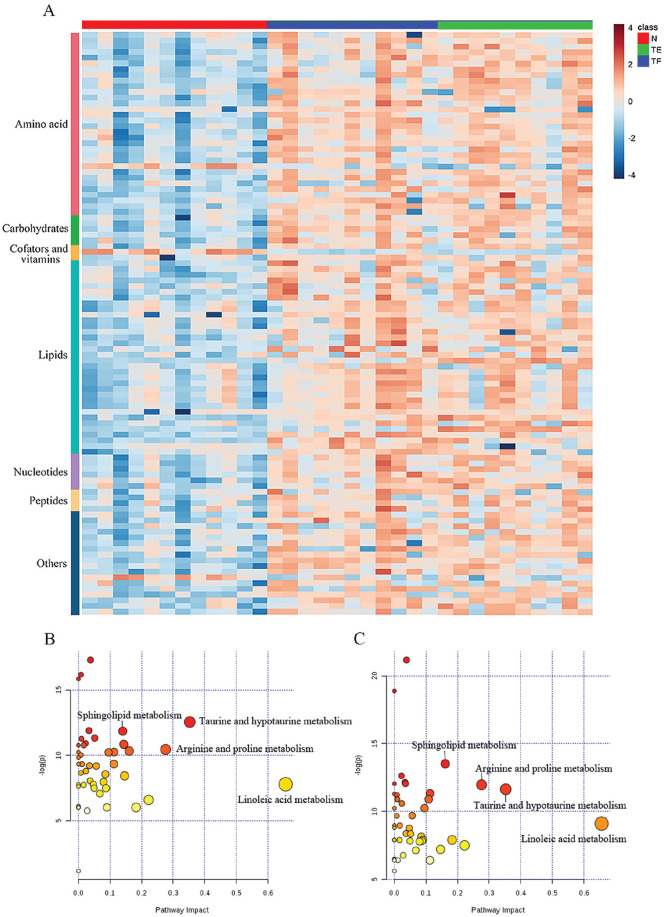
We selected the differentially expressed metabolites among the different time points based on the VIP, P values, and fold change. From the MS match and MS/MS match, a total of 103 differentially expressed metabolites (76 from HILIC and 40 from RP, which are shown in Table S2 in the Supplementary Material) were found between the controls and the ACL-injured knees based on thresholds set at a P value less than 0.05, a VIP value exceeding 1.0 and fold change more than 1.5 or less than −1.5. Among the identified 103 metabolites, 100 were elevated and 3 were decreased in the two cohorts of the ACL-injured group. All these differential variations are shown in a heat map ( Fig. 1A ).
After data processing, only the metabolites with KEGG IDs were included in the pathway analysis to determine the possible metabolic pathways that were affected in the ACLT models. Eleven metabolic pathways were found to be significantly altered (false discovery rate adjusted P < 0.05, impact value >0.1). The distribution of the metabolic pathways that differentiated the controls and ACL-injured knees is presented in Table S3a and Table S3b in the Supplementary Material. The majority of the differentially expressed metabolites were involved in lipid and amino acid metabolism. There seemed to be a notable change in lipid metabolism in the ACL-injured knees, with significant increases in linoleic acid (which includes derivatives of linoleic acids) and sphingolipids. In addition, amino acid metabolism, including taurine and hypotaurine metabolism, arginine and proline metabolism, phenylalanine metabolism, and tryptophan metabolism, was significantly perturbed and considered to be largely responsible for cartilage degeneration. The perturbation of these pathways was found to be primarily involved in inflammation, oxidative stress and the bone formation process.13-15 Furthermore, metabolites with the capability to cause metabolic pathway disturbances were selected as potential biomarkers for further analysis. The most relevant identified pathways are displayed in Figure 2B and C , where the size and color of each circle indicates the pathway impact value and the P value, respectively.
Figure 2.
(A) the Data refers to the relative synovial fluid (SF) concentration level observed in the training set. Metabolic patterns were visualized using heat maps based on the 103 differential metabolites generated from the N, TF, and TE groups. A blue/red color scheme was used to denote a decrease/increase in a metabolite relative level. Red, sham surgery knees; blue, anterior cruciate ligament (ACL)–injured knees at 4 weeks postsurgery; green, ACL-injured knees at 8 weeks postsurgery. (B, C) Pathway analysis of the altered metabolites in the SF of the ACL transection models using MetaboAnalyst (http://www.metaboanalyst.ca/). The significantly dysregulated metabolites in the ACL transection models at 4 weeks (N metabolites = 59, B) and 8 weeks (N metabolites = 58, C) postsurgery were analyzed in MetaboAnalyst to assess the association of the respective metabolites to the defined pathways. Biochemical pathway impact analysis revealed disturbed metabolic pathways. N = sham surgery model at 4 weeks; TF = ACL transection model at 4 weeks; TE = ACL transection model at 8 weeks.
Selection of Potential Biomarkers
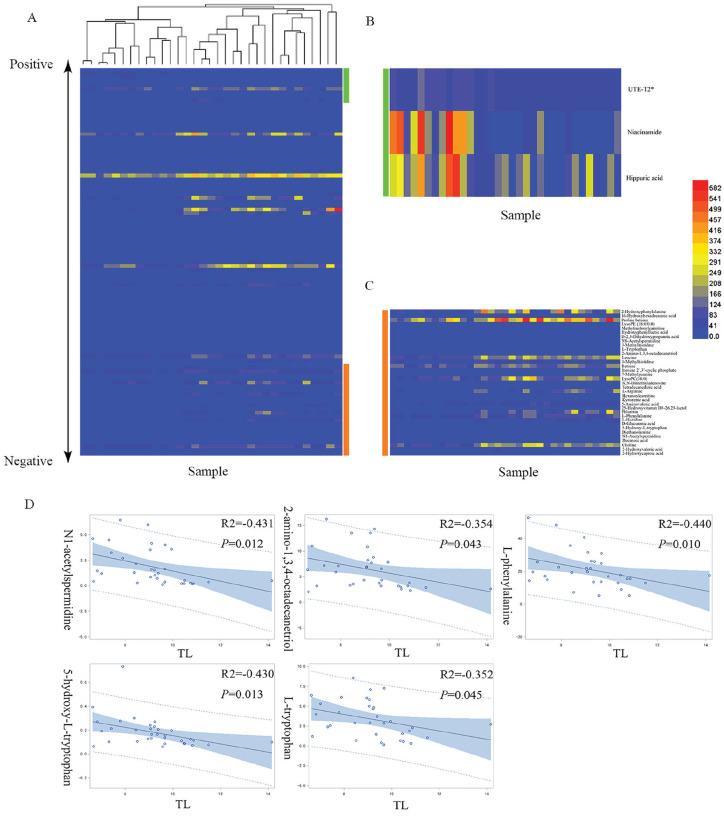
To provide insight into cartilage degeneration-associated differences in global metabolism, the relationships between differential metabolites and the UTE-T2* values of the TL cartilage compartment were assessed by Pearson correlation analysis. Fifteen metabolites involved in a perturbed pathway were found to be correlated with the UTE-T2* values and were selected to be the best candidates for further analysis. The other 10 metabolites without KEGG IDs also showed significant correlations with the UTE-T2* values and were included among the biomarker candidates (Table S4 in the Supplementary Material). In addition, a hierarchical cluster plot is provided in Figure 3 and is based on the Pearson correlation distances of each pair of metabolites among the 103 differential metabolites. The metabolites are shown in descending order according to the Pearson correlation coefficients between the metabolite levels (linear normalization) and the UTE-T2* values of the TL cartilage compartment. Subsequently, ROC analysis was performed to assess how well the levels of the aforementioned 25 metabolites could efficiently differentiate knees affected by cartilage degeneration from controls based on the AUC results. Overall, 25 metabolites had a potentially useful diagnostic value with an AUC above 0.7, and 17 metabolites had a good diagnostic value with an AUC greater than 0.9 ( Table 2 ). Among them, N1-acetylspermidine, 2-amino-1,3,4-octadecanetriol, l-phenylalanine, 5-hydroxy-l-tryptophan, and l-tryptophan showed the best AUC values and Pearson correlation coefficients.
Figure 3.
(A) The heatmap plot based on the Pearson correlation analysis is ordered according to the Pearson correlation coefficient (from low [bottom] to high [top]). (B, C) Zoomed-in view of the metabolites that were positively (green) and negatively (orange) correlated with the TL (lateral tibia) cartilage compartment UTE-T2* values. (D) Five metabolites (N1-acetylspermidine, 2-amino-1,3,4-octadecanetriol, l-phenylalanine, 5-hydroxy-l-tryptophan, l-tryptophan) that were inversely correlated with the UTE-T2* values are shown. The full list of metabolites correlated with the UTE-T2* values is provided in Table S4 in the Supplementary Material.
Table 2.
Receiver Operating Characteristic Analysis of 25 Potential Biomarkers Useful for Determining Cartilage Degeneration.
| Metabolites | Area Under the Curve | Standard Error | 95% CI | Sensitivity | Specificity | |
|---|---|---|---|---|---|---|
| Lower | Upper | |||||
| N1-Acetylspermidine | 1.00 | 0.00 | 1.00 | 1.00 | 1.00 | 1.00 |
| 2-Amino-1,3,4-octadecanetriol | 0.98 | 0.02 | 0.93 | 1.00 | 0.90 | 1.00 |
| l-Phenylalanine | 0.95 | 0.04 | 0.88 | 1.00 | 0.81 | 1.00 |
| 5-Hydroxy-l-tryptophan | 0.94 | 0.04 | 0.86 | 1.00 | 0.90 | 0.92 |
| Phloretin | 0.94 | 0.04 | 0.86 | 1.00 | 0.86 | 1.00 |
| 5-Aminovaleric acid | 0.93 | 0.04 | 0.85 | 1.00 | 0.81 | 0.92 |
| Methylmalonylcarnitine | 0.93 | 0.04 | 0.84 | 1.00 | 0.71 | 1.00 |
| Hexanoylcarnitine | 0.92 | 0.04 | 0.84 | 1.00 | 0.81 | 0.92 |
| 25-Hydroxyvitamin D3-26,23-lactol | 0.92 | 0.05 | 0.83 | 1.00 | 0.81 | 1.00 |
| l-Tryptophan | 0.92 | 0.05 | 0.81 | 1.00 | 0.81 | 1.00 |
| 2-Hydroxyphenylalanine | 0.91 | 0.05 | 0.82 | 1.00 | 0.81 | 0.92 |
| Inosine 2′,3′-cyclic phosphate | 0.91 | 0.06 | 0.80 | 1.00 | 0.86 | 1.00 |
| 1-Methylhistidine | 0.90 | 0.06 | 0.79 | 1.00 | 0.86 | 0.83 |
| l-Histidine | 0.90 | 0.05 | 0.81 | 1.00 | 0.76 | 0.92 |
| N8-Acetylspermidine | 0.90 | 0.05 | 0.80 | 1.00 | 0.86 | 0.83 |
| 16-Hydroxyhexadecanoic acid | 0.90 | 0.06 | 0.79 | 1.00 | 0.86 | 0.92 |
| Kynurenic acid | 0.90 | 0.06 | 0.79 | 1.00 | 0.81 | 0.92 |
| LysoPE(18:0/0:0) | 0.88 | 0.06 | 0.76 | 1.00 | 0.95 | 0.75 |
| 2-Hydroxycaproic acid | 0.87 | 0.07 | 0.73 | 1.00 | 0.86 | 0.83 |
| Hippuric acid | 0.87 | 0.06 | 0.74 | 0.99 | 0.62 | 1.00 |
| 3-Methylhistidine | 0.86 | 0.08 | 0.71 | 1.00 | 0.90 | 0.83 |
| Proline betaine | 0.85 | 0.07 | 0.72 | 0.98 | 0.86 | 0.75 |
| l-Arginine | 0.84 | 0.08 | 0.69 | 0.99 | 0.76 | 0.83 |
| 2-Hydroxyvaleric acid | 0.83 | 0.07 | 0.68 | 0.97 | 0.71 | 0.83 |
| N,N-Dimethyladenosine | 0.80 | 0.08 | 0.63 | 0.96 | 0.67 | 0.92 |
Discussion
In this study, we examined the disturbed metabolite profile in SF and identified promising biomarkers of cartilage degeneration in the rabbit ACLT model using an untargeted metabolite screening approach. Untargeted metabolic analysis could clearly discriminate between ACL-injured knees with potential cartilage degeneration and the controls, indicating that ACL injury caused a dynamic perturbation of the biochemical homeostasis in SF, which was in line with the results of a recent study in rats. 16 Pathway analysis showed that the dysregulated metabolites were related to eleven metabolic pathways involved lipid, amino acid and energy metabolism. After evaluating the relationship between the differentially expressed metabolites in SF and the UTE-T2* values of TL cartilage and calculating the diagnostic power of the candidate metabolites using ROC, we proposed that 5 metabolites (N1-acetylspermidine, 2-amino-1,3,4-octadecanetriol, l-phenylalanine, 5-hydroxy-l-tryptophan, and l-tryptophan) might represent a potential panel of small molecule biomarkers for the early diagnosis of cartilage degeneration. To the best of our knowledge, this is one of the first studies to reveal longitudinal changes in metabolite concentrations in SF and their correlation with the cartilage UTE-T2* relaxation time in a rabbit ACLT model. Furthermore, our selected biomarkers had better performance than 3 traditional biomarkers (urinary c-terminal telopeptide, serum cartilage oligomeric protein and matrix metalloprotease degraded type 2 collagen) with an AUC value of 0.631 in a large-scale meta-analysis of the detection of cartilage degeneration. 17
UTE-T2* measurements can reflect the laminar collagen ultrastructure in the articular cartilage as determined by polarized light microscopy and are sensitive to the early cartilage subsurface degeneration occurring acutely after ACL injury.3,18,19 We found significantly decreased UTE-T2* relaxation values in the TL cartilage compartment in TF and TE groups compared with the N group. Moreover, ACL-injured knees demonstrated lower UTE-T2* values in the medial tibiofemoral and lateral femoral condyle cartilage compartments than observed in sham surgery knees, although the difference was not significant. These results indicate abnormalities of the cartilage matrix in ACL-injured knees at 4 and 8 weeks postsurgery and suggest early cartilage degeneration. Consistent with the present results, early osteoarthritic changes in cartilage, including the loss of proteoglycans, reduced cartilage stiffness and fibrillations of the cartilage surface, were observed in the lateral compartments of rabbit ACL-injured knees at 4 weeks postsurgery based on histology and T2 measurements. 8
Pathway analysis found that ACL injury caused a significant disturbance in the biochemical homeostasis of the knee joint, which was potentially related to cartilage degeneration. Amino acid and linoleic acid are 2 classes of metabolites that have been found to be notably disrupted in SF. Linoleic acid metabolism is the most greatly affected pathway in the early course of ACL injury. Linoleic acid is a major component of omega-6 fatty acids and is one of the most prominent fatty acids in SF. Its level in SF depends on the degree of joint disease and the fatty acid levels in serum.20,21 This could result in the production of PGE2, one of the major catabolic mediators involved in cartilage degradation in stimulated chondrocytes.20,22 In addition, previous studies have found that omega-6 fatty acids play a critical role in the pathogenesis of OA due to their pro- or anti-inflammatory effects. 23 Meanwhile, 8 metabolites involved in arginine and proline metabolism were observed to have significant accumulation in ACL-injured knees. As important products of arginine and proline metabolism, phosphocreatine and creatinine were also dramatically increased in ACL-injured knees, which indicated enhanced ATP production and utilization. This can be explained by the profound ATP depletion in chondrocytes in OA, which is due to the increased production of reactive oxygen species and nitric oxide in chondrocytes.24,25 Thus, we hypothesized that the upregulation of arginine and proline metabolism revealed the metabolic adaptation of knee joints to oxygen stress in degenerative conditions.
Among the 5 identified metabolic biomarker candidates, we found that the UTE-T2* values of TL cartilage were significantly correlated with the N1-acetylspermidine levels in SF. Thus, we propose that the increase in N1-acetylspermidine might be related to the presence of the early stage of cartilage degeneration. Additionally, the levels of N8-acetylspermidine in ACL-injured knees were also observed to be significantly higher than those in controls. In eucaryotic cells, N1-acetylspermidine is a polyamine derivative synthesized via spermidine acetylation by spermine and spermidine acetyltransferase (SSAT). Excess polyamines are converted to acetyl forms and released into extracellular sites. 26 The significant elevation of acetylated polyamine derivations found in our study suggests the potential increase of SSAT and the acceleration of polyamine catabolism in ACL-injured knees. Accelerated polyamine catabolism results in the consumption of energy and produces potentially harmful compounds, such as H2O2, in cells. 27 Oxidative stress induced by H2O2 is capable of upsetting cartilage homeostasis and accelerating catabolism in chondrocytes, which may induce a vicious cycle and aggravate cartilage breakdown. 28 Therefore, we speculated that the high extracellular concentration of N1-acetylspermidine, which indicated the activation of SSAT and the entire catabolic pathway, was correlated with chondrocyte injury in early cartilage degeneration.
We found that the 2-amino-1,3,4-octadecanetriol concentration was higher in ACL-injured knees, and its level was inversely correlated with the UTE-T2* values in TL cartilage. 2-Amino-1,3,4-octadecanetriol is structurally similar to sphingosine. It is well known to be involved in many significant cellular responses, including apoptosis, differentiation, and migration. 29 However, the physiological role of 2-amino-1,3,4-octadecanetriol in chondrocytes is largely unknown according to the literature. Based on our findings, 2-amino-1,3,4-octadecanetriol might have an effect on cartilage degeneration and may be potentially useful in diagnosis. However, more studies are necessary to investigate the effects of 2-amino-1,3,4-octadecanetriol on cartilage degeneration.
Additionally, the levels of l-phenylalanine and its derivatives (2-hydroxyphenylalanine) were also increased, while the levels of hippuric acid obtained from l-phenylalanine were decreased in ACL injury models. It is known that type-II collagen is the main component of the articular cartilage extracellular matrix and is mainly composed of l-phenylalanine, proline, and hydroxyproline. 30 Thus, we believe that the elevated l-phenylalanine level in SF can be explained by the partial breakdown of type-II collagen in the early course of cartilage degeneration. Of note, 2-hydroxyphenylalanine can be generated by oxidative stress through the destruction by the hydroxyl radical of the benzyl ring of l-phenylalanine. 31 2-Hydroxyphenylalanine is therefore considered to be a biochemical marker of oxidative stress.31,32 Taken together, the results indicating the presence of l-phenylalanine metabolic alterations in ACL injury models may show that l-phenylalanine could serve as a sensitive biomarker for cartilage degeneration by revealing matrix breakdown and oxidative stress.
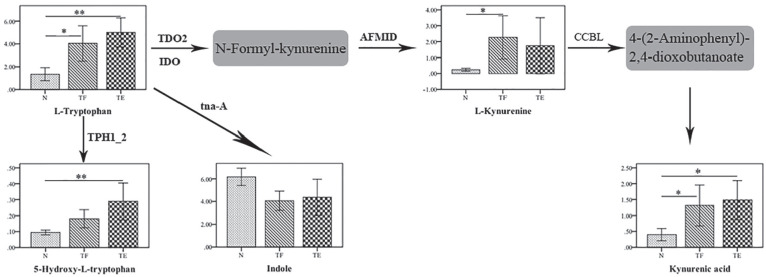
Furthermore, we found significant increases in the levels of tryptophan and its derivative (5-hydroxy-l-tryptophan) in SF, which was associated with cartilage degeneration. Interestingly, we also found that kynurenine and kynurenic acid, which are important products of the kynurenine pathway, were significantly increased. Tryptophan is an essential precursor for several biologically important metabolic pathways, especially the kynurenine pathway ( Fig. 4 ). Thus, the accumulation of kynurenine and kynurenic acid in SF can be explained by the activation of kynurenine metabolism in ACL-injured knees. Metabolism of tryptophan through the kynurenine metabolic pathway has been linked to inflammation in a previous study. 33 Wei et al. 34 found that there is a close relationship between the kynurenine metabolic pathway and the activation of cytokines, such as interleukin (IL)-4, IL-23, tumor necrosis factor–α (TNF-α). 34 It was reported that the overproduction of cytokines is a major irritant factor in the pathophysiology of OA. 35 These cytokines can stimulate their own expression and activate chondrocytes, inducing them to synthesize molecules related to extracellular matrix destruction.36,37 These results indicated that the activation of tryptophan and kynurenine metabolism observed in our study potentially reflects the inflammatory response involved in the early course of cartilage degeneration.
Figure 4.
Bar charts showing the concentrations of the 5 metabolites in the tryptophan pathway identified among the 3 groups. The vertical axis represents the levels; the horizontal axis represents the group; the whiskers represent the 95% confidence level. *P < 0.05, **P < 0.001. The gray box represents the metabolites that were not measured. The enzymes involved in the individual chemical reactions are denoted next to the arrows connecting the 2 metabolites.
There were certain limitations in this study. First, the sample sizes were not equal among the three groups due to the presence of joint infection indicated in the MRI PD images. The sample sizes of the TF and TE groups were eleven and ten, respectively. Despite this limitation, statistical analysis was performed on the data, and statistically significant conclusions were drawn. This minimized the interanimal variation. Additionally, only 2 time points were chosen for this analysis. However, previous studies have found that degenerative changes may occur as early as 4 to 12 weeks after intervention, and moderate/severe OA was shown to be already present at 8 weeks after ACL injury.8,38 Last, MRI in this study was performed on a 3-T scanner, which limited the resolution of images of the thin cartilage in the rabbit knee. Further studies are needed using higher scanner field.
In conclusion, the pathway analysis and biomarker panel used in our study revealed inflammatory and oxidation-related properties, indicating the involvement of inflammatory activity and oxidative stress in the course of cartilage degeneration in the rabbit ACLT model. This study also found that a panel composed of N1-acetylspermidine, 2-amino-1,3,4-octadecanetriol, l-phenylalanine, 5-hydroxy-l-tryptophan, and l-tryptophan showed high specificity and sensitivity for the early diagnosis of cartilage degeneration. Our results indicate that further investigation should be conducted in human participants, including the targeted analysis of identified biomarkers.
Supplemental Material
Supplemental material, Revised_Supplemental_table for Disturbances in Metabolic Pathways and the Identification of a Potential Biomarker Panel for Early Cartilage Degeneration in a Rabbit Anterior Cruciate Ligament Transection Model by Yiwen Hu, Qian Wu, Yang Qiao, Peng Zhang, Wentao Dai, Hongyue Tao and Shuang Chen in CARTILAGE
Supplemental material, Supplementary_FigureS1 for Disturbances in Metabolic Pathways and the Identification of a Potential Biomarker Panel for Early Cartilage Degeneration in a Rabbit Anterior Cruciate Ligament Transection Model by Yiwen Hu, Qian Wu, Yang Qiao, Peng Zhang, Wentao Dai, Hongyue Tao and Shuang Chen in CARTILAGE
Supplemental material, Supplementary_Materials_and_Method for Disturbances in Metabolic Pathways and the Identification of a Potential Biomarker Panel for Early Cartilage Degeneration in a Rabbit Anterior Cruciate Ligament Transection Model by Yiwen Hu, Qian Wu, Yang Qiao, Peng Zhang, Wentao Dai, Hongyue Tao and Shuang Chen in CARTILAGE
Footnotes
Supplementary material for this article is available on the Cartilage website at https://journals.sagepub.com/home/car.
Authors’ Note: The current study was done in Huashan Hospital, Fudan University, Shanghai, China.
Acknowledgments and Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the National Natural Science Foundation for Young Scholars, China (No. 81501440), and the Project of the Shanghai Municipal Science and Technology Commission, China (16ZR1404600). The funding organization had no role in the study design or conduct, data collection, analysis or interpretation of data, preparation or review of the manuscript, or the decision to submit this manuscript for publication.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Ethical Approval: The Animal Care and Ethics Committee of our institution approved all experimental procedures using animals (Application No.: 1000805).
Animal Welfare: All experiments comply with the ARRIVE (Animal Research: Reporting of In Vivo Experiments) guidelines.
ORCID iD: Shuang Chen  https://orcid.org/0000-0001-6716-1763
https://orcid.org/0000-0001-6716-1763
References
- 1. Lattermann C, Jacobs CA, Bunnell MP, Huston LJ, Gammon LG, Johnson DL, et al. A multicenter study of early anti-inflammatory treatment in patients with acute anterior cruciate ligament tear. Am J Sports Med. 2017;45:325-33. [DOI] [PubMed] [Google Scholar]
- 2. Pedoia V, Russell C, Randolph A, Li X, Majumdar S, Consortium AA. Principal component analysis-T1rho voxel based relaxometry of the articular cartilage: a comparison of biochemical patterns in osteoarthritis and anterior cruciate ligament subjects. Quant Imaging Med Surg. 2016;6:623-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Williams AA, Titchenal MR, Andriacchi TP, Chu CR. MRI UTE-T2* profile characteristics correlate to walking mechanics and patient reported outcomes 2 years after ACL reconstruction. Osteoarthritis Cartilage. 2018;26:569-79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Sun EY, Fleck AKM, Abu-Hakmeh AE, Kotsakis A, Leonard GR, Wan LQ. Cartilage metabolism is modulated by synovial fluid through metalloproteinase activity. Ann Biomed Eng. 2018;46:810-8. [DOI] [PubMed] [Google Scholar]
- 5. Kaplan DJ, Cuellar VG, Jazrawi LM, Strauss EJ. Biomarker changes in anterior cruciate ligament-deficient knees compared with healthy controls. Arthroscopy. 2017;33:1053-61. [DOI] [PubMed] [Google Scholar]
- 6. Cardoso MR, Santos JC, Ribeiro ML, Talarico MCR, Viana LR, Derchain SFM. A metabolomic approach to predict breast cancer behavior and chemotherapy response. Int J Mol Sci. 2018;19(2_suppl):E617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bai B, Li Y. Danshen prevents articular cartilage degeneration via antioxidation in rabbits with osteoarthritis. Osteoarthritis Cartilage. 2016;24:514-20. [DOI] [PubMed] [Google Scholar]
- 8. Rautiainen J, Nissi MJ, Liimatainen T, Herzog W, Korhonen RK, Nieminen MT. Adiabatic rotating frame relaxation of MRI reveals early cartilage degeneration in a rabbit model of anterior cruciate ligament transection. Osteoarthritis Cartilage. 2014;22:1444-52. [DOI] [PubMed] [Google Scholar]
- 9. Kumagai K, Toyoda F, Staunton CA, Maeda T, Okumura N, Matsuura H, et al. Activation of a chondrocyte volume-sensitive Cl− conductance prior to macroscopic cartilage lesion formation in the rabbit knee anterior cruciate ligament transection osteoarthritis model. Osteoarthritis Cartilage. 2016;24:1786-94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Gao SG, Cheng L, Zeng C, Wei LC, Zhang FJ, Tian J, et al. Usefulness of specific OA biomarkers, thrombin-cleaved osteopontin, in the posterior cruciate ligament OA rabbit model. Osteoarthritis Cartilage. 2013;21:144-50. [DOI] [PubMed] [Google Scholar]
- 11. Lindhorst E, Wachsmuth L, Kimmig N, Raiss R, Aigner T, Atley L, et al. Increase in degraded collagen type II in synovial fluid early in the rabbit meniscectomy model of osteoarthritis. Osteoarthritis Cartilage. 2005;13:139-45. [DOI] [PubMed] [Google Scholar]
- 12. Yuan M, Kremer DM, Huang H, Breitkopf SB, Ben-Sahra I, Manning BD, et al. Ex vivo and in vivo stable isotope labelling of central carbon metabolism and related pathways with analysis by LC-MS/MS. Nat Protoc. 2019;14:313-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Yang B, Gao H, Stanton C, Ross RP, Zhang H, Chen YQ, et al. Bacterial conjugated linoleic acid production and their applications. Prog Lipid Res. 2017;68:26-36. [DOI] [PubMed] [Google Scholar]
- 14. Maceyka M, Spiegel S. Sphingolipid metabolites in inflammatory disease. Nature. 2014;510:58-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Jurkowska H, Stipanuk MH, Hirschberger LL, Roman HB. Propargylglycine inhibits hypotaurine/taurine synthesis and elevates cystathionine and homocysteine concentrations in primary mouse hepatocytes. Amino Acids. 2015;47:1215-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Maerz T, Sherman E, Newton M, Yilmaz A, Kumar P, Graham SF, et al. Metabolomic serum profiling after ACL injury in rats: a pilot study implicating inflammation and immune dysregulation in post-traumatic osteoarthritis. J Orthop Res. 2018;36(7):1969-79. [DOI] [PubMed] [Google Scholar]
- 17. Valdes AM, Meulenbelt I, Chassaing E, Arden NK, Bierma-Zeinstra S, Hart D, et al. Large scale meta-analysis of urinary C-terminal telopeptide, serum cartilage oligomeric protein and matrix metalloprotease degraded type II collagen and their role in prevalence, incidence and progression of osteoarthritis. Osteoarthritis Cartilage. 2014;22:683-9. [DOI] [PubMed] [Google Scholar]
- 18. Titchenal MR, Williams AA, Chehab EF, Asay JL, Dragoo JL, Gold GE, et al. Cartilage subsurface changes to magnetic resonance imaging UTE-T2* 2 years after anterior cruciate ligament reconstruction correlate with walking mechanics associated with knee osteoarthritis. Am J Sports Med. 2018;46:565-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Williams A, Qian Y, Golla S, Chu CR. UTE-T2* mapping detects sub-clinical meniscus injury after anterior cruciate ligament tear. Osteoarthritis Cartilage. 2012;20:486-94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Bastiaansen-Jenniskens YM, Siawash M, van de Lest CH, Verhaar JA, Kloppenburg M, Zuurmond AM, et al. Monounsaturated and saturated, but not n-6 polyunsaturated fatty acids decrease cartilage destruction under inflammatory conditions: a preliminary study. Cartilage. 2013;4:321-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Oliviero F, Lo Nigro A, Bernardi D, Giunco S, Baldo G, Scanu A, et al. A comparative study of serum and synovial fluid lipoprotein levels in patients with various arthritides. Clin Chim Acta. 2012;413:303-7. [DOI] [PubMed] [Google Scholar]
- 22. Li X, Ellman M, Muddasani P, Wang JH, Cs-Szabo G, van Wijnen AJ, et al. Prostaglandin E2 and its cognate EP receptors control human adult articular cartilage homeostasis and are linked to the pathophysiology of osteoarthritis. Arthritis Rheum. 2009;60:513-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Valdes AM, Ravipati S, Pousinis P, Menni C, Mangino M, Abhishek A, et al. Omega-6 oxylipins generated by soluble epoxide hydrolase are associated with knee osteoarthritis. J Lipid Res. 2018;59:1763-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Dave M, Attur M, Palmer G, Al-Mussawir HE, Kennish L, Patel J, et al. The antioxidant resveratrol protects against chondrocyte apoptosis via effects on mitochondrial polarization and ATP production. Arthritis Rheum. 2008;58:2786-97. [DOI] [PubMed] [Google Scholar]
- 25. Mobasheri A, Rayman MP, Gualillo O, Sellam J, van der Kraan P, Fearon U. The role of metabolism in the pathogenesis of osteoarthritis. Nat Rev Rheumatol. 2017;13:302-11. [DOI] [PubMed] [Google Scholar]
- 26. Smirnova OA, Bartosch B, Zakirova NF, Kochetkov SN, Ivanov AV. Polyamine metabolism and oxidative protein folding in the ER as ROS-producing systems neglected in virology. Int J Mol Sci. 2018;19:E1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Janne J, Alhonen L, Pietila M, Keinanen TA, Uimari A, Hyvonen MT, et al. Genetic manipulation of polyamine catabolism in rodents. J Biochem. 2006;139:155-60. [DOI] [PubMed] [Google Scholar]
- 28. Hwang HS, Kim HA. Chondrocyte apoptosis in the pathogenesis of osteoarthritis. Int J Mol Sci. 2015;16:26035-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Jang EJ, Shin Y, Park HJ, Kim D, Jung C, Hong JY, et al. Anti-melanogenic activity of phytosphingosine via the modulation of the microphthalmia-associated transcription factor signaling pathway. J Dermatol Sci. 2017;87:19-28. [DOI] [PubMed] [Google Scholar]
- 30. Xu Z, Chen T, Luo J, Ding S, Gao S, Zhang J. Cartilaginous metabolomic study reveals potential mechanisms of osteophyte formation in osteoarthritis. J Proteome Res. 2017;16:1425-35. [DOI] [PubMed] [Google Scholar]
- 31. Kun S, Mikolás E, Molnár GA, Sélley E, Laczy B, Csiky B, et al. Association of plasma ortho-tyrosine/para-tyrosine ratio with responsiveness of erythropoiesis-stimulating agent in dialyzed patients. Redox Rep. 2014;19:190-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Matayatsuk C, Poljak A, Bustamante S, Smythe GA, Kalpravidh RW, Sirankapracha P, et al. Quantitative determination of ortho- and meta-tyrosine as biomarkers of protein oxidative damage in beta-thalassemia. Redox Rep. 2007;12:219-28. [DOI] [PubMed] [Google Scholar]
- 33. Morris JK, Piccolo BD, Shankar K, Thyfault JP, Adams SH. The serum metabolomics signature of type 2 diabetes is obscured in Alzheimer’s disease. Am J Physiol Endocrinol Metab. 2018;314:E584-E596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Wei M, Ma Y, Liu Y, Zhou Y, Men L, Yue K, et al. Urinary metabolomics study on the anti-inflammation effects of flavonoids obtained from Glycyrrhiza. J Chromatogr B Analyt Technol Biomed Life Sci. 2018;1086:1-10. [DOI] [PubMed] [Google Scholar]
- 35. Pelletier JP, Martel-Pelletier J, Abramson SB. Osteoarthritis, an inflammatory disease: potential implication for the selection of new therapeutic targets. Arthritis Rheum. 2001;44:1237-47. [DOI] [PubMed] [Google Scholar]
- 36. Baker M, Brook BS, Owen MR. Mathematical modelling of cytokines, MMPs and fibronectin fragments in osteoarthritic cartilage. J Math Biol. 2017;75:985-1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Rahmati M, Mobasheri A, Mozafari M. Inflammatory mediators in osteoarthritis: a critical review of the state-of-the-art, current prospects, and future challenges. Bone. 2016;85:81-90. [DOI] [PubMed] [Google Scholar]
- 38. Batiste DL, Kirkley A, Laverty S, Thain LM, Spouge AR, Holdsworth DW. Ex vivo characterization of articular cartilage and bone lesions in a rabbit ACL transection model of osteoarthritis using MRI and micro-CT. Osteoarthritis Cartilage. 2004;12:986-96. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, Revised_Supplemental_table for Disturbances in Metabolic Pathways and the Identification of a Potential Biomarker Panel for Early Cartilage Degeneration in a Rabbit Anterior Cruciate Ligament Transection Model by Yiwen Hu, Qian Wu, Yang Qiao, Peng Zhang, Wentao Dai, Hongyue Tao and Shuang Chen in CARTILAGE
Supplemental material, Supplementary_FigureS1 for Disturbances in Metabolic Pathways and the Identification of a Potential Biomarker Panel for Early Cartilage Degeneration in a Rabbit Anterior Cruciate Ligament Transection Model by Yiwen Hu, Qian Wu, Yang Qiao, Peng Zhang, Wentao Dai, Hongyue Tao and Shuang Chen in CARTILAGE
Supplemental material, Supplementary_Materials_and_Method for Disturbances in Metabolic Pathways and the Identification of a Potential Biomarker Panel for Early Cartilage Degeneration in a Rabbit Anterior Cruciate Ligament Transection Model by Yiwen Hu, Qian Wu, Yang Qiao, Peng Zhang, Wentao Dai, Hongyue Tao and Shuang Chen in CARTILAGE