Key Points
Question
Is open-label placebo effective in the treatment of disorders of gut-brain interactions (eg, functional abdominal pain and irritable bowel syndrome) in children and adolescents?
Findings
In this randomized clinical trial that included 30 children or adolescents with functional abdominal pain or irritable bowel syndrome, the mean pain scores were significantly lower during the open-label placebo period compared with the control period.
Meaning
The findings suggest that open-label placebo may be used to reduce pain and decrease the use of rescue medications for children and adolescents with functional abdominal pain or irritable bowel syndrome.
Abstract
Importance
Although it is widely believed that concealment or deception is required to elicit a placebo response, recent studies with adults suggest that open-label placebo (OLP) (ie, honestly prescribed placebos) can yield significant benefits. No studies of OLP have been performed with children.
Objective
To evaluate the efficacy of OLP for the treatment of children and adolescents with functional abdominal pain or irritable bowel syndrome.
Design, Setting, and Participants
This multicenter crossover randomized clinical trial was conducted from July 1, 2015, to June 15, 2018, at 3 US centers among children and adolescents aged 8 to 18 years with functional abdominal pain or irritable bowel syndrome defined per Rome III criteria. Statistical analysis was performed from March 1, 2019, to September 30, 2020, on an intention-to-treat basis.
Interventions
Patients completed 1 week of observation prior to randomization to 1 of 2 counterbalanced groups: OLP for 3 weeks followed by a 3-week control period or control period for 3 weeks followed by OLP for 3 weeks. During the OLP period, participants took 1.5 mL of an inert liquid placebo twice a day. A standardized method for explaining the OLP was used, and the interaction with clinicians had the same duration and style for both time periods. Hyoscyamine was allowed as a rescue medication.
Main Outcomes and Measures
The primary outcome was the mean daily pain score during each of the interventions, measured on a 0- to 100-mm visual analog scale, where higher scores indicated greater pain. The number of rescue medications taken during each intervention served as an objective secondary measure.
Results
Thirty patients (mean [SD] age, 14.1 [3.4] years; 24 female participants [80.0%]; 16 [53.3%] with functional abdominal pain and 14 [46.7%] with irritable bowel syndrome) completed the study. The mean (SD) pain scores were significantly lower during OLP treatment compared with the control period (39.9 [18.9] vs 45.0 [14.7]; difference, 5.2; 95% CI, 0.2-10.1; P = .03). Patients took nearly twice as many hyoscyamine pills during the control period compared with during the OLP period (mean [SD] number, 3.8 [5.1] pills vs 2.0 [3.0] pills; difference, 1.8 pills; 95% CI, 0.5-3.1 pills).
Conclusions and Relevance
During OLP, patients with functional abdominal pain or irritable bowel syndrome reported significantly less pain and took significantly fewer pain medications. Open-label placebo may be an effective treatment for children and adolescents with functional abdominal pain or irritable bowel syndrome.
Trial Registration
ClinicalTrials.gov Identifier: NCT02389998
This randomized clinical trial evaluates the efficacy of open-label placebo for the treatment of children and adolescents with functional abdominal pain or irritable bowel syndrome.
Introduction
Children with pain-predominant disorders of gut-brain interaction (DGBI) can have severely disabling symptoms, resulting in poor quality of life, high use of health care resources, and social isolation.1,2,3 The optimum therapy for DGBIs has not been established.1,2 One of the major difficulties to identifying effective treatments is the high placebo response rate that has been observed in this population.1,3 However, the high placebo response rate also presents opportunities for clinical practice. It has been suggested that, instead of mitigating the placebo response, an alternative approach may be to harness it.4
Although placebo responses probably play a role in virtually every clinical intervention, the use of deceptive placebos as stand-alone treatments has largely been discouraged because of ethical concerns about patient deception.4,5,6 Until recently, it has been widely believed that patient blinding (via deception or concealment) is required to elicit placebo effects, but recent studies with adults suggest that the open-label placebo honestly prescribed treatment can yield positive effects, including in patients with DGBI.7,8 No similar studies have been performed for children, to our knowledge. The aim of the present study was to assess the efficacy of open-label placebo (OLP) in the treatment of children and adolescents with DGBIs.
Methods
Study Design
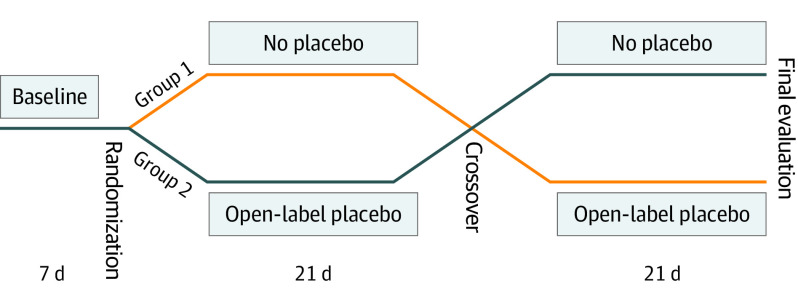
A multicenter prospective crossover randomized clinical trial of OLP vs no treatment control for children with functional abdominal pain or irritable bowel syndrome was conducted at 3 centers from July 1, 2015, to June 15, 2018: Boston Children’s Hospital, Boston, Massachusetts; Nationwide Children’s Hospital, Columbus, Ohio; and Children’s Mercy Hospital, Kansas City, Missouri. Written informed consent was obtained from parents and written assent from all children. The protocol was approved by the institutional review boards of Boston Children’s Hospital, Nationwide Children’s Hospital, and Children’s Mercy Hospital (trial protocol in Supplement 1). The study is registered in ClinicalTrials.gov (NCT02389998). The design of the study is shown in Figure 1. This study followed the Consolidated Standards of Reporting Trials (CONSORT) reporting guideline.
Figure 1. Study Design.
Inclusion Criteria
Children 8 to 18 years of age with functional abdominal pain or irritable bowel syndrome defined per Rome III criteria9 were included. All had normal laboratory test reults, negative lactose breath test results, or lack of response to a lactose-free diet for 14 days. Patients with organic disease were excluded. Patients were allowed to continue other treatments as long as they had been treated with stable regimens for 30 days.
Study Visits
Initial Visit: Observation Period
A standardized method for explaining placebo effects and the gut-brain connection was used (eAppendix 1 in Supplement 2). Participants were introduced to the general concept of placebo, which was described as an inert suspension, like “sugar pills,” without any medication in it, that in blinded randomized clinical trials including adults with DGBI often produces benefit. Patients then started a 7-day observation period in which they recorded their daily pain using a 0- to 100-mm visual analog scale,10 where higher scores indicated greater pain (Figure 1).
Randomization Visit
Patients were randomized in a 1:1 ratio to 1 of the 2 groups if they had a minimum mean daily pain score of 25 mm (out of 100 mm) during the observation week. The code was created by the respective pharmacy of each institution using a computer-generated random number sequence. Until this point, the patient-clinician interactions and the style for both groups were the same. Once the envelope was opened, the instructions and conversation varied according to group assignment because a distinct and well-defined script was then followed for each group (eAppendix 1 in Supplement 2). The characteristics of the interaction with clinicians were identical during both periods of the study.
In group 1, which started with the control period, the participants completed initial questionnaires and were provided with a daily symptom diary and the rescue medication. In group 2, which started with the OLP period, the clinician explained placebos and the nature and concepts related to its use (eAppendix 1 in Supplement 2). Patients took the first dose of placebo in the office. They then received the bottle of rescue medication, the initial questionnaires, and daily diaries.
Crossover Visit
Patients returned after 3 weeks. In group 1 (crossover to OLP), the daily diaries and the bottle of rescue medication were collected. Patients then received the placebo following the same script as outlined. In group 2 (crossover to control), the diaries, rescue medication, and placebo bottles were collected. The rescue medication was given, together with the diaries.
End of Study Visit
Patients returned 3 weeks after crossover. Final questionnaires and assessments were performed. They returned their unused rescue medications, diaries, and placebo.
Placebo, Rescue Medication, Daily Diary, Blinding, and Compliance
The placebo mimicked the appearance of medications used in pediatric care. We used an inert suspension (Simple Syrup; HUMCO) containing 85% sucrose, citric acid, purified water, and methyl paraben as a preservative. It was prepared by the research pharmacy of each institution. Patients were instructed to take 1.5 mL twice a day, given with a 3-mL plastic syringe.
After randomization, 0.125-mg dissolvable hyoscyamine tablets (Virtus Pharmaceutical) were allowed as an as-needed rescue medication for pain, up to 4 tablets per day. Pain was recorded daily in a specially designed diary that included the visual analog scale and specific questions about well-being, bowel movements, symptoms, adverse events, and use of rescue medication.
Given the nature of the study, patients and physicians were aware of treatment assignment. However, all assessments were performed by a blinded research assistant. Compliance was measured by a count of the remaining tablets of the rescue medication and measurement of the residual placebo suspension.
Outcome Measures
The primary outcome was the mean daily pain score during each intervention. The secondary outcome was the use of rescue medications measured by the number of tablets used, which was assessed by pill count. Global improvement was assessed by the following validated question11: “Overall, how do you feel your problem is (better, same, or worse)?” To assess expectations of success, we asked the following: “How well do you think the treatment will work (excellent, good, fair, poor, or not at all)?”
Additional Psychological and Symptom Measures
We also included the following questionnaires: Questionnaire on Pediatric Gastrointestinal Symptoms: Rome III version,12 Functional Disability Inventory,13 Pediatric Quality of Life Inventory,14 Pediatric Quality of Life Gastrointestinal Symptom Module,15 Pediatric Pain Questionnaire,16 Revised Child Anxiety and Depression Scale,17 and Children’s Depression Inventory 2.18
Statistical Analysis
Statistical analysis was performed from March 1, 2019, to September 30, 2020. Descriptive statistics are expressed as mean (SD) values or percentages. All tests were 2-tailed with results deemed statistically significant at P < .05. With 1 exception (see Results), the analyses were intention to treat. When participants failed to complete daily diaries, the mean for the remaining days was used. For the primary outcome, we compared the mean daily pain scores during the 3-week OLP period with the mean daily pain scores during the 3-week no-treatment control period using a repeated-measures analysis of covariance. We controlled for treatment order as well as mean daily abdominal pain score during the 1-week baseline period by including those variables in the model as covariates. The model also included the interaction between order and period, as well as the interaction between baseline pain and period. Effect sizes were computed using partial η2 (η2p), which is the percentage of variance in the dependent variable that can be accounted for by the independent variable, after controlling for all other variables in the model. By convention, η2p = 0.01 is considered a small effect, η2p = 0.06 is considered medium, and η2p = 0.14 is considered large.19,20
For nonparametric and ordinal variables, we used the Wilcoxon signed rank test. Dichotomous outcomes were analyzed by using the McNemar test. For between-groups comparisons, we used the independent-samples t test or the nonparametric Mann-Whitney test as indicated and either the χ2 test or the Fisher exact test as appropriate.
Power Analysis
We used G*Power 3 software to conduct a power analysis for the primary outcome.21 Assuming a 2-tailed test with results deemed statistically significant at P < .05 and a 0.50 correlation between the mean pain score in the 2 conditions (OLP period vs control period), we calculated that a repeated-measures analysis of variance with a sample size of 30 would provide 99% power to detect large effects (eg, η2p = 0.14) and 75% power to detect medium effects (eg, η2p = 0.06).
Results
Participants
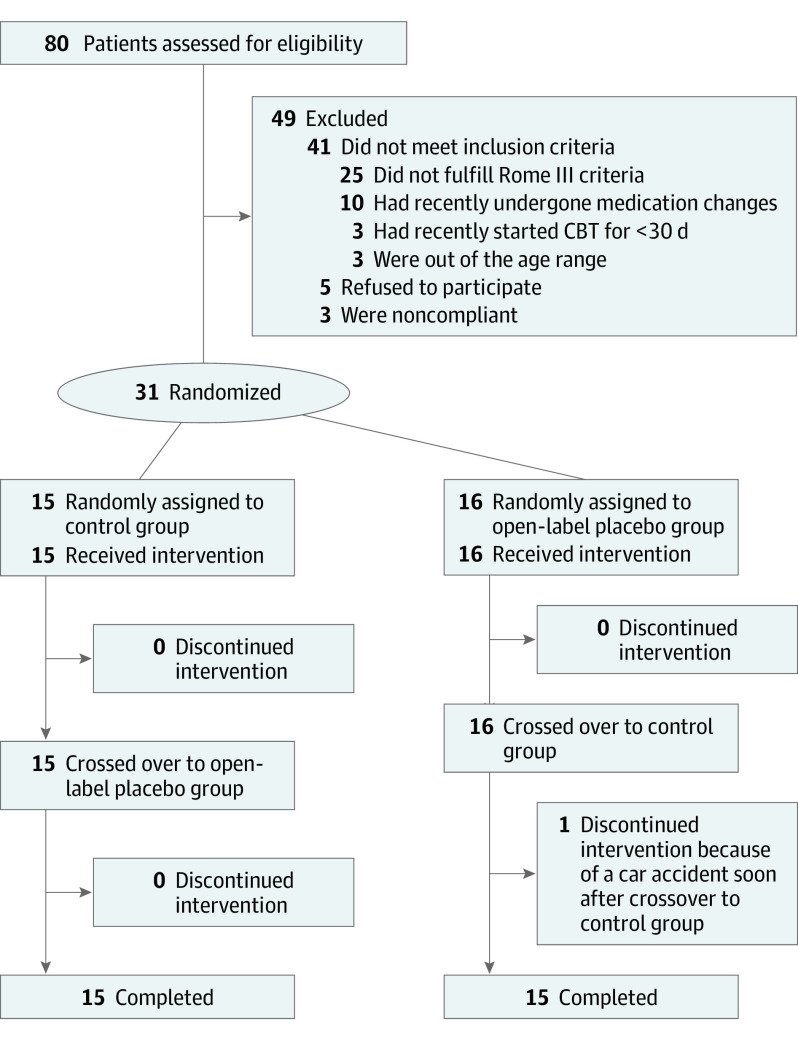
A total of 31 patients were randomized, and 30 patients completed the study (Figure 2). One patient who was initially randomly assigned to group 2 completed the OLP period but withdrew just after starting the control period because of a car accident. Even though the patient showed substantial improvement during the OLP period, we adopted the conservative approach to remove their data from the analysis, given that its inclusion would have improved our results. Therefore, the analysis included only the 30 patients who completed both phases of the study (15 per group). Twenty-five patients were recruited from Boston Children’s Hospital, and 5 patients were recruited from Nationwide Children’s Hospital.
Figure 2. Study Flow and Disposition of the Patients.
CBT indicates cognitive behavioral therapy.
Baseline characteristics are shown in Table 1. There were no clinically meaningful differences between the treatment groups. There were no clinically meaningful differences between patients with functional abdominal pain and those with irritable bowel syndrome, except for the expected differences in bowel movement frequency and laxative use (eTable 1 in Supplement 2).
Table 1. Baseline and Demographic Characteristics of the Patientsa.
| Characteristic | Patients, No. (%) | ||
|---|---|---|---|
| All (N = 30) | Group 1 (n = 15) | Group 2 (n = 15) | |
| Age, mean (SD), y | 14.1 (3.4) | 14.4 (3.5) | 13.8 (3.2) |
| Sex | |||
| Female | 24 (80.0) | 12 (80.0) | 12 (80.0) |
| Male | 6 (20.0) | 3 (20.0) | 3 (20.0) |
| Diagnosis | |||
| Functional abdominal pain | 16 (53.3) | 8 (53.3) | 8 (53.3) |
| Irritable bowel syndrome | 14 (46.7) | 7 (46.7) | 7 (46.7) |
| Duration of symptoms, mean (SD), y | 3.3 (2.0) | 2.5 (0.6) | 4.0 (0.9) |
| Pain intensity at baseline, mean (SD) | 45.8 (2.8) | 47.5 (3.9) | 44.1 (4.1) |
| Moderate or severe disability | 10 (33.3) | 5 (33.3) | 5 (33.3) |
| Anxiety | 13 (43.3) | 5 (33.3) | 8 (53.3) |
| CDI score, mean (SD) | 11.3 (1.3) | 9.6 (1.9) | 13.1 (1.6) |
| CSI score, mean (SD) | |||
| Total | 26.1 (2.7) | 27.5 (5.0) | 24.9 (2.3) |
| Somatization | 13.7 (2.1) | 16.0 (3.9) | 11.6 (1.7) |
| PedsQL score, mean (SD) | 56.7 (1.6) | 57.3 (2.3) | 56.2 (2.04) |
| PedsQL GI score, mean (SD) | 73.9 (1.8) | 76.5 (2.6) | 71.3 (2.3) |
| CBT | 11 (36.7) | 4 (26.7) | 7 (46.7) |
| No. of medications, mean (SD) | 2.2 (2) | 1.9 (0.4) | 4.0 (0.6) |
| Cyproheptadine | 5 (16.7) | 1 (6.7) | 4 (26.7) |
| Antidepressants | 3 (10.0) | 2 (13.3) | 1 (6.7) |
| Laxatives | 9 (30.0) | 3 (20.0) | 6 (40.0) |
| Proton pump inhibitors | 8 (26.7) | 5 (33.3) | 3 (20.0) |
| Probiotics | 6 (20.0) | 2 (13.3) | 4 (26.7) |
| Use of hyoscyamine before the trial | 10 (33.3) | 5 (33.3) | 5 (33.3) |
Abbreviations: CBT, cognitive behavioral therapy; CDI, Children’s Depression Inventory; CSI, Children’s Somatization Inventory; PedsQL, Pediatric Quality of Life Inventory; PedsQL GI, Pediatric Quality of Life Inventory Gastrointestinal Symptom Module.
Results shown for the total and according to randomization order.
Primary Outcome: Mean Pain Scores
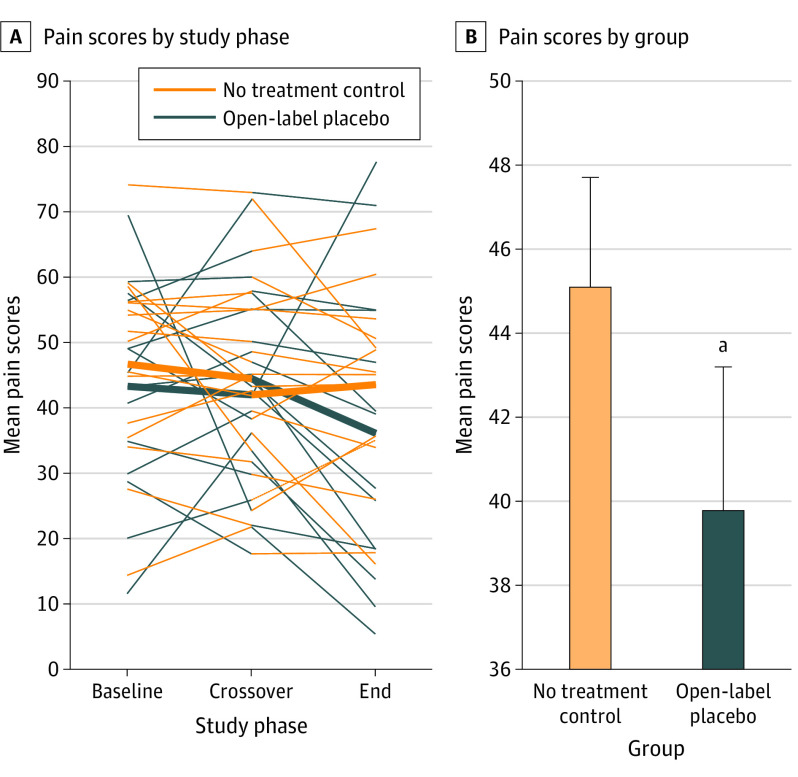
The mean (SD) pain scores are shown in Figure 3. They were significantly lower during the OLP period compared with the control period (39.9 [18.9] vs 45.0 [14.7]; difference, 5.2; 95% CI, 0.2-10.1; P = .03). The standardized effect size was η2p = 0.16.19 In simple terms, 21 of the 30 patients (70.0%) reported higher pain scores during the control period than during the OLP period.
Figure 3. Mean Pain Scores by Treatment Condition.
A, Observed patient-specific profiles (thin lines) and mean trend for each treatment group (thick lines) during the study period. B, Mean pain scores after open-label placebo or no treatment control. Errors bars indicate the standard error of the mean.
aP = .03.
Secondary Outcome: Rescue Medication
Patients took nearly twice as many hyoscyamine tablets during the control perod compared with the OLP period (mean [SD] number, 3.8 [5.1] tablets vs 2.0 [3.0] tablets; difference, 1.8 tablets; 95% CI, 0.5-3.1 tablets; P < .001). In addition, 16 of the 30 patients (53.3%) took more rescue medication during the control period, whereas only 2 (6.7%) took more rescue medication during the OLP period (P = .001). The full data are provided in eTable 2 in Supplement 2.
Secondary Outcome: Global Improvement
Although 14 of the 30 patients (46.7%) reported global improvement during the OLP period vs 9 (30.0%) during the control period, the difference was not significant (Wilcoxon signed rank test z = 1.00; P = .32). The full data are provided in eTable 3 in Supplement 2. In addition, the eFigure in Supplement 2 depicts the overall trajectory throughout both periods of the study.
Other Outcomes
There were no adverse effects associated with the OLP. Overall, 22 of the 30 patients (73.3%) reported that the OLP improved the pain score by more than 30%, and 15 patients (50.0%) reported that the OLP improved the pain score by more than 50%. There were no differences in bowel movement characteristics, and there were significant improvements in functional disability and quality of life (eAppendix 2 in Supplement 2). There were no clinically meaningful differences when comparing patients who responded to the placebo with those who did not (Table 2).
Table 2. Demographic and Baseline Characteristics of Patients Who Responded to Placeboa.
| Characteristic | Response to placebo, No. (%) | |
|---|---|---|
| Yes (n = 14) | No (n = 16) | |
| Age, mean (SD), y | 13.8 (0.9) | 14.3 (0.8) |
| Female | 12 (85.7) | 12 (75.0) |
| Diagnosis | ||
| Functional abdominal pain | 7 (50.0) | 9 (56.3) |
| Irritable bowel syndrome | 7 (50.0) | 7 (43.8) |
| Duration of symptoms, mean (SD), y | 2.4 (0.6) | 3.9 (0.8) |
| Pain intensity at baseline, mean (SD) | 46.8 (3.4) | 44.9 (4.4) |
| FDI score, mean (SD) | 10.9 (2.2) | 8.8 (1.5) |
| Moderate or severe disability | 5 (35.7) | 4 (25.0) |
| Anxiety | 4 (28.6) | 9 (56.3) |
| PedsQL score, mean (SD) | 56.2 (2.7) | 57.2 (2.0) |
| PedsQL GI score, mean (SD) | 72.7 (3.3) | 75.0 (1.8) |
| CDI score, mean (SD) | 11.4 (1.9) | 11.1 (1.8) |
| CSI score, mean (SD) | 25.6 (3.3) | 26.6 (3.9) |
| CBT | 6 (42.9) | 5 (31.3) |
| No. of medications, mean (SD) | 2.6 (0.6) | 3.2 (0.6) |
| Neuromodulation | 3 (21.4) | 5 (31.3) |
| Use of hyoscyamine before the trial | 4 (28.6) | 6 (37.5) |
| Expectation that placebo will work | 13 (92.9) | 10 (62.5) |
Abbreviations: CBT, cognitive behavioral therapy; CDI, Children’s Depression Inventory; CSI, Children’s Somatization Inventory; FDI, Functional Disability Inventory; PedsQL, Pediatric Quality of Life Inventory; PedsQL GI, Pediatric Quality of Life Inventory Gastrointestinal Symptom Module.
There were no clinically meaningful differences when comparing both groups. There was a tendency for those who did not respond to placebo to have more anxiety (P = .07).
Expectations
For children, there was a significantly higher expectation that the OLP period would have a beneficial effect compared with the control period (13 [43%] vs 4 [13%]; P = .045) (eAppendix 2 in Supplement 2). There was no association between the response to the placebo and the initial expectation, as 64% of those (9 of 14) who improved with OLP expected it would be beneficial, while 44% of those (7 of 16) who did not expect an improvement showed improvement (P = .30). Expectations of parents also showed no association with outcomes.
Discussion
To our knowledge, this is the first study to assess the efficacy of nondeceptive, nonconcealed OLP for the treatment of children and adolescents with pain, focusing on patients with DGBIs. Our study suggests that OLP has a beneficial effect. We found a significant reduction in the use of rescue medications as well improvement in daily pain scores during the OLP period. Our study met the criteria for minimum clinically important difference,22,23,24 and improvement in pain intensity is also the main end point recommended by the Rome Foundation Pediatric Subcommittee25,26 and the European and US regulatory agencies.25,26
Information on OLP response in children is limited. To our knowledge, the only other trial of OLP-like treatment for children was conducted for children with attention-deficit/hyperactivity disorder.27 However, that study first exposed the children to daily pairing of amphetamine salts with placebo pills in a conditioning paradigm. Although their design and methods were different from ours, they also showed a clear benefit of OLP.28 The only other trials of OLP for DGBI were 2 studies conducted for adults with irritable bowel syndrome.7,8 In both studies, OLP produced improvement and significantly lower symptom severity. Our findings are also consistent with other studies of OLP for adults with different conditions.4,29
Was the response to OLP a genuine placebo effect or the result of other factors? Our control period controlled for spontaneous improvement, natural fluctuations, and regression to the mean. Furthermore, the patient-physician relationships and all interactions were the same in both groups. Nonetheless, a major unavoidable methodological weakness exists in our trial: physicians, children, and their families were not blinded to treatment allocation. The children may have wanted to please the researchers, and physicians may have unconsciously treated patients differently. We cannot rule out such behaviors, but we suspect they were not very critical in our study; all patients had a history of prior medical failure, and study physicians worked hard to use identical interactions during the 2 phases of the trial. Importantly, all assessments were made in a blinded fashion.
The exact nature of which is the best “placebo” is not clear. We tried to enhance the effect by developing a ritual in which we used medication bottles and a label generated by the pharmacy (similar to a standard prescription), and we chose a dose that required some measurement. We used a suspension to avoid the possibility that some patients would not be able to swallow a pill. Finally, we chose a preparation that had small quantities of sucrose to give some flavor. The administration of the sucrose could have had a deleterious effect, but the amounts were so small that we did not expect an adverse outcome. In fact, we did not see any exacerbation of the pain after the OLP period.
Another noteworthy aspect is that most previous studies have compared OLP with no treatment.29 Because of ethical constraints not to leave children with DGBI without any therapy for 7 weeks, both groups were allowed to receive an antispasmodic medication as rescue therapy. To our knowledge, there are no clinical trials of antispasmodic therapy for children with DGBI, but 2 open small studies of adults have shown some benefit,30,31 while the only randomized clinical trial of hyoscyamine showed no benefit.32 The fact that the medications were used as rescue therapy and initiated by the individual patient mitigated the response that we could have seen. We found no difference in bowel movement characteristics between the OLP period and the control period, suggesting that improvements observed were associated with changes in pain modulation.4,5,23,24
We were unable to identify any factors associated with the success of the placebo. We also found no association between outcomes and the expectation of the response to the placebo from participants and their parents.4 Although positive baseline expectations are generally associated with placebo responses in acute laboratory experiments among healthy volunteers, for individuals with chronic pain conditions, positive baseline expectations are not consistently associated with placebo responses.4 Another potential factor is the effect of nonconscious expectations, as it has been shown that external influences may have an association with placebo effects.4 That parents in our study expected placebos to “work” confirms previous observations,28,33,34 and it has been argued that some pediatric placebo effects are due to parents’ expectations (placebo effect by proxy).35,36 Given that expectations were not associated with the response to the placebo, this possibility seems less likely. Our findings suggest that the ethical use of OLP may be acceptable to families.
Strengths and Limitations
Our study has several strengths. Importantly, the crossover design allowed each patient to serve as his or her own control, which substantially increased statistical power. Additional strengths include (1) careful attention to scripting the clinical interactions, (2) our primary outcome measure was assessed daily throughout the study, and (3) our study included both a subjective self-report outcome measure (ie, daily pain) and a more objective outcome measure (ie, use of rescue medication). This is particularly important because, to our knowledge, this is one of the very few OLP randomized clinical trials to include an objective outcome measure.
Our study has several limitations. First, crossover designs may result in carryover effects, which can be minimized by including a washout period. However, given that we were not administering an active treatment, we chose not to include one. Therefore, any carryover effects were likely to work against our hypothesis. Second, in studies of OLP, it is not possible to blind participants and clinicians to treatment; therefore, we cannot rule out the possibility that this resulted in some bias in the outcome assessments. Therefore, we designed the study so that all assessments were done blindly. Third, it is not clear whether our results can be applied to the general population of children with DGBI. We enrolled children with moderate to severe pain, and there may be some selection bias in those who decided to enroll in our trial. However, this limitation applies to all clinical trials—they can be generalized only to patients willing to be treated with the intervention. A fourth limitation is the short follow-up, which did not allow us to determine whether the observed effects will have a long-term impact.37
Conclusions
The findings of our study could have important implications. To our knowledge, this is the first study of children that provides further support to the previous adult findings suggesting that placebos can be used in a transparent way without compromising the therapeutic effect.7,8,29 Taking advantage of these therapeutic responses, it is possible that such treatments might translate to reduced use of pharmacologic treatments and their corresponding adverse effects. From a clinical perspective, one cannot ignore the fact that, in randomized clinical trials using drugs, symptoms significantly improved after placebo administration in close to 50% of children.3 The successful use of OLP circumvents the ethical dilemma of deception, and OLP may provide an inexpensive, easy-to-administer, safe, and effective way to achieve therapeutic success in patients with DGBI and other conditions. Our results suggest that we need to focus on understanding the underlying mechanism responsible for high placebo response rates among children to maximize therapeutic benefit. This study has shown that OLP can significantly reduce pain in children and adolescents with DGBI as well as decrease their use of rescue medications. Our findings suggest that OLP may provide an ethical way to harness the placebo effect as a therapeutic tool in the clinic. More research is required to confirm and extend these findings.
Trial Protocol
eTable 1. Demographic and Baseline Characteristics of FAP vs IBS Patients
eTable 2. Number of Hyoscyamine Pills Taken as Rescue Medication During the Control and Placebo Periods
eTable 3. Global Improvement at the End of the Placebo and Control Periods
eFigure. Individual Responses According to the Initial Randomization
eAppendix 1. Specifics of Placebo Implemenation and Explanations
eAppendix 2. Other Outcomes
Data Sharing Statement
References
- 1.Santucci NR, Saps M, van Tilburg MA. New advances in the treatment of paediatric functional abdominal pain disorders. Lancet Gastroenterol Hepatol. 2020;5(3):316-328. doi: 10.1016/S2468-1253(19)30256-0 [DOI] [PubMed] [Google Scholar]
- 2.Thapar N, Benninga MA, Crowell MD, et al. Paediatric functional abdominal pain disorders. Nat Rev Dis Primers. 2020;6(1):89. doi: 10.1038/s41572-020-00222-5 [DOI] [PubMed] [Google Scholar]
- 3.Hoekman DR, Zeevenhooven J, van Etten-Jamaludin FS, et al. The placebo response in pediatric abdominal pain-related functional gastrointestinal disorders: a systematic review and meta-analysis. J Pediatr. 2017;182:155-163. doi: 10.1016/j.jpeds.2016.12.022 [DOI] [PubMed] [Google Scholar]
- 4.Kaptchuk TJ, Hemond CC, Miller FG. Placebos in chronic pain: evidence, theory, ethics, and use in clinical practice. BMJ. 2020;370:m1668. doi: 10.1136/bmj.m1668 [DOI] [PubMed] [Google Scholar]
- 5.Trogen B, Caplan A, Klass P. The ethics of open-label placebos in pediatrics. Pediatrics. 2017;140(2):e20164328. doi: 10.1542/peds.2016-4328 [DOI] [PubMed] [Google Scholar]
- 6.Faria V, Linnman C, Lebel A, Borsook D. Harnessing the placebo effect in pediatric migraine clinic. J Pediatr. 2014;165(4):659-665. doi: 10.1016/j.jpeds.2014.06.040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kaptchuk TJ, Friedlander E, Kelley JM, et al. Placebos without deception: a randomized controlled trial in irritable bowel syndrome. PLoS One. 2010;5(12):e15591. doi: 10.1371/journal.pone.0015591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lembo A, Kelley JM, Nee J, et al. Open-label placebo vs double-blind placebo for irritable bowel syndrome: a randomized clinical trial. Pain. 2021;162(9):2428-2435. doi: 10.1097/j.pain.0000000000002234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hyams JS, Di Lorenzo C, Saps M, Shulman RJ, Staiano A, van Tilburg M. Functional disorders: children and adolescents. Gastroenterology. 2016;150(6):P1456-P1468.E2. doi: 10.1053/j.gastro.2016.02.015 [DOI] [PubMed] [Google Scholar]
- 10.Tesler MD, Savedra MC, Holzemer WL, Wilkie DJ, Ward JA, Paul SM. The word-graphic rating scale as a measure of children’s and adolescents’ pain intensity. Res Nurs Health. 1991;14(5):361-371. doi: 10.1002/nur.4770140507 [DOI] [PubMed] [Google Scholar]
- 11.Novick J, Miner P, Krause R, et al. A randomized, double-blind, placebo-controlled trial of tegaserod in female patients suffering from irritable bowel syndrome with constipation. Aliment Pharmacol Ther. 2002;16(11):1877-1888. doi: 10.1046/j.1365-2036.2002.01372.x [DOI] [PubMed] [Google Scholar]
- 12.van Tilburg MA, Rouster A, Silver D, Pellegrini G, Gao J, Hyman PE. Development and validation of a Rome III functional gastrointestinal disorders questionnaire for infants and toddlers. J Pediatr Gastroenterol Nutr. 2016;62(3):384-386. doi: 10.1097/MPG.0000000000000962 [DOI] [PubMed] [Google Scholar]
- 13.Walker LS, Greene JW. The Functional Disability Inventory: measuring a neglected dimension of child health status. J Pediatr Psychol. 1991;16(1):39-58. doi: 10.1093/jpepsy/16.1.39 [DOI] [PubMed] [Google Scholar]
- 14.Bastiaansen D, Koot HM, Bongers IL, Varni JW, Verhulst FC. Measuring quality of life in children referred for psychiatric problems: psychometric properties of the PedsQL 4.0 generic core scales. Qual Life Res. 2004;13(2):489-495. doi: 10.1023/B:QURE.0000018483.01526.ab [DOI] [PubMed] [Google Scholar]
- 15.Varni JW, Bendo CB, Nurko S, et al. Health-related quality of life in pediatric patients with functional and organic gastrointestinal diseases. J Pediatr. 2015;166(1):85-90. doi: 10.1016/j.jpeds.2014.08.022 [DOI] [PubMed] [Google Scholar]
- 16.Varni JW, Thompson KL, Hanson V. The Varni/Thompson Pediatric Pain Questionnaire, I: chronic musculoskeletal pain in juvenile rheumatoid arthritis. Pain. 1987;28(1):27-38. doi: 10.1016/0304-3959(87)91056-6 [DOI] [PubMed] [Google Scholar]
- 17.Chorpita BF, Moffitt CE, Gray J. Psychometric properties of the Revised Child Anxiety and Depression Scale in a clinical sample. Behav Res Ther. 2005;43(3):309-322. doi: 10.1016/j.brat.2004.02.004 [DOI] [PubMed] [Google Scholar]
- 18.Kovacs M. Children’s Depression Inventory (CDI and CDI 2). In: The Encyclopedia of Clinical Psychology. Wiley; 2014:1-5. doi: 10.1002/9781118625392 [DOI] [Google Scholar]
- 19.Cohen J. Statistical Power Analysis for the Behavioral Sciences. 2nd ed. Lawrence Erlbaum Associates Inc; 1988. [Google Scholar]
- 20.Cohen J. A power primer. Psychol Bull. 1992;112(1):155-159. doi: 10.1037/0033-2909.112.1.155 [DOI] [PubMed] [Google Scholar]
- 21.Faul F, Erdfelder E, Lang AG, Buchner A. G*Power 3: a flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav Res Methods. 2007;39(2):175-191. doi: 10.3758/BF03193146 [DOI] [PubMed] [Google Scholar]
- 22.Hirschfeld G, Wager J, Schmidt P, Zernikow B. Minimally clinically significant differences for adolescents with chronic pain-variability of ROC-based cut points. J Pain. 2014;15(1):32-39. doi: 10.1016/j.jpain.2013.09.006 [DOI] [PubMed] [Google Scholar]
- 23.Krasaelap A, Sood MR, Li BUK, et al. Efficacy of auricular neurostimulation in adolescents with irritable bowel syndrome in a randomized, double-blind trial. Clin Gastroenterol Hepatol. 2020;18(9):1987-1994. doi: 10.1016/j.cgh.2019.10.012 [DOI] [PubMed] [Google Scholar]
- 24.Kovacic K, Hainsworth K, Sood M, et al. Neurostimulation for abdominal pain–related functional gastrointestinal disorders in adolescents: a randomised, double-blind, sham-controlled trial. Lancet Gastroenterol Hepatol. 2017;2(10):727-737. doi: 10.1016/S2468-1253(17)30253-4 [DOI] [PubMed] [Google Scholar]
- 25.Saps M, Lavigne JV, van Tilburg MA, et al. Endpoints, reliability, and meaningful changes in clinical trials for children with irritable bowel syndrome: the Rome Foundation Pediatric Subcommittee on Clinical Trials. Neurogastroenterol Motil. 2018;30(5):e13308. doi: 10.1111/nmo.13308 [DOI] [PubMed] [Google Scholar]
- 26.Saps M, van Tilburg MA, Lavigne JV, et al. Recommendations for pharmacological clinical trials in children with irritable bowel syndrome: the Rome Foundation Pediatric Subcommittee on Clinical Trials. Neurogastroenterol Motil. 2016;28(11):1619-1631. doi: 10.1111/nmo.12896 [DOI] [PubMed] [Google Scholar]
- 27.Sandler AD, Bodfish JW. Open-label use of placebos in the treatment of ADHD: a pilot study. Child Care Health Dev. 2008;34(1):104-110. doi: 10.1111/j.1365-2214.2007.00797.x [DOI] [PubMed] [Google Scholar]
- 28.Sandler A, Glesne C, Geller G. Children’s and parents’ perspectives on open-label use of placebos in the treatment of ADHD. Child Care Health Dev. 2008;34(1):111-120. [DOI] [PubMed] [Google Scholar]
- 29.von Wernsdorff M, Loef M, Tuschen-Caffier B, Schmidt S. Effects of open-label placebos in clinical trials: a systematic review and meta-analysis. Sci Rep. 2021;11(1):3855. doi: 10.1038/s41598-021-83148-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Matts SG. An assessment of dicyclomine hydrochloride (“Merbentyl”) in the irritable colon syndrome. Br J Clin Pract. 1967;21(11):549-551. [PubMed] [Google Scholar]
- 31.Page JG, Dirnberger GM. Treatment of the irritable bowel syndrome with Bentyl (dicyclomine hydrochloride). J Clin Gastroenterol. 1981;3(2):153-156. doi: 10.1097/00004836-198106000-00009 [DOI] [PubMed] [Google Scholar]
- 32.Carling L, Svedberg LE, Hulten S. Short term treatment of irritable bowel syndrome: a placebo-controlled trial of peppermint oil against hyoscyamine. OPMEAR. 1989;34:55–57. [Google Scholar]
- 33.Faria V, Kossowsky J, Petkov MP, et al. Parental attitudes about placebo use in children. J Pediatr. 2017;181:272-278. doi: 10.1016/j.jpeds.2016.10.018 [DOI] [PubMed] [Google Scholar]
- 34.Whalley B, Hyland ME. Placebo by proxy: the effect of parents’ beliefs on therapy for children’s temper tantrums. J Behav Med. 2013;36(4):341-346. doi: 10.1007/s10865-012-9429-x [DOI] [PubMed] [Google Scholar]
- 35.Czerniak E, Oberlander TF, Weimer K, Kossowsky J, Enck P. “Placebo by proxy” and “nocebo by proxy” in children: a review of parents’ role in treatment outcomes. Front Psychiatry. 2020;11:169. doi: 10.3389/fpsyt.2020.00169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Grelotti DJ, Kaptchuk TJ. Placebo by proxy. BMJ. 2011;343:d4345. doi: 10.1136/bmj.d4345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Carvalho C, Pais M, Cunha L, Rebouta P, Kaptchuk TJ, Kirsch I. Open-label placebo for chronic low back pain: a 5-year follow-up. Pain. 2021;162(5):1521-1527. doi: 10.1097/j.pain.0000000000002162 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial Protocol
eTable 1. Demographic and Baseline Characteristics of FAP vs IBS Patients
eTable 2. Number of Hyoscyamine Pills Taken as Rescue Medication During the Control and Placebo Periods
eTable 3. Global Improvement at the End of the Placebo and Control Periods
eFigure. Individual Responses According to the Initial Randomization
eAppendix 1. Specifics of Placebo Implemenation and Explanations
eAppendix 2. Other Outcomes
Data Sharing Statement