Abstract
Background
Gliomas are the most common type of malignant brain and other CNS tumors, accounting for 80.8% of malignant primary brain and CNS tumors. They cause significant morbidity and mortality. This study investigates the intersection between age and sex to better understand variation of incidence and survival for glioma in the United States.
Methods
Incidence data from 2000 to 2017 were obtained from CBTRUS, which obtains data from the NPCR and SEER, and survival data from the CDC’s NPCR. Age-adjusted incidence rate ratios (IRR) per 100 000 were generated to compare male-to-female incidence by age group. Cox proportional hazard models were performed by age group, generating hazard ratios to assess male-to-female survival differences.
Results
Overall, glioma incidence was higher in males. Male-to-female incidence was lowest in ages 0-9 years (IRR: 1.04, 95% CI: 1.01-1.07, P = .003), increasing with age, peaking at 50-59 years (IRR: 1.56, 95% CI: 1.53-1.59, P < .001). Females had worse survival for ages 0-9 (HR: 0.93, 95% CI: 0.87-0.99), though male survival was worse for all other age groups, with the difference highest in those 20-29 years (HR: 1.36, 95% CI: 1.28-1.44). Incidence and survival differences by age and sex also varied by histological subtype of glioma.
Conclusions
To better understand the variation in glioma incidence and survival, investigating the intersection of age and sex is key. The current work shows that the combined impact of these variables is dependent on glioma subtype. These results contribute to the growing understanding of sex and age differences that impact cancer incidence and survival.
Keywords: age, CBTRUS, glioma, incidence, sex differences, survival
Key Points.
Male-to-female incidence increases with age in primary gliomas.
Female survival is worse in children 0-9 years but worse for males at all other ages in primary gliomas.
Importance of the Study.
Age and sex differences in incidence and survival for primary malignant gliomas are well documented, independently. However, in order to better understand this differences, analysis of the intersection of these 2 variables are key. The current work shows that age and sex interact and influence glioma incidence and survival. Males had significantly higher incidence rates overall, and by most glioma subtypes, as compared to females for all age groups. Male-to-female incidence was lowest in children but increased into adulthood. Males with malignant gliomas also had significantly higher risk of death as compared to females, which differed by age group. These results contribute to the growing understanding and impact that both sex and age have on cancer incidence and survival. Identifying disadvantaged sex and age groups is critical in influencing further research, and in developing individual care plans for patients with these lethal tumors.
Gliomas are tumors that arise from glial or precursor cells and include astrocytoma, oligodendroglioma, ependymoma, and other rare histologies.1 Gliomas are the most common type of primary malignant brain and other CNS tumor, accounting for approximately 25.1% of all primary brain and other CNS tumors and 80.8% of malignant brain and other CNS tumors, and cause significant morbidity and mortality.1 The most commonly occurring primary malignant brain and other CNS tumor is glioblastoma multiforme (GBM), accounting for the majority of gliomas (57.7%) and comprising 14.5% of all tumors and 48.6% of malignant tumors.1
Age is a key factor associated with incidence and survival for all cancers and therefore is also an important factor in the incidence and survival of gliomas.2 Gliomas are more common in older adults, and peak in incidence between the ages of 45 and 65 years old.3 Despite this, gliomas are one of the most common solid tumor types in children and account for approximately 45.5% of tumors in children and adolescents age 0-19 years.1 Studies have shown that the median age for diagnosis of high-grade glioma is higher in females as compared to males.4 Interest in sex differences in cancer has grown in recent years,5–8 especially now that all National Institutes of Health (NIH) grants require an assessment of sex as a biological variable.9 Population-based studies demonstrate that the incidence of gliomas varies significantly by sex, with males having a 30%-50% higher incidence for most glioma subtypes.10 In addition, a recent study showed that among patients diagnosed with high-grade gliomas, there were significantly more female long-term survivors, with mean survival being 742 vs 628 days (P = .03).4
Age or sex differences in incidence and survival for primary malignant gliomas are now well validated.1,5–8,11 While differences in survival and incidence based on sex and age have been studied individually, there have been no studies looking at the intersection of the 2 key variables and their interactive role on incidence and survival for gliomas. This study investigates the intersection between age and sex to better understand the variation of incidence and survival for glioma in the United States. Identifying disadvantaged sex and age groups is critical to providing individualized care for these devastating tumors.
Methods
Data Sources
Incidence data.
Incidence data were obtained for the years 2000-2017 from the Central Brain Tumor Registry of the United States (CBTRUS), which receives data in collaboration with the Centers for Disease Control and Prevention’s (CDC) National Program of Cancer Registries (NPCR) and the National Cancer Institute’s (NCI) Survival Epidemiology and End Results Program (SEER).12 This is the largest population-based registry focused exclusively on primary brain and other central nervous system tumors in the United States, covering the entire US population.1
Survival data.
Overall survival data from 2001 to 2016 used for analysis were obtained from the NPCR.13 This dataset provides population-based information for 93.6% of the US population and is a subset of the data used for the incidence calculations. Overall survival information is derived from both active and passive follow-up.
Selection Criteria
Patients with primary brain and other CNS gliomas from individuals with no prior cancer diagnosis who were either microscopically or radiographically confirmed were included in the analysis. International Classification of Diseases for Oncology, Third Edition (ICD-O-3) codes were used for identification, and tumors were defined according to the CBTRUS histologic grouping scheme.1 Only malignant cases (ICD-O-3 behavior code of 3) were included in this analysis. ICD-O-3 codes used are as follows: anaplastic astrocytoma (ICD-O-3 code 9401), anaplastic oligodendroglioma (9451, 9460), diffuse astrocytoma (9400, 9410, 9411, 9420), ependymal tumors (9391, 9392, 9393), GBM (9440, 9441, 9442), glioma malignant, not otherwise specified (NOS) (9380), neuronal and mixed neuronal-glial tumors (8680, 8693, 9505, 9522, 9523), oligoastrocytic tumors (9382), oligodendroglioma (9450), other neuroepithelial tumors (9423, 9430), pilocytic astrocytoma (9421, 9425), and unique astrocytoma variants (9381, 9424). While pilocytic astrocytoma is not a clinically malignant tumor, cancer registries have historically coded it as malignant (ICD-O-3 behavior code of 3). CBTRUS therefore reports pilocytic astrocytoma as malignant per guidelines set by the International Agency for Research on Cancer (IARC) and International Association of Cancer Registries (IACR).14
Statistical Analysis
The average annual age-adjusted incidence rates (AAIR) and 95% confidence intervals (95% CI) were estimated per 100 000 population using the methodology described in Tiwari et al.15 All rates were age-standardized to the 2000 US population and are reported per 100 000 population. We used 10-year age interval to create age groups (0-9, 10-19, 20-29, 30-39, 40-49, 50-59, 60-69, 70-79, and 80 years and older) based on age at primary cancer diagnosis. Age-adjusted incidence rate ratios (IRR) for sex (male:female) with 95% CI were also generated from these standardized rates. For incidence data, counts and rates are not presented when fewer than 16 cases were reported for the specific category, or where the inclusion of the count and rate would allow for back-calculation of suppressed values. For example, if the remaining cells in a category can be used to calculate the value of a suppressed cell (n < 16), an additional cell will be suppressed.
Survival differences were assessed using age-stratified Cox proportional hazards models, to generate hazard ratios (HRs) with 95% CIs of male vs female by age group. Both AAIRs/IRRs and HRs were estimated for overall gliomas, and separately for anaplastic oligodendroglioma, anaplastic oligodendroglioma, diffuse astrocytoma, GBM, and oligodendroglioma. Survival time was recorded in months and was defined from the date of diagnosis to the date of death or last known contact. The Cox proportional hazards assumption was tested for survival models, and no variables were found in violation. For survival data, counts and rates are not presented when fewer than 20 events were reported for the specific category. Additional Cox proportional hazards models were assessed on a subset of patients who had received a gross total resection (SEER Site-Specific Surgery Codes: 30, 55) to adjust for surgical treatment.
Chi-square tests were performed to assess demographic and histology differences between males and females. All analyses were performed using R (version 4.0.4) and SEER*Stat 8.3.8.
Results
Descriptive Statistics
Overall, from 2000 to 2017, there were 294 886 patients with primary malignant brain and other CNS gliomas (Table 1). Of these, 130 051 (44.1%) were females and 164 835 (55.9%) were males. Overall, malignant glioma incidence was highest in the age group 60-69 years (60 198 cases, 20%), and lowest in 10-19 years (15 005 cases, 5.1%). Age distribution differed significantly between males and females (P < .001). Additional patient characteristics by sex are presented in Table 1. GBM was the most predominant histology with 161 237 (55%) of the overall patients. Among GBM, 68 759 (53%) were female, and 92 478 (56%) were male.
Table 1.
Descriptive Statistics of Patients With Primary, Malignant Gliomas by Sex (CBTRUS: Data provided by CDC’s National Program of Cancer Registries and NCI’s Surveillance, Epidemiology and End Results Program, 2000-2017)
| Characteristic | Overall, N = 294 886a | Female, N = 130 051a | Male, N = 164 835a | P Valueb |
|---|---|---|---|---|
| Age group, yr | ||||
| 0-9 | 19 707 (6.7%) | 9425 (7.2%) | 10 282 (6.2%) | <.001 |
| 10-19 | 15 005 (5.1%) | 7090 (5.5%) | 7915 (4.8%) | |
| 20-29 | 16 945 (5.7%) | 7541 (5.8%) | 9404 (5.7%) | |
| 30-39 | 24 595 (8.3%) | 10 472 (8.1%) | 14 123 (8.6%) | |
| 40-49 | 36 206 (12%) | 14 678 (11%) | 21 528 (13%) | |
| 50-59 | 56 027 (19%) | 22 586 (17%) | 33 441 (20%) | |
| 60-69 | 60 198 (20%) | 25 524 (20%) | 34 674 (21%) | |
| 70-79 | 44 959 (15%) | 21 121 (16%) | 23 838 (14%) | |
| 80+ | 21 244 (7.2%) | 11 614 (8.9%) | 9630 (5.8%) | |
| Race | ||||
| White | 262 643 (89%) | 115 239 (89%) | 147 404 (89%) | <.001 |
| Black | 20 275 (6.9%) | 9509 (7.3%) | 10 766 (6.5%) | |
| American Indian/Alaska Native | 1531 (0.5%) | 687 (0.5%) | 844 (0.5%) | |
| Asian or Pacific Islander | 5859 (2.0%) | 2714 (2.1%) | 3145 (1.9%) | |
| Other/Unknown | 4578 (1.6%) | 1902 (1.5%) | 2676 (1.6%) | |
| Ethnicity | ||||
| Non-Hispanic or Latino | 266 735 (90%) | 117 238 (90%) | 149 497 (91%) | <.001 |
| Hispanic or Latino | 28 151 (9.5%) | 12 813 (9.9%) | 15 338 (9.3%) | |
| Histology | ||||
| Anaplastic astrocytoma | 19 983 (6.8%) | 8853 (6.8%) | 11 130 (6.8%) | <.001 |
| Anaplastic oligodendroglioma | 6218 (2.1%) | 2763 (2.1%) | 3455 (2.1%) | |
| Diffuse astrocytoma | 27 519 (9.3%) | 12 144 (9.3%) | 15 375 (9.3%) | |
| Ependymal tumors | 13 053 (4.4%) | 6211 (4.8%) | 6842 (4.2%) | |
| Glioblastoma | 161 237 (55%) | 68 759 (53%) | 92 478 (56%) | |
| Glioma malignant, NOS | 21 718 (7.4%) | 10 775 (8.3%) | 10 943 (6.6%) | |
| Neuronal and mixed neuronal glial tumors | 2698 (0.9%) | 1109 (0.9%) | 1589 (1.0%) | |
| Oligoastrocytic tumors | 8481 (2.9%) | 3606 (2.8%) | 4875 (3.0%) | |
| Oligodendroglioma | 14 001 (4.7%) | 6226 (4.8%) | 7775 (4.7%) | |
| Other neuroepithelial tumors | 215 (<0.1%) | 147 (0.1%) | 68 (<0.1%) | |
| Pilocytic astrocytoma | 17 594 (6.0%) | 8450 (6.5%) | 9144 (5.5%) | |
| Unique astrocytoma variants | 2169 (0.7%) | 1008 (0.8%) | 1161 (0.7%) | |
| Surgery type | ||||
| No surgery | 114 752 (39%) | 52 295 (40%) | 62 457 (38%) | <.001 |
| Partial or subtotal resection | 60 071 (20%) | 25 458 (20%) | 34 613 (21%) | |
| Gross total resection | 74 889 (25%) | 32 395 (25%) | 42 494 (26%) | |
| Unknown | 45 174 (15%) | 19 903 (15%) | 25 271 (15%) |
Abbreviations: CDC, Centers for Disease Control and Prevention; NCI, National Cancer Institute; NOS, not otherwise specified.
an (%).
bPearson’s chi-square test.
Incidence
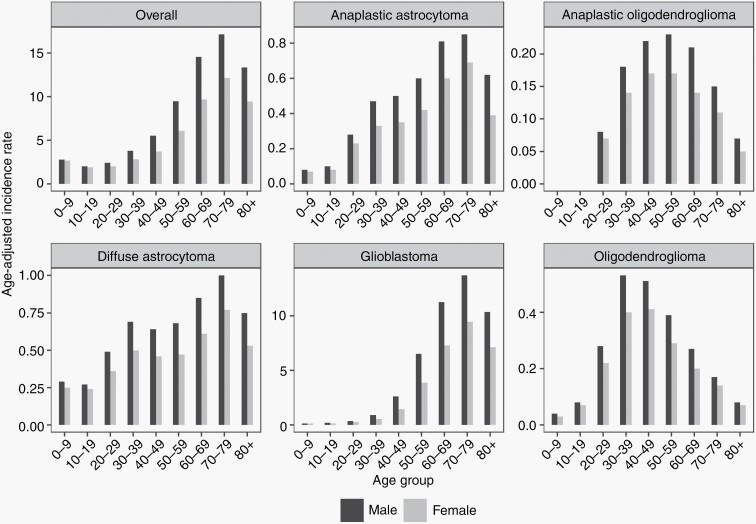
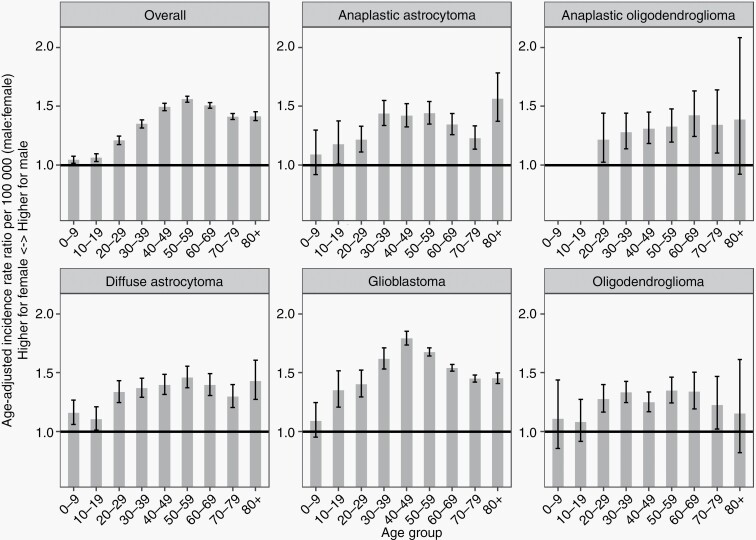
The AAIRs and IRRs of male vs female with 95% CIs by age groups for all gliomas are displayed in Figures 1 and 2 and Supplementary Table 1. The age group with the highest AAIR for overall patients was 70-79 years (AAIR = 14.36, 95% CI: 14.23-14.5), followed by 60-69 years (AAIR = 11.98, 95% CI: 11.88-12.08) and 80+ years (AAIR = 10.86, 95% CI: 10.71-11). Overall, the incidence of glioma in all age groups was higher in males. The age group with the smallest IRR was 0-9 years (IRR = 1.04, 95% CI: 1.01-1.07, P = .003). The IRR continued to increase with age, peaking at 50-59 years old (IRR = 1.56, 95% CI: 1.53-1.59, P < .001) (Figure 2).
Fig. 1.
Age-adjusted incidence rate by sex for primary, malignant gliomas by age groups (CBTRUS: Data provided by CDC’s National Program of Cancer Registries and NCI’s Surveillance, Epidemiology and End Results Program, 2000-2017). Abbreviations: CDC, Centers for Disease Control and Prevention; NCI, National Cancer Institute.
Fig. 2.
Age-adjusted incidence rate ratio (male vs female) with 95% CI for primary, malignant gliomas by age groups (CBTRUS: Data provided by CDC’s National Program of Cancer Registries and NCI’s Surveillance, Epidemiology and End Results Program, 2000-2017). Abbreviations: CDC, Centers for Disease Control and Prevention; NCI, National Cancer Institute.
AAIRs and IRRs by sex and age are also shown for the following glioma subtypes: anaplastic oligodendroglioma, anaplastic oligodendroglioma, diffuse astrocytoma, GBM, and oligodendroglioma (Figures 1 and 2, Supplementary Table 3). For males, the age group with the highest AAIR for anaplastic astrocytoma, diffuse astrocytoma, and GBM was 70-79 years. In males, the highest incidence for anaplastic oligodendroglioma and oligodendroglioma occurred earlier, at ages 50-59 and 30-39 years, respectively. Females showed peaks in incidence that differed from males for anaplastic oligodendroglioma and oligodendroglioma, with the highest AAIR occurring between ages 40-49 years. In addition, for anaplastic oligodendroglioma and diffuse astrocytoma, the corresponding IRRs of all age groups were significantly higher in males. For anaplastic astrocytoma and GBM, the corresponding IRRs of all age groups were significant for higher incidence in males except for age 0-9 years.
Survival
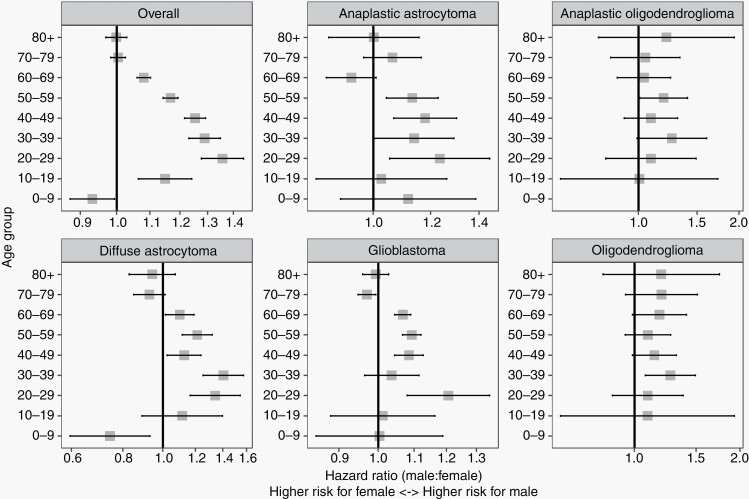
Cox proportional hazards models were used to generate HRs of male vs female sex with 95% CIs by age groups overall are shown in Figure 3 and Supplementary Table 2. The HR for male sex for those 0-9 years old was 0.93 (95% CI: 0.87-0.99), showing that females in this age group with malignant gliomas were significantly at higher risk of death. For all other age groups (10-69 years), male sex was associated with increased hazard of death. Patients age 10-19 years or 20-29 years had the highest HR for male sex compared to other age groups (20-29 years HR: 1.36, 95% CI: 1.28-1.44; 30-39 years HR: 1.29, 95% CI: 1.23-1.35).
Fig. 3.
Hazard ratio (male vs female) for overall survival with 95% CI for primary, malignant gliomas by age groups (CBTRUS: Data provided by CDC’s National Program of Cancer Registries, 2001-2016). Abbreviation: CDC, Centers for Disease Control and Prevention.
Subtype-specific HRs by age groups were also generated (Figure 3, Supplementary Table 4). The HR for male was highest in age groups 30-39 and 50-59 years oligodendroglioma and anaplastic oligodendroglioma, respectively. Male HR was significantly increased in age groups 20-29, 30-39, 40-49, and 50-59 years for anaplastic astrocytoma, diffuse astrocytoma, GBM, with the exception of GBM patients in the 30- to 39-year age group.
In order to assess the impact of extent of resection on survival differences between male and female by age group and by histology, we also looked at the subset of patients who had complete extent of resection data (n = 215 944 (87.63% of patients included in survival analysis). Overall, 64 087 (29.68%) patients had gross total resection, 51 771 (23.97%) had subtotal or partial resection, and 100 086 (46.35%) had no surgery. The survival trends among male vs female by age groups and histology were not significantly different after adjusting for extent of resection in the multivariable Cox model (Supplementary Figure 1).
Discussion
In this study, we provide evidence to show that incidence and overall survival of primary malignant gliomas vary by sex and age. To our knowledge, this is the first analysis investigating the intersection of sex and age to better understand variation in incidence and survival for glioma. We found that the incidence of glioma was higher for males across all age groups and in general males had a higher risk of death as compared to females.
Gliomas are the most common malignant brain tumor and can cause significant morbidity and mortality. For example, median survival of GBM cases diagnoses from 2001 to 2016 in the United States was only 8 months. Anaplastic ependymoma had a reported median survival of 18 months and 3 years for diffuse astrocytoma.1 These tumors are often diagnosed through either neuroimaging techniques, such as CT or MRI scans, and pathologic confirmation from tumor tissue received at biopsy or resection.16 The use of molecular biomarkers is also used to help differentiate between glioma types, such as between oligodendrogliomas and astrocytomas, and to identify clinically significant subgroups within certain histologies, such as glioblastoma vs anaplastic astrocytoma.16 Molecular biomarker data are not currently available in tumor registries, such as CBTRUS, as tumor registries did not collect the 2016 WHO Classification biomarkers until 2018.17 This information will be available in future data releases.
Age is a well-validated independent factor associated with incidence and survival for all cancers.1 Some cancers, including brain and other CNS tumors and leukemia, are common in children while others are more common in adults.18 Specific glioma subtypes are well documented to be quite common in childhood and very rare in adulthood and vice versa.1 Age is one of the primary risk factors for cancer, with individuals ≥65 years of age accounting for 60% of newly diagnosed malignancies and 70% of all cancer-related deaths.19,20 Previous studies highlighted an age-adjusted cancer mortality rate for persons ≥65 at ~16 times higher than the mortality rate for those <65.20,21
Aging is a complex process that could affect cancer risk through multiple mechanisms. Among these, are the effects of aging on nearly all aspects of the immune system,20,22 which has been strongly implicated in cancer prevention, development, and defense.20,23 Aging negatively impacts apoptotic cell clearance,24 the numbers of naïve T cells,25 and the wound healing response.26 Neuro-immune interactions are also affected by aging in the brain,20,27 and research has shown that aging progressively suppresses normal immunosurveillance and decreases immunotherapeutic efficacy against malignant glioma.20 Immunosuppression increases in the brain with advanced age, and there is an inhibition of anti-glioma immunity in older adults.28 These results suggest that cellular immunity may play an age-dependent role in the development of brain and other CNS tumors and should be integrated with emerging evidence for sex differences in immune suppression and tumor microenvironment interactions.29,30 Studies have shown that the incidence of primary brain and other CNS tumors increases with age, and our updated study further confirms such a trend for primary brain and other CNS glioma as well as its association with sex.1,31 An increasing incidence of primary malignant gliomas with increasing age likely suggests that malignant transformation of these cells is a chronic process with sequential changes of genetic alteration occurring with age.1,32 Glioma incidence is at its nadir between the ages of 10-19 and peaks at 70-79 years, which suggests that both genetic and environmental factors may have a role, with genetic factors playing a relatively larger role in early life risk and the environment being the larger contributor for older age groups.31,32 Analyzing these patterns of glioma incidence and survival with age and sex allows us to evaluate these patterns by age and generate hypotheses about their etiopathogenesis.
Sexual differentiation during development results in sex differences in cellular and systems biology,6,33,34 including sexually dimorphic traits such as body size, metabolism, and disease risk.35–38 Sex differences are evident in brain size, rates of myelination, and risk for neurodevelopmental disorders and psychiatric diseases.39–41 In cancer, significant sex differences exist in incidence of most tumor types that arise in both sexes, suggesting fundamental biological differences between males and females impact cancer incidence.5 Historically, sex-based differences in complex diseases have been understudied. Multiple studies have been conducted to examine the potential role of either endogenous or exogenous hormone exposures and risk of brain and other CNS tumors, with mixed results.6 Prior research has shown that overall incidence of cancer has increased in males42 including GBM.8,43 Females with GBM have a significant overall survival advantage that is further enhanced after adjustment for known independent prognostic factors such as age, Karnofsky performance status (KPS), extent of resection, and receipt of standard therapy.8 Though, this is not seen for lower grade glioma.44
Sex differences in glioma incidence may be partially due to the genetic differences between males and females.10 A sex-specific genome-wide association study identified 3 sex-specific glioma risk loci (7p11.2, 8q24.21, 3p21.31).10 DNA methylation differences between males and females in 4 glioma molecular subtypes suggest an important sex-specific role for DNA methylation in epigenetic regulation of gliomagenesis.45 In addition, research has shown that the standard treatment for GBM might be more effective for females than for males.46 With respect to the molecular basis of this sex difference in treatment response, male- and female-specific gene expression clusters were identified that uncovered long-term survival signatures, with male long-term survivors having alterations in cell cycle genes compared to female long-term survivors that had alterations in integrin signaling genes.7 However, our understanding of the many factors that determine sex-based differences in complex disease remains limited. Using population-based cancer registry data to better understand age/sex variation in incidence and survival is critical to moving forward our understanding of the intersection of these key variables and the role they play in tumorigenesis and treatment.
There is growing information on the role of sex and age on cancer incidence and survival. Most prior research has analyzed the impact of age and sex separately. The aim of our study was to examine the interaction between age and sex influence incidence and survival of gliomas in the United States. We found that glioma incidence is higher in males and adults, accurately aligning with the previously stated literature. Importantly, we found that this male-to-female difference in incidence was influenced by age. Male-to-female IRR of glioma was lowest in those 0-9 years old (IRR = 1.04, 95% CI: 1.01-1.07, P = .003), and increased over time, with the highest IRR present in those 50-59 years old (IRR = 1.56, 95% CI: 1.53-1.59, P < .001). Additionally, we found age and sex differences to be present in glioma survival as well, where females had overall worse survival in ages 0-9 years (HR: 0.93, 95% CI: 0.87-0.99), but better survival in all other age groups. These findings in this study imply an interaction between age and sex in glioma incidence and survival, the first study to present these findings at this scale. These data are integral in guiding future research to analyze and better understand the mechanisms between age and sex in cancer.
Cancer registry data represent the most complete dataset for characterizing patterns of cancer incidence and survival, nevertheless, some limitations are present. The data used for this analysis are limited to variables available through cancer registry datasets. Furthermore, there was no central pathology review in place for registry procedures, meaning that each case had been classified at the diagnosing institution with no central confirmation of histology at the state or national levels. Also, we currently have very limited information on molecular data. For example, it has been documented that IDH-mutant GBM is diagnosed at a younger age than IDH-wildtype GBM.47 Future analyses with access to information would help further assess the relationships documented in our current study. Many of the predictors associated with differences in glioma survival, including extent of resection, KPS, and treatment pattern were not included in the Cox regression model for overall survival.11 It is also possible that the treatment information available, may not be consolidated at the central registry level, and multiple reports from different facilities may not lead to the most extensive procedure being reflected.48 However, the CBTRUS dataset, provided in collaboration with NPCR and SEER, encapsulates almost 100% of glioma cases in the United States, and the 18-year time period of the study (2000-2017) allows for this in-depth analysis of variation in incidence and survival by age and sex for this rare tumor.
Conclusion
Age and sex differences in incidence and survival or primary malignant gliomas have been independently described. However, in order to better understand this differences, analysis of the intersection of these 2 variables are key. The current work shows that age and sex interact and influence glioma incidence and survival. Males had significantly higher incidence rates overall, and by most glioma subtypes, as compared to females for all age groups. Male-to-female incidence was lowest in children but increased into adulthood. Males with malignant gliomas also had significantly higher risk of death as compared to females, which differed by age group, for both overall data and the subset of patients who had complete extent of resection data. These results contribute to the growing understanding and impact that both sex and age have on cancer incidence and survival. Identifying disadvantaged sex and age groups is critical in influencing further research, and in developing individual care plans for patients with these lethal tumors.
Supplementary Material
Acknowledgments
This study was approved by the University Hospitals Cleveland Medical Center Institutional Review Board.
Funding
The CBTRUS data were provided through an agreement with the Centers for Disease Control’s (CDC) National Program of Cancer Registries. In addition, CBTRUS used data from the research data files of the National Cancer Institute’s (NCI) Surveillance, Epidemiology, and End Results Program, and the National Center for Health Statistics National Vital Statistics System. CBTRUS acknowledges and appreciates these contributions to this report and to cancer surveillance in general. Funding for CBTRUS was provided by the CDC under Contract No. 75D30119C06056 Amendment /Modification No: 00001, the American Brain Tumor Association, The Sontag Foundation, Novocure, the Musella Foundation, National Brain Tumor Society, the Pediatric Brain Tumor Foundation, the Uncle Kory Foundation, the Zelda Dorin Tetenbaum Memorial Fund, as well as private and in-kind donations. Contents are solely the responsibility of the authors and do not necessarily represent the official views of the CDC or the NCI.
Conflict of interest statement. None declared.
References
- 1. Ostrom QT, Patil N, Cioffi G, Waite K, Kruchko C, Barnholtz-Sloan JS. CBTRUS statistical report: primary brain and other central nervous system tumors diagnosed in the United States in 2013–2017. Neuro Oncol. 2020;22(12 Suppl 2):iv1–iv96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Lin Z, Yang R, Li K, et al. . Establishment of age group classification for risk stratification in glioma patients. BMC Neurol. 2020;20(1):310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Brennan CW, Verhaak RG, McKenna A, et al. . The somatic genomic landscape of glioblastoma. Cell. 2013;155(2):462–477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Malmström A, Akesson L, Asklund T. Gender differences in glioma – findings from the Swedish national quality registry for primary brain tumors. Neuro Oncol. 2018;20:267. [Google Scholar]
- 5. Dong M, Cioffi G, Wang J, et al. . Sex differences in cancer incidence and survival: a pan-cancer analysis. Cancer Epidemiol Biomarkers Prev. 2020;29(7):1389–1397. [DOI] [PubMed] [Google Scholar]
- 6. Sun T, Plutynski A, Ward S, Rubin JB. An integrative view on sex differences in brain tumors. Cell Mol Life Sci. 2015;72(17):3323–3342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Yang W, Warrington NM, Taylor SJ, et al. . Sex differences in GBM revealed by analysis of patient imaging, transcriptome, and survival data. Sci Transl Med. 2019;11(473): eeao5253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ostrom QT, Rubin JB, Lathia JD, Berens ME, Barnholtz-Sloan JS. Females have the survival advantage in glioblastoma. Neuro Oncol. 2018;20(4):576–577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ostrom QT, Gittleman H, Liao P, et al. . CBTRUS Statistical Report: primary brain and other central nervous system tumors diagnosed in the United States in 2010-2014. Neuro Oncol. 2017;19(suppl_5):v1–v88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ostrom QT, Kinnersley B, Wrensch MR, et al. ; GliomaScan Consortium . Sex-specific glioma genome-wide association study identifies new risk locus at 3p21.31 in females, and finds sex-differences in risk at 8q24.21. Sci Rep. 2018;8(1):7352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ostrom QT, Cote DJ, Ascha M, Kruchko C, Barnholtz-Sloan JS. Adult glioma incidence and survival by race or ethnicity in the United States from 2000 to 2014. JAMA Oncol. 2018;4(9):1254–1262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Central Brain Tumor Registry of the United States SEER*Stat Database. CDC National Program of Cancer Registries and NCI Surveillance, Epidemiology and End Results Incidence Data. 2019. submission (2000–2017).
- 13. National Program of Cancer Registries SEER*Stat Database: NPCR Survival Analytic file (2001–2016). United States Department of Health and Human Services, Centers for Disease Control and Prevention. Released June 2020, based on the 2019. submission.
- 14. Ostrom QT, Kruchko C, Barnholtz-Sloan JS. Pilocytic astrocytomas: where do they belong in cancer reporting? Neuro Oncol. 2020;22(2): 298–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Tiwari RC, Clegg LX, Zou Z. Efficient interval estimation for age-adjusted cancer rates. Stat Methods Med Res. 2006;15(6):547–569. [DOI] [PubMed] [Google Scholar]
- 16. Ahmed R, Oborski MJ, Hwang M, Lieberman FS, Mountz JM. Malignant gliomas: current perspectives in diagnosis, treatment, and early response assessment using advanced quantitative imaging methods. Cancer Manag Res. 2014;6:149–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kruchko C, Gittleman H, Ruhl J, et al. . Cancer collection efforts in the United States provide clinically relevant data on all primary brain and other CNS tumors. Neurooncol Pract. 2019;6(5):330–339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer Statistics, 2021. CA Cancer J Clin. 2021;71(1):7–33. [DOI] [PubMed] [Google Scholar]
- 19. Ries LAG, Harkins D, Krapcho M, et al. . SEER Cancer Statistics Review, 1975–2003. Bethesda, MD: National Cancer Institute; 2006. [Google Scholar]
- 20. Ladomersky E, Scholtens DM, Kocherginsky M, et al. . The coincidence between increasing age, immunosuppression, and the incidence of patients with glioblastoma. Front Pharmacol. 2019;10:200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Berger NA, Savvides P, Koroukian SM, et al. . Cancer in the elderly. Trans Am Clin Climatol Assoc. 2006;117:147–155; discussion 155. [PMC free article] [PubMed] [Google Scholar]
- 22. Nikolich-Žugich J. The twilight of immunity: emerging concepts in aging of the immune system. Nat Immunol. 2018;19(1):10–19. [DOI] [PubMed] [Google Scholar]
- 23. Candeias SM, Gaipl US. The immune system in cancer prevention, development and therapy. Anticancer Agents Med Chem. 2016;16(1):101–107. [DOI] [PubMed] [Google Scholar]
- 24. Aprahamian T, Takemura Y, Goukassian D, Walsh K. Ageing is associated with diminished apoptotic cell clearance in vivo. Clin Exp Immunol. 2008;152(3):448–455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Cambier J. Immunosenescence: a problem of lymphopoiesis, homeostasis, microenvironment, and signaling. Immunol Rev. 2005;205:5–6. [DOI] [PubMed] [Google Scholar]
- 26. Wick G, Backovic A, Rabensteiner E, Plank N, Schwentner C, Sgonc R. The immunology of fibrosis: innate and adaptive responses. Trends Immunol. 2010;31(3):110–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Carson MJ, Doose JM, Melchior B, Schmid CD, Ploix CC. CNS immune privilege: hiding in plain sight. Immunol Rev. 2006;213:48–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Ladomersky E, Zhai L, Lauing KL, et al. . Advanced age increases immunosuppression in the brain and decreases immunotherapeutic efficacy in subjects with glioblastoma. Clin Cancer Res. 2020;26(19):5232–5245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Turaga SM, Silver DJ, Bayik D, et al. . JAM-A functions as a female microglial tumor suppressor in glioblastoma. Neuro Oncol. 2020;22(11):1591–1601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Bayik D, Zhou Y, Park C, et al. . Myeloid-derived suppressor cell subsets drive glioblastoma growth in a sex-specific manner. Cancer Discov. 2020;10(8):1210–1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Arora RS, Alston RD, Eden TO, Estlin EJ, Moran A, Birch JM. Age-incidence patterns of primary CNS tumors in children, adolescents, and adults in England. Neuro Oncol. 2009;11(4):403–413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Kleihues P, Louis DN, Scheithauer BW, et al. . The WHO classification of tumors of the nervous system. J Neuropathol Exp Neurol. 2002;61(3):215–225; discussion 226. [DOI] [PubMed] [Google Scholar]
- 33. Knoedler JR, Shah NM. Molecular mechanisms underlying sexual differentiation of the nervous system. Curr Opin Neurobiol. 2018;53:192–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. VanRyzin JW, Pickett LA, McCarthy MM. Microglia: driving critical periods and sexual differentiation of the brain. Dev Neurobiol. 2018;78(6):580–592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Zore T, Palafox M, Reue K. Sex differences in obesity, lipid metabolism, and inflammation-A role for the sex chromosomes? Mol Metab. 2018;15:35–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Montero D, Madsen K, Meinild-Lundby AK, Edin F, Lundby C. Sexual dimorphism of substrate utilization: differences in skeletal muscle mitochondrial volume density and function. Exp Physiol. 2018;103(6):851–859. [DOI] [PubMed] [Google Scholar]
- 37. Kiserud T, Benachi A, Hecher K, et al. . The World Health Organization fetal growth charts: concept, findings, interpretation, and application. Am J Obstet Gynecol. 2018;218(2S):S619–S629. [DOI] [PubMed] [Google Scholar]
- 38. Dearden L, Bouret SG, Ozanne SE. Sex and gender differences in developmental programming of metabolism. Mol Metab. 2018;15:8–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Chung WC, Auger AP. Gender differences in neurodevelopment and epigenetics. Pflugers Arch. 2013;465(5):573–584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Marrocco J, McEwen BS. Sex in the brain: hormones and sex differences. Dialogues Clin Neurosci. 2016;18(4):373–383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. McCarthy MM, Arnold AP. Reframing sexual differentiation of the brain. Nat Neurosci. 2011;14(6):677–683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Cronin KA, Lake AJ, Scott S, et al. . Annual report to the nation on the status of cancer, part I: national cancer statistics. Cancer. 2018;124(13):2785–2800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Ostrom QT, Gittleman H, Truitt G, Boscia A, Kruchko C, Barnholtz-Sloan JS. CBTRUS statistical report: primary brain and other central nervous system tumors diagnosed in the United States in 2011–2015. Neuro Oncol. 2018;20(suppl_4):iv1–iv86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Gittleman H, Ostrom QT, Stetson LC, et al. . Sex is an important prognostic factor for glioblastoma but not for nonglioblastoma. Neurooncol Pract. 2019;6(6):451–462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Johansen ML, Stetson LC, Vadmal V, et al. . Gliomas display distinct sex-based differential methylation patterns based on molecular subtype. Neurooncol Adv. 2020;2(1):vdaa002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Mao DD, Gujar AD, Mahlokozera T, et al. . A CDC20-APC/SOX2 signaling axis regulates human glioblastoma stem-like cells. Cell Rep. 2015;11(11):1809–1821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Yan H, Parsons DW, Jin G, et al. . IDH1 and IDH2 mutations in gliomas. N Engl J Med. 2009;360(8):765–773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Cioffi G, Cote DJ, Ostrom QT, Kruchko C, Barnholtz-Sloan JS. Association between urbanicity and surgical treatment among patients with primary glioblastoma in the United States. Neurooncol Pract. 2020;7(3):299–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.