Abstract
Statins have demonstrated to be effective for treating chondrodysplasia and its effects were believed to be associated with the fibroblast growth factor receptor 3 (FGFR3). Statins promoted the degradation of FGFR3 in studies using disease-specific induced pluripotent stem cells and model mice, however, recent studies using normal chondrocytes reported that statins did not degrade FGFR3. In order to further investigate the effects of statins in endochondral ossification, this study examined the influence of statins on Indian hedgehog (Ihh), another important component of endochondral ossification, and its related pathways. The chondrocyte cell line ATDC5 was used to investigate changes in cell proliferation, mRNA, and protein expression levels. In addition, an organ culture of a mouse metatarsal bone was performed followed by hematoxylin-eosin staining and fluorescent immunostaining. Results indicated that expression level of Ihh increased with the addition of statins, which activated the Ihh pathway and altered the localization of Ihh. Changes in cholesterol modification may have affected Ihh diffusibility; however, further experiments are necessary. A reactive increase in parathyroid hormone–related protein (PTHrP) was observed in addition to changes in the Wnt pathway through secreted-related protein 2/3 and low-density lipoprotein 5/6. This led to the promotion of cell proliferation, increase of the hypertrophic chondrocyte layer, inhibition of apoptosis, and decrease in mineralization. This study demonstrated that statins had an influence on Ihh, and that the hyperfunction of Ihh may prevent premature cell death caused by FGFR3-related chondrodysplasia through an indirect increase in the expression of PTHrP.
Keywords: statin, endochondral ossification, Indian hedgehog, parathyroid hormone–related protein, Wnt
Introduction
Statins are commonly used as therapeutic agents for hypercholesterolemia. 1 They interfere with cholesterol biosynthesis by inhibiting hydroxymethylglutaryl-CoA (HMG-CoA) reductase, the rate-limiting enzyme within the mevalonate pathway. For many years, statins were believed to be effective for treating fibroblast growth factor receptor 3 (FGFR3) gene-related diseases such as achondroplasia (ACH) and thanatophoric dysplasia (TD). 2 These diseases present genetic dwarfism due to the inhibition of cartilage proliferation and differentiation as a result of gain-of-function mutations in FGFR3. 3
Yamashita et al. 4 created a mouse model of human ACH from induced pluripotent stem (iPS) cells from ACH and TD patients and revealed that statins reduced the inhibition of cartilage differentiation improving bone length and stature of ACH mice. This study confirmed that statins promoted the recovery of chondrogenic differentiation induced by impaired function of FGFR3, however, the mechanism of statin effect on FGFR3 in the cholesterol pathway remained unclear. 4
On the contrary, Fafilek et al. 5 examined the influence of FGFR signaling on cultured chondrocytes and revealed statins had no significant effect. This study suggested that statins did not inhibit the signaling of FGFR3; however, they did influence other important signaling pathways that regulate changes in the growth plate. These changes may have resulted in the indirect suppression of FGFR3. The study revealed that factors other than FGFR3 were affected by statins, however the mechanism by which statin influences endochondral ossification remains unclarified.
The Indian hedgehog (Ihh), a signaling protein in the hedgehog signaling pathway, is an important component of endochondral ossification. Ihh is synthesized in its precursor form then excised into N-terminal and C-terminal fragments. The N-terminal fragment undergoes cholesterol modification and palmitoylation, then is later secreted from prehypertrophic chondrocytes. Its functions include the proliferation of chondrocytes in the perichondrium and proliferative zone and the promotion of parathyroid hormone–related protein (PTHrP) secretion. PTHrP controls the growth of endochondral ossification by adjusting the reciprocal secretion of Ihh through a negative feedback mechanism. 6 No studies have examined the effects of statins on Ihh during typical endochondral ossification.
The objective of this study was to investigate the effects of statins on Ihh and its related pathways. Determining the effects of statins on Ihh would be useful in order to better understand statin influence on endochondral ossification, as well as how statin treatment as therapeutic agents for chondroplasia can be improved.
Materials and Methods
Cell Culture
ATDC5 cells were cultured as a monolayer with a mixture of Dulbecco’s modified Eagle’s medium and Ham’s F-12 medium (DMEM/F12, GIBCO) supplemented with 5% fetal bovine serum (FBS, GE Healthcare) and 100 U/mL penicillin-streptomycin (GIBCO) in a humidified atmosphere of 5% CO2 at 37°C. 7 Inoculum sizes were 6 × 104 cells/well in a 6-multiwell plate, 1 × 104 cells/well in a 48-multiwell plate, and 2 × 103 cells/well in a 96-multiwell plate. Ascorbic acid and β-glycerophosphate, with concentrations of 50 ng/mL and 10 mM, respectively, were added in order to accelerate chondrogenic differentiation and produce a physiologically mineralized extracellular matrix. 8 The medium was replaced every other day. On reaching confluence, cells were stimulated with 10 µg/mL insulin, 5.5 µg/mL transferrin, and 6.7 ng/mL sodium selenite (ITS, GIBCO).
WST-1 Assay and BrdU Assay
ATDC5 cells were cultured in 96-well multiplates in the presence or absence of fluvastatin (statin, 10, 100, and 1000 nM, Cayman chemical). Statin was dissolved in dimethyl sulfoxide (DMSO); the same volume of DMSO was used as the control. On days 1, 7, 14, and 21 after differentiation, 10 μL of WST-1 (TAKARA) was added, and the absorbance was measured at a wavelength of 450 nm using SpectraMaxM (Molecular Devices). The control wavelength was established at 650 nm. Data from days 7, 14, and 21 were normalized to that of day 1.
BrdU cell proliferation ELISA kit (Sigma-Aldrich) was used under the same conditions. After culturing cells, BrdU was incorporated into the DNA and reacted with anti-BrdU-POD Fab fragment after cell fixation with FixDenat. Chemiluminescence of the substrate solution was measured using SpectraMaxM (Molecular Devices).
Alkaline Phosphatase Activity
ATDC5 cells were cultured in 48-well plates in the presence or absence of statin (10, 100, and 1000 nM). An ALP laboratory assay kit (WAKO) was used to determine alkaline phosphatase (ALP) activity on day 7 after differentiation. After washing the cells with phosphate buffer solution (PBS), lysate was retrieved using 0.05% Triton X-100, and a carbonate buffer solution (pH 9.8) containing P-nitrophenylphosphoric acid was reacted to measure change in color. Absorbance was measured at a wavelength of 405 nm using SpectraMaxM (Molecular Devices). The data was normalized to total protein which was measured using a Pierce BCA Protein Assay Kit. The 1-Step NBT (Thermo Scientific) was used to perform ALP staining.
Alcian Blue and Alizarin Red Staining
ATDC5 cells were cultured in 48-well plates in the presence or absence of statin (10, 100, and 1000 nM). On day 21 after differentiation, cells were fixed with 10% formalin. Alcian Blue solution (0.1%, WAKO) was used for Alcian Blue staining. Cells were washed with distilled water before capturing images. For quantitative analyses, cells were lysed in 6 M guanidine HCl for 6 hours at room temperature. The absorbance was measured at a wavelength of 650 nm using SpectraMaxM (Molecular Devices). Alizarin Red solution (1%, Sigma-Aldrich) was used for Alizarin Red staining. Cells were washed with distilled water before capturing images. Cells were extracted with 10% cetylpyridinium chloride for 10 minutes. The absorbance was measured at a wavelength of 425 nm using SpectraMaxM (Molecular Devices). 8
Total RNA Extraction and Real-Time Polymerase Chain Reaction analysis
ATDC5 cells were cultured in 6-well plates in the presence or absence of statin (1000 nM). Total RNA was extracted using TRIzol (Invitrogen) on days 7, 14, and 21. cDNA was synthesized using ReverTra Ace qPCR RT Master Mix with gDNA Remover (TOYOBO). The cDNA was subjected to real-time polymerase chain reaction (RT-PCR) by the SYBR Green method using the THUNDERBIRD SYBR qPCR Mix (Toyobo) and 7500 Fast Real-Time PCR system (Applied Biosystems). Hypoxanthine-guanine phosphoribosyltransferase (Hprt) was used as a housekeeping gene. 9 The primers used are shown in Table 1 . The procedure was done in triplicate and the level of mRNA expression was calculated by theΔΔCt method. Sex-determining region Y box9 (Sox9), type 2 collagen (Col2), and aggrecan (ACAN) as early differentiation markers. Runt-related transcription factor 2 (Runx2), type 10 collagen (Col10), and Osteopontin (OPN) were used as late differentiation markers. For the Ihh pathway Ihh, PTHrP, and smoothened (Smo) protein Patched homolog 1 (Ptch1) were used.
Table 1.
Primer Sequences.
| mRNA | Forward (F)/Reverse (R) | 5′ → 3′ | Product Size |
|---|---|---|---|
| Hprt | F | CTGGTGAAAAGGACCTCTCGAA | 110 |
| R | CTGAAGTACTCATTATAGTCAAGGGCAT | ||
| Col2a1 | F | GGCCAGGATGCCCGAAAATTAG | 156 |
| R | CGCACCCTTTTCTCCCTTGT | ||
| Col10 | F | GCATCTCCCAGCACCAGAATC | 126 |
| R | TTATGCCTGTGGGCGTTTGG | ||
| Sox9 | F | TGAAGATGACCGACGAGCAG | 198 |
| R | GGATGCACACGGGGAACTTA | ||
| Runx2 | F | AATTAACGCCAGTCGGAGCA | 70 |
| R | CACTTCTCGGTCTGACGACG | ||
| Ihh | F | GCTTCGACTGGGTGTATTACG | 231 |
| R | GCTCACGGTCCAGGAAAAT | ||
| PTHrP | F | AGCAGTGGAGTGTCCTGGTA | 167 |
| R | ATGGTGGAGGAAGAAACGGC | ||
| Smo | F | AAGACAGCTTCGATCTCCAGG | 189 |
| R | CGGAGGCTACTTAGGCGACC | ||
| OPN | F | GGCTGAATTCTGAGGGACTAACT | 211 |
| R | ACAGCATTCTGTGGCGCAAG | ||
| ACAN | F | GCTGGCTGACCAGACAGTCA | 100 |
| R | CCGGATTCCGTAGGTTCTCA | ||
| Ptch | F | CCAGCGGCTACCTACTGATG | 151 |
| R | TGCCAATCAAAGGAGCAGAGG |
Western Blot Analysis
ATDC5 cells were cultured in 6-well plates in the presence or absence of statin (1000 nM). Proteins were extracted using RIPA Buffer (Wako); protein concentrations were determined using the Pierce BCA Protein Assay Kit. Equal amounts of protein were separated by SDS-PAGE (sodium dodecyl sulfate–polyacrylamide gel electrophoresis) and transferred onto nitrocnellulose membranes with an iBlot2 Dry Blotting System (Thermo Scientific) according to the manufacturer’s protocol. Avidin/Biotin Blocking Kit (VECTOR) was used for biotin blocking. BSA (2%) in TBS-Tween (TBS-T) was used for blocking. Table 2 shows the list of primary antibodies used derived from rats, goats, and donkeys. These primary antibodies were reacted with secondary antibodies (mouse biotinylated anti-rat IgG(H + L), donkey biotinylated anti-rabbit IgG(H + L), and donkey biotinylated anti-goat IgG(H + L) (2 μg/mL, Jackson)) and corresponding tertiary antibodies. Finally, tertiary antibodies were reacted with HRP streptavidin (2 μg/mL, BioLegend) and proteins were visualized using Luminata Forte Western HRP Substrate (Merck Millipore). Fluorescence was captured using ImageQuant Las (GE Healthcare) and ImageJ was quantified. This procedure was triplicated for every protein. α-Tubulin was used for loading control.
Table 2.
Antibodies.
| Antibody | Clonality | Clone Name | Company Code | Dilution Factor | Company |
|---|---|---|---|---|---|
| Ihh | Rat monoclonal | 3A11 | GTX53235 | 2 μg/mL (WB)■20 μg/mL (IHC) | Genetex |
| PTHrP | Rabbit polyclonal | PA5-40799 | 1 μg/mL (WB) · 8 μg/mL (IHC) | Thermofisher Scientific | |
| Gli1 | Rabbit polyclonal | ab167388 | 1 μg/mL | Abcam | |
| Gli2 | Rabbit polyclonal | ab167389 | 1 μg/mL | Abcam | |
| Gli3 | Rabbit polyclonal | ab69838 | 1 μg/mL | Abcam | |
| Lrp5/6 | Rabbit polyclonal | ab51910 | 2 μg/mL | Abcam | |
| βcatenin | Mouse monoclonal | 12F7 | ab22656 | 2 μg/mL | Abcam |
| βcatenin(phos) | Rabbit polyclonal | ab27798 | 2 μg/mL | Abcam | |
| sfrp2 | Mouse monoclonal | C-4 | sc365524 | 2 μg/mL | Santa Cruz Biotechnology |
| sfrp3 | Goat polyclonal | PA5-47793 | 0.1 μg/mL | Thermofisher Scientific | |
| Nkx3.2 | Rabbit polyclonal | PA5-21108 | 2 μg/mL | Thermofisher Scientific | |
| Col2a1 | Rabbit polyclonal | SC28887 | 20 μg/mL (IHC) | Santa Cruz Biotechnology | |
| Col10 | Mouse monoclonal | X53 | 14-9771-82 | 10 μg/mL (IHC) | Invitrogen |
WB = Western blot; IHC = immunohistochemistry.
Total Cholesterol Assay
Proteins were extracted and adjusted to the same concentration using the procedure discussed above. Total cholesterol assay was performed using the Total Cholesterol Assay Kit Colorimetric (Cell Biolabs) according to the manufacturer’s protocol. Cholesterol esterase and cholesterol oxidase were added to all samples. Cholesterol ester and cholesterol were broken down into cholest-4-ene-3-one and hydrogen peroxide. The generated hydrogen peroxide was reacted with colorimetric probe and absorbance was measured at 550nm wavelength to quantify samples from control values.
Organ Culture and Immunohistochemistry
Four-day-old postnatal (P4) C57BL/6 metatarsals were dissected under sterile conditions and cultured in the same medium used for cell cultures. One long bone was cultured per well in 300 μL of complete medium in a 24-well tissue plate in humidified atmosphere of 5% CO2 at 37°C. The medium was replaced every other day. The metatarsals were cultured with or without 1000 nM statin. Experiments were set up for left-right paired observations, with one long bone serving as a control to the other experimental samples. After 7 days, the bones were fixed in 4% paraformaldehyde for 48 hours, washed with PBS, and stored in 70% ethanol. The bones were decalcified using 10% 2Na EDTA (pH 7.0) at 37°C for 10 days. Bone tissue was cut into 4 µm sections with microtome after paraffin embedding. After deparaffinization, PBS was substituted and antigen activation with an immunosaver (Nisshin) was performed. Endogenous peroxidase and endogenous biotin blocking were also performed. Normal blocking buffer was performed in 5% BSA in PBS. The anti-mouse monoclonal antibody from the Mouse On Mouse (M.O.M) immunodetection kit (VECTOR) was used. Primary antibodies were incubated overnight at concentrations of 2 µg in blocking buffer using PTHrP, Ihh, Col2, and Col10 ( Fig. 2 ). Secondary antibodies were incubated at room temperature for 2 hours after diluting concentration to 2000:1 using Anti-Rabbit Alexa Fluor 546, Anti-Mouse Alexa Fluor 633 (Life Technology), Anti-Rat Alexa Fluor 633 (Life technology) and washing with PBS. For nuclear staining, Hochest 33342 (Dojindo) was used. This procedure was repeated on 3 samples.
Figure 2.
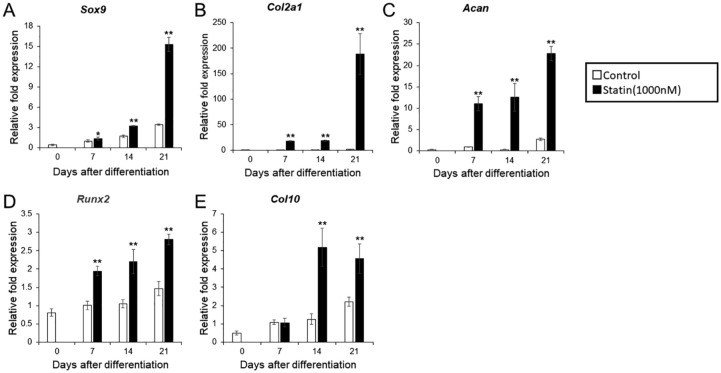
mRNA expression of the cartilage marker in differentiation-induced ATDC5 cells with the addition of statin. (A) Sox 9. (B) Col2. (C) ACAN. (D) Runx2. (E) Col10. ITS (insulin, transferrin, sodium selenite) was added on day 0 and normalized according to the control value of day 7. The graph values denote the average ± SD (n = 3), *P < 0.05 and **P < 0.01, estimated by analysis of variance followed by post hoc Tukey-Kramer and compared with control group at same time point.
This study was approved by the Institutional Animal care and Use Committee (Permission number: 282802) and conducted according to Tokyo Dental Collage Animal Experimentation Regulations.
Statistical Analyses
Statistical analyses were performed using SPSS statistics 23 (IBM). Significance was assessed using 1-way analysis of variance; the adjusted P values were obtained using Tukey-Kramer post hoc comparisons. P values >0.05 were considered to be significant.
Results
Influence of Statin on ATDC5 Cell Proliferation and Mineralization
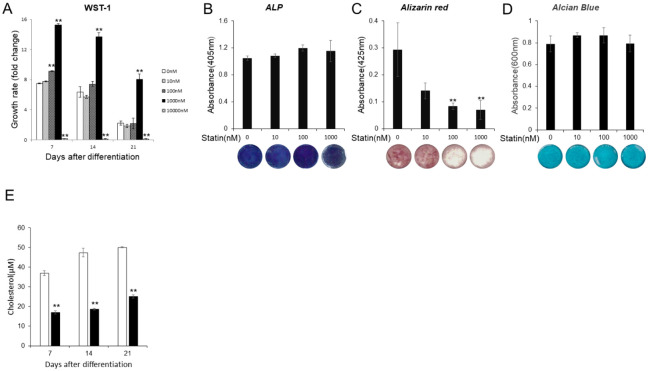
WST-1 was used to examine the effects of statin on the proliferative capacity of ATDC5 cells ( Fig. 1A ). After addition of ITS, the group with statin concentration of 1000 nM displayed nearly twice the proliferation capacity compared with control. The cells died when the concentration was greater than 10 μM. The effects of statin on ATDC5 cell calcification and cartilage matrix secretion was also investigated. No change in ALP activity was indicated for all concentrations ( Fig. 1B ). A change in cell number was found during Alizarin Red staining where the formation of calcified matrix was suppressed at 100 and 1000 nM ( Fig. 1C ). Alcian Blue staining indicated that the addition of statin had no effect on cartilage matrix secretion ( Fig. 1D ). Statin concentration of 1000 nM was used in subsequent experiments based on the above results. Total cholesterol level was decreased with the addition of statin at a concentration of 1000 nM ( Fig. 1E .).
Figure 1.
Various tests with statins at different concentration. (A) Comparison of cell proliferation ability by, WST-1. The growth rates were compared by absorbance before ITS (insulin, transferrin, sodium selenite) treatment as a control at each, concentration on day 0. (B) The comparison of alkaline phosphatase (ALP) activity on day 7 and its image. (C) Comparison of the, dyeability of Alizarin Red on day 21 and its image. (D) Comparison of the dyeability of Alcian Blue on day 21, and its image. (E) To check statin effect for cholesterol . The graph values denote the average ± SD (n = 3), *P < 0.05 and **P < 0.01, estimated by analysis of variance followed by post hoc Tukey-Kramer and compared with, control group at same time point.
Influence of Statins on mRNA Expression of Early and Late Differentiation Markers
After addition of 1% ITS, the mRNA levels of early and late differentiation markers were measured and compared at 7, 14, and 21 days between the experimental (1000 μM statin) and control group. In the experimental group, expression levels of early chondrocyte differentiation markers Sox9, Col2, and Acan, increased between days 7 and 21 compared with the control group. In particular, Sox9 sharply increased on day 21, whereas the increase of Col2 was constant ( Fig. 2A-C ). With regard to late differentiation markers Runx2 and Col10, Runx2 expression increased between days 7 and 21, reaching a maximum on day 21. Col10 expression levels increased after day 14 ( Fig. 2D and E ). In the control group, both the early and late differentiation markers showed an increase in expression over time ( Fig. 2A-E ).
The Influence of Statins on Ihh and PTHrP Expression
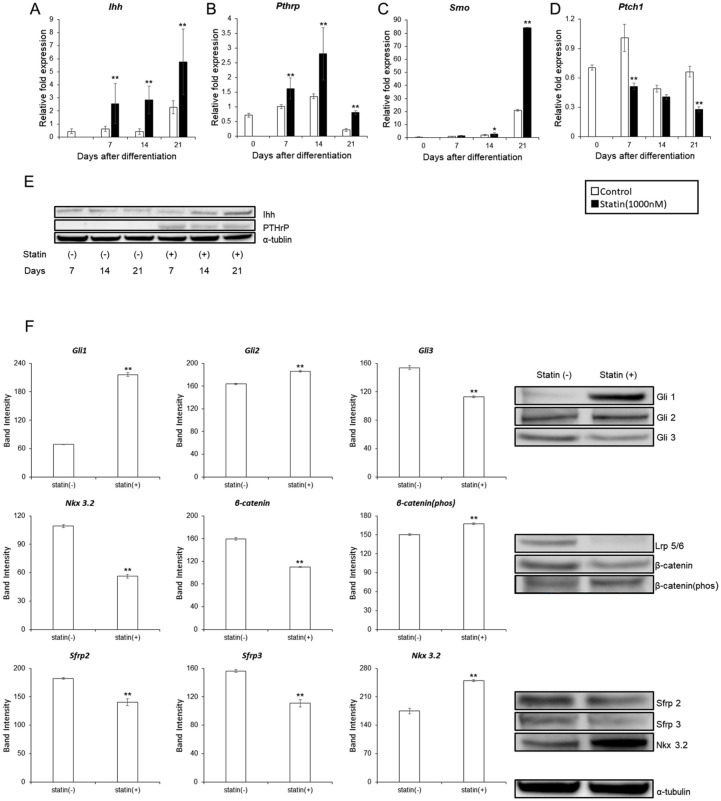
ATDC5 cells were used to examine statin-induced changes in Ihh, PTHrP, and Wnt pathway, which are important pathways in endochondral ossification. The expression levels of Ihh, PTHrP, Smo, and Ptch1 with the addition of 1000 nM statin were determined. In the experimental group, the expression of Ihh was greater on days 7, 14, and 21 ( Fig. 3A ). In the control group, the expression level of Ihh was greatest on day 21. PTHrP expression was elevated on day 14 for both control and experimental groups, however, reduced by day 21. In the experimental group, expression levels were greater on days 7, 14, and 21 compared with the control group ( Fig. 3B ). Smo, expression reached a maximum on day 21 in both the control and experimental group. However, in the experimental group, expression level increased on days 14 and 21 compared with the control group ( Fig. 3C ). Ptch1 expression decreased on days 7 and 21 in the experimental group compared with the control group ( Fig. 3D ).
Figure 3.
Changes in the signaling pathways of Ihh, PTrP, and Wnt in ATDC5 cells with the addition of statin. (A) Ihh. (B) PTHrP. (C) Smo. (D) Ptch1. ITS (insulin, transferrin, sodium selenite) was added on day 0 and was normalized according to the control value of day 7. For the significant difference test, the Tukey-Kramer test was conducted. (E) Comparison of protein amounts of Ihh and PTHrP by Western blot on days 7, 14, and 21 after induction of differentiation with 1% ITS. (F) Comparison of protein amounts related to Ihh pathway and Wnt pathway by western blot on day 21 after induction of differentiation with 1% ITS. The graph values denote the average ± SD (n = 3), *P < 0.05 and **P < 0.01, estimated by analysis of variance followed by post hoc Tukey-Kramer and compared with control group at same time point.
Changes in the protein amount of Ihh and PTHrP with the addition of 1000 nM statin was determined using the western blot. Ihh amount increased in on days 14 and 21, while PTHrP amount increased on days 7, 14, and 21 ( Fig. 3E ). The proteins involved in the Ihh and Wnt pathway on day 21 were determined, because the amount of Ihh protein increased the most significantly.
Regarding the Hedgehog pathway, Gli 1 and 2 increased whereas Gli 3 decreased, confirming the activation of the Ihh pathway. For the Canonical Wnt pathway, LRP 5/6 decreased, β-catenin decreased, and β-catenin (Phos) increased. This suggested that expression of LRP 5/6 was suppressed by increased expression of Ihh, and canonical Wnt pathway was suppressed as a result. In Noncanonical Wnt pathway, expression of Sfrp 2/3 was suppressed due to increased expression of Ihh and the activation of non-canonical Wnt pathway was suggested; however, Nkx 3.2 increased ( Fig. 3F ).
Influence of Statins on Function of Ihh
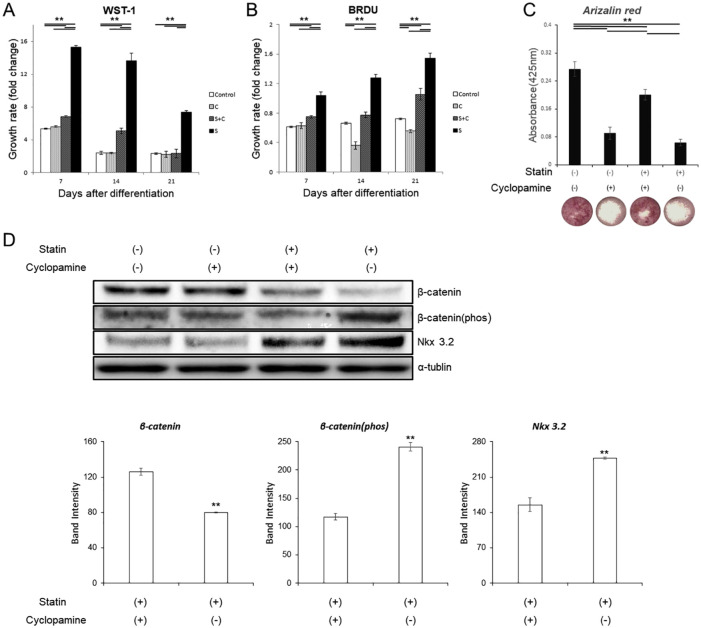
To further confirm the results above, experiments were conducted using cyclopamine, an inhibitor of the hedgehog signaling pathway. In the WST-1 assay, the addition of cyclopamine significantly suppressed statin-induced cell proliferation on all days ( Fig. 4A ). The same results were obtained for the BrdU assay ( Fig. 4B ).
Figure 4.
Various tests with statins and cyclopamine. (A, B) Comparison of cell proliferation ability by WST-1 and BrdU. The growth rates were compared by absorbance before ITS (insulin, transferrin, sodium selenite) treatment as a control at each concentration on day 0. (C) Comparison of the dyeability of Alizarin Red on day 21 and its image. (D) Western blot for Wnt pathway. The graph values denote the average ± SD (n = 3), *P < 0.05 and **P < 0.01, estimated by analysis of variance followed by post hoc Tukey-Kramer.
For alizarin staining, cyclopamine improved the suppression of mineralization caused by statins ( Fig. 4C ). For the Wnt pathway, changes in β-catenin, β-catenin (phos), Nkx 3.2 caused by the addition of statin was recovered and values were brought close to the control group with the addition of cyclopamine ( Fig. 4D ).
Influence of Statin on Location of Ihh
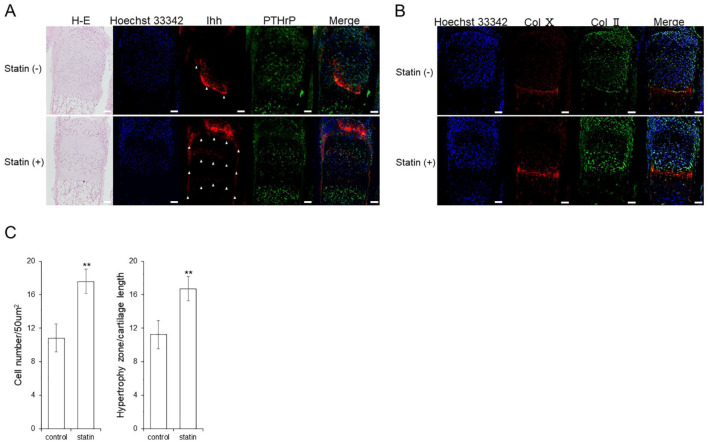
Although Ihh exists in the form of a band near the prehypertrophic chondrocyte, it is also found in the central part of the epiphysis, particularly in the perichondrium, which was observed after the addition of statin. The localization of PTHrP did not significantly change ( Fig. 5A ), and Col10 showed no change in localization from the addition of the statin. However, Col2 showed an increase in expression in the proliferating zone ( Fig. 5B ).
Figure 5.
Changes in Ihh location. Immunostaining image of the cultured metatarsal bone of a mouse (A) Ihh, PTHrP, and HE. Cell polarity was not changed by statins. Ihh expression location was changed by statin. The white triangle indicates the luminescent spot of Ihh. (B) Col2 and Col10. Col2 and Col10 expression location are unchanged by statins. Scale bar indicates 50 μm. (C) The comparison of cell density in proliferating cell layers by HE staining, and the comparison in length ratios of the cartilage part in hypertrophic chondrocyte layers. The graph values denote the average ± SD (n = 3), *P < 0.05 and **P < 0.01, estimated by analysis of variance followed by post hoc Tukey-Kramer.
During hematoxylin–eosin (HE) staining, the thickness of the hypertrophic chondrocyte layer and changes in the number of proliferating chondrocytes were examined. As a result, the addition of the statin increased the thickness of the hypertrophic zone and number of cells in the proliferative zone ( Fig. 5C ).
Discussion
Statins are known to be effective for treating FGFR3 gene–related diseases such as achondroplasia (ACH) and thanatophoric dysplasia (TD). 2 As discussed, Yamashita et al. 2 demonstrated that statins assisted in the recovery of chondrogenic differentiation induced by impaired function of FGFR3 but the mechanism was unclarified. In addition, the growth of wild-type mice in the control group was not affected by statin and the reason was also undetermined. 4 Fafilek et al. 5 studied the effect of statin on FGFR3 protein expression in rats and revealed that statin did not improve the reduction of cell number, bone length, or cartilage differentiation in rat chondrosarcoma chondrocyte (RCS) cells, ex vivo embryonal tibia cultures, or limb bud micro-mass cultures, respectively. Furthermore, ectopically expressed FGFR3 in RCS cells did not decrease even when statin was added to RCS cells with mutations introduced to ACH (G380R) and TD (K650M). The study proposed the possibility that statin influenced alternative signaling pathways that regulated changes in the growth plate, which consequentially resulted in the indirect suppression of FGFR3. 5
Mundy et al 10 discovered that statins were also involved in osteogenesis; this idea was supported by many other studies.11-13 Later research confirmed that statins also had a positive effect on osteoporosis, osteoarthritis, and periodontal diseases.14-16 These findings supplemented the idea that statins influence endochondral ossification via pathways unrelated to FGFR3. Therefore, this study investigated the effects of statin on Ihh, another essential component of chondrocyte differentiation in order to better understand the role of statins on endochondral ossification.
The cell culture used was the ATDC5 cell line derived from mouse embryonal calcinoma (EC), which is widely used in studies of endochondral ossification. 7 The following discusses the increased function of Ihh by fluvastatin caused by the suppression of cholesterol biosynthesis. In addition, findings indicated a corresponding increase in PTHrP secretion, as well as changes in canonical and noncanonical pathways associated with NK3 homeobox 2 (Nkx3.2), and the wingless-int (Wnt) pathway via secreted frizzled-related protein 2/3 (Sfrp 2/3) and low-density lipoprotein receptor–related protein 5/6 (LRP 5/6).
Influence of Statin on Ihh Expression and Function
Results indicated that statins enhanced mRNA and protein expression of Ihh ( Fig. 3A and E ). Many studies have reported that statins induce BMP2 in chondrocytes, 17 and that BMP2 promotes the secretion of Ihh. 18 The Hh family, including Ihh, undergoes cleavage after synthesis and the N-terminal fragment is secreted by cholesterol modification and palmitoylation. The effects of cholesterol-unmodified Hh have been widely studied in mice, flies, and other organisms.19-21 Despite some differences in mechanism, the suppression of cholesterol modification has shown to affect diffusivity. In this study, the result of IHC ( Fig. 5A ) suggested that statins may change the diffusivity of Ihh. However, additional experiments are necessary to confirm the effects of statins on the distribution of Ihh.
Cellular Proliferation
The membrane protein Smo and transcription factor Gli, located upstream and downstream of the Hh signaling system, respectively, were analyzed. The addition of statin enhanced the expression level of Ihh, which led to an increase in Gli1/2 through the activation of Smo, and decrease in Gli3, resulting in cellular proliferation of chondrocytes ( Fig. 3C and E ). 22 Moreover, the addition of statin to chondrocytes also increased the production of PTHrP as a response to enhanced diffusibility of Ihh into perichondrocytes and chondrocytes. As a result, PTHrP suppressed the differentiation of chondrocytes into hypertrophic chondrocytes, and the number of proliferating chondrocytes increased.Nkx 3.2, an early-stage chondrogenic factor, on Ihh and reported that Ihh signaling triggers Nkx 3.2 protein degradation during maturation of chondrocytes. This study indicated that Ihh suppressed the expression of Sfrp 2/3, leading to the activation of the non-canonical Wnt pathway, which resulted in the degradation of Nkx 3.2. 23 The expression of Nkx 3.2 increased despite the suppressed expression of Sfrp 2/3 due to the increased expression of PTHrP in response to Ihh and its diffusibility. The suppression of hypertrophy and the increase in proliferating chondrocytes occurred following an increase in Nkx 3.2. 24
The Wnt/β catenin pathway, a canonical pathway, is inactivated by Ihh via the suppression of LRP 5/6. 25 Conversely, Sfrp 2/3 suppresses not only non-canonical but also the canonical Wnt pathway. In this study, suppression of LRP5/6 was superior to suppression of Sfrp 2/3. Studies reported that the activation of the Wnt/β catenin pathway causes dwarfism, dysplasia, and early closure of the growth plate. 26 The decreased β-catenin caused by Ihh and the increase in phosphorylated β-catenin may have suppressed early chondrocyte differentiation.
These findings were supported by the fact that cellular proliferation was promoted ( Figs. 1A and 4A and B ). The IHC results ( Fig. 5B ) indicated that Col II was strongly expressed in proliferating cell layers and that expression levels of early differentiation markers increased with the addition of statin ( Fig. 2A and C ).
Cellular Hypertrophy, Death, and Calcification
PTHrP and Nkx3.2 inhibit hypertrophication of chondrocytes during chondrocyte differentiation as discussed earlier. In contrast, Ihh has shown to increase the expression of Runx2 and Col10, promoting chondrocyte hypertrophy independent of PTHrP. 23 This information suggested that differentiation of proliferating cells to hypertrophic cartilage was antagonized by increased expression of Ihh and subsequent enhanced expression of PTHrP and Nkx3.2 following the addition of statin.
PTHrP inhibited apoptosis in hypertrophic chondrocytes.27,28 Apoptosis was also suppressed by an increase in the expression level of Nkx3.2. 29 In this study, the addition of statin caused an increased expression of Ihh ( Fig. 3E ), as well Col10 and Runx2 ( Fig. 2D and E ). With regard to differentiation, magnified effects of Ihh was strongly indicated from the addition of statin.
On the contrary, calcification was suppressed despite the increase in Col10 and Runx2 with the addition of statins ( Fig. 1C ). This was because apoptosis of hypertrophic chondrocytes was prevented due to the increase of PTHrP and Nkx3.2 ( Fig. 3E and F ). As a result, the number of cells was retained ( Figs. 1A and 4A ) and the calcification associated with cell death did not appear as a phenotype. This reason was also supported by the increase in thickness and cell number of the hypertrophic chondrocyte layer in organ culture ( Fig. 5C ).
The expression of late differentiation markers increased despite the fact that calcification did not occur ( Fig. 2D and E ). A report had shown that BMP2 causes an increase in Runx2, suggesting that alternative factors may also be involved. 30
Conclusion
This study investigated the effects of statins on Ihh and its related pathways during endochondral ossification. Results indicated that expression level of Ihh increased with the addition of statins, which activated the Ihh pathway and altered the localization of Ihh. With regard to the change in localization, changes in cholesterol modification may have affected Ihh diffusibility, however further experiments are necessary to confirm such results. These findings suggested a novel idea that statin influences endochondral ossification through a pathway involving Ihh. The increase in Ihh also led to enhanced PTHrP reactivity indicating the possible existence of pathway where statin increased PTHrP via Ihh, inhibiting the ossification in early chondrocytes. 31
Footnotes
Author Contributions: KS and TI conceived and supervised the study; TI, TM and YI designed experiments; MI and TI performed experiments.
Acknowledgments and Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Ethical Approval: Not applicable.
Informed Consent: Not applicable.
Trial Registration: No applicable.
ORCID iD: Munetada Ishikawa  https://orcid.org/0000-0002-9035-9820
https://orcid.org/0000-0002-9035-9820
References
- 1. Endo A, Kuroda M, Tsujita Y. ML-236A, ML-236B, and ML-236C, new inhibitors of cholesterogenesis produced by Penicillium citrinium. J Antibiot (Tokyo). 1976;29:1346-8. [DOI] [PubMed] [Google Scholar]
- 2. Yamashita A, Morioka M, Kishi H, Kimura T, Yahara Y, Okada M, et al. Statin treatment rescues FGFR3 skeletal dysplasia phenotypes. Nature. 2014;513:507-11. [DOI] [PubMed] [Google Scholar]
- 3. Tavormina PL, Shiang R, Thompson LM, Zhu YZ, Wilkin DJ, Lachman RS, et al. Thanatophoric dysplasia (types I and II) caused by distinct mutations in fibroblast growth factor receptor 3. Nat Genet. 1995;9:321-8. [DOI] [PubMed] [Google Scholar]
- 4. Bush JR, Bérubé NG, Beier F. A new prescription for growth? Statins, cholesterol and cartilage homeostasis. Osteoarthritis Cartilage. 2015;23:503-6. [DOI] [PubMed] [Google Scholar]
- 5. Fafilek B, Hampl M, Ricankova N, Vesela I, Balek L, Bosakova MK, et al. Statins do not inhibit the FGFR signaling in chondrocytes. Osteoarthritis Cartilage. 2017;25:1522-30. [DOI] [PubMed] [Google Scholar]
- 6. Vortkamp A, Lee K, Lanske B, Segre GV, Kronenberg HM, Tabin CJ. Regulation of rate of cartilage differentiation by Indian Hedgehog and PTH-related protein. Science. 1996;273:613-22. [DOI] [PubMed] [Google Scholar]
- 7. Shukunami C, Shigeno C, Atsumi T, Ishizeki K, Suzuki F, Hiraki Y. Chondrogenic differentiation of clonal mouse embryonic cell line ATDC5 in vitro. J Cell Biol. 1996;133:457-68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Newton PT, Staines KA, Spevak L, Boskey AL, Teixeira CC, Macrae VE, et al. Chondrogenic ATDC5 cells: an optimised model for rapid and physiological matrix mineralisation. Int J Mol Med. 2012;30:1187-93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Zhai Z, Yao Y, Wang Y. Importance of suitable reference gene selection for quantitative RT-PCR during ATDC5 cells chondrocyte differentiation. PLoS One. 2013;8:e64786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Mundy G, Garrett R, Harris S, Chan J, Chen D, Rossini G, et al. Stimulation of bone formation in vitro and in rodents by statins. Science. 1999;286:1946-9. [DOI] [PubMed] [Google Scholar]
- 11. Maeda T, Matsunuma A, Kawane T, Horiuchi N. Simvastatin promotes osteoblast differentiation and mineralization in MC3T3-E1 cells. Biochem Biophys Res Commun. 2001;280:874-7. [DOI] [PubMed] [Google Scholar]
- 12. Ohnaka K, Shimoda S, Nawata H, Shimokawa H, Kaibuchi K, Iwamoto Y, et al. Pitavastatin enhanced BMP-2 and osteocalcin expression by inhibition of Rho-associated kinase in human osteoblasts. Biochem Biophys Res Commun. 2001;287:337-42. [DOI] [PubMed] [Google Scholar]
- 13. Ruiz-Gaspa S, Nogues X, Enjuanes A, Monllau JC, Blanch J, Carreras R, et al. Simvastatin and atorvastatin enhance gene expression of collagen type 1 and osteocalcin in primary human osteoblasts and MG-63 cultures. J Cell Biochem. 2007;101:1430-8. [DOI] [PubMed] [Google Scholar]
- 14. An T, Hao J, Sun S, Li R, Yang M, Cheng G, et al. Efficacy of statins for osteoporosis: a systematic review and meta-analysis. Osteoporos Int. 2017;28:47-57. doi: 10.1007/s00198-016-3844-8 [DOI] [PubMed] [Google Scholar]
- 15. Baker JF, Walsh P, Mulhall KJ. Statins: a potential role in the management of osteoarthritis? Joint Bone Spine. 2011;78:31-4. doi: 10.1016/j.jbspin.2010.02.035 [DOI] [PubMed] [Google Scholar]
- 16. Bertl K, Steiner I, Pandis N, Buhlin K, Klinge B, Stavropoulos A. Statins in nonsurgical and surgical periodontal therapy. A systematic review and meta-analysis of preclinical in vivo trials, J Periodontal Res. 2018;53:267-87. doi: 10.1111/jre.12514 [DOI] [PubMed] [Google Scholar]
- 17. Lazzerini PE, Capecchi PL, Selvi E, Lorenzini S, Bisogno S, Baldari CT, et al. Statins and the joint: multiple targets for a global protection? Semin Arthritis Rheum. 2011;40:430-46. [DOI] [PubMed] [Google Scholar]
- 18. Minina E, Kreschel C, Naski MC, Ornitz DM, Vortkamp A. Interaction of FGF, Ihh/Pthlh, and BMP signaling integrates chondrocyte proliferation and hypertrophic differentiation. Dev Cell. 2002;3:439-49. [DOI] [PubMed] [Google Scholar]
- 19. Bumcrot DA, Takada R, McMahon AP. Proteolytic processing yields two secreted forms of sonic hedgehog. Mol Cell Biol. 1995;15:2294-303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Porter JA, von Kessler DP, Ekker SC, Young KE, Lee JJ, Moses K, et al. The product of hedgehog autoproteolytic cleavage active in local and long-range signalling. Nature. 1995;374:363-6. [DOI] [PubMed] [Google Scholar]
- 21. Porter JA, Ekker SC, Park WJ, Von Kessler DP, Young KE, Chen CH, et al. Hedgehog patterning activity: Role of a lipophilic modification mediated by the carboxy-terminal autoprocessing domain. Cell. 1996;86:21-34. [DOI] [PubMed] [Google Scholar]
- 22. Long F, Zhang XM, Karp S, Yang Y, McMahon AP. Genetic manipulation of hedgehog signaling in the endochondral skeleton reveals a direct role in the regulation of chondrocyte proliferation. Development. 2001;128:5099-108. [DOI] [PubMed] [Google Scholar]
- 23. Choi SW, Jeong DU, Kim JA, Lee B, Joeng KS, Long F, et al. Indian Hedgehog signalling triggers Nkx3.2 protein degradation during chondrocyte maturation. Biochem J. 2012;443:789-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Church V, Yamaguchi K, Tsang P, Akita K, Logan C, Francis-West P. Expression and function of Bapx1 during chick limb development. Anat Embryol (Berl). 2005;209:461-9. [DOI] [PubMed] [Google Scholar]
- 25. Tamamura Y, Otani T, Kanatani N, Koyama E, Kitagaki J, Komori T, et al. Developmental regulation of Wnt/β-catenin signals is required for growth plate assembly, cartilage integrity, and endochondral ossification. J Biol Chem. 2005;280:19185-95. [DOI] [PubMed] [Google Scholar]
- 26. Mak KK, Kronenberg HM, Chuang PT, Mackem S, Yang Y. Indian hedgehog signals independently of PTHrP to promote chondrocyte hypertrophy. Development. 2008;135:1947-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Amizuka N, Henderson JE, Hoshi K, Warshawsky H, Ozawa H, Goltzman D, et al. Programmed cell death of chondrocytes and aberrant chondrogenesis in mice homozygous for parathyroid hormone-related peptide gene deletion. Endocrinology. 1996;137:5055-67. [DOI] [PubMed] [Google Scholar]
- 28. Henderson JE, Amizuka N, Warshawsky H, Biasotto D, Lanske BM, Goltzman D, et al. Nucleolar localization of parathyroid hormone-related peptide enhances survival of chondrocytes under conditions that promote apoptotic cell death. Mol Cell Biol. 1995;15:4064-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Park M, Yong Y, Choi SW, Kim JH, Lee JE, Kim DW. Constitutive RelA activation mediated by Nkx3.2 controls chondrocyte viability. Nat Cell Biol. 2007;9:287-98. [DOI] [PubMed] [Google Scholar]
- 30. Takazawa Y, Tsuji K, Nifuji A, Kurosawa H, Ito Y, Noda M. An osteogenesis-related transcription factor, core-binding factor A1, is constitutively expressed in the chondrocytic cell line TC6, and its expression is upregulated by bone morphogenetic protein-2. J Endocrinol. 2000;165:579-586. [DOI] [PubMed] [Google Scholar]
- 31. Yamanaka Y, Tanaka H, Koike MIO, Nishimura R, Seino Y. PTHrP rescues ATDC5 cells from apoptosis induced by FGF receptor 3 mutation. J Bone Min Res. 2003;18:1395-1403. [DOI] [PubMed] [Google Scholar]