Abstract
Objective
Chondrogenic differentiation of mesenchymal stem cells (MSCs) into hyaline cartilage is complicated by terminal hypertrophic differentiation. In growth plate, parathyroid hormone–related peptide (1-34) (PTHrP) plays a crucial role in maintaining chondrocytes in their proliferation state by counteracting the hypertrophic differentiation. This study aims to test the effect of PTHrP supplementation at different time points on chondrogenic differentiation of MSCs and assess the final quality of differentiated chondrocytes.
Methods
Human periosteum and bone marrow MSCs isolated from 3 patient samples (donor unmatched) were characterized by flow cytometry and multilineage differentiation. The cells were differentiated into chondrocytes in the presence of transforming growth factor-β (TGF-β) and the PTHrP (1-34) was added from 4th or 14th day of culture. The outcome was analyzed by histology, immunohistochemistry, and gene expression.
Results
Flow cytometry and multilineage differentiation confirmed that the cells isolated from periosteum and bone marrow exhibited the phenotype of MSCs. During chondrogenic differentiation, pellets that received PTHrP from the 4th day of culture showed a significant reduction in hypertrophic markers (COL10A1 and RUNX) than the addition of PTHrP from the 14th day and TGF-β alone treated samples. Furthermore, 4th day supplementation of PTHrP significantly improved the expression of cartilage-specific markers (COL2A1, SOX9, ACAN) in both periosteum and bone marrow-derived MSCs. Histology and immunostaining with collagen type X data corroborated the gene expression outcomes.
Conclusion
The outcome showed that supplementing PTHrP from the 4th day of chondrogenic differentiation produced better chondrocytes with less hypertrophic markers in both bone marrow and periosteal-derived MSCs.
Keywords: periosteum MSCs, bone marrow MSCs, parathyroid hormone–related peptide, chondrogenic differentiation, chondrocyte hypertrophy
Introduction
Chondrocytes are the main cell source for cartilage tissue engineering. In adults, the limited availability of high-quality chondrocytes and the need for invasive harvesting procedure has resulted in a quest for an alternative cell source.1,2 Mesenchymal stem cells (MSCs) with their ability to self-renew, proliferate, and undergo multilineage differentiation are a promising candidate for cartilage tissue engineering. MSCs, when primed into chondrogenic lineage prior to transplantation, generate better quality hyaline cartilage than the undifferentiated cells. 3 In vitro differentiation of human MSCs derived from various cell sources into chondrocytes has been well explored. 4 However, one of the major limitations associated with the current protocols is the formation of hypertrophic chondrocytes; this transient cartilage when transplanted in vivo is primed more to osteogenic lineage rather than retaining its hyaline cartilage phenotype. 5
In the articular cartilage, growth factors present in the extracellular matrix play a pivotal role in cartilage development and maintenance of hyaline phenotype. The addition of growth factors such as transforming growth factor-β (TGF-β), bone morphogenetic proteins, insulin-like growth factors influences the quality of chondrocytes produced. 6 The use of TGF-β to promote chondrogenesis remains the gold standard; this, however, promotes hypertrophic differentiation in MSCs derived from various origins including the one from induced pluripotent stem cells. 7
An additional growth factor such as parathyroid hormone–related peptide (1-34) (PTHrP (1-34)) has been shown to upregulate the genes responsible for chondrogenesis by suppressing the hypertrophic markers in bone marrow MSCs. 8 This growth factor is known to be expressed in both articular as well as growth plate cartilage. It plays a vital role in the maintenance of cartilage phenotype by controlling the hypertrophic differentiation. It functions through the parathyroid hormone/parathyroid hormone–related peptide receptor (PTH1R) on chondrocytes and activates many intracellular signaling pathways including protein kinase A (PKA)/protein kinase C pathway. The activation of PKA signaling pathway inhibits the translocation of myocyte enhancer factor-2 and counteracts the Indian hedgehog pathway, which would otherwise lead to hypertrophy.9,10 The PTHrP signaling also activates the SOX9 transcription factor and induces collagen type 2 expression. 11 Recent studies have shown that intermittent PTHrP(1-34) supplementation stimulates the chondrogenic differentiation of MSCs, while the continuous supplementation of PTHrP(1-34) inhibits chondrogenesis. 10 The existing literature suggests that the effect of growth factors is not only dependent on the isoform used but rather the parameters such as concentration and timing of administration during differentiation.8,12,13 So far, the effect of PTHrP(1-34) supplementation at different time points has not been explored. This study aims at the differentiation of human MSCs to chondrocytes using TGF-β3 (control) and studies the synergistic effect of PTHrP(1-34) supplementation on different time points in the maintenance of chondrocyte phenotype and the subsequent effect on cartilage hypertrophy. This will help us arrive at a clinically relevant protocol for maintaining the hyaline phenotype of chondrocytes derived from MSCs.
Materials and Methods
This study was approved by the institutional review board of Christian Medical College, Vellore, and conducted as per the recommendation of the Declaration of Helsinki. The patient samples were collected after obtaining informed written consent.
Cell Source, Isolation, Expansion, and Characterization
Isolation and Culture of Periosteal MSCs (P-MSCs)
Periosteum samples were harvested from 3 patients (range: 5-15 years) undergoing femoral shortening osteotomies. The samples were transported to the laboratory within an hour for processing. The mean tissue weight obtained was 423.3 mg (range: 330-540 mg). Tissue was processed according to a published protocol. 14 Briefly, the sample was minced into small pieces and incubated with 1% collagenase type 2 (Worthington, Freehold, NJ) for 3 hours at 37°C. After digestion, the sample was filtered through 100-µm cell strainer and then the isolated cells were cultured in DMEM/F12 medium supplemented with 10% fetal bovine serum (FBS), 50 µg/mL gentamicin, and 2 µg/mL of amphotericin B. The mean cell yield obtained after culture was 1.74 × 106 cells (1.025 × 106 to 2.7 × 106 cells).
Isolation and Culture of Bone Marrow MSCs (BM-MSCs) from Trabecular Bone
Three patients (range: 6-12 years) who underwent bone grafting surgeries were enrolled and the trabecular bone was harvested and collected in a 50-mL centrifuge tube containing αMEM medium. The samples were washed with PBS and treated with 3 mg/mL collagenase type 2 for 3 hours at 37°C. After digestion, the sample was filtered through a 100-µm cell strainer and then centrifuged at 2,000 rpm for 10 minutes at room temperature. The cell pellet was resuspended in MSC culture medium (containing αMEM supplemented with 10% FBS, 5 ng/mL FGF-2 [PeproTech, Rocky Hill, NJ], 50 µg/mL gentamicin, and 2 µg/mL amphotericin B) and plated in the 25-cm2 tissue culture flask. The mean cell yield obtained after primary culture was 1.23 × 106 cells (0.9 × 106 to 1.6 × 106 cells).
Characterization and Chondrogenic Differentiation of Periosteal and Bone Marrow MSCs
The MSCs at passage 3 were used for the study after characterization by flow cytometry and multilineage differentiation assay. Cells at a density of 2.5 × 105 per well were cultured in a 96-well plate with chondrogenic differentiation medium (Cyagen, Santa Clara, CA) containing dexamethasone, ascorbate, insulin transferrin selenium, sodium pyruvate, proline, and 10 ng/mL TGF-β3 (R&D Systems, Minneapolis, MN). 14 The plate was centrifuged at 500g for 5 minutes and was placed in the incubator. Twenty-four hours later, the cell pellets were detached by gentle pipetting. Pellet cultures were carried out in 3 groups of 6 pellets in each for both P-MSCs and BM-MSCs. The groups were named as P4th, P14th, and TGF-β alone. In the P4th group, the chondrogenic differentiation medium was supplemented with PTHrP(1-34) (100 ng/mL; BACHEM, Bubendorf, Switzerland) at every media change from the 4th day of culture onwards. In the P14th group, the supplementation with PTHrP(1-34) (100 ng/mL) was done from the 14th day of differentiation onwards, and in TGF-β alone group, the cells were cultured with standard chondrogenic differentiation medium without PTHrP(1-34) supplement. The differentiation was carried out for 4 weeks and then the pellets were analyzed.
Biochemical Analyses
The cartilage pellets at 14th and 28th days were digested in papain (125 µg/mL) for 16 hours at 60°C. After digestion, it was centrifuged at 12,000 rpm for 10 minutes at 4°C and the supernatant was used for glycosaminoglycan (GAG) and DNA quantification. Sulfated GAGs were measured with the help of a metachromatic dye 1,9-dimethylmethylene blue as per a previously published protocol. 15 The DNA was quantitated using a Quant-iT PicoGreen dsDNA Assay Kit (Invitrogen, Carlsbad, CA). The results were expressed as total GAG per µg of DNA (GAG/DNA).
Gene Expression
Total RNA was isolated from the pellet samples using TRI reagent (Sigma-Aldrich, St. Louis, MO) by following the manufacturer’s protocol. Total RNA (500 ng) was reverse transcribed into cDNA using Prime Script cDNA First-strand synthesis kit (Takara, Otsu, Japan). Expression of parathyroid hormone/parathyroid hormone–related peptide receptor (PTH1R), cartilage-specific and cartilage hypertrophic markers were quantitated using SYBR Premix Ex Taq (Takara, Otsu, Japan) with specific forward and reverse primers. Primers specific to the target genes were designed using Primer-Blast software and listed in the Supplementary Table 1. Data were analyzed using the ΔΔCt method. Beta-actin (housekeeping gene) was used to normalize the target gene expression and the results were expressed as fold change.
Histological Analysis and Immunohistochemistry
The pellets after 4 weeks of culture were preserved overnight in 4% paraformaldehyde and were processed for routine paraffin embedding. The sections were stained for Alcian blue stain to view the secretion of GAGs. For immunohistochemistry, tissue sections were deparaffinized and enzymatic antigen retrieval with trypsin was performed. Sections were incubated (overnight at 4°C) with collagen type 10 (Rabbit polyclonal, Millipore, Burlington, MA) antibody followed by goat anti-rabbit (Abcam, Cambridge, UK) secondary antibody for 1 hour at room temperature. EnVision FLEX DAB+ chromogen and substrate buffer (Dako, Glostrup, Denmark) were used to develop the color. Finally, the sections were counterstained with hematoxylin and studied under a light microscope (Leica DM 2000, Leica Microsystems, Wetzlar, Germany).
Statistical Analysis
Statistical analyses were performed using GraphPad Prism 6.01 (GraphPad Software, Inc., La Jolla, CA, USA). Data were represented as mean ± SD. One-way ANOVA was performed to calculate the significance between the 3 groups. A calculated P value less than 0.05 was considered as significant.
Results
Characterization of Human P-MSCs and BM-MSCs
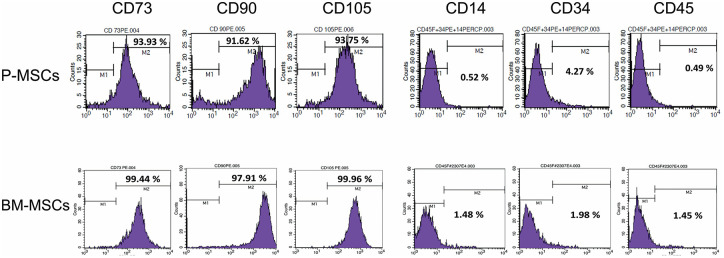
Periosteum and bone marrow MSCs were isolated from 3 patient samples (donor unmatched). Both the cell types were plastic adherent and appeared spindle-like in morphology during culture. Flow cytometric analysis showed that MSCs stained >90% positive for human MSCs markers CD73, CD90, and CD105 and stained negative (<5%) for the hematopoietic markers CD14, CD34, and CD45 ( Fig. 1 ).
Figure 1.
Flow cytometry analysis of MSCs surface markers expression in P-MSCs and BM-MSCs. The histogram indicates >90% of cells stained positive for MSC surface markers (CD73, CD90, and CD105) and <5% cells stained for negative markers (CD14, CD34, and CD45; n = 3 in each group). MSC = mesenchymal stem cell; P-MSC = periosteum MSC; BM = bone marrow.
Multilineage Differentiation Assay
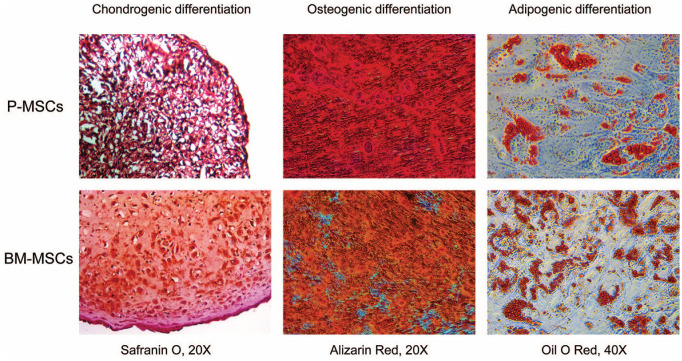
The functional characteristics of P-MSCs and BM-MSCs were confirmed by a multilineage differentiation assay ( Fig. 2 ). After differentiation, histological staining confirmed that MSCs from both sources produced lipid droplets, deposited calcium crystals, and secreted extracellular matrix, which demonstrates adipogenic, osteogenic, and chondrogenic differentiation, respectively. Chondrogenic differentiation was qualitatively better in cartilage pellets derived from BM-MSCs.
Figure 2.
The multilineage differentiation potential of P-MSCs and BM-MSCs. The differentiated cells stained with Safranin O, Alizarin red, and Oil O red staining confirmed the chondrogenic, osteogenic, and adipogenic differentiation, respectively. The cartilage derived from BM-MSCs stained higher with Safranin O dye, which indicates higher GAG deposition compared with the P-MSCs (n = 3 in each group). MSC = mesenchymal stem cell; P-MSC = periosteum MSC; BM = bone marrow; GAG = glycosaminoglycan.
Biochemical Assay
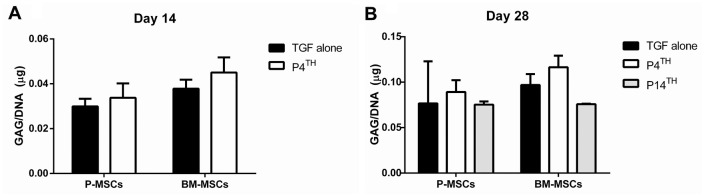
Estimation of GAG content in 14th and 28th day cartilage pellets showed that there was no difference (P = ns) in GAG content between the groups in both P-MSCs and BM-MSCs compared with P14th and TGF-β alone treated groups ( Fig. 3 ).
Figure 3.
Estimation of GAG/DNA content in the cartilage pellets derived from P-MSCs and BM-MSCs. Samples from 14th (A) and 28th (B) day of chondrogenic differentiation showed no significant difference in GAG/DNA content between the PTHrP(1-34) treated groups and TGF-β alone (n = 2 in each group). MSC = mesenchymal stem cell; P-MSC = periosteum MSC; BM = bone marrow; GAG = glycosaminoglycan; PTHrP(1-34) = parathyroid hormone–related peptide (1-34).
Effect of PTHrP(1-34) on Chondrogenesis of P-MSCs
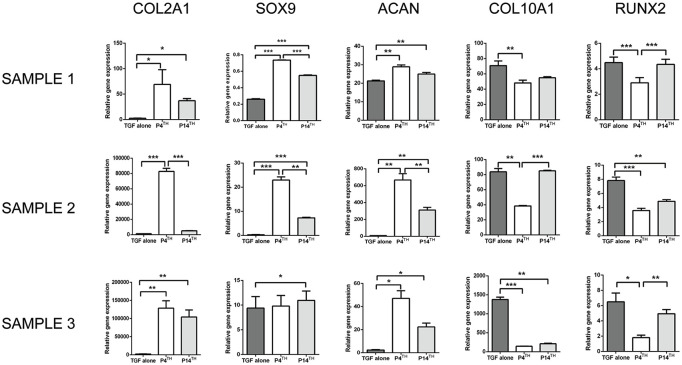
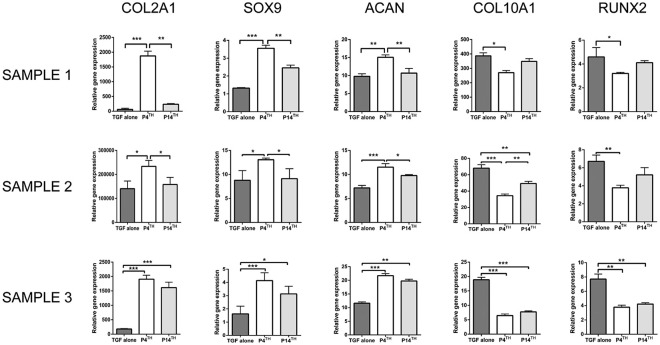
Gene expression analysis was performed to investigate the role of PTHrP(1-34) in regulating the hypertrophic chondrocyte differentiation of MSCs. In a previous study by us, P-MSCs had shown higher expression of COL10A1 (hypertrophic marker) compared with BM-MSCs. Therefore, we first studied the effect of PTHrP(1-34) on P-MSCs in the 3 study groups mentioned, that is, P4th, P14th, and TGF-β alone. The outcome of 3 human samples was expressed individually instead of mean due to heterogeneity in gene expression between the samples. After 4 weeks of chondrogenic differentiation, the gene expression analysis showed a significant increase in COl2A1, SOX9, and ACAN expression in P4th treated group compared to the P14th and TGF-β alone group. In all 3 samples, COL10A1 and RUNX2 expression was significantly decreased on P4th treated when compared with the P14th and TGF-β alone treated samples ( Fig. 4 ). In sample 3, SOX9 expression was significantly higher in the P14th group than the TGF-β alone group; however, there was no significant difference between P4th and P14th groups.
Figure 4.
RT-PCR analysis of cartilage-specific and hypertrophic markers’ expression in the cartilage derived from P-MSCs. Supplementing PTHrP(1-34) from the 4th day of culture significantly increased the expression of COL2A1, SOX9, and ACAN (in samples 1 and 2) and reduced the expression of COL10A1 and RUNX2 (in all samples) than the other 2 groups (number of sample = 3), *P < 0.05; **P < 0.01; ***P < 0.001. RT-PCR = real time polymerase chain reaction; P-MSC = periosteum mesenchymal stem cell; PTHrP(1-34) = parathyroid hormone–related peptide (1-34).
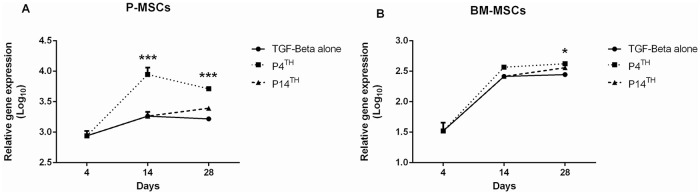
Analysis of PTH1R expression showed a 3-fold increase in its expression on the 4th day of chondrogenesis and the expression was further increased on day 14 (P < 0.001) in the P4th sample compared with TGF-β alone. After 4 weeks of differentiation, the PTH1R expression was higher in the P4th (P < 0.001) than the TGF-β alone and P14th samples ( Fig. 5A ).
Figure 5.
RT-PCR analysis of parathyroid hormone/parathyroid hormone-related peptide receptor (PTH1R) expression in P-MSCs and BM-MSCs during chondrogenic differentiation. In both (A) P-MSCs and (B) BM-MSCs, P4th treatment exhibited higher expression of PTH1R compared with the other 2 groups (n = 3 in each group). RT-PCR = real time polymerase chain reaction; MSC = mesenchymal stem cell; P-MSC = periosteum MSC; BM = bone marrow.
Effect of PTHrP(1-34) on Chondrogenesis of BM-MSCs
The PTH1R expression was significantly higher on the 4th day of chondrogenesis in BM-MSCs similar to P-MSCs, and the expression was further increased on the 14th day of culture in the P4th sample (P = ns) compared with others. On the 28th day, the PTH1R expression was significantly higher in the P4th sample (P < 0.05) compared with the P14th and TGF-β alone treated groups ( Fig. 5B ).
Real-time PCR (polymerase chain reaction) analysis exhibited significantly higher expression of cartilage-specific genes COL2A1, SOX9, and ACAN in sample 1 and sample 2 ( Fig. 6 ) in the P4th treated group compared with controls. In sample 3, the cartilage-specific gene expression was significantly higher in the P4th and P14th treated groups compared with TGF-β alone control. The expression of hypertrophic markers COL10A1 and RUNX2 were significantly downregulated in all 3 samples in the P4th group, and in sample 3 alone there was a significant reduction of hypertrophic markers expression in the P14th group as well. Collectively, this outcome demonstrated that PTHrP(1-34) supplementation from the 4th day of culture onwards could significantly control the hypertrophic differentiation of MSCs.
Figure 6.
RT-PCR analysis of cartilage-specific and hypertrophic chondrocytes gene expression in PTHrP(1-34) treated and untreated cartilage pellets from BM-MSCs. Adding PTHrP(1-34) from the 4th day of culture (P4th) significantly increased the expression of COL2A1, SOX9, and ACAN compared with both P14th and TGF-β alone treated groups. The level of COL10A1 and RUNX2 expression was significantly reduced in the P4th group. *P < 0.05; **P < 0.01; ***P < 0.001 (n = 3 in each group). RT-PCR = real time polymerase chain reaction; PTHrP(1-34) = parathyroid hormone–related peptide (1-34); BM-MSC = bone marrow mesenchymal stem cell.
Histology
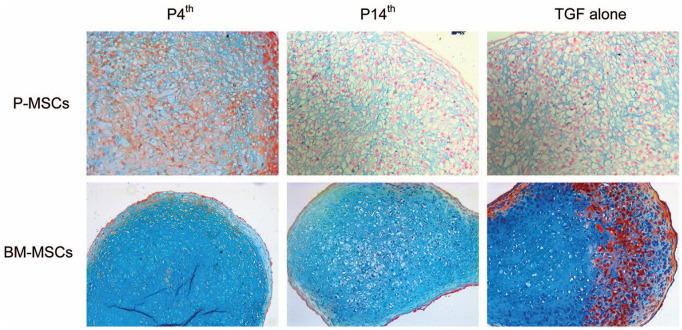
After 4 weeks of chondrogenic differentiation, cartilage derived from BM-MSCs in the P4th group showed more intense staining with Alcian blue than the P14th and TGF-β alone groups ( Fig. 7 ). However, in P-MSCs, there was no difference observed in the staining between the 3 groups. Hypertrophic chondrocytes were bigger in size, seen more in P14th and TGF-β alone groups in MSCs from both sources when compared with the P4th group.
Figure 7.
Alcian blue staining on cartilage pellets derived from P-MSCs (20× magnification) and BM-MSCs (10× magnification). In the BM-MSC sample, the staining in P4th is more intense than P14th though there is less counterstaining with Neutral Red in both groups. The staining results indicate that supplementing PTHrP(1-34) from the fourth day of culture improved the chondrogenic differentiation of MSCs from both sources compared with other groups (n = 3 in each group). MSC = mesenchymal stem cell; P-MSC = periosteum MSC; BM = bone marrow; GAG = glycosaminoglycan; PTHrP(1-34) = parathyroid hormone–related peptide (1-34).
Immunohistochemistry
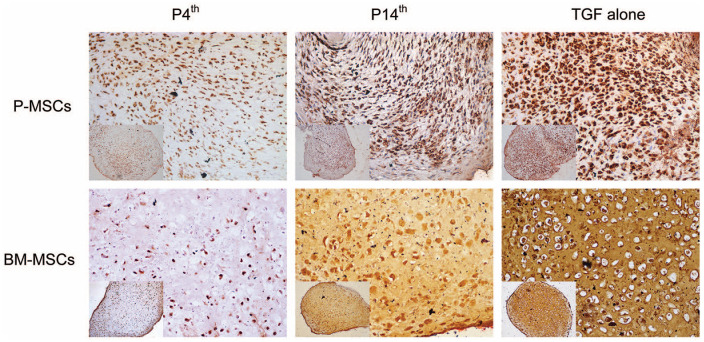
Immunostaining with anti-collagen type 10 antibody confirmed that PTHrP(1-34) treatment from the 4th day of culture inhibits the hypertrophic differentiation and showed reduced collagen type 10 staining in both P-MSCs and BM-MSCs compared with P14th and TGF-β alone treated groups ( Fig. 8 and Fig. S1). The histology and immunohistochemistry outcomes are in agreement with the gene expression data that the PTHrP(1-34) treatment from the 4th day of culture exhibit better chondrogenic differentiation with less hypertrophic cartilage markers expression.
Figure 8.
Collagen type 10 immunostaining on cartilage pellets derived from P-MSCs and BM-MSCs (20× magnification). A reduced collagen type 10 staining in P4th samples indicates a decrease in the hypertrophic differentiation compared with the other 2 treated samples. Insets show a low magnification (10× magnification) image of the respective cartilage pellet (n = 3 in each group). MSC = mesenchymal stem cell; P-MSC = periosteum MSC; BM = bone marrow.
Discussion
The techniques for differentiation of MSC to chondrocyte requires a good understanding of the microenvironment 16 as the in vivo regenerated cartilage has a tendency to become fibroblastic (fibrocartilage) or hypertrophic (leading to ossification) at the end of the differentiation process.5,17 The growth factors such as TGF-β1, BMP-2, and IGF-2 have been used to improve the early chondrogenic differentiation of MSCs. Studies have shown that supplementing the additional growth factor (BMP-7 or PTHrP) enhanced the late chondrogenesis of MSCs with less hypertrophic differentiation.8,18
The choice of MSC source also plays a pivotal role in the quality of the resultant chondrocytes. 19 Among the various sources of MSCs, P-MSCs are the ones that exhibit maximum hypertrophy when tested by us and this is probably due to its natural function of bone formation. 20 In this study, to prove the role of PTHrP(1-34) in suppressing hypertrophy, we first used P-MSCs for chondrogenic differentiation and demonstrated the ability of PTHrP(1-34) to suppress hypertrophic cartilage, and then tested it on BM-MSCs, which is most commonly used in clinical trials.
Previous reports suggest that when periosteal explants are exposed to TGF-β1 (at 100 ng/mL for 30 minutes) there is an increase in the rate of chondrogenesis. 21 Mara et al. in their study used chondrogenic markers to identify the quality of chondrocytes from P-MSCs in a TGF-β3 supplemented culture medium. 14 However, they failed to study the effect of TGF-β3 on the hypertrophic markers.14,21
In this study, we looked at the independent effect of TGF-β3 as well as the synergistic effect of TGF-β3 with PTHrP(1-34) added from the 4th and 14th days of culture on the suppression of hypertrophic markers of the differentiated chondrocytes. The choice of the 4th day PTHrP(1-34) supplementation was based on a preliminary study (data not shown) of ours where we found the expression of hypertrophic markers (COL10A1 and RUNX2) as early as the 7th day of differentiation in periosteal MSCs. This was in agreement with previous reports, where the hypertrophic markers were expressed on the 7th day of bone marrow and adipose-derived MSCs to chondrocyte differentiation.22,23 This persuaded us to supplement PTHrP(1-34) from the 4th day of culture before the manifestation of hypertrophy markers. Previously, Weiss et al. have reported that the addition of PTHrP(1-34) from the beginning of differentiation (0th day) inhibited the chondrogenesis of MSCs. 13 So we delayed the supplementation to the 4th day of culture. The supplementation of PTHrP(1-34) from the 14th day as a comparison group was based on a previous publications.8,24 In the present study, we found early addition (day 4) of PTHrP(1-34) inhibited the chondrocyte hypertrophy, suggesting that the timing of supplementation of the growth factors during chondrogenesis is crucial.
The strategy of providing PTHrP(1-34) on the 4th day seems to have worked favorably as it not only induced a higher expression of chondrocyte-specific markers but also produced superior cartilage as demonstrated by histology and immunohistochemistry. Previous studies have shown the addition of PTHrP(1-34) during early chondrogenesis was contradictory. Histologically, Weiss et al. showed poor staining when chondrogenic pellets were treated with PTHrP(1-34) from day 0; interestingly, gene expression studies demonstrated inhibition of hypertrophic markers. 13 Whereas Zhang et al. demonstrated the addition of parathyroid hormone (1-34) (PTH(1-34)) from initiation of chondrogenic differentiation promoted chondrogenesis with no effect on hypertrophy. 25 Both PTH(1-34) and PTHrP(1-34) are similar in structure and function through a common PTH1R receptor to inhibit chondrocyte hypertrophy.
Fischer et al. demonstrated that the intermittent supplementation of PTHrP promotes chondrogenesis with a reduction in cartilage hypertrophy. 10 This intermittent regime is based on the superior outcome of the effect of PTH on osteoporosis when given intermittently. 26 This is not necessarily relevant in chondrogenesis as has been shown by another study where intermittent PTH is inhibitory to cartilage regeneration in a microfracture cartilage repair model in rabbits. 27 A previous study by Fischer’s group in 2010 and another by Lee et al. have shown improved chondrogenesis with continuous treatment of PTHrP in the bone marrow and adipose MSCs.8,28 Our idea of continuous supplementation of PTHrP(1-34) is also supported by a recently published study where MSCs were transduced with adenoviral vectors overexpressing PTHrP to enhance chondrogenesis. 29 The outcome of the above-mentioned study showed a higher expression of cartilage-specific markers (SOX9 and ACAN) with less hypertrophic differentiation. 29
The PTH1R receptor is essential for chondrogenic differentiation and normal cartilage development. Sarem et al. showed that the downregulation of PTH1R receptor inhibits the chondrogenesis of MSCs even in the presence of TGF-β growth factor. Consequently, the supplementation of PTH(1-34) rescued the PTH1R expression and partially restored the chondrogenic differentiation. 30 Furthermore, increasing the dose of PTH (0.1-10 nM) treatment concomitantly induced the PTH1R expression, which potentiates the chondrogenic differentiation through the PKA signaling pathway. 25 In this study, PTH1R expression was higher in the P4th treated group in both P-MSCs and BM-MSCs. Although the GAG deposition did not show any difference in the biochemical assay, the gene expression showed increased expression of cartilage-specific genes in the P4th treated group. This is in line with the previous finding where higher expression of PTH1R resulted in upregulation of cartilage-specific markers. 10
The P-MSCs used as a cell source for articular cartilage regeneration has been discussed in the literature as it is considered more stable phenotypically for chondrogenesis. 31 However, the periosteum being a tissue that normally forms bone in vivo may explain its tendency to differentiate more toward hypertrophic lineage in culture. 20 We provide evidence that early addition of growth factors during chondrogenesis of P-MSCs may facilitate the production of good-quality chondrocytes for translational use.
Our previous experiments (unpublished), as well as published literature, show that on comparison the BM-MSCs were better for chondrogenesis so the strategy was applied to BM-MSCs. 4 With BM-MSCs supplemented with PTHrP(1-34) on the 4th and 14th days, we found that superior cartilage phenotype and suppressed hypertrophy were achieved on the 4th day PTHrP(1-34) group. We found improvement in COL2A1, SOX9, and ACAN expression and decreased hypertrophy along with GAG production as shown in histology and gene expression. Our outcome is partly consistent with a previously published study, which demonstrated the suppression of hypertrophic and osteogenic markers in both bone marrow–derived MSCs and adipose-derived MSCs by PTHrP(1-34). However, they added PTHrP(1-34) from the 14th day of differentiation. These discrepancies could have been influenced by the use of different cell sources, different isoforms, and a different dose of TGF-β (TGF-β2 at 5 ng/mL).8,24 It is thus necessary to understand that the dose, type of growth factor, and the timing influences the process of differentiation and hence the time of introducing the PTHrP (1-34) to the culture may be of importance as demonstrated by us.
In this study, the bone marrow MSCs were cultured with FGF-2 supplementation mainly to avoid the cellular senescence. Literature has shown that without FGF-2, BM-MSCs undergo senescence as early as at second passage. 32 Whereas the P-MSCs exhibits age-independent high proliferative potential and it continues to grow up to 30 doublings without FGF supplementation. 31 In addition, the previously published studies that used P-MSCs were not supplemented FGF-2 so we followed the same protocol.14,31 Stevens et al. have shown that the addition of FGF-2 to periosteum explant before the induction of differentiation enhanced chondrogenesis with high GAG content. 33 And this could be a reason for showing less GAG deposition in P-MSCs compared with BM-MSCs. While the protocol used for BM-MSC showed superior outcomes in our study, it was not intended to compare the MSCs from 2 sources but to explore the effect of PTHrP(1-34).
The runt-related transcription factor 2 (RUNX2) is expressed during hypertrophic differentiation of chondrocytes. 34 A previous study in rat MSCs has shown that knockdown of RUNX2 during chondrogenesis led to the decrease in the formation of hypertrophic chondrocytes with a concomitant increase in the expression of cartilage-specific markers (Col2A1, SOX9, and ACAN). 35 Zhang et al. have shown that supplementation of PTH(1-34) during mouse chondrogenesis downregulated the RUNX2 protein expression and upregulated the level of SOX9 and PTH1R proteins. 25 Our results are in line with these findings where early supplementation of PTHrP(1-34) increased chondrogenesis with minimal hypertrophy (RUNX2 expression), suggesting that PTHrP(1-34) targets RUNX2 and controls the hypertrophic differentiation. 36
This study has a few limitations. First is the use of pediatric periosteal and bone marrow–derived MSCs. This study mainly demonstrates the role of PTHrP(1-34) in preventing hypertrophy and is applicable to both allogeneic and autologous MSCs. The allogenic source will preferably have to be younger individuals. Even when autologous MSCs are considered, there are a large number of young adults with sports injuries as well as children with cartilage defects secondary to osteochondritis dissecans and trauma for whom the results of this study would be relevant. Second, the use of patient samples with minimal replicates limits the power of the study, especially in the biochemical analysis.
In conclusion, bone marrow and periosteal-derived MSCs with appropriate growth factor combination may be a good source for cartilage tissue engineering. We herein show the significance of introducing PTHrP(1-34) at an early time point prior to the onset of hypertrophy to produce better quality chondrocytes. This protocol can be applied to the regeneration of articular cartilage using MSCs. However, further in vivo studies are required to confirm our findings.
Supplemental Material
Supplemental material, S1 for Early Addition of Parathyroid Hormone–Related Peptide Regulates the Hypertrophic Differentiation of Mesenchymal Stem Cells by Karthikeyan Rajagopal, Sowmya Ramesh and Vrisha Madhuri in CARTILAGE
Supplemental material, TableS1 for Early Addition of Parathyroid Hormone–Related Peptide Regulates the Hypertrophic Differentiation of Mesenchymal Stem Cells by Karthikeyan Rajagopal, Sowmya Ramesh and Vrisha Madhuri in CARTILAGE
Footnotes
Supplementary material for this article is available on the Cartilage website at https://journals.sagepub.com/home/CAR.
Acknowledgments and Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was supported by a grant from the Science and Engineering Research Board (PI: VM, Grant Number: SR/SO/HS/190/2012), Department of Science and Technology, Government of India and Department of Biotechnology (Grant Number: BT/IN/DENMARK/02/PDN/2011 DATED26/05/11), Government of India.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Ethics Approval: This study was approved by the institutional review board (Reference number: 8514 dated 30.10.2013 and 8964 dated 23.07.2014) of Christian Medical College, Vellore, and conducted as per the recommendation of the Declaration of Helsinki.
Informed Consent: The patient samples were collected after obtaining informed written consent.
References
- 1. Richardson SM, Kalamegam G, Pushparaj PN, Matta C, Memic A, Khademhosseini A, et al. Mesenchymal stem cells in regenerative medicine: focus on articular cartilage and intervertebral disc regeneration. Methods. 2016;99:69-80. [DOI] [PubMed] [Google Scholar]
- 2. Jones BA, Pei M. Synovium-derived stem cells: a tissue-specific stem cell for cartilage engineering and regeneration. Tissue Eng Part B Rev. 2012;18:301-11. [DOI] [PubMed] [Google Scholar]
- 3. Zscharnack M, Hepp P, Richter R, Aigner T, Schulz R, Somerson J, et al. Repair of chronic osteochondral defects using predifferentiated mesenchymal stem cells in an ovine model. Am J Sports Med. 2010;38:1857-69. [DOI] [PubMed] [Google Scholar]
- 4. Sakaguchi Y, Sekiya I, Yagishita K, Ichinose S, Shinomiya K, Muneta T. Suspended cells from trabecular bone by collagenase digestion become virtually identical to mesenchymal stem cells obtained from marrow aspirates. Blood. 2004;104:2728-35. [DOI] [PubMed] [Google Scholar]
- 5. Chen S, Fu P, Cong R, Wu H, Pei M. Strategies to minimize hypertrophy in cartilage engineering and regeneration. Genes Dis. 2015;2:76-95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Indrawattana N, Chen G, Tadokoro M, Shann LH, Ohgushi H, Tateishi T, et al. Growth factor combination for chondrogenic induction from human mesenchymal stem cell. Biochem Biophys Res Commun. 2004;320:914-9. [DOI] [PubMed] [Google Scholar]
- 7. Nejadnik H, Diecke S, Lenkov OD, Chapelin F, Donig J, Tong X, et al. Improved approach for chondrogenic differentiation of human induced pluripotent stem cells. Stem Cell Rev Rep. 2015;11:242-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Lee JM, Im GI. PTHrP isoforms have differing effect on chondrogenic differentiation and hypertrophy of mesenchymal stem cells. Biochem Biophys Res Commun. 2012;421:819-24. [DOI] [PubMed] [Google Scholar]
- 9. Zhang W, Chen J, Zhang S, Ouyang HW. Inhibitory function of parathyroid hormone-related protein on chondrocyte hypertrophy: the implication for articular cartilage repair. Arthritis Res Ther. 2012;14:221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Fischer J, Aulmann A, Dexheimer V, Grossner T, Richter W. Intermittent PTHrP (1-34) exposure augments chondrogenesis and reduces hypertrophy of mesenchymal stromal cells. Stem Cells Dev. 2014;23:2513-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Huang W, Chung UI, Kronenberg HM, de Crombrugghe B. The chondrogenic transcription factor Sox9 is a target of signaling by the parathyroid hormone-related peptide in the growth plate of endochondral bones. Proc Natl Acad Sci U S A. 2001;98:160-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Mueller MB, Fischer M, Zellner J, Berner A, Dienstknecht T, Kujat R, et al. Effect of parathyroid hormone-related protein in an in vitro hypertrophy model for mesenchymal stem cell chondrogenesis. Int Orthop. 2013;37:945-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Weiss S, Hennig T, Bock R, Steck E, Richter W. Impact of growth factors and PTHrP on early and late chondrogenic differentiation of human mesenchymal stem cells. J Cell Physiol. 2010;223:84-93. [DOI] [PubMed] [Google Scholar]
- 14. Mara CS, Sartori AR, Duarte AS, Andrade ALL, Pedro MAC, Coimbra IB. Periosteum as a source of mesenchymal stem cells: the effects of TGF-β3 on chondrogenesis. Clinics (Sao Paulo). 2011;66:487-92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Farndale RW, Buttle DJ, Barrett AJ. Improved quantitation and discrimination of sulphated glycosaminoglycans by use of dimethylmethylene blue. Biochim Biophys Acta. 1986;883:173-7. [DOI] [PubMed] [Google Scholar]
- 16. Djouad F, Delorme B, Maurice M, Bony C, Apparailly F, Louis-Plence P, et al. Microenvironmental changes during differentiation of mesenchymal stem cells towards chondrocytes. Arthritis Res Ther. 2007;9:R33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hubka KM, Dahlin RL, Meretoja VV, Kasper FK, Mikos AG. Enhancing chondrogenic phenotype for cartilage tissue engineering: monoculture and coculture of articular chondrocytes and mesenchymal stem cells. Tissue Eng Part B Rev. 2014;20:641-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Caron M, Emans P, Cremers A, Surtel D, Coolsen M, Van Rhijn L, et al. Hypertrophic differentiation during chondrogenic differentiation of progenitor cells is stimulated by BMP-2 but suppressed by BMP-7. Osteoarthritis Cartilage. 2013;21:604-13. [DOI] [PubMed] [Google Scholar]
- 19. Pelttari K, Steck E, Richter W. The use of mesenchymal stem cells for chondrogenesis. Injury. 2008;39(Suppl 1):S58-S65. [DOI] [PubMed] [Google Scholar]
- 20. Ferretti C, Mattioli-Belmonte M. Periosteum derived stem cells for regenerative medicine proposals: boosting current knowledge. World J Stem Cells. 2014;6:266-77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Miura Y, Parvizi J, Fitzsimmons JS, O’driscoll SW. Brief exposure to high-dose transforming growth factor-beta1 enhances periosteal chondrogenesis in vitro: a preliminary report. J Bone Joint Surg Am. 2002;84:793-9. [DOI] [PubMed] [Google Scholar]
- 22. Herlofsen SR, Küchler AM, Melvik JE, Brinchmann JE. Chondrogenic differentiation of human bone marrow-derived mesenchymal stem cells in self-gelling alginate discs reveals novel chondrogenic signature gene clusters. Tissue Eng Part A. 2010;17:1003-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Galeano-Garces C, Camilleri ET, Riester SM, Dudakovic A, Larson DR, Qu W, et al. Molecular validation of chondrogenic differentiation and hypoxia responsiveness of platelet-lysate expanded adipose tissue-derived human mesenchymal stromal cells. Cartilage. 2017;8:283-99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kim YJ, Kim HJ, Im GI. PTHrP promotes chondrogenesis and suppresses hypertrophy from both bone marrow-derived and adipose tissue-derived MSCs. Biochem Biophys Res Commun. 2008;373:104-8. [DOI] [PubMed] [Google Scholar]
- 25. Zhang Y, Kumagai K, Saito T. Effect of parathyroid hormone on early chondrogenic differentiation from mesenchymal stem cells. J Orthop Surg Res. 2014;9:68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Horwitz MJ, Augustine M, Kahn L, Martin E, Oakley CC, Carneiro RM, et al. A comparison of parathyroid hormone-related protein (1-36) and parathyroid hormone (1-34) on markers of bone turnover and bone density in postmenopausal women: the PrOP study. J Bone Miner Res. 2013;28:2266-76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Feeley BT, Doty SB, Devcic Z, Warren RF, Lane JM. Deleterious effects of intermittent recombinant parathyroid hormone on cartilage formation in a rabbit microfracture model: a preliminary study. HSS J. 2010;6:79-84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Fischer J, Dickhut A, Rickert M, Richter W. Human articular chondrocytes secrete parathyroid hormone-related protein and inhibit hypertrophy of mesenchymal stem cells in coculture during chondrogenesis. Arthritis Rheum. 2010;62:2696-706. [DOI] [PubMed] [Google Scholar]
- 29. Fahy N, Gardner OF, Alini M, Stoddart MJ. Parathyroid hormone-related protein gradients affect the progression of mesenchymal stem cell chondrogenesis and hypertrophy. Tissue Eng Part A. 2018;24:849-59. [DOI] [PubMed] [Google Scholar]
- 30. Sarem M, Heizmann M, Barbero A, Martin I, Shastri VP. Hyperstimulation of CaSR in human MSCs by biomimetic apatite inhibits endochondral ossification via temporal down-regulation of PTH1R. Proc Natl Acad Sci U S A. 2018;115:E6135-E6144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. De Bari C, Dell’Accio F, Vanlauwe J, Eyckmans J, Khan IM, Archer CW, et al. Mesenchymal multipotency of adult human periosteal cells demonstrated by single-cell lineage analysis. Arthritis Rheum. 2006;54:1209-21. [DOI] [PubMed] [Google Scholar]
- 32. Coutu DL, François M, Galipeau J. Inhibition of cellular senescence by developmentally regulated FGF receptors in mesenchymal stem cells. Blood. 2011;117:6801-12. [DOI] [PubMed] [Google Scholar]
- 33. Stevens MM, Marini RP, Martin I, Langer R, Shastri VP. FGF-2 enhances TGF-beta1-induced periosteal chondrogenesis. J Orthop Res. 2004;22:1114-9. [DOI] [PubMed] [Google Scholar]
- 34. Chen H, Ghori-Javed FY, Rashid H, Adhami MD, Serra R, Gutierrez SE, et al. Runx2 regulates endochondral ossification through control of chondrocyte proliferation and differentiation. J Bone Miner Res. 2014;29:2653-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Lu H, Lin Z, Yang Z, Chen M, Zhang K. Inhibition of RUNX2 expression promotes differentiation of MSCs correlated with SDF-1 up-regulation in rats. Int J Clin Exp Pathol. 2016;9:11388-95. [Google Scholar]
- 36. Zhang M, Xie R, Hou W, Wang B, Shen R, Wang X, et al. PTHrP prevents chondrocyte premature hypertrophy by inducing cyclin-D1-dependent Runx2 and Runx3 phosphorylation, ubiquitylation and proteasomal degradation. J Cell Sci. 2009;122(Pt 9):1382-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, S1 for Early Addition of Parathyroid Hormone–Related Peptide Regulates the Hypertrophic Differentiation of Mesenchymal Stem Cells by Karthikeyan Rajagopal, Sowmya Ramesh and Vrisha Madhuri in CARTILAGE
Supplemental material, TableS1 for Early Addition of Parathyroid Hormone–Related Peptide Regulates the Hypertrophic Differentiation of Mesenchymal Stem Cells by Karthikeyan Rajagopal, Sowmya Ramesh and Vrisha Madhuri in CARTILAGE