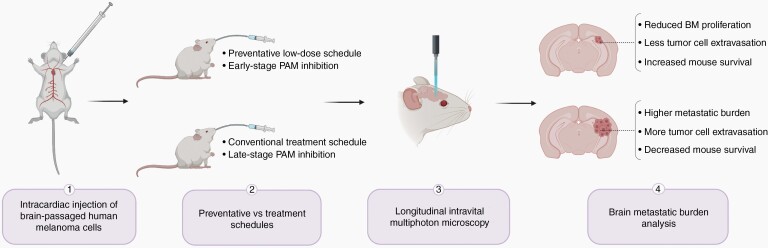
Over 50% of cancer patients with advanced melanoma develop brain metastases (BM) and ultimately succumb to this secondary disease within 12 months of diagnosis.1 The PI3K/Akt/mTOR (PAM) pathway has been widely implicated as a potential therapeutic target because it is upregulated in BM compared to primary tumors and extracranial metastases.2–4 However, PAM pathway inhibitors with promising preclinical results fail to achieve intracranial response in clinical trials when treating established BM.5 In this issue of Neuro-Oncology, Tehranian et al use advanced molecular in vivo imaging techniques to establish the importance of PAM pathway activation in early-stage BM formation and show that a preventative therapy is an effective alternative to treating established BM (Figure 1).2 The idea of BM prevention is already being explored within the field, with one active clinical trial using temozolomide to prevent BM in breast cancer patients.6 Findings from the present study could therefore have extensive implications for a disease that is currently largely incurable.
Fig. 1.
PAM pathway inhibition during early-stage brain colonization via a preventative low-dose schedule reduces the growth rate and survival of brain metastases compared to a conventional treatment schedule.
The metastatic cascade is a multistep process laden with many lethal barriers to circulating tumor cells, with the early steps of brain colonization being the most rate-limiting.7 It is therefore plausible that targeting early steps of BM, rather than attempting to treat an established tumor, could halt BM formation more effectively. Using a green fluorescent Akt biosensor with real-time in vivo monitoring, Tehranian et al observed that circulating tumor cells rapidly activate the PAM pathway during early brain colonization.2 Only cells with high Akt activity in the intravascular stage successfully extravasated and colonized the perivascular niche, while cells with low Akt activity exhibited permanent arrest and never extravasated. These findings are supported by other studies that implicate the PAM pathway in BM; Ippen et al showed that the dual PI3K/mTOR pathway inhibitor GDC-0084 achieves antitumor effects in breast BM,8 while Chen et al used molecular profiling to demonstrate an increased expression of PI3K/Akt activation markers in BM compared to extracranial metastases.9 The apparent essentiality for the activation of certain pathways to complete brain colonization adds another level of complexity to the metastatic cascade, supporting the notion that prevention may be easier to achieve than a cure. Since recent clinical data showed insufficient responses of PAM pathway inhibitors against established BM, the authors hypothesized that such inhibitors may show better, more consistent therapeutic effects in early-stage melanoma BM.
The authors addressed this hypothesis by using repetitive intravital multiphoton microscopy to investigate whether PAM inhibition is effective against early-stage brain colonization in vivo. GNE-317, a brain-penetrant dual PI3K/mTOR inhibitor, was administered 4-days post-intracardiac injection of brain metastatic melanoma cells. This preventative schedule significantly reduced the growth rate and survival of individual micrometastatic lesions compared to a treatment schedule. PI3K/mTOR inhibitors are generally associated with many adverse clinical side effects, most commonly hyperglycemia.2 The authors addressed this concern by administrating the drug at low-dose (10% of maximum-tolerated dose) and medium-dose (50% of maximum-tolerated dose) prevention schedules. While the medium-dose prevention schedule significantly increased blood glucose levels in their mouse model, the low-dose prevention schedule did not exhibit any significant side effects. Notably, both schedules resulted in comparable BM growth suppression, effectively showing that low-dose preventative inhibition can reduce common side effects of PAM pathway inhibitors while achieving comparable therapeutic benefit.
Remarkably, the low-dose prevention schedule was seen to target the earliest steps of brain colonization, affecting both extravasation and early colonization of the perivascular niche. However, Tehranian et al noted that stopping treatment caused accelerated brain metastatic growth and argue that continuous low-dose drug administration would be essential for maximal BM suppression. Since clinical trials using the maximum-tolerated dose of PI3K/mTOR inhibitors on established BM report severe side effects, careful pharmacokinetic studies, and low-dose preventive schedules can improve the translational success of these inhibitors. Indeed, Tehranian et al demonstrate therapeutic efficacy against BM formation but reduced hyperglycemia in treated mice when low-dose drug concentrations were administered. These encouraging results should reinforce efforts toward the clinical development of preventative BM therapies, particularly those that show therapeutic benefit in preliminary secondary prevention trials. Future CRISPR-Cas9 knock-out studies would further elucidate the essentiality of the PAM pathway during extravasation and any on-/off-target effects of PI3K/mTOR inhibitors.
It is important to address that PAM pathway inhibition was able to slow BM formation, rather than completely prevent it. Perhaps the most important next step in this research is addressing why some cells established BM even after the preventative inhibition, and how this cell population can be co-targeted to completely block BM. Furthermore, whether PAM pathway inhibition is specific for the early steps of brain colonization, or if it affects the viability of primary tumor cells too, remains to be seen. Pairing a preclinical primary melanoma model with preventative PAM pathway inhibition would test if BM were truly prevented. This proof-of-principle experiment might further indicate the importance of early prophylactic targeting of the PAM pathway in patients at risk of BM.
Collectively, these findings broaden our understanding of the early, crucial steps of brain colonization and could have important implications for at-risk patients. However, the clinical development of preventative therapies still faces logistical challenges. The authors address the most obvious and logical next step of identifying melanoma patients at a high risk of BM development. These patients would need to tolerate the drug for a long period of time at low enough dosage to maintain maximum therapeutic benefit while avoiding significant side effects. Although prognostic factors that identify high-risk patients remain to be fully elucidated, genomic profiling efforts report novel drivers of select metastatic disease.10 The next step would be to discern how long it is feasible to administer a preventative therapy and identifying the long-term effects of continuous administration. This high-risk, high-reward approach to unraveling the intricacies of a preventative therapy is crucial to address this unmet clinical need.
Disrupting the metastatic cascade during brain colonization could slow BM outgrowth with far-reaching implications for diagnostics and prognostics. The findings of this study could provide at-risk patients with less toxic therapy options and better prognostic outcomes, and ultimately translate into other types of anti-metastatic therapies.
Acknowledgments
We thank C. Venugopal, S. Chafe, and D. Mobilio for assisting in the review of the manuscript discussed here and contributing ideas toward this editorial. This text is the sole product of the authors and no third party had input or gave support to its writing.
References
- 1. Davies MA, Liu P, McIntyre S, et al. Prognostic factors for survival in melanoma patients with brain metastases. Cancer. 2011;117(8):1687–1696. [DOI] [PubMed] [Google Scholar]
- 2. Tehranian C, Fankhauser L, Harter PN, et al. The PI3K/Akt/mTOR pathway as a preventive target in melanoma brain metastasis. Neuro Oncol. 2022;24(2),213–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Brastianos PK, Carter SL, Santagata S, et al. Genomic characterization of brain metastases reveals branched evolution and potential therapeutic targets. Cancer Discov. 2015;5(11):1164–1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Chen G, Chakravarti N, Aardalen K, et al. Molecular profiling of patient-matched brain and extracranial melanoma metastases implicates the PI3K pathway as a therapeutic target. Clin Cancer Res. 2014;20(21):5537–5546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Amaral T, Niessner H, Sinnberg T, et al. An open-label, single-arm, phase II trial of buparlisib in patients with melanoma brain metastases not eligible for surgery or radiosurgery—the BUMPER study. Neurooncol Adv. 2020;2(1):1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Zimmer AS, Steinberg SM, Smart DD, et al. Temozolomide in secondary prevention of HER2-positive breast cancer brain metastases. Future Oncol. 2020;16(14):899–909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Singh M, Manoranjan B, Mahendram S, McFarlane N, Venugopal C, Singh SK. Brain metastasis-initiating cells: survival of the fittest. Int J Mol Sci. 2014;15(5):9117–9133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ippen FM, Grosch JK, Subramanian M, et al. Targeting the PI3K/Akt/mTOR pathway with the pan-Akt inhibitor GDC-0068 in PIK3CA-mutant breast cancer brain metastases. Neuro Oncol. 2019;21(11):1401–1411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Chen G, Chakravarti N, Aardalen K, et al. Molecular profiling of patient-matched brain and extracranial melanoma metastases implicates the PI3K pathway as a therapeutic target. Clin Cancer Res. 2014;20(21):5537–5546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Han CH, Brastianos PK. Genetic characterization of brain metastases in the era of targeted therapy. Front Oncol. 2017;7:230. [DOI] [PMC free article] [PubMed] [Google Scholar]