Abstract
The aim of this study is to evaluate the mechanical and biological performance of cartilage-like constructs produced by 3D printing. During the investigation, poly(ε-caprolactone) (PCL) and polyvinylpyrrolidone (PVP) were used as a matrix polymer and low-molecular-weight chitosan (CS), hyaluronic acid (HA), and alginic acid sodium salt (SA) were integrated separately with the polymer matrix to fabricate the constructs. Thermal, mechanical, morphology, and chemical properties and swelling, degradation, and biocompatibility behaviors were evaluated in detail. With the addition of 3 fillers, the melting temperature of the matrix increased with the addition of fillers, and PCL/3wt.%PVP/1wt.%HA had the highest melting temperature value. Mechanical characterization results demonstrated that the printed PCL/3wt.%PVP/1wt.%CS displayed the highest compressive strength of around 9.51 MPa. The compressive strength difference between the PCL/3wt.%PVP and PCL/3wt.%PVP/1wt.%CS was 5.38 MPa. Biocompatibility properties of the constructs were tested by mitochondrial dehydrogenase activity, and in vitro studies showed that the PCL/3wt.%PVP/1wt.%HA composite construct had more cell viability than the other constructs by making use of the mesenchymal stem cell line.
Keywords: cartilage tissue engineering, mesenchymal stem cell, polycaprolactone, polyvinylpyrrolidone
Introduction
Joint pain and chronic disability are caused mainly by osteoarthritis, aging, and joint damage. For self-healing of mature cartilage, blood vessels, nerves, and lymphatics must be present. Joint replacement surgery is the most common treatment for cartilage degeneration in an advanced stage, but this treatment is highly complicated and expensive.1,2 Although cell transplantation–based tissue engineering treatment for human cartilage repair has been practiced since almost 20 years, the new tissue generated is not yet been like a natural cartilage when using existing cartilage tissue engineering strategies, regional cloning, extracellular matrix composition. 1 Cartilage tissue engineering has been a developmental area for the last 20 years. However, there are still many clinical obstacles for treatment, even though there have been huge efforts on developing new biological solutions. In this period, a lot of developmental efforts ensued that have or could positively affect biomaterial technologies, cell sources, molecular mechanisms, and genetic manipulations for cartilage tissue engineering. 3 Inadequate success in tissue engineering has shown that it does not have complexity with regard to cell types and tissue patterns, so interest in the tissue engineering of the musculoskeletal system using 3-dimensional (3D) printing technology has increased. 4 Three-dimensional scaffolds, which can be produced with biodegradable, biocompatible polymers, and cells are linked in order to get in vitro tissues for cartilage regeneration. 5 Three-dimensional scaffolds have a significant role in articular cartilage tissue engineering approaches. 6 Biodegradable biomimetic structures are generated by using a 3D printer and functional structures are obtained for tissue engineering and organ replacement applications. 2 Moreover, biomaterials that have high resolution and different cells are used in order to resemble the microarchitecture of different tissues using 3D bioprinting.7-9 Three-dimensional printing technique can build the constructs without any vascularization problem and it also has a great advantage of operating automatically. 10 The computer-guided 3D printing technique is a novel and rapid fabrication technology that can produce scaffolds with complex geometries, 10 and it improves the functionality of scaffolds in 3D space. It is possible to fabricate scaffolds with personalized characteristics that have the fine shape and function of targeted tissues.11,12 Micro-extrusion, laser-assisted, and inkjet-printing are different types of 3D printing. The physical properties of the solutions can be a challenge for optimizing the structures in 3D printing. Therefore, the solid form of the solutions was used to prevent any effects caused by solutions’ viscosity, surface tension, and density effects. 13 Biodegradable and biocompatible polymers are important to form the cartilage structure. In this study, to determine the functional cartilage-like constructs, several polymers based on the same concentrations of hyaluronic acid, chitosan, and sodium alginate were investigated as potential cartilage-like tissue. Poly(ε-caprolactone) has several advantages in the human body, which are excellent thermal stability and biocompatibility, but it is hydrophobic, which is a disadvantage for cell adhesion. 13 Alginate has controllable degradation properties and good biocompatibility. 9 Additionally, Chitosan is nontoxic, and also it is preferable due to antibacterial properties. 14 Hyaluronic acid is a ubiquitous part of extracellular matrices, and it has important functions in cartilage. 15 Chitosan has been used for articular cartilage engineering, as its structure is similar to different glycosaminoglycans (GAGs) that are responsible for modulating chondrocyte morphology and differentiation.15-17 Sodium alginate is widely used in cartilage tissue engineering applications. 18 According to Daly et al., it was found that alginic acid sodium salt is the best supporting polymer for the development of hyaline-like cartilage compared with GelMA and BioINK. 4
Materials and Method
Materials
Poly(ε)caprolactone (PCL; molecular weight [MW] = 80,000 Da), polyvinylpyrrolidone (PVP; MW = 40,000 Da), chitosan (CS; MW = 50,000-190,000 Da), alginic acid sodium salt (SA; MW = 120,000-190,000 Da) were obtained from Sigma Aldrich, and hyaluronic acid (HA) was obtained from Yigitoglu Chemistry (Istanbul, Turkey).
Preparation of Digital Model and G-Code Export
Preparation of the 3D cartilage constructs consisted of 2 steps: (1) PCL/3wt.%PVP matrix formation and (2) formation of PCL/3wt.%PVP/1wt.%CS, PCL/3wt.%PVP/1wt.%HA, PCL/3wt.%PVP/1wt.%SA constructs. To form both constructs, solid model of the structures were drawn in 20 × 20 × 3 mm3 dimensions, and it was transferred in Simplify program to get G-code, which gave a command to the 3D device for printing the constructs.
Preparation of PCL/3wt.%PVP Solution and Constructs
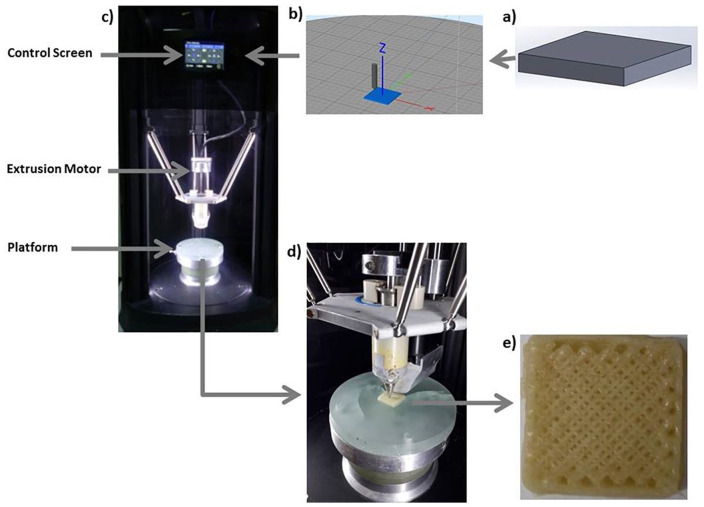
First, PCL (9.7 g) and PVP (0.3 g) were weighted by using a scientific balance (RADWAG). In the second step, PCL was put on a magnetic stirrer (Velp Scientifica) at 150°C to get the gel form, and after 20 minutes, PVP was added into this gel and mixed manually for forming a homogeneous mixture. After the mixing process was completed, the homogeneous structure was shaped to take the shape of the printer’s chamber and the mixture was put into this chamber. Constructs were built according to the G-code commands. For fabrication of PCL/3wt.%PVP constructs, 60% and 70% infill properties were adjusted to determine the ideal pore size distributions. Pore size values at these 2 infill rates were measured via SEM (scanning electron microscopy) and Optical Microscope Analysis program (Olympus Analysis, USA) by using an SEM image (50× magnification) and this image was transferred to the Analysis program to measure the pore dimensions theoretically. Almost 20 pores were measured for PCL/PVP constructs with 60% and 70% infill rates and the mean pore size values of the constructs were obtained. Another process parameter is temperature, which is a very crucial parameter for building the constructs. The device is also available for changing the print speeds and extrusion rates at the optimization stages. The fabrication process stages are given in Figure 1a-e .
Figure 1.
The process steps for cartilage construct fabrication: solid model (a), slicing process (b), 3D device (c), printing process (d), and printed cartilage construct (e).
Preparation of the PCL/3wt.%PVP/1wt.%(CS, SA, HA) Solutions and Constructs
First, PCL and PVP (3 wt.%) were mixed using the same method as described above. After that CS, SA, and HA powders were added into the matrix polymer as a filler with the same amount (1 wt.%). PCL/3wt.%PVP/1wt.%(CS, SA, HA) mixtures were printed with the same production parameters as in PCL/3wt.%PVP matrix construct to obtain the constructs.
Characterization of the Printed Constructs
To observe the characteristic properties of the printed constructs, characterization tests were performed. Fourier-transform infrared spectroscopy (FTIR, JASCO-4000) was used to detect the presence of typical functional groups of pure polymers. Scans were recorded in transmittance mode, between 4,000 and 400 cm−1 with a resolution of 4 cm−1. Printed constructs were shaped as plate and the test was performed for all constructs and compared with pure components. Morphological properties were obtained by using SEM (EVO MA10). The constructs were coated with gold for 90 seconds, 18 mA prior to imaging, to enable conductivity in the constructs. Differential scanning calorimetry (DSC, Shimadzu) was used to determine the thermal transitions of the constructs in the closed pan, and heating temperature ranges were adjusted from room temperature to 200°C and the heating rate was selected as 10°C/min. Mechanical properties of the constructs were determined with a compressive testing device (Shimadzu) to examine the mechanical behavior of the constructs. Test parameters, temperature, force, and speed, were adjusted to 20°C, 0.1 N, and 1 mm/min, respectively. Two experiments were performed for each constructs allowing for the mean values to be determined. The swelling test is similar to the method used in Correia et al. 19 First, 3 pieces were taken from each construct (with nearly the same amounts) and these pieces were placed in Eppendorf tubes. Phosphate buffer saline (PBS) at pH 7 (Merck) was added to the tubes and they were placed on the thermo-shaker (Biosan) with 250 revolutions per minute at 37°C. The weights were measured every 24 hours without changing the PBS. According to the same method as in the swelling test, the degradation test was performed. This test was completed in 1 month by measuring the weights of all constructs each week by changing the PBS after each measurement. To test the viability properties of the cells into the 3D scaffolds, MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide) assay was performed, and in order to see the cell morphology on the constructs, SEM analysis was used after cell fixation. The 3D printed constructs with round pieces (3 mm in thickness and discs of 6 mm in diameter) were transferred to 96-well plates and sterilized under ultraviolet (UV) light overnight, and then the constructs were incubated in cell culture medium for 2 hours before cell seeding. Mesenchymal stem cell (MSC; ATCC, PCS-500-011) suspensions (1 × 104 cells/well in Dulbecco’s modified Eagle’s medium supplemented with 10% fetal bovine serum and 1% penicillin/streptomycin) were seeded onto the scaffold for specific intervals at 37°C in a 5% CO2 incubator, followed by the examination of cell adhesion, proliferation, and cytotoxicity. An MTT assay was performed to analyze the cell viability during 3 days of cell culture on 3D-printed scaffolds. On the day of the test, cultured composite scaffolds were washed with cold PBS (pH 7.4), followed by addition of MTT solution at the concentration of 0.5 mg/mL (0.5 mL) and incubated for 4 hours at 37°C in a humid environment with 5 vol% CO2. 20 According to the manufacturer’s protocol, the supernatant was removed gently followed by the addition of 1.5 mL of dimethylsulfoxide. Plates were again incubated for 15 minutes at 37°C with 5% CO2 and the absorbance was measured using a microplate reader in 590 nm wavelength. In this study, only biocompatibility properties of the printed constructs were tested with MSC; thus, MSCs were not tested for MSC markers and their potential to generate chondrocytes. The attachment and spreading of MSCs on the 3D-printed constructs were evaluated by SEM. After culture day 4, cell-laden constructs were fixed with 4% glutaraldehyde (Sigma) for 10 minutes and then dehydrated through serial dilutions of ethanol and dried in air. Dried specimens were sputter-coated with gold and observed by SEM (EVO MA-10, Zeiss, Germany) with an accelerating voltage of 10 kV. All data were presented as the mean ± standard deviation. Statistical analyses were performed by Student’s t test with 95% confidence interval. All measurements were performed in duplicate and their mean values were taken as the final result. Differences were considered significant at P < 0.05.
Results and Discussion
Morphology and Pore Size Analysis of Printed Cartilage Constructs
Scaffold pore size is an important parameter in tissue regeneration, and pores enable cells to an unstable environment to control the formation of new tissues. 21 According to the SEM results in Figure 2a-d , it was observed that pore sizes were getting smaller when the infill rates were increased. Sixty percent infill rate gave ~528 µm and 70% infill rate gave ~400 µm mean pore size values. Additionally, it was determined that 60% infill rate gave a better distribution than 70% infill rate. Therefore, other constructs were produced with this infill rate value. As a result of the pore distribution for constructs fabricated with 60% infill rate, different pore sizes and structures were observed. The average pore sizes for PCL/PVP/CS (1 wt.%), PCL/PVP/HA (1 wt.%), and PCL/PVP/SA (1 wt.%) were 512 µm, 448 µm, and 436 µm, respectively. Pore size range in tissue engineering usually ranges from ~150 µm to ~500 µm.22-24 According to the Zhang et al., 24 results indicated that the pore size effect on tissue regeneration was increased as follows: 250 to 355 µm < 355 to 425 µm < 425 to 500 µm < 150 to 250 µm. It can be deduced from this that cells could distribute both larger and smaller pore regions homogeneously. Therefore, large pore sizes found in this study can increase the cell-cell interactions by delivering a sufficient number of cells and form a 3D microenvironment to increase cartilage tissue formation. 25
Figure 2.
SEM images of the cartilage construct with 60% (a, b) and 70% (c, d) infill rates.
FTIR Analysis
In Figure 3A , pure components of the composites were subjected to FTIR to compare the chemical structures of the composites with their components. In Figure 3(A, a) , 3 main absorption peaks were observed at ~2940 cm−1 (asymmetric CH2 stretching), ~2865 cm−1 (symmetric CH2 stretching), ~1720 cm−1 (carbonyl stretching) for PCL. 26 In Figure 3(A, b) , CH2 asymmetric stretching vibration occurred at ~2943 cm−1. The peaks at ~1016.3 cm−1 and ~1282 cm−1 refer to the CH2 rocking mode and twisting of CH2, respectively. The peak at ~1491 cm−1 refers to the CH2 scissor mode. 27 CS had main absorption peaks at ~3283 cm−1 (N-H stretching), ~2869 cm−1 (CH stretching), ~1589 cm−1 (C=O stretching; Fig. 3(A, c) ). 26 In Figure 3(A, d) , SA had absorption peaks at ~3227 cm−1 due to the O-H stretching, asymmetric stretching of COO observed at ~1592 cm−1, and symmetric stretching of COO observed at ~1404 cm−1. HA showed 3 main absorption peaks at ~2888 cm−1 (C-H stretching), ~1605 cm−1 (amid II group), ~1403 cm−1 (C-O; Figure 3(A, e) ). 28 The FTIR spectra of PCL/3wt.%PVP, PCL/3wt.%PVP/1wt.%CS, PCL/3wt.%PVP/SA, and PCL/3wt.%PVP/1wt.%HA are shown in Figure 3B(a, b, c, d) , respectively. In the PCL/3wt.%PVP spectrum, ~1720 cm−1 and ~2864 cm−1 belong to the PCL. The peak at ~2941 cm−1 was due to the interaction between the PCL (~2940.9 cm−1) and PVP (~2943.8 cm−1). Absorption peaks at ~1365 cm−1 and ~1160 cm−1 were observed after interaction between the polymers. The FTIR spectrum of the PCL/3wt.%PVP/1wt.%CS is shown in Figure 3B(b) . Small differences were observed between the PCL/3wt.%PVP and PCL/3wt.%PVP/1wt.%CS absorption graphics. The absorption peak for PCL/3wt.%PVP was shifted from ~1720 cm−1 to ~1721.2 cm−1 after CS addition. Another interaction peak changed from ~450 cm−1 to ~453 cm−1 with the addition of CS. In Figure 3B(c) , a similar spectrum was observed with the other composites except for 2 absorption peaks, which were ~1721 cm−1 and ~452 cm−1 after SA addition. In Figure 3B(d) , a similar tendency was observed for HA, and the peaks at ~2942 cm−1 and ~452 cm−1 were different from the PCL/3wt.%PVP absorption spectrum.
Figure 3.

FTIR spectrum of pure components: PCL (A, a), PVP (A, b), CS (A, c), SA (A, d), HA (A, e). Printed constructs: PCL/3wt.%PVP (B, a), PCL/3wt.%PVP/1wt.%CS (B, b), PCL/3wt.%PVP/1wt.%SA (B, c), PCL/3wt.%PVP/1wt.%HA (B, d).
Thermal Analysis of Constructs
The resulting DSC thermograms are shown in Figure 4 . Sharp single endothermic peaks were observed at around 62°C to 65°C for all groups. This temperature range correlates to the melting points of the PCL. 29 The melting temperature of the PCL/3wt.%PVP matrix was 62.66°C ( Fig. 4a ). This value was increased with the addition of the CS, HA, and SA to 65.71°C, 67.33°C, and 64.98°C, respectively ( Fig. 4a, b, c, d ). The melting temperature of PCL increased with the addition of the PVP, CS, HA, and SA. These changes might be due to the interactions between the components. According to the DSC results, it was observed that the 3D-printing method and high processing temperature did not cause a significant change in the chemical structure of PCL. 29 The melting point of the PVP (at ~179°C) was not seen in the curve, and this might be due to its dominant amorphous structure and its low amount in the mixture. 30
Figure 4.

DSC thermograph for all constructs with their melting points values and labels: PCL/3wt.%PVP (a), PCL/3wt.%PVP/1wt.%HA (b), PCL/3wt.%PVP/1wt.%SA (c), PCL/3wt.%PVP/1wt.%CS (d).
Compressive Strength of the Constructs
Mechanical properties of articular cartilage are vital due to its structure, which enables the body to smooth surfaces for movement of joints. 31 According to the compressive testing results given in Table 1 and Figure 5 , it can be said that with the addition of polymer, compressive strength values were increased and PCL/3wt.%PVP/1wt.%CS had the highest compressive strength value (9.51 MPa) under the same test conditions. PCL/3wt.%PVP had the lowest compressive strength value (4.13 MPa) compared with the other compressive strength values of the constructs. This result indicated that additive polymers increased the mechanical properties of PCL/3wt.%PVP. Although higher strength values were obtained with addition of the polymer into the matrix, elongation (%) values were nearly the same for all constructs. It means that they increased the resistance to impacts with the same elongation properties. Native cartilage had ~1.5 MPa compressive modulus value, 12 and the compressive strength values found in this study showed that printed cartilage constructs had high enough strength in terms of mechanical properties for cartilage tissue applications.
Table 1.
Compressive Testing Results of All Constructs.
| Constructs | Compressive Strength (MPa) | Strain (%) |
|---|---|---|
| PCL/3wt.%PVP | 4.13 ± 0.035 | 51 |
| PCL/3wt.%PVP/1wt.%CS | 9.51 ± 0.21 | 50 |
| PCL/3wt.%PVP/1wt.%HA | 8.12 ± 0.16 | 50 |
| PCL/3wt.%PVP/1wt.%SA | 7.51 ± 0.09 | 50 |
PCL = poly(ε-caprolactone); PVP = polyvinylpyrrolidone; CS = chitosan; HA = hyaluronic acid; SA = alginic acid sodium salt.
Figure 5.

The stress-strain curve obtained from the compressive test.
Swelling Behaviors of the Constructs
Swelling properties are related to the diffusion of nutrients and waste throughout the whole structures 20 and providing liquid absorption of secretions. 32 The swelling capacity of the constructs was examined for 7 days. The mean weights were taken between the 3 values for each construct and error bars were added as standard deviation. In Figure 6a , it was observed that PCL/3wt.%PVP/1wt.%HA had higher swelling rate than the other constructs. In the first 72 hours, all constructs increased their weight rapidly and this situation continued until 144 hours. In Figure 6a , the composites that consisted of CS and SA had a lower swelling rate than the control group. The swelling results showed that HA could improve the hydrophilicity and wetting properties of the PCL/3wt.%PVP construct. This aspect may indicate that the diffusion and absorption of solutes throughout the pores in PCL/3wt.%PVP are improved by the addition of HA. 21
Figure 6.

Swelling (a) and degradation (b) behaviors of the constructs as a function of time.
In Vitro Degradation of Constructs
The in vitro degradation of PCL, PVP correlation between CS, SA, and HA constructs were evaluated by soaking the constructs in PBS at 37°C for 4 weeks. The degradation test was done by measuring the weight loss of the constructs. 33 In Figure 6b , the same tendency was observed in the swelling test. PCL/3wt.%PVP/1wt.%HA, which had the highest swelling capacity, had the highest degradation rate due to its higher liquid uptake. After 7 days, the degradation rate of each construct decreased due to the deceleration of hydrolysis.
Cytocompatibility of Cartilage Constructs
The cell adhesion, proliferation, and cytotoxicity properties were evaluated with MTT assay. Figure 7 shows the MTT absorption (590 nm) values for MSCs seeded in the microplate during the cell culture period (3 days). According to the results after 72 hours of incubation, it can be said that constructs had non-cytotoxicity and each construct has an impact on the growth of cells distinctively. Although proliferation rates were different for all constructs, no statistically significant effect was observed. However, the highest viability (%) was observed for PCL/3wt.%PVP/1wt.%HA, and can be due to HA, which is the major component of the extracellular matrix, 34 and thus facilitates increase in cell aggregation, proliferation, and migration. 35 For further studies, we need to undertake molecular or protein preliminary analysis to determine the potential of printed constructs as a cartilage structured template.
Figure 7.
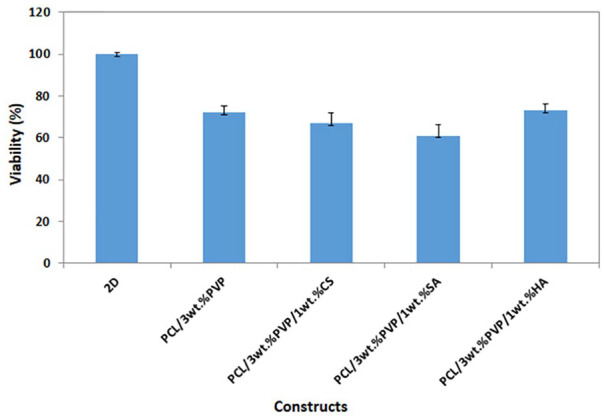
Cell viability graph of all constructs with the control group (2D cells).
Cell Attachment
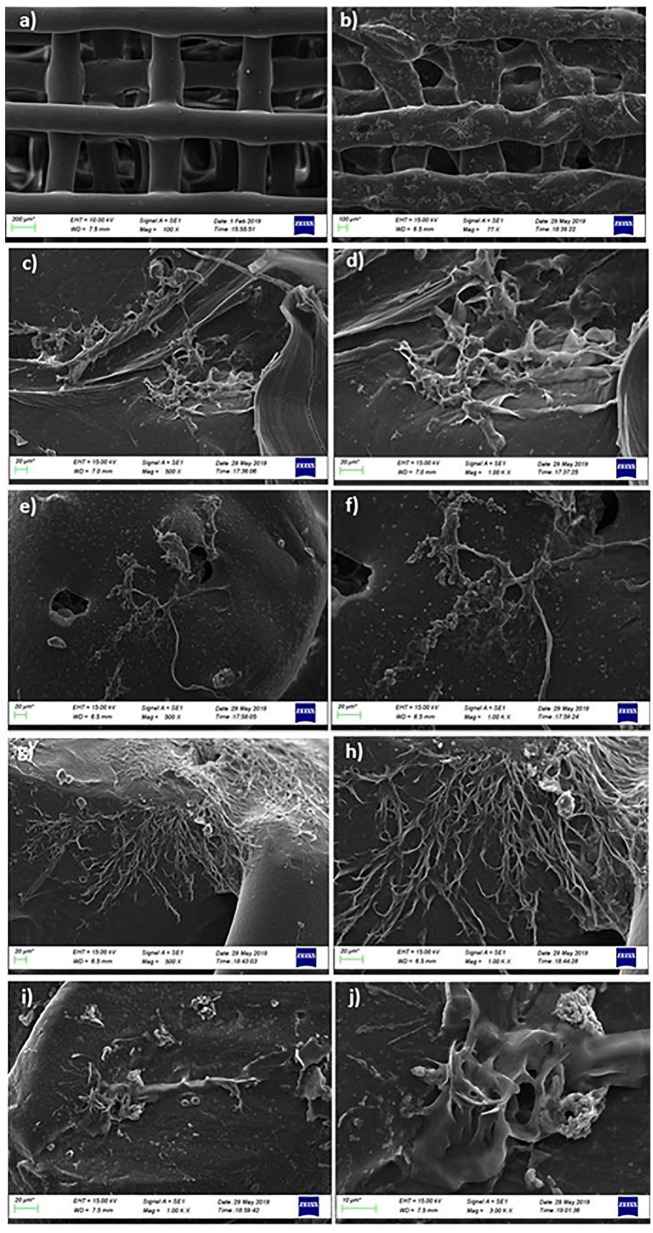
SEM analysis was performed to investigate the MSCs’ attachment and morphology on PCL/3wt.%PVP, PCL/3wt.%PVP/1wt.%CS, PCL/3wt.%PVP/1wt.%SA, and PCL/3wt.%PVP/1wt.%HA compared with the acellular structure ( Fig. 8a ). According to the results obtained by SEM, it was possible to observe the cells attached to the surface of scaffolds. Cell-seeded scaffolds demonstrated the presence of cellular networks for all scaffolds. This network can be associated with the extracellular matrix attached to the scaffolds. Figure 8b-h demonstrates the formation of some colonies of cells attached between the scaffolds. With the results obtained by SEM, it can be easily said that MSCs can attach and spread on all the scaffolds.36,37 Biocompatibility of the structures with MSCs can be considered as candidate and reference combinations in the differentiation of the stem cells into chondrocytes for further studies.
Figure 8.
SEM images of PCL/3wt.%PVP (a, b) without cells, PCL/3wt.%PVP/1wt.%CS (c, d) with cell attachment, PCL/3wt.%PVP/1wt.%CS (e, f), PCL/3wt.%PVP/1wt.%SA (g, h), PCL/3wt.%PVP/1wt.%HA (i, j) seeded with MSCs after 4 days of culturing.
Conclusions
In this study, PCL and PVP were combined to form the main component of the constructs. Chitosan, hyaluronic acid, and sodium alginate were integrated with this base construct separately in order to find the ideal correlations between them for advancing the biological alternatives that can restore the damaged tissue. 3D printing is the rapid and versatile technique that can provide the required geometry of the diseased tissues to mimic the exact structure of the tissues with adjustable porosity. Replacing diseased tissue is possible by mimicking the extracellular matrix structure. According to optimization results, it was found that the structure of the constructs with a 60% infill rate had a suitable and uniform pore distribution. With this infill rate, other composites were printed keeping this value fixed. The compressive strength values of the constructs increased with different additive polymers. The swelling ratios of the PCL/3wt.%PVP have been enhanced with the addition of the HA and also HA added composites had the highest viability value compared with the others. As a result, in vitro culture test using MSCs line showed that printed constructs are suitable to carry out further studies in order to improve their capacities for cartilage regeneration.
Footnotes
Acknowledgments and Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Ethical Approval: Ethical approval was not required for this article.
Informed Consent: Informed consent was not required for this article.
ORCID iD: Oguzhan Gunduz  https://orcid.org/0000-0002-9427-7574
https://orcid.org/0000-0002-9427-7574
References
- 1. Cui X, Breitenkamp K, Finn MG, Lotz M, D’Lima DD. Direct human cartilage repair using three-dimensional bioprinting technology. Tissue Eng Part A. 2012;18(11-12):1304-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Rosenzweig DH, Carelli E, Steffen T, Jarzem P, Hanglud L. 3D-printed ABS and PLA scaffolds for cartilage and nucleus pulposus tissue regeneration. Int J Mol Sci. 2015;16(7):15118-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Johnstone B, Alini M, Cucchiarini M, Dodge GR, Eglin D, Guilak F, et al. Tissue engineering for articular cartilage repair—the state of the art. Eur Cell Mater. 2013;25:248-67. [DOI] [PubMed] [Google Scholar]
- 4. Daly AC, Critchley SE, Rencsok EM, Kelly DJ. A comparison of different bioinks for 3D bioprinting of fibrocartilage and hyaline cartilage. Biofabrication. 2016;8(4):045002. [DOI] [PubMed] [Google Scholar]
- 5. Hung KC, Tseng CS, Dai LG, Hsu SH. Water-based polyurethane 3D printed scaffolds with controlled release function for customized cartilage tissue engineering. Biomaterials. 2016;83:156-68. [DOI] [PubMed] [Google Scholar]
- 6. Neves SC, Teixeira LSM, Moroni L, Reis RL, Van Blitterswijk CA, Alves NM, et al. Chitosan/poly(epsilon-caprolactone) blend scaffolds for cartilage repair. Biomaterials. 2011;32(4):1068-79. [DOI] [PubMed] [Google Scholar]
- 7. Nguyen D, Hägg DA, Forsman A, Ekholm J, Nimkingratana P, Brantsing C, et al. Cartilage tissue engineering by the 3D bioprinting of iPS cells in a nanocellulose/alginate bioink. Sci Rep. 2017;7(1):658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Utomo L, Pleumeekers MM, Nimeskern L, Nürnberger S, Stok KS, Hildner F, et al. Preparation and characterization of a decellularized cartilage scaffold for ear cartilage reconstruction. Biomed Mater. 2015;10(1):015010. [DOI] [PubMed] [Google Scholar]
- 9. Kim YB, Kim GH. PCL/alginate composite scaffolds for hard tissue engineering: fabrication, characterization, and cellular activities. ACS Comb Sci. 2015;17(2_suppl):87-99. [DOI] [PubMed] [Google Scholar]
- 10. Woodfield TB, Malda J, de Wijn J, Péters F, Riesle J, van Blitterswijk CA. Design of porous scaffolds for cartilage tissue engineering using a three-dimensional fiber-deposition technique. Biomaterials. 2004;25(18):4149-61. [DOI] [PubMed] [Google Scholar]
- 11. Li J, Chen M, Fan X, Zhou H. Recent advances in bioprinting techniques: approaches, applications and future prospects. J Transl Med. 2016;14:271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Yu Y, Moncal KK, Li J, Peng W, Rivero I, Martin JA, et al. Three-dimensional bioprinting using self-assembling scalable scaffold-free “tissue strands” as a new bioink. Sci Rep. 2016;6:28714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Songul U, Cevriye K, Faik NO, Muhammet U, Yesim MS, Betul K, et al. 3D printing artificial blood vessel constructs using PCL/chitosan/hydrogel biocomposites. ChemistrySelect. 2019;4(8):2387-91. [Google Scholar]
- 14. Jin RM, Sultana N, Baba S, Hamdan S, Ismail AF. Porous PCL/chitosan and nHA/PCL/chitosan scaffolds for tissue engineering applications: fabrication and evaluation. J Nanomater. 2015;16(1):138. [Google Scholar]
- 15. Peter AL, Dietmar WH, Jos M, Travis JK. Hyaluronic acid enhances the mechanical properties of tissue-engineered cartilage constructs. PLoS One. 2014; 9(12): e113216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Suh JK, Matthew HW. Application of chitosan-based polysaccharide biomaterials in cartilage tissue engineering: a review. Biomaterials. 2000;21:2589-98. [DOI] [PubMed] [Google Scholar]
- 17. Di Martino A, Sittinger M, Risbud MV. Chitosan: a versatile biopolymer for orthopaedic tissue-engineering. Biomaterials. 2005;26(30):5983-90. [DOI] [PubMed] [Google Scholar]
- 18. Neufurth M, Wang X, Schröder HC, Feng Q, Diehl-Seifert B, Ziebart T, et al. Engineering a morphogenetically active hydrogel for bioprinting of bioartificial tissue derived from human osteoblast-like SaOS-2 cells. Biomaterials. 2014;35(31):8810-9. [DOI] [PubMed] [Google Scholar]
- 19. Correia CR, Moreira-Teixeira LS, Moroni L, Reis RL, van Blitterswijk CA, Karperien M, et al. Chitosan scaffolds containing hyaluronic acid for cartilage tissue engineering. Tissue Eng Part C Methods. 2011;17(7):717-30. [DOI] [PubMed] [Google Scholar]
- 20. Saçak B, Certel F, Akdeniz ZD, Karademir B, Ercan F, Özkan N, et al. Repair of critical size defects using bioactive glass seeded with adipose-derived mesenchymal stem cells. J Biomed Mater Res B Appl Biomater. 2017;105(5):1002-8. [DOI] [PubMed] [Google Scholar]
- 21. Lu H, Kawazoe N, Kitajima T, Myoken Y, Tomita M, Umezawa A, et al. Spatial immobilization of bone morphogenetic protein-4 in a collagen-PLGA hybrid scaffold for enhanced osteoinductivity. Biomaterials. 2012;33:6140-6. [DOI] [PubMed] [Google Scholar]
- 22. Nuernberger S, Cyran N, Albrecht C, Red H, Vécsei V, Marlovits S. The influence of scaffold architecture on chondrocyte distribution and behavior in matrix-associated chondrocyte transplantation grafts. Biomaterials. 2011;32:1032-40. [DOI] [PubMed] [Google Scholar]
- 23. Hutmacher DW. Scaffolds in tissue engineering bone and cartilage. Biomaterials. 2000;21:2529-43. [DOI] [PubMed] [Google Scholar]
- 24. Zhang Q, Lu H, Kawazoe N, Chen G. Pore size effect of collagen scaffolds on cartilage regeneration. Acta Biomater. 2014;10(5):2005-13. [DOI] [PubMed] [Google Scholar]
- 25. Zheng P, Yao Q, Mao F, Liu N, Xu Y, Wei B, et al. Adhesion, proliferation and osteogenic differentiation of mesenchymal stem cells in 3D printed poly-ε-caprolactone/hydroxyapatite scaffolds combined with bone marrow clots. Mol Med Rep. 2017;16:5078-84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Zhong X, Ji C, Chan AK, Kazarian SG, Ruys A, Dehghani F. Fabrication of chitosan/poly(ε-caprolactone) composite hydrogels for tissue engineering applications. J Mater Sci Mater Med. 2011;22(2_suppl):279-88. [DOI] [PubMed] [Google Scholar]
- 27. Wang JC, Zheng H, Chang MW, Ahmad Z, Li JS. Preparation of active 3D film patches via aligned fiber electrohydrodynamic (EHD) printing. Sci Rep. 2017;7:43924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Mahapatra C, Jin GZ, Kim HW. Alginate-hyaluronic acid-collagen composite hydrogel favorable for the culture of chondrocytes and their phenotype maintenance. Tissue Eng Regen Med. 2016;13(5):538-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Gao M, Zhang H, Dong W, Bai J, Gao B, Xia D, et al. Tissue-engineered trachea from a 3D-printed scaffold enhances whole-segment tracheal repair. Sci Rep. 2017;7:5246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Saroj AL, Singh RK, Chandra S. Studies on polymer electrolyte poly(vinyl)pyrrolidone (PVP) complexed with ionic liquid: effect of complexation on thermal stability, conductivity and relaxation behaviour. Mater Sci Eng B. 2013;178:231-8. [Google Scholar]
- 31. Kerin AJ, Wisnom MR, Adams MA. The compressive strength of articular cartilage. Proc Inst Mech Eng H. 1998;212(4):273-80. [DOI] [PubMed] [Google Scholar]
- 32. Aydogdu MO, Altun E, Crabbe-Mann M, Brako F, Koc F, Ozen G, et al. Cellular interactions with bacterial cellulose: polycaprolactone nanofibrous scaffolds produced by a portable electrohydrodynamic gun for point-of-need wound dressing. Int Wound J. 2018;15:789-97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Dorati R, Colonna C, Genta I, Modena T, Conti B. Effect of porogen on the physico-chemical properties and degradation performance of PLGA scaffolds. Polym Degrad Stab. 2010;95(4):694-701. [Google Scholar]
- 34. Kochlamazashvili G, Henneberger C, Bukalo O, Dvoretskova E, Senkov O, Lievens PMJ, et al. The extracellular matrix molecule hyaluronic acid regulates hippocampal synaptic plasticity by modulating postsynaptic L-type Ca(2+) channels. Neuron. 2010;67(1):116-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Bastow ER, Byers S, Golub SB, Clarkin CE, Pitsillides AA, Fosang AJ. Hyaluronan synthesis and degradation in cartilage and bone. Cell Mol Life Sci. 2008;65(3):395-413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Siddiqi SA, Manzoor F, Jamal A, Tariq M, Ahmad R, Kamran M, et al. Mesenchymal stem cell (MSC) viability on PVA and PCL polymer coated hydroxyapatite scaffolds derived from cuttlefish. RSC Adv. 2016;6:32897-904. [Google Scholar]
- 37. Steffens D, Rezende RA, Santi B, Pereira FD, Neto PI, da Silva JV, et al. 3D-printed PCL scaffolds for the cultivation of mesenchymal stem cells. J Appl Biomater Funct Mater. 2016;14(1):e19-e25. [DOI] [PubMed] [Google Scholar]