This cross-sectional study investigates whether site-specific tau phosphorylation occupancy is associated with BDNF Val66Met in presymptomatic and symptomatic dominantly inherited Alzheimer disease.
Key Points
Question
To what extent does the BDNF Val66Met polymorphism moderate cognitive performance and tau levels in dominantly inherited Alzheimer disease?
Findings
In this cross-sectional cohort study with 374 participants, presymptomatic mutation carriers who also carry the BDNF Met66 allele showed significantly poorer episodic memory, smaller hippocampal volume, and higher p-tau217, p-tau181, and total tau, compared with Val66 homozygotes. In symptomatic mutation carriers, Met66 carriers showed significantly poorer global cognition and higher p-tau217, total tau, and p-tau205, when compared with Val66 homozygotes.
Meaning
BDNF Val66Met may be an important moderator of clinical and tau outcomes in dominantly inherited Alzheimer disease.
Abstract
Importance
Allelic variation in the brain-derived neurotrophic factor (BDNF) Val66Met polymorphism moderates increases in cerebrospinal fluid (CSF) levels of tau and phosphorylated tau 181 (p-tau181), measured using immunoassay, and cognitive decline in presymptomatic dominantly inherited Alzheimer disease (DIAD). Advances in mass spectrometry show that CSF tau phosphorylation occupancy at threonine 181 and 217 (p-tau181/tau181, p-tau217/tau217) increases with initial β-amyloid (Aβ) aggregation, while phosphorylation occupancy at threonine 205 (p-tau205/tau205) and level of total tau increase when brain atrophy and clinical symptoms become evident.
Objective
To determine whether site-specific tau phosphorylation occupancy (ratio of phosphorylated to unphosphorylated tau) is associated with BDNF Val66Met in presymptomatic and symptomatic DIAD.
Design, Setting, and Participants
This cross-sectional cohort study included participants from the Dominantly Inherited Alzheimer Network (DIAN) and Aβ-positive cognitively normal older adults in the Alzheimer’s Disease Neuroimaging Initiative (ADNI). Data were collected from 2009 through 2018 at multicenter clinical sites in the United States, United Kingdom, and Australia, with no follow-up. DIAN participants provided a CSF sample and completed clinical and cognitive assessments. Data analysis was conducted between March 2020 and March 2021.
Main Outcomes and Measures
Mass spectrometry analysis was used to determine site-specific tau phosphorylation level; tau levels were also measured using immunoassay. Episodic memory and global cognitive composites were computed.
Results
Of 374 study participants, 144 were mutation noncarriers, 156 were presymptomatic mutation carriers, and 74 were symptomatic carriers. Of the 527 participants in the network, 153 were excluded because their CSF sample, BDNF status, or both were unavailable. Also included were 125 Aβ-positive cognitively normal older adults in the ADNI. The mean (SD) age of DIAD participants was 38.7 (10.9) years; 43% were women. The mean (SD) age of participants with preclinical sporadic AD was 74.8 (5.6) years; 52% were women. In presymptomatic mutation carriers, compared with Val66 homozygotes, Met66 carriers showed significantly poorer episodic memory (d = 0.62; 95% CI, 0.28-0.95), lower hippocampal volume (d = 0.40; 95% CI, 0.09-0.71), and higher p-tau217/tau217 (d = 0.64; 95% CI, 0.30-0.97), p-tau181/tau181 (d = 0.65; 95% CI, 0.32-0.99), and mass spectrometry total tau (d = 0.43; 95% CI, 0.10-0.76). In symptomatic mutation carriers, Met66 carriers showed significantly poorer global cognition (d = 1.17; 95% CI, 0.65-1.66) and higher p-tau217/tau217 (d = 0.53; 95% CI, 0.05-1.01), mass spectrometry total tau (d = 0.78; 95% CI, 0.28-1.25), and p-tau205/tau205 (d = 0.97; 95% CI, 0.46-1.45), when compared with Val66 homozygotes. In preclinical sporadic AD, Met66 carriers showed poorer episodic memory (d = 0.39; 95% CI, 0.00-0.77) and higher total tau (d = 0.45; 95% CI, 0.07-0.84) and p-tau181 (d = 0.46; 95% CI, 0.07-0.85).
Conclusions and Relevance
In DIAD, clinical disease stage and BDNF Met66 were associated with cognitive impairment and levels of site-specific tau phosphorylation. This suggests that pharmacological strategies designed to increase neurotrophic support in the presymptomatic stages of AD may be beneficial.
Introduction
In dominantly inherited Alzheimer disease (DIAD), mutations in presenilin 1 (PSEN1), presenilin 2 (PSEN2), or the amyloid precursor protein (APP) cause aggregation of β-amyloid (Aβ),1,2 with consequent hyperphosphorylation of the microtubule-associated protein tau and formation of neurofibrillary tangles. While Aβ and tau aggregation are associated with loss of brain volume and cognitive decline, the processes involved remain unspecified.3 Studies of the effects of the brain-derived neurotrophic factor (BDNF) Val66Met (rs6265) polymorphism on the biomarkers Aβ, tau, hippocampal volume, and cognition in humans suggest that tau-induced neurotoxicity is accelerated if trophic support for neurons is reduced.4,5
In presymptomatic DIAD and preclinical sporadic AD, carriers of BDNF Met66 show faster loss of hippocampal volume and cognition over 3 years, relative to those who are Val66 homozygotes.6,7,8 In DIAD, faster neurodegeneration in Met66 carriers was accompanied by substantial increases in cerebrospinal fluid (CSF) levels of total tau and tau phosphorylated at threonine 181 (p-tau181), but not CSF Aβ42.6 These increases in CSF tau and p-tau181 could reflect greater tau hyperphosphorylation or passive increase in tau resulting from neuronal death.6
Accumulation of soluble and insoluble tau reflects different dimensions of tau accumulation, including site-specific phosphorylation, and are associated with different clinical aspects of the AD spectrum.9,10,11 Specifically, CSF tau phosphorylation occupancy at threonine 181 and 217 (p-tau181/tau181 and p-tau217/tau217) increases with initial Aβ aggregation, up to 2 decades before tau can be detected on positron emission tomography (PET).10,11,12 Conversely, phosphorylation occupancy at threonine 205 (p-tau205/tau205) and level of total tau increase when atrophy, hypometabolism, and clinical symptoms become evident, although still before detection of fibrillar tau by PET.11
Understanding how variation in site-specific tau phosphorylation occupancy (ratio of phosphorylated to unphosphorylated tau) is associated with BDNF in presymptomatic and symptomatic DIAD could increase understanding of how neurotrophic factors influence interactions between Aβ aggregation and the posttranslational modification of tau as AD develops. This study examined associations between BDNF Val66Met and site-specific tau hyperphosphorylation and total tau, in both presymptomatic and symptomatic carriers of DIAD mutations. For clinical context, the association of BDNF with episodic memory, global cognition, and hippocampal volume was also determined. These same associations were then investigated in Aβ-positive, cognitively normal older adults enrolled in the Alzheimer’s Disease Neuroimaging Initiative (ADNI).
Methods
Participants
Individuals at risk for carrying a DIAD mutation (ie, PSEN1, PSEN2, or APP mutations) were enrolled in the Dominantly Inherited Alzheimer Network (DIAN).1 Baseline data were included from 374 participants (144 mutation noncarriers, 156 presymptomatic mutation carriers, 74 symptomatic mutation carriers) who provided a CSF sample and completed clinical and cognitive assessments. For mutation carriers, only those with available BDNF Val66Met polymorphism data were included. Table 1 provides the demographic, clinical, and biological characteristics of each group. All participants provided written informed consent. DIAN was approved by the institutional research boards of each participating institution. In addition, baseline data were included from 125 Aβ-positive cognitively normal older adults, hereafter referred to as participants with preclinical sporadic AD, who were enrolled in ADNI and for whom BDNF polymorphism data were available. Demographic, clinical, and biological characteristics of this preclinical sporadic AD group are provided in eTable 1 in Supplement 1.
Table 1. Demographic, Clinical, and Biological Characteristics.
| NC (n = 144) | pMC Val (n = 100) | pMC Met (n = 56) | P value | sMC Val (n = 46) | sMC Met (n = 28) | P value | |
|---|---|---|---|---|---|---|---|
| Female sex, No. (%) | 54 (37.5) | 43 (43.0) | 25 (44.6) | .84 | 24 (52.2) | 13 (46.4) | .63 |
| APOE ε4, No. (%) | 52 (36.1) | 33 (33.0) | 13 (23.2) | .19 | 18 (39.1) | 7 (25.0) | .21 |
| PSEN1/PSEN2/APP, No. | 96/20/28 | 62/12/26 | 44/6/6 | .06 | 38/2/6 | 24/0/4 | .53 |
| Age, mean (SD), y | 37.97 (11.68) | 35.40 (9.29) | 34.14 (8.21) | .40 | 46.24 (8.23) | 47.86 (9.89) | .45 |
| DIAN EYO, mean (SD), y | −10.04 (12.16) | −12.74 (8.93) | −13.11 (8.11) | .79 | 1.83 (5.70) | 3.61 (4.59) | .17 |
| Education, mean (SD), y | 15.01 (2.51) | 14.95 (2.74) | 14.93 (3.29) | .96 | 13.27 (2.77) | 13.68 (3.84) | .59 |
| CDR score, mean (SD) | NA | 0.01 (0.09) | 0.01 (0.07) | .65 | 0.76 (0.49) | 0.89 (0.69) | .34 |
| CDR SB, mean (SD) | 0.01 (0.06) | 0.06 (0.21) | 0.07 (0.20) | .64 | 3.75 (3.15) | 4.48 (4.60) | .42 |
| MMSE score, mean (SD) | 28.98 (1.30) | 29.00 (1.23) | 29.00 (1.23) | .99 | 23.42 (5.55) | 20.21 (8.42) | .05 |
| PiB-PET SUVR, mean (SD) | 1.05 (0.16) | 1.67 (0.79) | 1.73 (0.83) | .65 | 2.73 (1.21) | 3.15 (1.49) | .27 |
| xMAP CSF, mean (SD), pg/mL | |||||||
| Aβ42 | 472.02 (139.76) | 387.55 (165.60) | 422.33 (191.19) | .24 | 242.12 (125.66) | 239.38 (112.13) | .93 |
| Total tau | 57.32 (24.15) | 79.80 (49.38) | 98.17 (56.06) | .04 | 163.86 (82.22) | 187.46 (125.85) | .33 |
| p-Tau181 | 29.41 (10.84) | 44.91 (25.78) | 58.90 (34.75) | .005 | 90.07 (37.50) | 90.48 (40.23) | .96 |
Abbreviations: CDR, Clinical Dementia Rating; CSF, cerebrospinal fluid; DIAN, Dominantly Inherited Alzheimer Network; EYO, estimated year of symptom onset; MMSE, Mini-Mental State Examination; NA, not applicable; NC, mutation noncarrier; PiB-PET, positron emission tomography using Pittsburgh compound B; pMC, presymptomatic (CDR 0) mutation carrier; p-tau181, phosphorylation occupancy at threonine 181; sMC, symptomatic (CDR ≥0.5) mutation carrier; SB, Sum of Boxes score; SUVR, standardized uptake value ratio.
Clinical Assessment
Without reference to neuropsychological test performance, a clinician assessed each participant for the presence and severity of clinical symptoms of dementia using the Clinical Dementia Rating (CDR), for which a CDR global score of 0 indicates cognitive normality.13,14 Participants also completed the Mini-Mental State Examination (MMSE)15 and the 15-item Geriatric Depression Scale.16
Neuropsychological Assessment
The DIAN neuropsychological test battery and its standardization have been detailed previously and were administered according to standard protocols.17 Outcome measures for each test were standardized against the baseline mean and SD of mutation noncarriers. Standardized scores were averaged to form a composite score for episodic memory (Logical Memory delayed recall, word list learning delayed recall) and for global cognition (Logical Memory delayed recall, word list learning delayed recall, Digit Symbol, MMSE).6,18 The ADNI episodic memory composite has also been described previously.19
Biochemical Analysis
Fasting CSF was collected in the morning via lumbar puncture. Samples were shipped on dry ice to the DIAN biomarker core laboratory. Cerebrospinal fluid concentrations of Aβ1-42, total tau, and p-tau181 were measured by Luminex bead-based multiplexed xMAP technology immunoassay (INNO-BIA AlzBio3; Innogenetics). All values met quality-control standards, including a coefficient of variation of 25% or less, kit “controls” within the expected range as defined by the manufacturer, and measurement consistency between plates of a common sample that was included in each run.
Detailed descriptions of the mass spectrometry analysis to determine each site-specific tau phosphorylation level have been provided elsewhere,11,20 and described in brief in the eMethods in Supplement 1.
Genotyping
Genotyping for pathogenic mutations in the APP, PSEN1, and PSEN2 genes was performed on DNA extracted from peripheral blood samples using methods described previously.21 Genotype data were cleaned by applying a minimum call rate for single-nucleotide variations (SNVs, formerly SNPs) and individuals (98%); SNVs not in Hardy-Weinberg equilibrium (P < 1 × 10−6) were excluded. No SNVs were removed because of low minor allele frequency. Genotype data for the BDNF Val66Met (rs6265) polymorphism were extracted using PLINK. Clinicians were blinded to all genetic information, and genetic polymorphisms were not used diagnostically.
Neuroimaging
DIAN neuroimaging protocols have been described previously.22,23 Briefly, images from PET using Pittsburgh compound B (PiB-PET) were co-registered with individual magnetic resonance images (MRIs) for region-of-interest (ROI) determination. Three Tesla volumetric T1-weighted MRI scans were used with data acquired and processed through FreeSurfer 5.3 (Martinos Center). Hippocampal volume was corrected for total intracranial volume. For PiB-PET, total neocortical standardized uptake value ratio (SUVR) was used to determine levels of cortical Aβ deposition, using cerebellar gray matter as the reference region and applying partial volume correction using a regional point spread function.23
Estimated Year of Onset
Estimated year of expected symptom onset (EYO)2 was computed using the age of the participant at the baseline assessment minus the mean age at onset of symptoms for all individuals with the same mutation type.
Data Analysis
All analyses were conducted in R version 4.0.2 between March 2020 and March 2021. Participants were grouped according to DIAD mutation status, BDNF Val66Met status, and CDR score (presymptomatic mutation carriers had a CDR global score of 0; symptomatic mutation carriers had a CDR global score ≥0.5).
A series of analyses of covariance were conducted, where group, EYO, and the group × EYO interaction were specified as predictive factors, and sex and apolipoprotein E (APOE) ε4 status were included as covariates, given their previously reported effects on tau in sporadic AD.24,25 Planned comparisons determined the association of BDNF Val66Met with each tau/cognitive outcome within presymptomatic mutation carriers and symptomatic mutation carriers, separately.
Associations between BDNF, EYO, and disease stage were explored using linear regressions, with age, sex, and ε4 as covariates. These analyses were restricted to the main cognitive and tau outcomes (ie, episodic memory, global cognition, p-tau217/tau217, p-tau205/tau205).
For all analyses, statistical significance was set at P < .05, with no adjustments for multiple comparisons, as measures of effect sizes (Cohen d; β estimates) were reported to guide interpretation of results. Effect sizes less than 0.2 were classified as trivial and not interpreted regardless of statistical significance. For clinical data, effect sizes greater than 1 were considered clinically important.26 This strategy balances risk of type I error against the identification of potentially important relationships in a novel area of research that may have important implications for understanding clinical disease progression in DIAD.
Data Availability
Data and biospecimens from DIAN can be accessed by a formal application to the DIAN Steering Committee. Data are systematically released after a 1-year delay to allow DIAN investigators the opportunity to publish main study findings. Data from ADNI are publicly accessible via http://adni.loni.usc.edu.
Results
Demographic, Clinical, and Biological Characteristics
Table 1 summarizes the demographic, clinical and biological characteristics of each DIAD clinical group. eTable 1 in Supplement 1 summarizes the demographic, clinical, and biological characteristics of the preclinical sporadic AD group. As reported previously in presymptomatic mutation carriers,6 Met66 carriers had significantly higher levels of CSF total tau and p-tau181 than Val66 homozygotes as measured by immunoassay (Table 1).
Characterization of BDNF Val66Met on Cognition and Hippocampal Volume
Significant group × EYO interactions were observed for both cognitive outcomes and for hippocampal volume (Table 2). Relative to mutation noncarriers, presymptomatic mutation carriers who were also Val66 homozygotes did not differ significantly on either cognitive outcome (both P > .99) (Figure 1). In presymptomatic mutation carriers, compared with Val66 homozygotes, Met66 carriers showed poorer episodic memory (d = 0.62; 95% CI, 0.28-0.95; P = .002), lower hippocampal volume (d = 0.40; 95% CI, 0.09-0.71; P = .01), but equivalent global cognition (d = 0.12; 95% CI, −0.21 to 0.45; P = .95). In symptomatic mutation carriers, compared with Val66 homozygotes, Met66 carriers performed equivalently on episodic memory (d = 0.22; 95% CI, −0.25 to 0.69; P = .09), but significantly worse on global cognition (d = 1.17; 95% CI, 0.65-1.66; P < .001). In symptomatic MCs, hippocampal volume in Met66 carriers was equivalent to that in Val66 homozygotes (d = 0.37; 95% CI, −0.11 to 0.83; P = .13).
Table 2. Association of Clinical Group and EYO With Cognitive Outcomes, Site-Specific Tau Phosphorylation Occupancies and Mass Spectrometry Total Tau Levels.
| F a , b | Estimated marginal mean (SE) | |||||||
|---|---|---|---|---|---|---|---|---|
| Group | EYO | Group × EYO | NC | pMC Val66 | pMC Met66 | sMC Val66 | sMC Met66 | |
| Episodic memory | 80.0134,357 | 18.5821,357 | 3.2054,357 | −0.048 (0.066) | −0.098 (0.087) | −0.639 (0.119) | −1.381 (0.240) | −1.799 (0.419) |
| Global cognition | 72.6304,360 | 10.1341,360 | 7.1034,360 | −0.132 (0.147) | −0.214 (0.179) | −0.427 (0.240) | −2.458 (0.271) | −4.599 (0.346) |
| Hippocampal volume | 47.9424,341 | 22.6611,341 | 5.2864,341 | 8.84 (0.069) | 8.89 (0.091) | 8.50 (0.126) | 8.73 (0.268) | 7.91 (0.482) |
| p-Tau217/tau217 (%) | 150.0824,362 | 38.6841,362 | 17.5344,362 | 1.29 (0.192) | 3.59 (0.255) | 5.22 (0.347) | 5.70 (0.695) | 8.59 (1.212) |
| p-Tau181/tau181 (%) | 88.2954,362 | 25.9211,362 | 10.5014,362 | 21.80 (0.446) | 26.40 (0.592) | 30.30 (0.808) | 31.50 (1.617) | 32.40 (2.818) |
| p-Tau205/tau205 (%) | 137.8374,362 | 34.6941,362 | 14.4194,362 | 0.347 (0.017) | 0.521 (0.022) | 0.509 (0.030) | 0.781 (0.061) | 1.242 (0.107) |
| MS total tau (tau181), ng/mL | 49.7834,362 | 20.7031,362 | 4.5124,362 | 0.407 (0.020) | 0.495 (0.026) | 0.607 (0.035) | 0.575 (0.071) | 1.002 (0.123) |
Abbreviations: EYO, estimated year of symptom onset; NC, mutation noncarriers; pMC, presymptomatic (CDR 0) mutation carrier; p-tau, phosphorylated tau; sMC, symptomatic (CDR ≥0.5) mutation carrier; MS, mass spectrometry.
P value for all <.001.
APOE and sex were included in all statistical models.
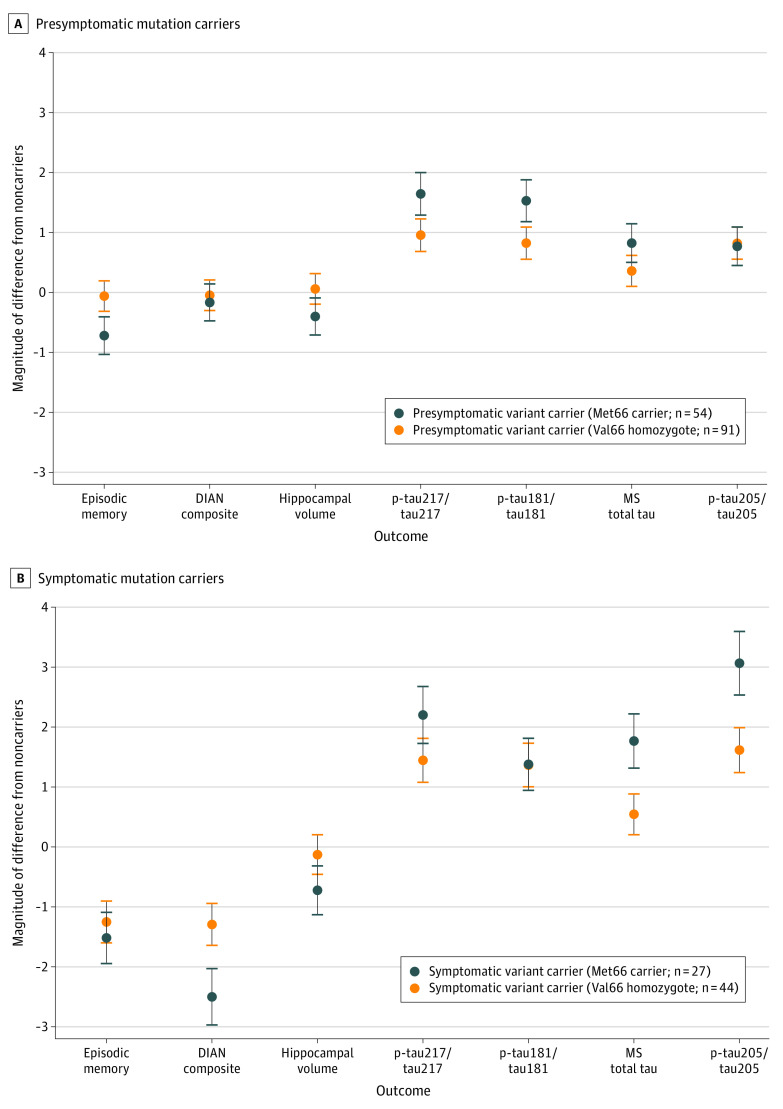
Figure 1. Magnitude of Difference on Each Cognitive and Tau Outcome and Hippocampal Volume Between Mutation Noncarriers and Presymptomatic and Symptomatic Mutation Carriers.
A, Magnitude of difference from noncarriers (at 0) with 95% CI for mutation carriers who were presymptomatic, as defined by Clinical Dementia Rating (CDR) global score of 0. B, Magnitude of difference from noncarriers (at 0) with 95% CI for mutation carriers who were symptomatic (CDR global score ≥0.5). DIAN indicates Dominantly Inherited Alzheimer Network; MS, mass spectrometry; p-tau, phosphorylated tau.
Characterization of BDNF Val66Met on Site-Specific Tau Phosphorylation
Both presymptomatic (Figure 1A) and symptomatic (Figure 1B) mutation carriers had higher levels of all tau phosphorylation occupancies and total tau compared with mutation noncarriers, irrespective of BDNF subgroup (Table 2).
In presymptomatic mutation carriers, compared with Val66 homozygotes, Met66 carriers showed significantly higher p-tau217/tau217 (d = 0.64; 95% CI, 0.30-0.97; P < .001), p-tau181/tau181 (d = 0.65; 95% CI, 0.32-0.99; P < .001), and mass spectrometry total tau level (d = 0.43; 95% CI, 0.10-0.76; P = .01), with the magnitude of differences between BDNF groups, by convention, moderate (Figure 1A). No difference between Met66 carriers and Val66 homozygotes were observed for p-tau205/tau205 (d = 0.05; 95% CI, −0.27 to 0.38; P = .99).
In symptomatic mutation carriers, compared with Val66 homozygotes, Met66 carriers showed significantly higher p-tau217/tau217 (d = 0.53; 95% CI, 0.05-1.01; P = .03), mass spectrometry total tau level (d = 0.78; 95% CI, 0.28-1.25; P = .002), and p-tau205/tau205 (d = 0.97; 95% CI, 0.46-1.45; P < .001), with the magnitude of these differences, by convention, moderate to large (Figure 1B). Met66 carriers and Val66 homozygotes had elevated but equivalent p-tau181/tau181 ratio (d = 0.07; 95% CI, −0.40 to 0.54; P = .99).
All analyses were repeated with PiB-PET SUVR as a covariate, and the magnitude of difference observed between groups remained similar (eTable 3 in Supplement 1). Similarly, restricting the sample to only PS1 mutation carriers did not alter the magnitude of differences observed between groups (eTable 4 in Supplement 1).
Associations of BDNF With EYO, Disease Stage, Cognition, and Site-Specific Tau Phosphorylation
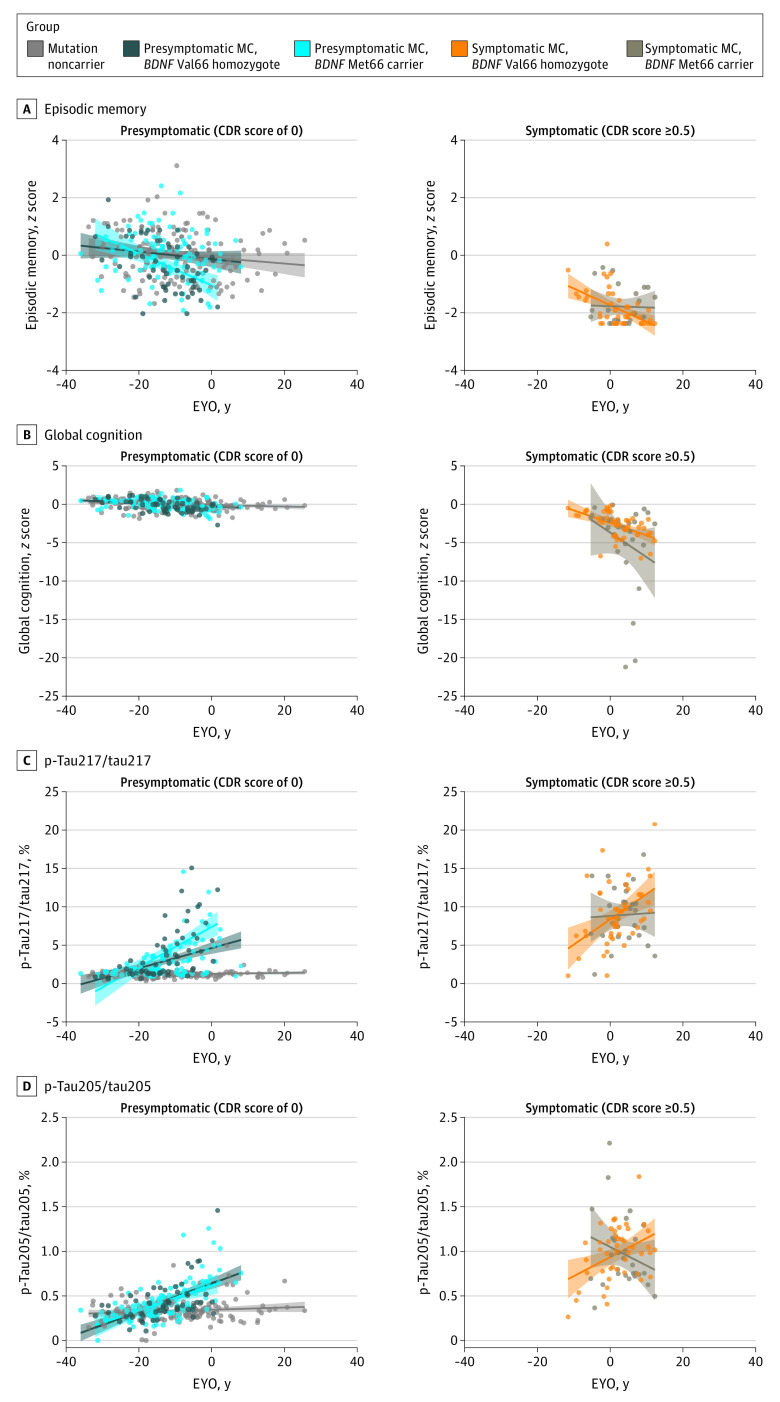
Figure 2 illustrates the association between EYO and cognition, and between EYO and each tau site-specific phosphorylation occupancy in each group. Table 3 summarizes the nature and strength of association between EYO and cognition, and between EYO and each site phosphorylation occupancy in each group. No significant associations between EYO and any measure of cognition or site-specific tau phosphorylation were observed in mutation noncarriers (Figure 2 and Table 3).
Figure 2. Associations Between Estimated Year of Expected Symptom Onset (EYO) and Cognitive Outcome and Tau Phosphorylation Occupancy.
The cognitive outcomes shown are episodic memory (A) and global cognition (B). Tau phosphorylation occupancy (C and D) is shown at threonine 217 (p-tau181/tau181) and threonine 205 (p-tau205/tau205). Shading represents 95% CI. CDR indicates Clinical Dementia Rating; MC, mutation carrier; p-tau, phosphorylated tau.
Table 3. Strength of Associations Between EYO and Main Cognitive and Tau Outcomes in Each Groupa.
| NC | pMC Val66 | pMC Met66 | sMC Val66 | sMC Met66 | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| β (SE) | P value | β (SE) | P value | β (SE) | P value | β (SE) | P value | β (SE) | P value | |
| Episodic memory | −0.108 (0.055) | .05 | −0.156 (0.091) | .10 | −0.580 (0.134) | <.001 | −0.478 (0.211) | .02 | −0.053 (0.342) | .88 |
| Global cognition | −0.043 (0.056) | .45 | −0.147 (0.094) | .12 | −0.240 (0.138) | .08 | −0.726 (0.217) | <.001 | −1.584 (0.344) | <.001 |
| p-Tau217/tau217 (%) | 0.012 (0.045) | .79 | 0.410 (0.076) | <.001 | 0.770 (0.111) | <.001 | 0.945 (0.175) | <.001 | 0.105 (0.278) | .71 |
| p-Tau205/tau205 (%) | 0.039 (0.046) | .41 | 0.570 (0.078) | <.001 | 0.469 (0.115) | <.001 | 0.659 (0.180) | <.001 | −0.731 (0.286) | .01 |
Abbreviations: CDR, Clinical Dementia Rating; EYO, estimated year of symptom onset; NC, mutation noncarrier; pMC, presymptomatic (CDR 0) mutation carrier; p-tau, phosphorylated tau; sMC, symptomatic (CDR ≥0.5) mutation carrier.
All β estimates have been standardized.
In presymptomatic mutation carriers, compared with Val66 homozygotes, Met66 carriers showed stronger associations between EYO and episodic memory (β [SE], −0.394 [0.163]; P = .02) than between EYO and general cognition (β [SE], −0.093 [0.167]; P = .58). Similarly, in presymptomatic mutation carriers, compared with Val66 homozygotes, Met66 carriers showed stronger associations between EYO and p-tau217/tau217 (β [SE], 0.360 [0.135]; P = .008) but equivalent associations between EYO and p-tau205/tau205 (β [SE], −0.100 [0.139]; P = .47).
When compared with symptomatic Val66 homozygotes, symptomatic Met66 carriers showed stronger associations between EYO and general cognition (β [SE], −0.858 [0.406]; P = .04) but not between EYO and episodic memory (β [SE], 0.426 [0.402]; P = .29). Associations between EYO and p-tau217/tau217 (β [SE], −0.840 [0.328]; P = .01) and EYO and p-tau205/tau205 (β [SE], −1.389 [0.338]; P < .001) were significantly different between symptomatic Val66 homozygotes and Met66 carriers (Figure 2).
To further challenge these results, an exploratory analysis was conducted to determine if the associations of BDNF Val66Met with p-tau explained differences in associations between EYO and cognition. Given the exploratory nature of this analysis, only associations between EYO and episodic memory were examined in presymptomatic mutation carriers, and EYO and global cognition were examined in symptomatic mutation carriers.
After accounting for p-tau217/tau217 levels, the association between EYO and episodic memory for presymptomatic Met66 carriers did not differ significantly from mutation noncarriers (β [SE], −0.252 [0.148]; P = .09). However, after accounting for p-tau205/tau205, the association between EYO and global cognition for symptomatic Met66 carriers remained significantly different from symptomatic Val66 homozygotes (β [SE], −0.954 [0.415]; P = .02).
Characterization of BDNF Val66Met on Cognitive and Tau Outcomes in Preclinical Sporadic AD
In the preclinical sporadic AD group, after accounting for the associations with age, sex, and ε4, and compared with Val66 homozygotes, Met66 carriers showed poorer episodic memory (d = 0.39; 95% CI, 0.00-0.77; P = .048), higher levels of CSF total tau (d = 0.45; 95% CI, 0.07-0.84; P = .02), and higher levels of CSF p-tau181 (d = 0.46; 95% CI, 0.07-0.85; P = .02) (eTable 2 in Supplement 1).
Discussion
In DIAD, both clinical disease stage and the BDNF Met66 allele moderated the extent to which levels of site-specific tau phosphorylation were increased, hippocampal volume was decreased, and cognition impaired. In presymptomatic mutation carriers, clinically important memory impairment (ie, performance <1 SD below mutation noncarriers) and smaller hippocampal volume were observed only in Met66 carriers (Figure 1A). There was no impairment in general cognition in Val66 homozygotes or Met66 carriers. In presymptomatic mutation carriers, site-specific tau isoforms were increased relative to mutation noncarriers. However, within presymptomatic mutation carriers, p-tau217 and p-tau181 phosphorylation were substantially greater in Met66 carriers than Val66 homozygotes, while total tau level and p-tau205 phosphorylation were equivalent between these groups. Thus, despite being classified clinically as presymptomatic, DIAD mutation carriers who also carry Met66 showed clinically important episodic memory impairment, lower hippocampal volume, and moderate elevation in p-tau181 and p-tau217 phosphorylation. Further, the results suggest that the episodic memory impairment observed in Met66 carriers may be due to the associations of BDNF Val66Met with p-tau217 and p-tau181. While total tau level and p-tau205 phosphorylation were also increased in presymptomatic mutation carriers relative to mutation noncarriers, these changes were unrelated to Met66 carriage.
In symptomatic DIAD mutation carriers, the associations of BDNF with cognition and site-specific tau phosphorylation occupancy changed. As expected, symptomatic mutation carriers showed a large impairment in general cognition and episodic memory (Figure 1B), although only symptomatic Met66 carriers showed smaller hippocampal volume (Figure 1B). While Met66 was not associated with the magnitude of memory impairment, symptomatic Met66 carriers showed greater impairment in general cognition than Val66 homozygotes. Compared with noncarriers, all site-specific tau phosphorylation occupancies were increased in symptomatic mutation carriers. However, within symptomatic mutation carriers, only total tau level and p-tau205 phosphorylation were substantially greater in Met66 carriers than in Val66 homozygotes (Figure 1B). Levels of p-tau217 and p-tau181 were equivalent between these BDNF groups. In addition, in symptomatic mutation carriers, associations between BDNF and p-tau205 did not wholly explain associations between EYO and general cognition, suggesting that other disease processes may influence cognitive outcomes in symptomatic DIAD.
These data reinforce earlier clinical and biomarker findings that BDNF Val66Met moderated the downstream association of Aβ with AD clinical disease progression.6,27,28,29 The absence of any impairment in memory or general cognition in presymptomatic DIAD mutation carriers who are Val66 homozygotes suggests these individuals have some resilience to AD, potentially through higher BDNF levels acting to minimize hyperphosphorylation of tau or its downstream consequences on neuronal and synaptic loss. As presymptomatic DIAD mutation carriers progress to symptomatic stages and neurodegeneration becomes more widespread,30 impairment in memory increases to clinically important levels in those who are Val66 homozygotes. In symptomatic disease, cognitive impairment becomes generalized, though still greater in magnitude in Met66 carriers than in Val66 homozygotes. This increased cognitive impairment in symptomatic Met66 carriers is also reflected in tau levels, with higher total tau level and p-tau205 hyperphosphorylation, but not in p-tau217 or p-tau181.
We have observed previously that increases in CSF p-tau181 and total tau, measured using immunoassay, in DIAD mutation carriers slow as neurodegeneration increases.30,31 The absence of any significant associations between EYO and p-tau217 and total tau observed here in symptomatic Met66 carriers is consistent with these observations. Thus, for any EYO, disease progression is greater in Met66 carriers than in Val66 homozygotes. This hypothesis is supported further by the observation that symptomatic Met66 carriers showed greater impairment in global cognition, but not in episodic memory, compared with Val66 homozygotes (Figure 1). In symptomatic mutation carrier Val66 homozygotes, a higher EYO was associated with greater impairment in episodic memory, while in Met66 carriers, memory impairment did not increase further. This observation is consistent with another in sporadic AD dementia, where Aβ-positive Val66 homozygotes showed memory decline over 126 months while memory performance remained impaired but stable in matched Aβ-positive Met66 carriers.29 These data suggest that resilience afforded by BDNF Val66 homozygosity in early disease is reduced as AD advances into the dementia stages. The data also suggest that BDNF influences amyloid-tau associations in DIAD and sporadic AD similarly. This hypothesis is supported by the current observation that in older adults with preclinical sporadic AD, Met66 carriers showed poorer episodic memory and higher CSF total tau and p-tau181 levels compared with matched Val66 homozygotes (eTable 2 in Supplement 1).
The current results also inform clinicopathological models of AD that link Aβ accumulation, tau hyperphosphorylation, neuronal death, and cognitive impairment. The current findings that p-tau181 and p-tau217 are elevated substantially in presymptomatic DIAD mutation carriers who also carry the Met66 allele, and in preclinical sporadic AD Met66 carriers, suggest that the reduced neurotrophic support for neurons, through BDNF Met66 carriage, may foster faster rates of neuronal death in early disease. In presymptomatic DIAD, the relatively specific impairment in episodic memory associated with higher p-tau181 and p-tau217 hyperphosphorylation in these individuals suggests that neuronal death is centered on medial temporal lobe areas. This is consistent with previous observations, where presymptomatic DIAD and preclinical sporadic AD Met66 carriers showed greater rates of hippocampal volume loss and reduction in hippocampal connectivity when compared with Val66 homozygotes.6,7,8 Once p-tau181 and p-tau217 reach their maximal hyperphosphorylation state in symptomatic DIAD, the degree of neurotrophic support afforded by Val66 homozygosity decreases. This is reflected in higher levels of total tau and p-tau205 as well as the greater general atrophy and general cognitive impairments that are characteristic of this disease stage. These data suggest that neuronal decline in early DIAD and sporadic AD may be mitigated, at least temporarily, by pharmacological strategies designed to increase neurotrophic support.32,33
Limitations
This study is limited by its cross-sectional design. Given the complete penetrance of DIAD, we expect that presymptomatic mutation carrier Val66 homozygotes will show higher p-tau181 and p-tau217, albeit later than Met66 carriers. Similarly, while these data suggest that despite equivalent levels of Aβ42, Met66 carriers demonstrate more advanced clinical symptoms compared with Val66 homozygotes, presymptomatic DIAD mutation carriers need to be followed up over longer periods of time to determine whether rates of progression from presymptomatic to symptomatic are higher in Met66 carriers relative to Val66 homozygotes. Notwithstanding these issues, the results of this study suggest strongly that BDNF Val66Met is an important moderator of clinical and tau outcomes in DIAD.
Conclusions
In DIAD, clinical disease stage and the BDNF Met66 allele were associated with moderate cognitive impairment and levels of site-specific tau phosphorylation. This suggests that pharmacological strategies designed to increase neurotrophic support in the presymptomatic stages of AD may be beneficial.
eMethods
eTable 1. Demographic, Clinical, and Biological Characteristics of the ADNI Preclinical Sporadic AD Sample
eTable 2. Effect of BDNF Val66Met on Baseline Episodic Memory, and Levels of CSF Total Tau and CSF p-Tau181 in Preclinical Sporadic AD
eTable 3. Effect of Clinical Group and EYO on Cognitive Outcomes, Site-Specific Tau Phosphorylation Occupancies, and MS Total Tau levels
eTable 4. Effect of Clinical Group and EYO on Each Cognitive and Biomarker Outcome in PS1 Mutation Carriers
Nonauthor Collaborators. Dominantly Inherited Alzheimer Network Investigators and Coordinators
References
- 1.Bateman RJ, Xiong C, Benzinger TLS, et al. ; Dominantly Inherited Alzheimer Network . Clinical and biomarker changes in dominantly inherited Alzheimer’s disease. N Engl J Med. 2012;367(9):795-804. doi: 10.1056/NEJMoa1202753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ryman DC, Acosta-Baena N, Aisen PS, et al. ; Dominantly Inherited Alzheimer Network . Symptom onset in autosomal dominant Alzheimer disease: a systematic review and meta-analysis. Neurology. 2014;83(3):253-260. doi: 10.1212/WNL.0000000000000596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chang CW, Shao E, Mucke L. Tau: enabler of diverse brain disorders and target of rapidly evolving therapeutic strategies. Science. 2021;371(6532):eabb8255. doi: 10.1126/science.abb8255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fahnestock M. Brain-derived neurotrophic factor: the link between amyloid-β and memory loss. Future Neurol. 2011;6:627-639. doi: 10.2217/fnl.11.44 [DOI] [Google Scholar]
- 5.Rosa E, Mahendram S, Ke YD, Ittner LM, Ginsberg SD, Fahnestock M. Tau downregulates BDNF expression in animal and cellular models of Alzheimer’s disease. Neurobiol Aging. 2016;48:135-142. doi: 10.1016/j.neurobiolaging.2016.08.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lim YY, Hassenstab J, Goate A, et al. ; Dominantly Inherited Alzheimer Network . Effect of BDNFVal66Met on disease markers in dominantly inherited Alzheimer’s disease. Ann Neurol. 2018;84(3):424-435. doi: 10.1002/ana.25299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Franzmeier N, Ren J, Damm A, et al. The BDNFVal66Met SNP modulates the association between beta-amyloid and hippocampal disconnection in Alzheimer’s disease. Mol Psychiatry. 2021;26(2):614-628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lim YY, Villemagne VL, Laws SM, et al. ; Australian Imaging, Biomarkers and Lifestyle (AIBL) Research Group . BDNF Val66Met, Aβ amyloid, and cognitive decline in preclinical Alzheimer’s disease. Neurobiol Aging. 2013;34(11):2457-2464. doi: 10.1016/j.neurobiolaging.2013.05.006 [DOI] [PubMed] [Google Scholar]
- 9.Gordon BA, Blazey TM, Christensen J, et al. Tau PET in autosomal dominant Alzheimer’s disease: relationship with cognition, dementia and other biomarkers. Brain. 2019;142(4):1063-1076. doi: 10.1093/brain/awz019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mattsson-Carlgren N, Andersson E, Janelidze S, et al. Aβ deposition is associated with increases in soluble and phosphorylated tau that precede a positive tau PET in Alzheimer’s disease. Sci Adv. 2020;6(16):eaaz2387. doi: 10.1126/sciadv.aaz2387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Barthélemy NR, Li Y, Joseph-Mathurin N, et al. ; Dominantly Inherited Alzheimer Network . A soluble phosphorylated tau signature links tau, amyloid and the evolution of stages of dominantly inherited Alzheimer’s disease. Nat Med. 2020;26(3):398-407. doi: 10.1038/s41591-020-0781-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gordon BA, Blazey TM, Su Y, et al. Spatial patterns of neuroimaging biomarker change in individuals from families with autosomal dominant Alzheimer’s disease: a longitudinal study. Lancet Neurol. 2018;17(3):241-250. doi: 10.1016/S1474-4422(18)30028-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Berg L, Miller JP, Storandt M, et al. Mild senile dementia of the Alzheimer type: 2. Longitudinal assessment. Ann Neurol. 1988;23(5):477-484. doi: 10.1002/ana.410230509 [DOI] [PubMed] [Google Scholar]
- 14.Morris JC. The Clinical Dementia Rating (CDR): current version and scoring rules. Neurology. 1993;43(11):2412-2414. doi: 10.1212/WNL.43.11.2412-a [DOI] [PubMed] [Google Scholar]
- 15.Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”: a practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12(3):189-198. doi: 10.1016/0022-3956(75)90026-6 [DOI] [PubMed] [Google Scholar]
- 16.Burke WJ, Roccaforte WH, Wengel SP. The short form of the Geriatric Depression Scale: a comparison with the 30-item form. J Geriatr Psychiatry Neurol. 1991;4(3):173-178. doi: 10.1177/089198879100400310 [DOI] [PubMed] [Google Scholar]
- 17.Storandt M, Balota DA, Aschenbrenner AJ, Morris JC. Clinical and psychological characteristics of the initial cohort of the Dominantly Inherited Alzheimer Network (DIAN). Neuropsychology. 2014;28(1):19-29. doi: 10.1037/neu0000030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lim YY, Hassenstab J, Cruchaga C, et al. ; Dominantly Inherited Alzheimer Network . BDNF Val66Met moderates memory impairment, hippocampal function and tau in preclinical autosomal dominant Alzheimer’s disease. Brain. 2016;139(pt 10):2766-2777. doi: 10.1093/brain/aww200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Crane PK, Carle A, Gibbons LE, et al. Development and assessment of a composite score for memory in the Alzheimer's Disease Neuroimaging Initiative (ADNI). Brain Imaging Behav. 2012;6(4):502-516. doi: 10.1007/s11682-012-9186-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Barthélemy NR, Fenaille F, Hirtz C, et al. Tau protein quantification in human cerebrospinal fluid by targeted mass spectrometry at high sequence coverage provides insights into its primary structure heterogeneity. J Proteome Res. 2016;15(2):667-676. doi: 10.1021/acs.jproteome.5b01001 [DOI] [PubMed] [Google Scholar]
- 21.Talbot C, Lendon C, Craddock N, Shears S, Morris JC, Goate A. Protection against Alzheimer’s disease with apoE epsilon 2. Lancet. 1994;343(8910):1432-1433. doi: 10.1016/S0140-6736(94)92557-7 [DOI] [PubMed] [Google Scholar]
- 22.Benzinger TL, Blazey T, Jack CR Jr, et al. Regional variability of imaging biomarkers in autosomal dominant Alzheimer’s disease. Proc Natl Acad Sci U S A. 2013;110(47):E4502-E4509. doi: 10.1073/pnas.1317918110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Su Y, Blazey TM, Snyder AZ, et al. ; Dominantly Inherited Alzheimer Network . Partial volume correction in quantitative amyloid imaging. Neuroimage. 2015;107:55-64. doi: 10.1016/j.neuroimage.2014.11.058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ramanan VK, Castillo AM, Knopman DS, et al. Association of apolipoprotein e ɛ4, educational level, and sex with tau deposition and tau-mediated metabolic dysfunction in older adults. JAMA Netw Open. 2019;2(10):e1913909. doi: 10.1001/jamanetworkopen.2019.13909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Buckley RF, Mormino EC, Rabin JS, et al. Sex differences in the association of global amyloid and regional tau deposition measured by positron emission tomography in clinically normal older adults. JAMA Neurol. 2019;76(5):542-551. doi: 10.1001/jamaneurol.2018.4693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cohen J. Statistical Power Analysis for the Behavioral Sciences. 2nd ed. Lawrence Erlbaum Associates; 1988. [Google Scholar]
- 27.Lim YY, Villemagne VL, Laws SM, et al. ; Australian Imaging, Biomarkers and Lifestyle (AIBL) Research Group . BDNF Val66Met, Aβ amyloid, and cognitive decline in preclinical Alzheimer’s disease. Neurobiol Aging. 2013;34(11):2457-2464. doi: 10.1016/j.neurobiolaging.2013.05.006 [DOI] [PubMed] [Google Scholar]
- 28.Lim YY, Villemagne VL, Laws SM, et al. ; AIBL Research Group . Effect of BDNF Val66Met on memory decline and hippocampal atrophy in prodromal Alzheimer’s disease: a preliminary study. PLoS One. 2014;9(1):e86498. doi: 10.1371/journal.pone.0086498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lim YY, Laws SM, Perin S, et al. ; AIBL Research Group . BDNF VAL66MET polymorphism and memory decline across the spectrum of Alzheimer’s disease. Genes Brain Behav. 2021;20(5):e12724. doi: 10.1111/gbb.12724 [DOI] [PubMed] [Google Scholar]
- 30.McDade E, Wang G, Gordon BA, et al. ; Dominantly Inherited Alzheimer Network . Longitudinal cognitive and biomarker changes in dominantly inherited Alzheimer disease. Neurology. 2018;91(14):e1295-e1306. doi: 10.1212/WNL.0000000000006277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fagan AM, Xiong C, Jasielec MS, et al. ; Dominantly Inherited Alzheimer Network . Longitudinal change in CSF biomarkers in autosomal-dominant Alzheimer’s disease. Sci Transl Med. 2014;6(226):226ra30. doi: 10.1126/scitranslmed.3007901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lu B, Nagappan G, Guan X, Nathan PJ, Wren P. BDNF-based synaptic repair as a disease-modifying strategy for neurodegenerative diseases. Nat Rev Neurosci. 2013;14(6):401-416. doi: 10.1038/nrn3505 [DOI] [PubMed] [Google Scholar]
- 33.Wang S, Yao H, Xu Y, et al. Therapeutic potential of a TrkB agonistic antibody for Alzheimer’s disease. Theranostics. 2020;10(15):6854-6874. doi: 10.7150/thno.44165 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eMethods
eTable 1. Demographic, Clinical, and Biological Characteristics of the ADNI Preclinical Sporadic AD Sample
eTable 2. Effect of BDNF Val66Met on Baseline Episodic Memory, and Levels of CSF Total Tau and CSF p-Tau181 in Preclinical Sporadic AD
eTable 3. Effect of Clinical Group and EYO on Cognitive Outcomes, Site-Specific Tau Phosphorylation Occupancies, and MS Total Tau levels
eTable 4. Effect of Clinical Group and EYO on Each Cognitive and Biomarker Outcome in PS1 Mutation Carriers
Nonauthor Collaborators. Dominantly Inherited Alzheimer Network Investigators and Coordinators
Data Availability Statement
Data and biospecimens from DIAN can be accessed by a formal application to the DIAN Steering Committee. Data are systematically released after a 1-year delay to allow DIAN investigators the opportunity to publish main study findings. Data from ADNI are publicly accessible via http://adni.loni.usc.edu.