Abstract
Arthropod-transmitted flaviviruses are responsible for considerable morbidity and mortality, causing severe encephalitic, hemorrhagic, and febrile illnesses in humans. Because there are no specific clinical symptoms for infection by a determined virus and because different arboviruses could be present in the same area, a genus diagnosis by PCR would be a useful first-line diagnostic method. The six published Flavivirus genus primer pairs localized in the NS1, NS3, NS5, and 3′ NC regions were evaluated in terms of specificity and sensitivity with flaviviruses (including the main viruses pathogenic for humans) at a titer of 105 50% tissue culture infectious doses (TCID50s) ml−1 with a common identification step by agarose gel electrophoresis. Only one NS5 primer pair allowed the detection of all tested flaviviruses with the sensitivity limit of 105 TCID50s ml−1. Using a heminested PCR with new primers designed in the same region after an alignment of 30 different flaviviruses, the sensitivity of reverse transcription-PCR was improved and allowed the detection of about 200 infectious doses ml−1 with all of the tick- and mosquito-borne flaviviruses tested. It was confirmed that the sequenced amplified products in the NS5 region allowed predictability of flavivirus species by dendrogram, including the New York 99 West Nile strain. This technique was successfully performed with a cerebrospinal fluid sample from a patient hospitalized with West Nile virus encephalitis.
Flaviviruses are arthropod-transmitted viruses that belong to the Flaviviridae family. The genus Flavivirus includes more than 70 single-stranded RNA viruses sharing common antigenic determinants, and the group is divided into eight serosubgroups and nine individual serotypes. Flaviviruses are responsible for considerable morbidity and mortality and may cause severe encephalitic, hemorrhagic, hepatic, and febrile illness in vertebrates, including humans. The pathogenic viruses in this genus include the Yellow fever virus (YF), Dengue viruses (DEN-1, -2, -3, and -4), Tick-borne encephalitis virus (TBE), Japanese encephalitis virus (JE), St. Louis encephalitis virus (SLE), and West Nile encephalitis virus (WN) (25).
Conventional flavivirus diagnosis is based on serology tests screening for the presence of virus-specific antibodies in the patient serum, which often require documentation of a rise in antibody concentration from an acute-phase blood sample to a convalescent-phase sample.
In August 1999, an outbreak of arboviral encephalitis was first recognized in New York City (2) and has since been identified in a neighboring state. This outbreak resulted in 61 human infections and seven deaths (5). Although initially attributed to SLE virus based on positive serologic findings in cerebrospinal fluid and serum samples using a virus-specific immunoglobulin M-capture enzyme-linked immunosorbent assay (ELISA), the cause of the outbreak has been confirmed as a WN virus (JE virus complex) based on the identification of the virus in human, avian, and mosquito samples by molecular tools (1, 18, 22).
The exceptional sensitivity of the PCR method allowed rapid detection and identification of flaviviruses (9) in mosquitoes and clinical samples (11, 21), in which virus culture is difficult or time-consuming and when early diagnosis is necessary for clinical treatment (28) and has implications for vaccination and mosquito control. The aim of this study was to develop, after comparing the specificity and sensitivity of the published primers for genus diagnosis of the main human pathogenic flaviviruses, a more sensitive technique with new primers. After having selected the best primers, we used the widely accepted heminested method to increase sensitivity, designing a new degenerate primer likely to be used in patient samples.
MATERIALS AND METHODS
Viruses and isolation of viral RNA.
All viruses were manipulated in a level 3 facility. With the exception of hepatitis C virus (HCV), all viral strains (Table 1) were obtained from mouse brain tissues and propagated in Vero cells as previously described (10). HCV was obtained from an HCV-positive patient's plasma, GR416. Its genotype, determined with the Inno Lipa HCV II kit (Innogenetics, Zwijndrecht, Belgium), was 1b, and its viral load, quantified with the Monitor HCV RNA assay (Roche Diagnostics System, Meylan, France), was 0.5 × 106 copies/ml. RNA was extracted with silica gel membrane spin columns (QIAmp Viral RNA 250; Qiagen SA, Courtaboeuf, France) from 280-μl samples obtained either from cell culture supernatant for arboviruses or from human serum for HCV. The extracted nucleic acid was stored at −80°C and eventually diluted with ultrapure water (pretreated with diethyl procarbonate [DEPC] in a 1:1,000 dilution; Sigma, St. Quentin Fallavier, France).
TABLE 1.
Arboviruses used, virus titer before extraction, and equivalent after dilution
| Virus | Strain | Origina | Virus titer (log TCID50 ml−1)
|
|
|---|---|---|---|---|
| Before extraction | Calculated | |||
| Flavivirus | ||||
| DEN-1 | Hawaii | PI, Dakar | 5.5 | 5.0 |
| DEN-2 | NGC | PI, Paris | 6.6 | 5.0 |
| DEN-4 | YUNH | PI, Paris | 6.0 | 5.0 |
| JE | Nakayama | PI, Paris | 6.9 | 5.0 |
| YF 17D | 17D | Vaccineb | 6.7 | 5.0 |
| YF FNV | FNV | PI, Dakar | 7.5 | 5.0 |
| Langat | Langat | PI, Paris | 7.0 | 5.0 |
| Usutu | Dak Ar D19848 | PI, Dakar | 7.9 | 5.0 |
| Wesselsbron | Wesselsbron | PI, Paris | 6.3 | 5.0 |
| WN a | E101 | PI, Paris | 6.6 | 5.0 |
| WN b | Local | Patient | 7.9 | 5.0 |
| Zika | Dak Ar B11514 | PI, Paris | 7.5 | 5.0 |
| Bunyaviridae | ||||
| Bunyamwera | Dak BUN73 | PI, Dakar | 6.4 | 6.4 |
| Rift Valley fever | MP12 | PI, Paris | 6.5 | 6.5 |
| Sandfly fever Sicilian | Sicile | PI, Paris | 5.9 | 5.9 |
| Togaviridae | ||||
| Chikungunya | Ross C347 | PI, Dakar | 7.5 | 7.5 |
| Semliki Forest | ASFV 64/79 | PI, Dakar | 9.1 | 9.1 |
| Sindbis | Dak Ar B489 | PI, Paris | 6.9 | 6.9 |
PI, Pasteur Institute (Dakar, Senegal, or Paris, France).
Stamaril, Pasteur Merieux, Lyon, France.
Primers.
The primers used in this study (Table 2) were either published previously or were designed with Primer Premier software (Primer Premier v4.1; Premier Biosoft International, Palo Alto, Calif.) after alignment of the flavivirus sequences with DNASIS software (DNASIS v2.6 for networks; Hitachi Software Engineering Europe S.A., Ardon, France). The locations of heminested primers are given according to the 17D YF virus sequence (GenBank accession no. X03700). They were synthesized by GIBCO-BRL (Life Technologies SARL, Cergy Pontoise, France).
TABLE 2.
Sequences and locations of primers used in this study
| Primer | Sequence | Annealing temp (°C) | Location | Source or reference |
|---|---|---|---|---|
| DJA | TCCATCCCATACCTGCA | 55 | NS1 | |
| DJS | GACATGGGGTATTGGAT | 24 | ||
| DV1 | GGRACKTCAGGWTCTCC | 50 | NS3 | |
| DV3 | AARTGIGCYTCRTCCAT | 8 | ||
| FG1 | TCAAGGAACTCCACACATGAGATGTACT | 45 | NS5 | |
| FG2 | GTGTCCCATCCTGCTGTGTCATCAGCATACA | 15 | ||
| CFDJ 9977 | GCATGTCTTCCGTCGTCATCC | 55 | NS5 | |
| FUDJ 9166 | GATGACACAGCAGGATGGGAC | 7 | ||
| VD8 | GGGTCTCCTCTAACCTCTAG | 53 | 3′ Nontranslated region | |
| EMF1 | TGGATGACSACKGARGAYATG | NS5 | 26 | |
| cFD2 | GTGTCCCAGCCGGCGGTGTCATCAGC | 53 | NS5 | |
| MA | CATGATGGGRAARAGRGARRAG | 19 | ||
| MAMD | AACATGATGGGRAARAGRGARAA | 53 | NS5 | This article |
| FS 778 | AARGGHAGYMCDGCHATHTGGT | 54 | NS5 | This article |
RT-PCR.
Reverse transcription (RT) reactions were performed in 30 μl containing 6 μl of RT buffer, 2 μl of 0.1 M dithiothreitol, 2.5 μl of 2.5 mM deoxynucleoside triphosphate (dNTP; final concentration, 200 μM), 1 μl of RNase (RNase out; GIBCO BRL), 10 μl of RNA template, 2.5 μl of reverse primer (10 μM; final concentration, 0.75 μM) and 5 μl of ultrapure DEPC-pretreated water.
Each sample was boiled for 5 min and cooled on wet ice. One microliter of Moloney murine leukemia virus (MMLV) reverse transcriptase (GIBCO BRL) was added. The reaction mixture was incubated at 37°C for 1 h and at 95°C for 10 min.
The PCRs were performed in 100 μl containing 10 μl of 10× Taq buffer, 3 μl of 50 mM MgCl2 (final concentration, 1.5 mM), 2 μl of 10 mM dNTP (final concentration, 200 μM), 2.5 μl of 10 μM reverse primer, and 2.5 μl of 10 μM sense primer (final concentration, 500 nM), 3 U of Taq DNA polymerase (GIBCO BRL), and 70 μl of ultrapure DEPC-pretreated H2O. Then, 10 μl of cDNA was added. The heminested PCRs were performed with 100 μl of mixture containing 10 μl of 10× Taq buffer, 3 μl of 50 mM MgCl2 (final concentration, 1.5 mM), 2 μl of 10 mM dNTP (final concentration, 200 μM), 2.5 μl of 10 μM reverse primer, 2.5 μl of 10 μM sense primer (final concentration, 500 nM), 3 U of Taq DNA polymerase (GIBCO BRL), and 75 μl of pure H2O. Then, 5 μl of PCR products was added.
The PCR thermal cycling incubations used for cFD2 and MAMD amplimers were performed as follows: initial amplification of 25 cycles of incubation at 94, 53, and 72°C for 1 min each; and amplification with nested primers cFD2 and FS 778 with 35 cycles of incubation at 94, 54, and 72°C for 1 min each. All thermal cycling was performed with PE Applied Biosystems 2400 machines. The amplification products were identified by their molecular weights analyzed by electrophoresis in a 2% agarose gel, and the separated fragments were stained with ethidium bromide and visualized under UV light transillumination (28).
Light cycler amplification.
A PCR quantitative instrument (LightCycler instrument, Roche Molecular Biochemicals, Meylan, France) PCR amplification was performed by using the same primers, but with a rapid cycle (denaturation, 1 s; annealing, 5 s; extension, 10 s), with results given in real time and with a master mix optimized for this machine containing the following in a final 20-μl volume: a 0.2 mM concentration of each of the dNTPs, 4 mM MgCl2, 0.6 μM concentrations of the cFD2 and FS 778 primers, and 0.16 μl of Taq DNA polymerase (Taq Start antibody; Ozyme, St. Quentin/Yvelines, France), stained with 2 μl of Sybr Green (LightCycler-DNA Master Sybr Green 1; Roche Molecular Biochemicals, Meylan, France). Analysis of the melting curve of specific PCR products was performed by slowly raising the temperature of the thermal chamber from 54°C to 95°C by means of regular fluorescence measurements.
Sequencing.
Products of PCR amplification in the laboratory were sequenced. The sequencing reaction was performed by PCR amplification in a final volume of 20 μl with 100 ng of PCR products, 5 pmol of primer, and 8 μl of BigDye Terminators premix according to the Applied Biosystems protocol. After being heated to 94°C for 2 min, the reaction mixture underwent 25 cycles of 30 s at 94°C, 30 s at 55°C, and 4 min at 60°C (Perkin-Elmer 9600 thermal cycler). Excess of BigDye Terminators was removed with exclusion columns. The samples were dried in a vacuum centrifuge and dissolved with 2 μl of deionized formamide-EDTA (5/1 ratio) (pH 8.0). The samples were loaded onto an Applied Biosystems 373XL sequencer and run for 12 h on a 4.5% denaturing acrylamide gel. Other sequences were obtained from GenBank. All sequences were compared by using DNASIS software that contains the Higgins and Sharp algorithm CLUSTAL 4 (16). This program takes a dendrogram as input, produced by applying the unweighted pair group method with arithmetic mean (UPGMA) to a matrix of similarity scores for all of the aligned sequences. The similarity scores are calculated as the number of exactly matched residues (top diagonals = 5) in a Wilbur and Lipman alignment between two sequences, minus a fixed penalty of 10 for every gap (23). The floating gap penalty was 10, and the K-tuple was 2.
RESULTS
Comparison between different published Flavivirus genus RT-PCR assays.
The flaviviruses were tested with six different primer pairs, and each result was confirmed three times. One HCV strain, three viruses of the Bunyaviridae family, and three viruses of the Togaviridae family were used as negative controls, and nonspecific amplifications were not found. DEN viruses, one WN strain, and Zika virus were amplified (Table 3) by amplimers of Meiyu (DJA and DJS, Table 2) and Chow (DV1 and DV3, Table 2). Using the primer pair of Chang (CFDJ 9977 and FUDJ 9166, Table 2), we detected DEN-1, DEN-2, and WN viruses; the Fulop (FG1 and FG2, Table 2) primer pair allowed us to amplify DNA fragments for many viruses, except JE, WN, Wesselsbron, and YF FNV. The best result was obtained with the primer pair proposed by Kuno (cFD2 and MA, Table 2), since we amplified every flavivirus tested (both mosquito- and tick-transmitted flaviviruses). However, the sensitivity limit is about 105 50% tissue culture infectious doses (TCID50s) ml−1: there is no detection of a virus titer lower than 104 TCID50s ml−1.
TABLE 3.
Comparison between different RT-PCR assays for detection of the Flavivirus genus
| Primer pair | Titer (log TCID50 ml−1) | Amplification of Flavivirus genus virusa
|
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| DEN-1 | DEN-2 | DEN-4 | JE | USU | WN a | WN b | WSL | YF FNV | YF 17D | Zika | ||
| DJA/DJS | 5 | + | + | + | − | − | + | − | − | − | − | + |
| DV1/DV3 | 5 | + | + | + | − | − | + | − | − | − | − | + |
| CFDJ 9977/FUDJ 9166 | 5 | + | + | − | − | − | − | + | − | − | − | − |
| VD8/EMF1 | 5 | − | − | − | − | − | − | − | − | − | − | − |
| FG1/FG2 | 5 | + | + | + | − | + | − | + | − | − | + | + |
| cFD2/MA | 5 | + | + | + | + | + | + | + | + | + | + | + |
| cFD2/MA | 4 | − | − | − | − | − | − | − | − | − | − | − |
+, positive amplification; −, negative amplification. USU, Usutu virus; WN a, strain E101; WN b, local strain; WSL, Wesselsbron virus.
New sensitive heminested technique for Flavivirus genus detection.
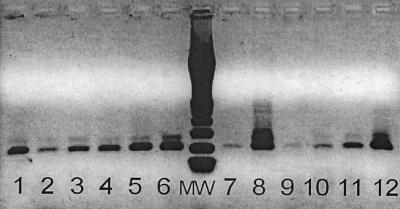
NS5 gene sequences of 30 different flaviviruses were aligned in order to design new primers allowing a heminested PCR (Table 4). cFD2 (location, NS5, 9232 to 9258) was not changed because it allowed the detection of every flavivirus in vitro. MA was modified in MAMD (location, NS5, 9006 to 9029): the primer was shifted toward the 5′-end region and was extended to 23 bases to increase the melting temperature. The inner sense primer (location, NS5, 9044 to 9066) allowing the heminested PCR was designed in an internal consensus region. After a cFD2 RT step, the first PCR was performed with the cFD2 reverse primer (6) and MAMD sense primer. The fragments of the expected 250-bp target size were successfully amplified from all mosquito- and tick-transmitted flaviviruses tested. The heminested PCR, performed with cFD2 reverse primer and a new FS 778 sense primer, allowed the detection of 200 infectious doses ml−1 (Fig. 1). The PCR in real time with a PCR quantitative instrument confirmed the DNA amplification of every flavivirus, and in a shorter time, the amplified products with the same titer of 105 TCID50 ml−1 were detected between 20 and 40 min after 30 and 55 cycles. The melting curve confirms that the heterogeneity of the amplified product depends on the degenerate primers on the different sequences targeted: peaks were not observed at the same temperature.
TABLE 4.
Sequence alignment of oligonucleotides MAMD, FS 778, and cFD2 with 30 flavivirus NS5 gene conserved regions
| Region | Sequence of oligonucleotidea:
|
||||
|---|---|---|---|---|---|
| MAMD | FS 778 | cFD2 | |||
| Primer | AACATGATGGGRAARAGRGARAA | (…) | AARGGHAGYMCDGCHATHTGGT | (…) | GTGTCCCAGCCGGCGGTGTCATCAGC |
| WN33G | AACATGATGGGAAAGAGAGAGAA | (…) | AAAGGCAGCAGAGCCATCTGGT | (…) | GCTGATGATACCGCAGGCTGGGACAC |
| WNNY99 | ––––––––––––––––––––––– | (…) | ––G––A–––––––––––T–––– | (…) | ––––––––C––A––T––––––––––– |
| YFVISN | –––––––––––G––A–––––––– | (…) | ––G––A–––C–T–––––A–––– | (…) | ––G–––––C–––––T––A–––––––– |
| YFTRIN | –––––––––––G––A–––––––– | (…) | ––G––A–––C–T–––––A–––– | (…) | ––G–––––C–––––T––A–––––––– |
| YFNEU | –––––––––––G––A–––––––– | (…) | ––G––A–––C–T–––––A–––– | (…) | ––G–––––C–––––T––A–––––––– |
| YF85N | –––––––––––G––A–––––––– | (…) | –––––A–––C–T–––––––––– | (…) | ––G–––––––––––T––G–––––––– |
| YF17DD | –––––––––––G––A–––––––– | (…) | ––G––A–––C–T–––––A–––– | (…) | ––G–––––C–––––T––A–––––––– |
| YF17DN | –––––––––––G––A–––––––– | (…) | ––G––A–––C–T–––––A–––– | (…) | ––G–––––C–––––T––A–––––––– |
| DE1WPA | ––––––––––––––––––––––– | (…) | –––––A––TC–C––A––A–––– | (…) | ––A–––––C––A––C––A–––––––– |
| DE2166 | ––––––––––––––A–––––––– | (…) | –––––––––––––––––A–––– | (…) | ––C–––––C––––––––A–––––T–– |
| DE2C04 | ––––––––––––––––––––A–– | (…) | –––––T–––––––––––A–––– | (…) | ––C––––––––A–––––A–––––––– |
| DE2NGC | ––––––––––––––A–––––––– | (…) | –––––––––––––––––A–––– | (…) | ––C–––––C––––––––A–––––––– |
| DE2PUO | ––––––––––––––A–––––––– | (…) | ––––––––T––G––T––A–––– | (…) | ––C–––––C––––––––A–––––––– |
| DE3H87 | –––––––––––C––––––––––– | (…) | –––––A–––––G–––––T–––– | (…) | ––––––––C––A––C––T–––––––– |
| JEBEI | ––––––––––––––A–––––A–– | (…) | –––––A–––––G–––––––––– | (…) | ––––––––––––––T––G–––––––– |
| JEG78 | ––––––––––––––A–––––––– | (…) | –––––A–––––G–––––T–––– | (…) | ––––––––C–––––C––G–––––––– |
| JEHVI | ––––––––––––––A–––––––– | (…) | –––––A–––––G–––––T–––– | (…) | ––––––––––––––C––G–––––––– |
| JEJAG | ––––––––––––––A–––––––– | (…) | –––––A–––––G–––––T–––– | (…) | ––––––––––––––C––G–––––––– |
| JEJAO | ––––––––––––––A–––––––– | (…) | –––––A–––––G–––––T–––– | (…) | ––––––––C–––––C––A–––––––– |
| JEK94 | ––––––––––––––A–––––––– | (…) | –––––A–––––G–––––T–––– | (…) | ––C–––––C–––––C––G–––––––– |
| JEP3N | ––––––––––––––A–––––––– | (…) | –––––A–––––G–––––T–––– | (…) | ––––––––––––––C––G–––––––– |
| JES14 | ––––––––––––––A–––––––– | (…) | –––––A–––––G–––––T–––– | (…) | ––––––––––––––C––G–––––––– |
| JESAA | ––––––––––––––A–––––––– | (…) | –––––A–––––G–––––T–––– | (…) | ––––––––––––––C––G–––––––– |
| JESAV | ––––––––––––––A–––––––– | (…) | –––––A–––––G–––––T–––– | (…) | ––––––––––––––C––G–––––––– |
| JETCN | –––––––––––G––A–––––––– | (…) | –––––A–––––G–––––T–––– | (…) | ––––––––C–––––C––G–––––––– |
| JETLN | –––––––––––G––A–––––––– | (…) | –––––A–––––G–––––T–––– | (…) | ––––––––C–––––C––G–––––––– |
| JEVEL | ––––––––––––––A–––––––– | (…) | –––––A–––––G–––––T–––– | (…) | ––––––––C–––––C––G–––––––– |
| TBE263 | –––––––––––C––––––––––– | (…) | –––––A––TC–G–––––T–––– | (…) | ––A–––––C––A––T––––––––––– |
| TBEHYP | –––––––––––C––––––––––– | (…) | ––G––A––TC–G–––––T–––– | (…) | ––A–––––C––A––T––––––––––– |
| TBENEU | –––––––––––C––––––––A–– | (…) | –––––A––TC–G–––––T–––– | (…) | ––A–––––C––A––T––––––––––– |
DNA sequences were obtained from the EMBL/GenBank databases. Accession numbers are as follows: WN33G, M12294; WNNY99 (WN New York 99 human), AF 202541; YFVISN, U21056; YFTRIN, AF094612; YFNEU, U21055; YF85N, U54798; YF17DD, U17066; YF17DN, U17067; DE1WPA (DEN-1 West Pac), U88535; DE2166 (DEN-2-166/81), U87411; DE2CO4 (DEN-2 CO4), AF119661; DE2NGC (DEN-2 NGC), AF038403; DE2PUO (DEN-2 PUO), AF038402; DE3H87 (DEN-3 H87), M93130; JEBEI, L48961; JEG78, AF075723; JEHVI, AF098735; JEJAG, AF 069076; JEJAO, M18370; JEK94, AF045551; JEP3N, U03695; JES14, M55506; JESAA, D90195; JESAV, D90194; JETCN, AF098736; JETLN, AF098737; JEVEL, AF080251; TBE263, U27491; TBEHYP, U39292; and TBENEU, U27495.
FIG. 1.
Results of RT-PCR with primer pairs cFD2 and MAMD and cFD2 and FS 778 in heminested PCR for Flavivirus genus detection. The figure shows the detection of 2 × 102 infectious doses ml−1 for all viruses tested. Lanes: 1, DEN-1 virus; 2, DEN-2 virus; 3, DEN-4 virus; 4, JE virus; 5, Usutu virus; 6, WN (a) virus; 7, WN (b) virus; 8, Wesselsbron virus; 9, YF FNV; 10, YF 17 D; 11, Zika virus; 12, Langat virus; MW, molecular weight marker (from top to bottom: 1,000, 700, 500, 400, 300, 200, and 100 bp).
Alignment of 12 sequences amplified by the cFD2-FS 778 primer pair and construction of the dendrogram
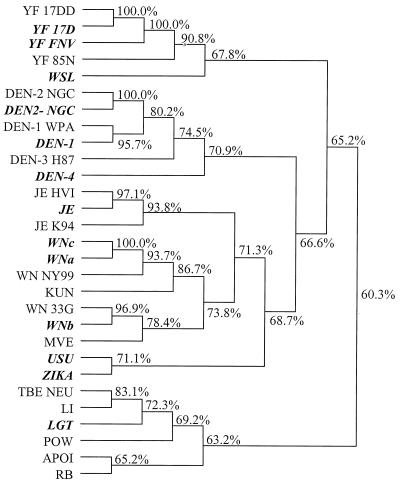
The alignment of the different sequences of PCR products did not show any complete homology between the different species, confirming the absence of cross-contamination. It also permitted us to verify that amplimers had amplified the expected products in NS5 location. In drawing the phylogenetic tree, it was possible to predict all tested Flavivirus serocomplexes and species (Fig. 2), including the most pathogenic viruses, such as the DEN-1, DEN-2, DEN-4, JE, YF, WN, and TBE viruses (20, 25).
FIG. 2.
Comparison of the sequences of the cFD2 and FS778 amplimer positions of flaviviruses according to the CLUSTAL algorithm. The program takes as input a dendrogram produced by the UPGMA method. Viruses amplified in our laboratory are shown in boldface (Table 1). Other sequences were obtained from GenBank (Table 4). The alignment allowed us to find all of the complexes of medical interest: the DEN, JE, WN, YF, TBE, and Rio Bravo groups.
DISCUSSION
Several RT-PCRs have been developed for detection of flavivirus RNA by using different pairs of primers for differentiating between species of viruses (12), including flavi-universal primers for mosquito-borne flaviviruses (29) and six published primer pairs permitting complete detection of the Flavivirus genus (7, 8, 15, 19, 24, 26). Our purpose was first to compare these flavivirus genus diagnosis references. To clarify their ability to detect the genus, we used both mosquito- and tick-borne viruses, including some strains of all of the virus groups of major public health importance, at a virus titer of 105 TCID50s ml−1. We tested specificity by using other viruses, either because of their potential epidemiological or clinical homology (other arboviruses) or because of a close relationship in the Flaviviridae family (HCV). In order to compare the actual levels of performance of the primers, we chose to use agarose gel electrophoresis and simple ethidium bromide staining to eliminate any possible identification variation factor.
Sequence similarity calculations revealed that the NS5 proteins are the most highly conserved of the flavivirus nonstructural proteins (17). The best result was obtained with the cFD2-MA pair (designed on the basis of the NS5 gene), which was the only pair able to detect all of the targeted viruses, and there was no problem of specificity towards other tested viruses. Tests carried out with cFD2 and FG1 primers gave positive results, except for the Wesselsbron and WN viruses.
These results do not mean that the other primers will completely fail when their own detection methods are used: only three groups (15, 19, 24) proposed an analysis of amplification products by electrophoresis on an agarose gel, while some others used a more sensitive technique, e.g., ELISA revelation of amplification products stained by digoxigenin (7, 26).
However, even with the cFD2-MA amplimers described by Kuno (19), the detection limit was only 105 TCID50s ml−1. This is sufficient to ensure virus identification by cell culture, because this titer is usually obtained after a few passages on Vero or C6/36 cells. However, this sensitivity is often insufficient to detect the virus in a serum (or cerebrospinal fluid) sample from symptomatic patients, whose samples are often obtained after the viremia peak. Consequently, we tried to increase this sensitivity by using a heminested approach.
MAMD and FS 778 were designed after determining the consensus base region on the basis of an alignment of 30 NS5 flavivirus sequences extracted from GenBank. The Langat virus was used to complete the specificity analysis with a detection of a tick-borne virus. The percentage of similarities of Langat virus NS5 amino acid sequences and those of other flaviviruses exceed 70% (17). These amplimers allowed the detection of the 12 viruses at a minimum of 200 infectious doses ml−1. This sensitivity could be improved by using a better detection method.
The detection of the positive specimens (Table 1) was accomplished by determining the size of amplified DNA by agarose gel electrophoresis. Each product amplified by the cFD2 and MAMD primers was sequenced to eliminate any false-positive result by cross-contamination (data not shown). Phylogenetic trees of flaviviruses derived from NS5 gene sequences have been described previously (20, 22). Furthermore, the phylogenetic tree designed by comparison of the amplification of the heminested products in the NS5 location (220 bp) allowed us to find all of the complexes of medical interest: the DEN, JE, YF, and TBE groups. Interestingly, by using this method, we were able to identify flaviviruses in two patient samples.
In the first case, a virus was isolated on Vero cells from serum from a febrile patient in Senegal. The virus obtained from the cell culture was difficult to identify because the polyclonal hyperimmune antisera showed anti-DEN virus cross-reaction, but the virus was able to kill 3-week-old Swiss albino mice after intraperitoneal infection, which contradicted the DEN virus characteristics. The specific PCR product was visible at approximately 220 bp with the heminested cFD2-MAMD and cFD2-FS778 pairs (Fig. 1). In the second case, the amplified product was directly obtained from a cerebrospinal fluid sample from a patient suffering from severe encephalitis. In these two cases, sequencing and comparison of the NS5 amplified products classified the viruses among the WN species on the phylogenetic tree (WNc; Fig. 2). The identification was then confirmed by a species-specific PCR targeting the envelope gene of WN viruses (3).
An outbreak of arboviral encephalitis associated with mosquitoes was recognized in New York City in late August 1999. SLE virus was identified initially as the causative agent because of compatible clinical symptoms (neurological disease including fatal encephalitis) and positive serological laboratory tests (13, 14). However, RT-PCR and sequencing first permitted the identification of Kunjin-WN-like flavivirus in brains of deceased patients (4), before identifying the WN lineage (18, 22). We confirmed that the NS5 amplification, sequencing, and phylogenetic analysis of the heminested amplified product would be able to provide a first key to identify the New York 99 WN strain (Fig. 2), even though these products are too short to ensure a definitive phylogenetic analysis. This genus PCR procedure could be used as a first-line diagnostic PCR screening test for an unknown virus, indicating the relatedness of the poorly characterized viruses to the pathogenic members of genus Flavivirus after cell culture. A definitive identification obviously requires both complete sequencing and the appropriate expertise in flavivirus identification. Because it would take only a few hours, PCR detection of a flavivirus directly from patient samples could help the physician choose the appropriate first-line treatment. The few successes obtained with amplification directly from patient samples without a cell culture step must be confirmed on a larger scale. The use of a single-step RT-PCR can shorten the reaction time. The PCR amplification in real time allowed a quicker diagnosis and could allow quantitative detection with fluorogenic hybridization probes. A quick and ultrasensitive technique is key to allowing diagnosis directly from patient samples.
Diagnosis of flavivirus infections by this method requires a subsequent stage to allow rapid identification of the virus species. This could be achieved by species-specific PCR or by hybridization (27) of specific probes of each serogroup (30) with fragments produced by these RT-PCR procedures.
ACKNOWLEDGMENTS
This work was supported by research grants from the Service de Santé des Armées (SSA).
We thank Corinne Rothlisberger, Danielle Gratier, Josette Guimet, and Henri Blancquaert for technical assistance.
REFERENCES
- 1.Anderson J F, Andreadis T G, Vossbrinck C R, Tirrell S, Wakem E M, French R A, Garmendia A E, Van Kruiningen H J. Isolation of West Nile Virus from mosquitoes, crows and a cooper's hawk in Connecticut. Science. 1999;286:2331–2333. doi: 10.1126/science.286.5448.2331. [DOI] [PubMed] [Google Scholar]
- 2.Asnis D, Contta R, Waldmon G. Outbreak of West Nile-like viral encephalitis: New York. Morb Mortal Wkly Rep. 1999;48:845–849. [PubMed] [Google Scholar]
- 3.Berthet F X, Zeller H G, Drouet M T, Rauzier J, Digoutte J P, Deubel V. Extensive nucleotide changes and deletions within the envelope glycoprotein gene of Euro-African West Nile viruses. J Gen Virol. 1997;78:2293–2297. doi: 10.1099/0022-1317-78-9-2293. [DOI] [PubMed] [Google Scholar]
- 4.Briese T, Jia X Y, Huang C, Grady L J, Lipkin W I. Identification of a Kunjin/West Nile-like flavivirus in brains of patients with New York encephalitis. Lancet. 1999;354:1261–1262. doi: 10.1016/s0140-6736(99)04576-6. [DOI] [PubMed] [Google Scholar]
- 5.Centers for Disease Control and Prevention. Guidelines for surveillance, prevention and control of West Nile Virus infection—United States. Morb Mortal Wkly Rep. 2000;49:25–28. [PubMed] [Google Scholar]
- 6.Chambers T J, Hahn C S, Galler R, Rice C M. Flavivirus genome organisation, expression, and replication. Annu Rev Microbiol. 1990;44:649–688. doi: 10.1146/annurev.mi.44.100190.003245. [DOI] [PubMed] [Google Scholar]
- 7.Chang G J, Trent D W, Vorndam A V, Vergne E, Kinney R M, Mitchell C J. An integrated target sequence and signal amplification assay, reverse transcriptase–PCR–enzyme-linked immunosorbent assay, to detect and characterize flaviviruses. J Clin Microbiol. 1994;32:477–483. doi: 10.1128/jcm.32.2.477-483.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chow V T K, Seah C L K, Chan Y C. Use of NS3 consensus primers for the polymerase chain reaction amplification and sequencing of dengue viruses and other flaviviruses. Arch Virol. 1993;133:157–170. doi: 10.1007/BF01309751. [DOI] [PubMed] [Google Scholar]
- 9.Clement J, Heyman P. PCR for diagnosis of viral infections of the central nervous system. Nature. 1997;349:1256. doi: 10.1016/S0140-6736(05)62455-5. [DOI] [PubMed] [Google Scholar]
- 10.Crance J M, Gratier D, Guimet J, Jouan A. Inhibition of Sandfly Fever Sicilian virus (Phlebovirus) replication in vitro by antiviral compounds. Res Virol. 1997;148:353–365. doi: 10.1016/s0923-2516(97)89132-7. [DOI] [PubMed] [Google Scholar]
- 11.Deubel V, Huerre M, Cathomas G, Drouet M T, Wuscher N, Le Guenno B, Widmer A F. Molecular detection and characterisation of Yellow fever virus in blood and liver specimens of a non-vaccinated fatal human case. J Med Virol. 1997;53:212–217. [PubMed] [Google Scholar]
- 12.Eldadah Z A, Asher D M, Godec M S, Pomeroy K L, Goldfarb L G, Feinstone S M, Levitan H, Gibbs C J, Carleton Gajdusek D. Detection of flavivirus by reverse-transcriptase polymerase chain reaction. J Med Virol. 1991;33:260–267. doi: 10.1002/jmv.1890330410. [DOI] [PubMed] [Google Scholar]
- 13.Enserink M. Groups race to sequence and identify New York virus. Science. 1999;286:206–207. doi: 10.1126/science.286.5438.206. [DOI] [PubMed] [Google Scholar]
- 14.Enserink M. New York's lethal virus came from Middle East, DNA suggests. Science. 1999;286:1450–1451. doi: 10.1126/science.286.5444.1450. [DOI] [PubMed] [Google Scholar]
- 15.Fulop L, Barrett A D T, Phillpotts R, Martin K, Leslie D, Titball R W. Rapid identification of flaviviruses based on conserved NS5 gene sequences. J Virol Methods. 1993;44:179–188. doi: 10.1016/0166-0934(93)90053-t. [DOI] [PubMed] [Google Scholar]
- 16.Higgins D G, Sharp P M. Clustal: a package for performing multiple sequence alignments on a microcomputer. Gene. 1988;73:237–244. doi: 10.1016/0378-1119(88)90330-7. [DOI] [PubMed] [Google Scholar]
- 17.Iacono-Connors L C, Schmaljohn C S. Cloning and sequence analysis of the genes encoding the non-structural proteins of Langat Virus and comparative analysis with other flaviviruses. Virology. 1992;188:875–880. doi: 10.1016/0042-6822(92)90545-z. [DOI] [PubMed] [Google Scholar]
- 18.Jia X-Y, Briese T, Jordan I, Rambaut A, Chi H C, Mackenzie J S, Hall R A, Scherret J, Lipkin W I. Genetic analysis of West Nile New York 1999 encephalitis virus. Lancet. 1999;354:1971–1972. doi: 10.1016/s0140-6736(99)05384-2. [DOI] [PubMed] [Google Scholar]
- 19.Kuno G. Universal diagnostic RT-PCR protocol for arboviruses. J Virol Methods. 1998;72:27–41. doi: 10.1016/s0166-0934(98)00003-2. [DOI] [PubMed] [Google Scholar]
- 20.Kuno G, Chang G-J, Tsuchiya K R, Karabatsos N, Cropp C B. Phylogeny of the genus Flavivirus. J Virol. 1998;72:73–83. doi: 10.1128/jvi.72.1.73-83.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lanciotti R S, Calisher C H, Gubler D J, Chang G J, Vorndam A V. Rapid detection and typing of dengue viruses from clinical samples by using reverse transcription-polymerase chain reaction. J Clin Microbiol. 1992;30:545–551. doi: 10.1128/jcm.30.3.545-551.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lanciotti R S, Roehrig J T, Deubel V, Smith J, Parker M, Steele K, Crise B, Volpe K E, Crabtree M B, Scherret J H, Hall R A, MacKenzie J S, Cropp C B, Panigrahy B, Ostlund E, Schmitt B, Malkinson M, Banet C, Weissman J, Komar N, Savage H M, Stone W, McNamara T, Gubler D J. Origin of the West Nile Virus responsible for an outbreak of encephalitis in the Northeastern United States. Science. 1999;286:2333–2337. doi: 10.1126/science.286.5448.2333. [DOI] [PubMed] [Google Scholar]
- 23.Lipman D J, Pearson W R. Rapid and sensitive protein similarity searches. Science. 1985;227:1435–1441. doi: 10.1126/science.2983426. [DOI] [PubMed] [Google Scholar]
- 24.Meiyu F, Huosheng C, Cuihua C, Xiaodong T, Lianhua J, Yifei P, Weijun C, Huiyu G. Detection of flaviviruses by reverse transcriptase-polymerase chain reaction with the universal primer set. Microbiol Immunol. 1997;41:209–213. doi: 10.1111/j.1348-0421.1997.tb01192.x. [DOI] [PubMed] [Google Scholar]
- 25.Monath T P, Heinz F X. In: Flaviviruses. Fields B N, Knipe D M, Howley P M, editors. Philadelphia, Pa: Lippincott-Raven Publishers; 1996. pp. 961–1034. [Google Scholar]
- 26.Pierre V, Drouet M T, Deubel V. Identification of mosquito-borne flavivirus sequences using universal primers and reverse transcription/polymerase chain reaction. Res Virol. 1994;145:93–104. doi: 10.1016/s0923-2516(07)80011-2. [DOI] [PubMed] [Google Scholar]
- 27.Ruiz B H, Zamora M P, Liu S. Detection of viral RNA by microplate hybridisation. J Virol Methods. 1995;54:97–108. doi: 10.1016/0166-0934(95)00026-q. [DOI] [PubMed] [Google Scholar]
- 28.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. pp. 6.1–6.19. [Google Scholar]
- 29.Tanaka M. Rapid identification of Flavivirus using the polymerase chain reaction. J Virol Methods. 1993;41:311–322. doi: 10.1016/0166-0934(93)90020-r. [DOI] [PubMed] [Google Scholar]
- 30.Zanotto P, Gould E A, Gao G F, Harvey P H, Holmes E C. Population dynamics of flaviviruses revealed by molecular phylogenies. Proc Natl Acad Sci USA. 1996;93:548–553. doi: 10.1073/pnas.93.2.548. [DOI] [PMC free article] [PubMed] [Google Scholar]