Abstract
Study Objectives:
Persons > 65 years with short sleep duration (≤ 6 hours) are at risk for adverse outcomes, but the accuracy of self-reported sleep duration may be affected by reduced symptom awareness. We evaluated the performance characteristics of self-reported vs objectively measured sleep duration in this age group.
Methods:
In 2,980 men from the Osteoporotic Fractures in Men Sleep Study and 2,855 women from the Study of Osteoporotic Fractures we examined the agreement and accuracy of self-reported vs actigraphy-measured short and normal (> 6 but < 9 hours) sleep duration. We evaluated associations of select factors (demographics; medical, physical, and neuropsychiatric conditions; medication and substance use; and sleep-related measures) with risk of false-negative (normal sleep duration by self-report but short sleep duration by actigraphy) and false-positive (short sleep duration by self-report and normal sleep duration by actigraphy) designations, respectively, using logistic regression.
Results:
Average ages were 76.3 ± 5.5 and 83.5 ± 3.7 years in men and women, respectively. There was poor agreement between self-reported and actigraphic sleep duration (kappa ≤ 0.24). False negatives occurred in nearly half and false positives in over a quarter of older persons. In multivariable models in men and women, false negatives were independently associated with obesity, daytime sleepiness, and napping, while false positives were significantly lower with obesity.
Conclusions:
Under- and overreporting of short sleep is common among older persons. Reliance on self-report may lead to missed opportunities to prevent adverse outcomes or unnecessary interventions. Self-reported sleep duration should be objectively confirmed when evaluating the effect of sleep duration on health outcomes.
Citation:
Miner B, Stone KL, Zeitzer JM, et al. Self-reported and actigraphic short sleep duration in older adults. J Clin Sleep Med. 2022;18(2):403–413.
Keywords: aging, sleep duration, actigraphy, sleep disorders
BRIEF SUMMARY
Current Knowledge/Study Rationale: Older persons with short sleep duration are at risk for adverse cognitive and functional outcomes, but reduced symptom awareness may affect how they report their sleep. The performance characteristics of self-reported vs objective (actigraphic) measures of short sleep have not been examined in this group.
Study Impact: The accuracy of self-reported compared to actigraphy-measured short sleep duration was poor, with nearly half of older persons having false-negative designations (normal sleep duration by self-report but short sleep by actigraphy) and over 25% having false-positive designations (short sleep duration by self-report and normal sleep duration by actigraphy). Objective confirmation is needed when evaluating the effect of short sleep duration on health outcomes to avoid missed diagnoses or unnecessary interventions.
INTRODUCTION
Short sleep duration, most often defined as 6 hours or less of total overnight sleep, is a strong risk factor for mortality as well as adverse cardiovascular, metabolic, immunologic, cognitive, and functional outcomes.1–4 Older persons are a particularly vulnerable group since they are already at risk for aging-related declines in cognition and physical function, which may worsen as a result of short sleep duration.5,6 Thus, identifying older persons with short sleep duration may allow us to recognize a high-risk group in need of further assessment and treatment.
In the clinical setting, sleep duration is usually assessed by self-report. However, a growing body of research suggests that the accuracy of self-report in older persons may be affected by reduced symptom awareness.7 Older persons report milder levels of insomnia and daytime sleepiness than younger persons, despite having more severe sleep disorders.8 Given concerns about reduced symptom awareness in older persons, there is a need to evaluate the performance characteristics of self-reported short sleep duration as compared to objective sleep measures and to determine whether age-related factors are associated with disagreement between these measures. Prior work has shown poor agreement between self-reported and objective measures of sleep duration in various populations.9–11 However, the frequency and direction of disagreement (ie, false-negative or “overestimation” vs false-positive or “underestimation” of self-reported as compared to objective sleep duration) and characteristics associated with these disagreements have received little attention, especially among older adults (ie, after the eighth decade of life).
In the current study, using two well-established cohorts,12–14 we evaluated agreement between self-reported and objectively (actigraphy) measured short (≤ 6 hours) and normal (> 6 but < 9 hours) sleep duration in community-dwelling older men and women, respectively, and identified factors that were associated with disagreement. These factors, which may contribute to short sleep duration and/or modify sleep–wake awareness, include demographics, medical comorbidities, physical impairments, neuropsychiatric conditions, medication and substance use, and sleep-related impairments. Because of reduced symptom awareness, we hypothesized that agreement between self-reported and actigraphic short sleep duration would be poor and that several clinical factors would be associated with disagreement. The results of our study may inform methods of evaluating short sleep duration in older persons.
METHODS
Study population
This is a secondary analysis of cross-sectional data from the Osteoporotic Fractures in Men (MrOS) Sleep Study and the Study of Osteoporotic Fractures (SOF).12–14 For both studies, inclusion criteria were age ≥ 65 years and ability to ambulate without the assistance of another person.12–14 The study protocols were approved by the institutional review boards at all participating centers and included written informed consent.
The investigators for MrOS recruited 5,994 community-dwelling men from 2000–2002 at six clinical centers in the United States.12,13 The MrOS Sleep Study, an ancillary study conducted from 2003–2005, recruited 3,135 participants for a comprehensive sleep assessment. The sleep assessment included an in-clinic interview with validated sleep questionnaires, a series of clinical measures, wrist actigraphy, and overnight in-home polysomnography (PSG). The men had wrist actigraphy for an average of 5 days. Of the 3,135 men who participated in the comprehensive sleep assessment, 3,055 (97.4%) had usable actigraphy (see Figure S1 in the supplemental material).
SOF was a prospective cohort study of community-dwelling older women from four geographic areas in the United States.14 From 1986 to 1988, 9,704 Caucasian participants were recruited into the original cohort. From 1997 to 1998, 662 African American women were purposefully recruited to increase diversity. A comprehensive sleep assessment was performed (2002–2004). This assessment included an in-clinic interview with validated sleep questionnaires, clinical measures, and a minimum of 3 days of wrist actigraphy. Of the 3,676 women who had clinic or in-home visits, 3,052 (83.0%) had usable actigraphy (see Figure S2 in the supplemental material).
For our analytical samples, we included only those participants with short or normal sleep duration, as defined by self-report or actigraphy. Participants with self-reported or actigraphic long sleep duration (> 9 hours) could not be examined separately due to small sample sizes and were excluded from the normal sleep duration group given the association of long sleep duration with adverse health outcomes.1 In MrOS, of the 3,055 men with usable actigraphy, 75 (2.4%) were excluded (44 had long sleep duration by self-report, 29 had long sleep duration by actigraphy, and 2 had missing data on self-reported sleep duration), yielding a final analytical sample of n = 2,980. In SOF, of the 3,052 women with usable actigraphy, 197 (6.9%) were excluded (105 had long sleep duration by self-report, 84 had long sleep duration by actigraphy, and 8 had missing data on self-reported sleep duration), yielding a final analytical sample of n = 2,855.
Demographic and clinical characteristics
Demographic information included age, race, education level (< high school education vs other), living situation (living alone vs other), and marital status (widowed vs other). Medical conditions included obesity (body mass index ≥ 30 kg/m2), self-reported conditions available in men and women (diabetes mellitus, osteoarthritis, chronic obstructive pulmonary disease, hypertension, myocardial infarction, congestive heart failure [CHF], and stroke), ≥ 3 chronic conditions15 (based on a count of the 7 self-reported conditions), and benign prostatic hypertrophy (in men only). Physical characteristics were evaluated objectively and included physical impairment (gait speed < 0.8 m/s at participant’s usual pace),16 vision impairment (worse than 20/40 on the Bailey-Lovie test of visual acuity),17 and physical inactivity (percent actigraphy wear time during the day with activity < 5,000 counts/min [see Validation of Definition of Sedentary Behavior Based on Actigraphy Data in the supplemental material], averaged over all days of monitoring, including at least 10 hours on each day).18 Neuropsychiatric conditions included depression (Geriatric Depression Scale ≥ 6),19 anxiety (Goldberg Anxiety Scale score > 5),20 and cognitive impairment (Modified Mini-Mental Status Examination score 1.5 standard deviations below the cohort-specific mean [≤ 22 in MrOS and ≤ 21 in SOF]).21,22 All prescription and nonprescription medications used within the preceding 30 days were entered into an electronic database; each medication was matched to its ingredient(s) based on the Iowa Drug Information Service Drug Vocabulary (College of Pharmacy, University of Iowa, Iowa City, IA).23 Medications included use of an antidepressant (tricyclics, selective serotonin reuptake inhibitors, serotonin-norepinephrine reuptake inhibitors, or trazodone)24 and use of a central nervous system–active medication (benzodiazepines, anticonvulsants, narcotics, or antipsychotics).24 Substance use included alcohol (drinks per week), caffeine intake ≥ 190 mg/d (ie, the equivalent of at least two cups of coffee),25 and current smoking.
Sleep-related measures included self-reported sleep quality, daytime sleepiness, napping, restless legs syndrome, and sleep-disordered breathing (SDB; in men only). Self-reported sleep quality was evaluated by Pittsburgh Sleep Quality Index (PSQI) item 9 (“During the past month, how would you rate your sleep quality overall?”), with scores ranging from 0 (very good) to 3 (very bad).26 Poor sleep quality was defined by a score > 1 (ie, fairly bad or very bad) on PSQI item 9. Because an additional item from the PSQI was used to define self-reported sleep duration (see the section on sleep duration below) we did not use total PSQI score to evaluate sleep quality. Daytime sleepiness was evaluated by the Epworth Sleepiness Scale (ESS).27 The ESS score ranges 0–24, with higher scores indicating more severe daytime sleepiness. An ESS score > 10 is a validated cutoff for patient-reported hypersomnia associated with adverse health outcomes in older persons.27 Restless legs syndrome was established by self-report of a physician diagnosis. SDB was established in MrOS by an apnea-hypopnea index (at ≥ 4% desaturation) per hour of sleep ≥ 15 on PSG.28 A polysomnographic assessment for the presence of SDB was available in a convenience sample of n = 461 (16.1%) women. Given the large amount of missing data for this variable, which were not missing at random, multiple imputation was not possible. Thus, we examined bivariate associations of SDB in n = 461 women from SOF with false-negative and false-positive designations, respectively (described under statistical analysis). We did not include SDB in multivariable models in SOF, which allowed us to have a larger sample with which to evaluate a greater number of clinical correlates.
Sleep duration
Overnight sleep duration was defined as normal (> 6 but < 9 hours) or short (≤ 6 hours), based on the joint consensus statement from the American Academy of Sleep Medicine and the Sleep Research Society on the recommended amount of sleep for a healthy adult.29 Our operational definition for short sleep duration is supported by several reports among older persons that have demonstrated adverse outcomes (mortality, impaired cognition, and impaired functional capacity) with sleep durations at or below 6 hours.2–4,30 In addition, the average sleep duration, whether self-reported or measured by actigraphy, was below 7 hours in men and women (as shown in Table S1 in the supplemental material), supporting use of a lower cutoff in our study population. Self-reported duration was assessed using PSQI item 4a: “During the past month, how many hours of actual sleep did you get at night?”.26 In MrOS and SOF, PSQI forms required participants to provide an integer response for estimated sleep duration.
Objective sleep duration was evaluated by wrist actigraphy. While PSG is the gold standard for measurement of sleep, meta-analyses of PSG vs actigraphy have shown narrow ranges in the mean difference in sleep duration between these two measures,31 with actigraphy overestimating sleep duration by 13–18 minutes on average in older men and women.32,33 In addition, the American Academy of Sleep Medicine suggests that actigraphy is appropriate for estimation of sleep duration in a variety of sleep disorders.31 Participants in MrOS and SOF wore the SleepWatch-O actigraph (Ambulatory Monitoring, Inc., Ardsley, New York) on the nondominant hand. Average use (standard deviation) was 5.2 (0.9) nights in MrOS and 4.1 (0.8) nights in SOF. Actigraph data were analyzed using Action W-2 software with proportional integration mode and the University of California San Diego scoring algorithm.34 Full details of the validation of these methods have been published previously.32,33 The software algorithm and sleep diaries were used to edit the raw data and generate variables for different sleep measures, including sleep duration.32,33 Prior analyses of actigraphy data from SOF and MrOS have shown high interscorer reliability.32,33 In both the MrOS and SOF studies, 95% of participants initiated their actigraphy recordings within a week of completing the PSQI.
Statistical analysis
MrOS (men) and SOF (women) were evaluated separately but in parallel fashion due to differences in clinical characteristics and availability of PSG-measured SDB. Distributions of clinical characteristics were summarized as means (± standard deviations) or frequencies (%). Frequency distributions for normal and short sleep duration were calculated according to measurement method (self-report or actigraphy; actigraphic sleep duration was recorded in minutes and was rounded to the nearest hour for comparison with self-reported sleep duration). Considering actigraphy as the gold standard, we calculated the frequency of four groups: (1) true negatives (normal sleep duration by self-report and actigraphy), (2) false negatives (normal sleep duration by self-report but short sleep duration by actigraphy), (3) true positives (short sleep duration by self-report and actigraphy), and (4) false positives (short sleep duration by self-report and normal sleep duration by actigraphy). Characteristics of participants were compared by group using chi-square tests for homogeneity for categorical variables and analysis of variance for normally distributed continuous variables.
Next, the kappa coefficient was calculated to examine the degree of agreement between self-reported and actigraphic measures of short sleep duration. With actigraphy serving as the reference standard, the sensitivity, false-negative rate, specificity, false-positive rate, positive predictive value, negative predictive value, and accuracy (ie, the proportion of true positives and true negatives in all evaluated cases) of self-reported sleep duration were calculated. The primary analyses used a duration ≤ 6 hours to define short sleep. In sensitivity analyses, we used a duration ≤ 7 hours (see Table S2 in the supplemental material). Concordance between continuous measures of self-reported and actigraphic sleep duration was analyzed with the estimate and 95% confidence interval of the two-way fixed-effects intraclass correlation coefficient for absolute agreement. Bland-Altman plots with 95% limits of agreement were presented to display the distribution of differences between the two measures of sleep duration and to assess systematic bias in these differences.35 Formal tests of systematic bias were performed using simple linear regression models to examine whether the scatter in the Bland-Altman plots was heteroscedastic.36
Using logistic regression, we calculated the odds of a false-negative or a false-positive designation. False-negative designations (normal sleep duration by self-report but short sleep [≤ 6 hours] duration by actigraphy) were evaluated among the subgroup of men and women who reported normal sleep duration (1,996 men and 1,626 women). The reference group for these models included those with a true-negative designation (normal sleep duration by self-report and actigraphy). False-positive designations (short sleep duration by self-report and normal sleep duration by actigraphy) were evaluated among the subgroup of men and women who reported short sleep duration (984 men and 1,229 women). The reference group for false-positive designations included those with a true-positive designation (short sleep duration by self-report and actigraphy). In bivariate analyses, logistic regression models estimated unadjusted odds ratios (ORs) and 95% confidence intervals of a false-negative or a false-positive designation for each demographic and clinical characteristic (see Table S3 and Table S4 in the supplemental material). Multivariate models, derived using backward selection with a requirement of a P value ≤ .20 to remain in the model, estimated adjusted ORs (adjORs) and 95% confidence intervals of a false-negative or a false-positive designation. This methodology was chosen based on the large number of variables examined and the exploratory nature of the analysis. Age, race, and education level were kept in all models regardless of P value due to their known associations with sleep duration and self-reported sleep.37 For verification purposes, we also performed forward selection with a P value ≤ .20 for entry into the model, which displayed similar results (data not shown). Model fit was inspected using collinearity diagnostics and residual plots. All analyses were conducted using SAS version 9.4 software (SAS Institute Inc., Cary, NC).
RESULTS
As compared with SOF, MrOS participants were younger (average ages were 76.3 ± 5.5 and 83.5 ± 3.7 years in men and women, respectively), more educated, and less likely to live alone or be widowed; reported fewer comorbidities, physical impairments, and neuropsychiatric conditions; and had less medication use. Actigraphy-measured short sleep was more prevalent in MrOS (32.4% and 26.1% in men and women, respectively), while self-reported short sleep was more prevalent in SOF (33% and 43.1% in men and women, respectively) (see Table S1).
Table 1 and Table 2 show clinical characteristics of MrOS (men) and SOF (women), respectively, for the four groups, where false negatives represent normal sleep duration by self-report but short sleep duration by actigraphy and false positives represent short sleep duration by self-report but normal sleep duration by actigraphy. Among men, sociodemographic factors (race, education, living alone, marital status), medical factors (obesity, diabetes mellitus, CHF, ≥ 3 chronic conditions), physical impairment, neuropsychiatric factors (depression, anxiety), medication and substance use (central nervous system–active medications, alcohol use, caffeine intake), and sleep-related factors (PSQI item 9 score > 1, ESS score > 10, daily napping, restless legs syndrome, SDB) differed significantly across the four groups. Among women, significant differences across the four groups were observed for sociodemographic factors (age, race, education), medical factors (obesity, chronic obstructive pulmonary disease, hypertension, CHF, ≥ 3 chronic conditions), physical impairment, neuropsychiatric factors (depression, anxiety, cognitive impairment), medication use (antidepressant), and sleep-related factors (PSQI item 9 score > 1, ESS score > 10, daily napping, restless legs syndrome).
Table 1.
Clinical characteristics in MrOS (men) according to sleep category.
| Characteristics | True Negatives (n = 1,504) |
False Negatives (n = 492) |
True Positives (n = 474) |
False Positives (n = 510) |
Pa |
|---|---|---|---|---|---|
| Sociodemographics | |||||
| Age, y | 79.4 ± 5.6 | 76.7 ± 5.6 | 76.1 ± 5.4 | 76.0 ± 5.3 | .155 |
| Non-White race | 115 (7.6) | 44 (8.9) | 79 (16.7) | 58 (11.4) | <.001 |
| Less than high school education | 60 (4.0) | 26 (5.3) | 40 (8.4) | 30 (5.9) | .002 |
| Living alone | 179 (11.9) | 75 (15.3) | 85 (18.1) | 59 (11.7) | .002 |
| Widowed | 114 (7.6) | 58 (11.8) | 55 (11.7) | 38 (7.5) | .003 |
| Medical | |||||
| Obesityb | 244 (16.2) | 151 (30.7) | 134 (28.3) | 78 (15.3) | <.001 |
| Diabetesc | 170 (11.3) | 79 (16.1) | 70 (14.8) | 70 (13.7) | .024 |
| Osteoarthritisc | 329 (21.9) | 124 (25.2) | 124 (26.2) | 129 (25.3) | .124 |
| COPDc | 68 (4.5) | 26 (5.3) | 33 (7.0) | 31 (6.1) | .169 |
| Hypertensionc | 716 (47.6) | 249 (50.6) | 247 (52.1) | 273 (53.5) | .076 |
| Myocardial infarctionc | 249 (16.6) | 75 (15.2) | 94 (19.8) | 99 (19.4) | .126 |
| CHFc | 60 (4.0) | 42 (8.5) | 46 (9.7) | 38 (7.5) | <.001 |
| Strokec | 58 (3.8) | 9 (1.8) | 21 (4.4) | 23 (4.5) | .088 |
| BPHc | 708 (47.1) | 224 (45.6) | 240 (50.9) | 263 (51.9) | .110 |
| ≥ 3 Chronic conditionsd | 144 (9.6) | 59 (12.0) | 72 (15.2) | 69 (13.5) | .003 |
| Physical | |||||
| Physical impairmente | 82 (5.5) | 42 (8.7) | 40 (8.6) | 34 (6.7) | .026 |
| Vision impairmentf | 50 (3.3) | 24 (4.9) | 17 (3.6) | 19 (3.7) | .475 |
| Physical inactivityg | 68.2 ± 11.0 | 67.2 ± 11.5 | 67.9 ± 11.3 | 68.9 ± 11.5 | .131 |
| Neuropsychiatric | |||||
| Depressionh | 70 (4.7) | 30 (6.1) | 42 (8.9) | 51 (10.0) | <.001 |
| Anxietyi | 80 (5.3) | 27 (5.5) | 74 (15.7) | 88 (17.3) | <.001 |
| Cognitive impairmentj | 165 (11.0) | 61 (12.4) | 71 (15.0) | 68 (13.3) | .105 |
| Medication and substance use | |||||
| Antidepressantk | 117 (7.8) | 32 (6.5) | 32 (6.8) | 46 (9.0) | .415 |
| CNS-active medicationl | 136 (9.0) | 61 (12.4) | 60 (12.7) | 66 (12.9) | .017 |
| Alcohol (drinks per week) | 3.7 ± 4.3 | 3.4 ± 4.2 | 3.3 ± 4.2 | 2.9 ± 3.9 | <.001 |
| Caffeine intake ≥ 190 mg/d | 677 (45.0) | 251 (51.0) | 240 (50.6) | 219 (42.9) | .011 |
| Current smoking | 25 (1.7) | 10 (2.0) | 17 (3.6) | 10 (2.0) | .085 |
| Sleep-related factors | |||||
| Self-reported sleep durationm | 7.6 ± 0.6 | 7.5 ± 0.6 | 5.5 ± 0.7 | 5.6 ± 0.7 | <.001 |
| Actigraphic sleep durationn | 7.1 ± 0.7 | 5.1 ± 0.9 | 5.0 ± 0.9 | 6.8 ± 0.6 | <.001 |
| Poor sleep qualityo | 92 (6.1) | 43 (8.7) | 147 (31.0) | 182 (35.7) | <.001 |
| Daytime sleepinessp | 152 (10.1) | 80 (16.3) | 85 (18.0) | 69 (13.5) | <.001 |
| Daily napping | 163 (10.8) | 109 (22.2) | 106 (22.4) | 75 (14.7) | <.001 |
| RLSc | 19 (1.3) | 15 (3.1) | 15 (3.3) | 16 (3.2) | .007 |
| SDBq | 308 (21.7) | 165 (35.7) | 145 (33.7) | 119 (24.7) | <.001 |
Values are presented as mean ± SD or n (%). Those with long sleep duration (> 9 hours on self-report or actigraphy; n = 73 men) were excluded. True negative = normal sleep duration by self-report and actigraphy, false negative = normal sleep duration by self-report but short sleep duration by actigraphy, true positive = short sleep duration by self-report and actigraphy, false positive = short sleep duration by self-report and normal sleep duration by actigraphy. aDerived from one-way analysis of variance for continuous variables and chi-square test for categorical variables comparing the 4 groups. bBody mass index ≥ 30 kg/m2. cSelf-reported, physician-diagnosed. dFrom 7 medical conditions available in men and women (diabetes, osteoarthritis, COPD, hypertension, myocardial infarction, CHF, and stroke). eGait speed < 0.8 m/s. fVisual acuity 20/40 or worse. gPercent actigraphy wear time with activity < 5,000 counts/min, averaged over all days of monitoring, including 10 hours on each day. hGeriatric Depression Scale score ≥ 6. iGoldberg Anxiety Scale score > 5. jModified Mini-Mental Status Examination score 1.5 SD below the mean (≤ 22). kIncludes use of tricyclics, selective serotonin reuptake inhibitors, serotonin reuptake inhibitors, or trazodone. lIncludes use of benzodiazepines, anticonvulsants, narcotics, or antipsychotics. mFrom PSQI item 4a (“How many actual hours of sleep did you get at night?”). nAveraged over 5.2 (0.9) nights in MrOS and 4.1 (0.8) nights in SOF. oSelf-rated sleep quality over the previous month > 1 (range = 0 [very good] to 3 [very bad]). pESS score > 10. qApnea-hypopnea index ≥ 15. BPH = benign prostatic hypertrophy, CHF = congestive heart failure, CNS = central nervous system, COPD = chronic obstructive pulmonary disease, ESS = Epworth Sleepiness Scale, MrOS = Osteoporotic Fractures in Men Sleep Study, PSQI = Pittsburgh Sleep Quality Index, RLS = restless legs syndrome, SD = standard deviation, SDB = sleep-disordered breathing, SOF = Study of Osteoporotic Fractures.
Table 2.
Clinical characteristics in SOF (women) according to sleep category.
| Characteristics | True Negatives (n = 1,303) |
False Negatives (n = 323) |
True Positives (n = 421) |
False Positives (n = 808) |
Pa |
|---|---|---|---|---|---|
| Sociodemographics | |||||
| Age, y | 83.7 ± 3.6 | 83.5 ± 4.1 | 82.9 ± 3.7 | 83.4 ± 3.8 | .001 |
| African American | 93 (7.1) | 45 (13.9) | 71 (16.9) | 93 (11.5) | <.001 |
| Less than high school education | 205 (15.7) | 62 (19.2) | 80 (19.0) | 168 (20.8) | .023 |
| Living alone | 778 (59.7) | 183 (56.7) | 268 (63.7) | 506 (62.6) | .136 |
| Widowed | 797 (61.2) | 199 (61.6) | 261 (62.0) | 512 (63.4) | .791 |
| Medical | |||||
| Obesityb | 278 (21.7) | 108 (34.8) | 143 (34.6) | 178 (22.3) | <.001 |
| Diabetesc | 128 (9.8) | 43 (13.3) | 53 (12.6) | 93 (11.5) | .184 |
| Osteoarthritisc | 483 (37.1) | 129 (40.0) | 180 (42.8) | 315 (39.0) | .203 |
| COPDc | 131 (10.1) | 63 (19.5) | 58 (13.8) | 94 (11.6) | <.001 |
| Hypertensionc | 741 (56.9) | 201 (62.2) | 269 (63.9) | 515 (63.7) | .004 |
| Myocardial infarctionc | 146 (11.2) | 43 (13.3) | 46 (10.9) | 96 (11.9) | .712 |
| CHFc | 89 (6.8) | 41 (12.7) | 40 (9.5) | 66 (8.2) | .005 |
| Strokec | 164 (12.6) | 45(13.9) | 55 (13.1) | 87 (10.8) | .410 |
| ≥ 3 chronic conditionsd | 206 (15.8) | 82 (25.4) | 89 (21.1) | 152 (18.8) | <.001 |
| Physical | |||||
| Physical impairmente | 481 (39.0) | 148 (51.9) | 174 (46.0) | 278 (36.8) | <.001 |
| Vision impairmentf | 255 (22.6) | 64 (23.6) | 75 (20.5) | 134 (18.7) | .159 |
| Physical inactivityg | 66.7 ± 12.9 | 66.9 ± 14.4 | 66.8 ± 13.0 | 66.6 ± 12.3 | .989 |
| Neuropsychiatric | |||||
| Depressionh | 130 (10.0) | 28 (8.7) | 57 (13.5) | 106 (13.1) | .025 |
| Anxietyi | 108 (8.3) | 20 (6.2) | 92 (21.9) | 181 (22.5) | <.001 |
| Cognitive impairmentj | 122 (9.8) | 40 (13.2) | 33 (8.2) | 53 (6.7) | .006 |
| Medication and substance use | |||||
| Antidepressantk | 174 (13.4) | 53 (16.5) | 52 (12.4) | 80 (9.9) | .015 |
| CNS-active medicationl | 229 (17.6) | 66 (20.4) | 85 (20.2) | 177 (21.9) | .095 |
| Alcohol (drinks per week) | 1.2 ± 2.8 | 1.1 ± 2.9 | 1.0 ± 2.7 | 1.0 ± 2.6 | .600 |
| Caffeine intake ≥ 190 mg/d | 528 (40.5) | 124 (38.4) | 151 (35.9) | 324 (40.1) | .364 |
| Current smoking | 35 (2.7) | 14 (4.3) | 13 (3.1) | 13 (1.6) | .062 |
| Sleep-related factors | |||||
| Self-reported sleep durationm | 7.6 ± 0.7 | 7.5 ± 0.6 | 5.5 ± 0.7 | 5.5 ± 0.7 | <.001 |
| Actigraphic sleep durationn | 7.3 ± 0.7 | 5.1 ± 0.9 | 5.1 ± 0.8 | 7.1 ± 0.7 | <.001 |
| Poor sleep qualityo | 55 (4.2) | 17 (5.3) | 119 (28.3) | 259 (32.1) | <.001 |
| Daytime sleepinessp | 117 (9.0) | 52 (16.1) | 78 (18.5) | 72 (8.9) | <.001 |
| Daily napping | 163 (12.5) | 67 (20.9) | 78 (18.6) | 101 (12.5) | <.001 |
| RLSc | 34 (2.6) | 16 (5.0) | 23 (5.5) | 48 (5.9) | .001 |
Values are presented as mean ± SD or n (%). Those with long sleep duration (> 9 hours on self-report or actigraphy; n = 189 women) were excluded. True negative = normal sleep duration by self-report and actigraphy, false negative = normal sleep duration by self-report but short sleep duration by actigraphy, true positive = short sleep duration by self-report and actigraphy, false positive = short sleep duration by self-report and normal sleep duration by actigraphy. aDerived from one-way analysis of variance for continuous variables and chi-square test for categorical variables comparing the 4 groups. bBody mass index ≥ 30 kg/m2. cSelf-reported, physician-diagnosed. dFrom 7 medical conditions available in men and women (diabetes, osteoarthritis, COPD, hypertension, myocardial infarction, CHF, and stroke). eGait speed < 0.8 m/s. fVisual acuity 20/40 or worse. gPercent actigraphy wear time with activity < 5,000 counts/min, averaged over all days of monitoring, including 10 hours on each day. hGeriatric Depression Scale score ≥ 6. iGoldberg Anxiety Scale score > 5. jModified Mini-Mental Status Examination score 1.5 SD below the mean (≤ 21). kIncludes use of tricyclics, selective serotonin reuptake inhibitors, serotonin reuptake inhibitors, or trazodone. lIncludes use of benzodiazepines, anticonvulsants, narcotics, or antipsychotics. mFrom PSQI item 4a (“How many actual hours of sleep did you get at night?”). nAveraged over 5.2 (0.9) nights in MrOS and 4.1 (0.8) nights in SOF. oSelf-rated sleep quality over the previous month > 1 (range = 0 [very good] to 3 [very bad]). pESS score > 10. CHF = congestive heart failure, CNS = central nervous system, COPD = chronic obstructive pulmonary disease, ESS = Epworth Sleepiness Scale, MrOS = Osteoporotic Fractures in Men Sleep Study, PSQI = Pittsburgh Sleep Quality Index, RLS = restless legs syndrome, SD = standard deviation, SDB = sleep-disordered breathing, SOF = Study of Osteoporotic Fractures.
Table 3 shows the agreement and accuracy of self-reported vs actigraphy-measured short sleep. Kappa statistics were low in men and women (0.24 and 0.15, respectively), indicating poor to no agreement. Using actigraphy as the reference standard, the sensitivity of self-reported short sleep was poor in both sexes (0.49 and 0.57 in men and women, respectively), leading to false-negative rates of 50.9% in men and 43.4% in women. Positive predictive values were also low for both sexes (0.48 and 0.34 in men and women, respectively), with false-positive rates of 25.3% in men and 38.3% in women. Otherwise, specificity and negative predictive values were fair to good in men and women. Overall accuracy was 0.66 in men and 0.60 in women, below the acceptable range for model discrimination.38 When a cutoff of ≤ 7 hours was used to define short sleep duration in men and women, kappa statistics showed similarly poor agreement. Sensitivity increased in both cohorts, while specificity decreased, and overall accuracy did not change substantially (see Table S2).
Table 3.
Agreement and accuracy of self-reported vs actigraphy-measured short sleep.
| Actigraphy: Short Sleep | |||
|---|---|---|---|
| Yes | No | Total | |
| A. MrOS (men) | |||
| Self-report: short sleep | |||
| Yes | 474 (15.9%) | 510 (17.1%) | 984 (33.0%) |
| No | 492 (16.5%) | 1504 (50.5%) | 1,996 (67.0%) |
| Total | 966 (32.4%) | 2,014 (67.6%) | 2,980 (100%) |
| Kappa statistic | 0.24 (95% CI: 0.20, 0.27) | ||
| Sensitivity | 0.49 (95% CI: 0.46, 0.52) | ||
| False-negative rate | 50.9% (95% CI: 47.7%, 54.1%) | ||
| Specificity | 0.75 (95% CI: 0.73, 0.77) | ||
| False-positive rate | 25.3% (95% CI: 23.4%, 27.2%) | ||
| PPV | 0.48 (95% CI: 0.45, 0.52) | ||
| NPV | 0.75 (95% CI: 0.73, 0.77) | ||
| Accuracy | 0.66 (95% CI: 0.65, 0.68) | ||
| B. SOF (women) | |||
| Self-report: short sleep | |||
| Yes | 421 (14.7%) | 808 (28.3%) | 1,229 (43.0%) |
| No | 323 (11.3%) | 1,303 (45.6%) | 1,626 (57.0%) |
| Total | 744 (26.0%) | 2,110 (73.9%) | 2,855 (100%) |
| Kappa statistic | 0.15 (95% CI: 0.12, 0.19) | ||
| Sensitivity | 0.57 (95% CI: 0.53, 0.60) | ||
| False-negative rate | 43.4% (95% CI: 39.8%, 47.1%) | ||
| Specificity | 0.62 (95% CI: 0.60, 0.64) | ||
| False-positive rate | 38.3% (95% CI: 36.2%, 40.4%) | ||
| PPV | 0.34 (95% CI: 0.32, 0.37) | ||
| NPV | 0.80 (95% CI: 0.78, 0.82) | ||
| Accuracy | 0.60 (95% CI: 0.58, 0.62) | ||
(A) MrOS (n = 2,980) and (B) SOF (n = 2,855). Range of sleep time is 0 to 9 hours, wherein short sleep duration is 0–6 hours and normal sleep duration is > 6 hours (up to 9 hours). CI = confidence interval, MrOS = Osteoporotic Fractures in Men Sleep Study, NPV = negative predictive value, PPV = positive predictive value, SOF = Study of Osteoporotic Fractures.
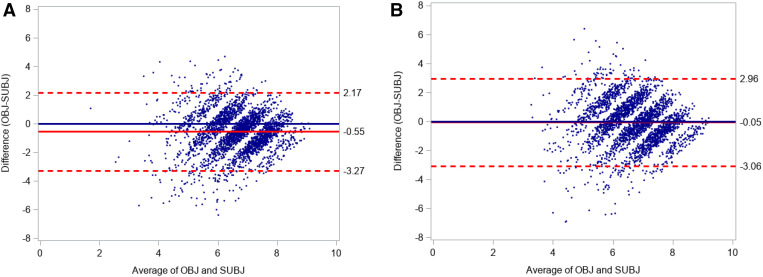
Figure 1 shows Bland-Altman plots comparing continuous measures of self-reported and actigraphic sleep duration in MrOS (Figure 1A) and SOF (Figure 1B). Mean differences do not appear to vary by the size of the average value of the two sleep measures. However, both plots display wide 95% limits of agreement, outside of a range that would be considered clinically negligible. In addition, the Bland-Altman plot for MrOS shows a fixed systematic bias for overestimation of self-reported sleep duration to actigraphy of 33 minutes (P < .001). The Pearson correlation coefficients for continuous measures of self-reported and actigraphic sleep duration were poor (0.30 in MrOS and 0.21 in SOF). The intraclass correlation coefficients and corresponding confidence intervals for agreement of the self-reported and actigraphic measures of sleep duration were 0.22 (0.19, 0.26) and 0.20 (0.17, 0.24) respectively, indicating poor reliability. Using a consistency measure, which accounts for bias, the intraclass correlation coefficient for the MrOS sample increased to 0.26.
Figure 1. Bland-Altman plots showing the agreement between objective (actigraphy) and self-reported measures of sleep duration in MrOS and SOF.
(A) MrOS, men. (B) SOF, women. Dotted lines represent upper and lower 95% limits of agreement. The unbroken red line represents mean differences. MrOS = Osteoporotic Fractures in Men Sleep Study, OBJ = actigraphic sleep duration, SOF= Study of Osteoporotic Fractures, SUBJ = self-reported sleep duration.
Table 4 and Table 5 show the adjORs after backward selection for correlates of a false-negative designation, relative to a true-negative designation (normal sleep duration by self-report and actigraphy). Multivariable models in MrOS and SOF were adjusted for all clinical characteristics shown in Table 1 and Table 2, respectively. As shown in Table 4, the adjOR of a false-negative designation was significantly higher in men who were widowed, obese, had a history of CHF, had daytime sleepiness, were daily nappers, and had SDB (adjORs of 1.56–2.34). The adjusted odds of a false-negative designation were significantly lower in men with a history of stroke or physical inactivity (adjORs 0.42–0.79). As shown in Table 5, the adjOR of a false-negative designation was significantly higher in women with obesity, physical impairment, daytime sleepiness, and daily napping (adjORs of 1.62–1.93) but significantly lower in women with depression or low physical activity (adjORs 0.56–0.85). In bivariate associations, the unadjusted OR for a false-negative designation in women with SDB was 1.68 (0.88, 3.22).
Table 4.
MrOS (men): adjusted odds of a false-negative designation (normal sleep duration by self-report but short sleep by actigraphy).
| Characteristicsa | Adjusted OR (95% CI)b |
|---|---|
| Age (per year) | 1.01 (0.99, 1.03) |
| Non-White race | 1.21 (0.80, 1.83) |
| Less than high school | 1.01 (0.59, 1.73) |
| Widowed | 1.67 (1.15, 2.42) |
| Obesity | 2.03 (1.55, 2.66) |
| Diabetes | 1.23 (0.89, 1.70) |
| Myocardial infarction | 0.85 (0.62, 1.16) |
| CHF | 2.01 (1.26, 3.19) |
| Stroke | 0.42 (0.19, 0.91) |
| Physical inactivity (per SD increase) | 0.79 (0.70, 0.89) |
| Antidepressant use | 0.65 (0.41, 1.04) |
| Use of CNS-active medication | 1.39 (0.96, 2.02) |
| Caffeine intake ≥ 190 mg/d | 1.17 (0.94, 1.47) |
| Poor sleep quality | 1.22 (0.80, 1.86) |
| Daytime sleepiness (ESS score > 10) | 1.56 (1.12, 2.16) |
| Daily napping | 2.34 (1.74, 3.15) |
| SDB | 1.72 (1.35, 2.20) |
Included men with normal sleep duration by self-report (n = 1,996). Participants having a false-negative designation (n = 492) were compared with those having a true-negative designation (normal sleep duration by self-report and actigraphy; n = 1,504). aSee footnotes to Table 1 regarding sample sizes for clinical characteristics. bFrom a multivariable model, including all demographic and clinical characteristics listed in Table 1, that was derived using backward selection with P ≤ .20 to remain in the model. The model maxRSQCv_PCT was 11.77, explaining 11% of total variance in the outcome. CHF = congestive heart failure, CI = confidence interval, CNS = central nervous system, ESS = Epworth Sleepiness Scale, MrOS = Osteoporotic Fractures in Men Sleep Study, OR = odds ratio, SD = standard deviation, SDB = sleep-disordered breathing.
Table 5.
SOF (women): adjusted odds of a false-negative designation (normal sleep duration by self-report but short sleep by actigraphy).
| Characteristicsa | Adjusted OR (95% CI)b |
|---|---|
| Age (per year) | 0.99 (0.94, 1.03) |
| African American | 1.41 (0.89, 2.25) |
| Less than high school | 1.03 (0.72, 1.49) |
| Obesity | 1.62 (1.18, 2.22) |
| CHF | 1.50 (0.92, 2.45) |
| Physical impairment | 1.77 (1.33, 2.37) |
| Physical inactivity (per SD increase) | 0.85 (0.73, 0.98) |
| Depression | 0.56 (0.31, 0.99) |
| Anxiety | 0.67 (0.36, 1.23) |
| Current smoking | 1.67 (0.79, 3.54) |
| Daytime sleepiness (ESS score > 10) | 1.93 (1.29, 2.90) |
| Daily napping | 1.69 (1.16, 2.44) |
| RLS | 1.80 (0.90, 3.63) |
Included women with normal sleep duration by self-report (n = 1,626). Participants having a false-negative designation (n = 323) were compared with those having a true-negative designation (normal sleep duration by self-report and actigraphy; n = 1,303). aSee footnotes to Table 2 regarding sample sizes for clinical characteristics. bFrom a multivariable model, including all demographic and clinical characteristics listed in Table 2, that was derived using backward selection with P ≤ .20 to remain in the model. The model maxRSQCv_PCT was 8.09, explaining 8% of total variance in the outcome. CHF = congestive heart failure, CI = confidence interval, ESS = Epworth Sleepiness Scale, OR = odds ratio, RLS = restless legs syndrome, SD = standard deviation, SOF = Study of Osteoporotic Fractures.
Table 6 and Table 7 show adjORs after backward selection of a false-positive designation, relative to a true-positive designation (short sleep by self-report and actigraphy). As shown in Table 6, the adjOR of a false-positive designation was significantly higher in men with poor sleep quality (adj OR 1.39 [1.03, 1.86]) and significantly lower in men with obesity, daily napping, and SDB (adjORs of 0.46–0.68). As shown in Table 7, the adjOR of a false-positive designation was significantly higher in women per each additional year of age (adjOR 1.05 [1.01, 1.10]) and significantly lower in women with obesity, physical impairment, and daytime sleepiness (adjOR of 0.46–0.65). In bivariate associations, the unadjusted OR for a false-positive designation in women with SDB was 0.52 (0.28, 0.95).
Table 6.
MrOS (men): adjusted odds of a false-positive designation (short sleep by self-report but normal sleep duration by actigraphy).
| Characteristicsa | Adjusted OR (95% CI)b |
|---|---|
| Age (per year) | 0.99 (0.96, 1.01) |
| Non-White race | 0.74 (0.49, 1.12) |
| Less than high school | 0.65 (0.37, 1.13) |
| Living alone | 0.68 (0.46, 1.01) |
| Obesity | 0.46 (0.32, 0.65) |
| Hypertension | 1.20 (0.91, 1.58) |
| CHF | 0.76 (0.46, 1.27) |
| Physical inactivity (per SD increase) | 1.13 (0.99, 1.30) |
| Caffeine intake ≥ 190 mg/d | 0.78 (0.59, 1.03) |
| Alcohol use (drinks per week) | 0.97 (0.94, 1.01) |
| Poor sleep quality (PSQI item 9 score > 1) | 1.39 (1.03, 1.86) |
| Daytime sleepiness (ESS score > 10) | 0.75 (0.51, 1.10) |
| Daily napping | 0.64 (0.45, 0.92) |
| SDB | 0.68 (0.50, 0.92) |
Included men with short sleep duration by self-report (n = 984). Participants having a false-positive designation (n = 510) were compared with those having a true-positive designation (short sleep duration by self-report and actigraphy; n = 474). aSee footnotes to Table 1 regarding sample sizes for clinical characteristics. bFrom a multivariable model, including all demographic and clinical characteristics listed in Table 1, that was derived using backward selection with P ≤ .20 to remain in the model. The model maxRSQCv_PCT was 9.34, explaining 9% of total variance in the outcome. CHF = congestive heart failure, CI = confidence interval, MrOS = Osteoporotic Fractures in Men Sleep Study, OR = odds ratio, PSQI = Pittsburgh Sleep Quality Index, SD = standard deviation, SDB = sleep-disordered breathing.
Table 7.
SOF (women): adjusted odds of a false-positive designation (short sleep by self-report but normal sleep duration by actigraphy).
| Characteristicsa | Adjusted OR (95% CI)b |
|---|---|
| Age (per year) | 1.05 (1.01, 1.10) |
| African American | 0.93 (0.62, 1.38) |
| Less than high school | 1.22 (0.87, 1.71) |
| Obesity | 0.61 (0.46, 0.82) |
| Myocardial infarction | 1.20 (0.79, 1.82) |
| Physical impairment | 0.65 (0.49, 0.85) |
| Daytime sleepiness (ESS score > 10) | 0.46 (0.32, 0.67) |
| RLS | 1.37 (0.77, 2.46) |
Included women with short sleep duration by self-report (n = 1,229). Participants having a false-positive designation (n = 808) were compared with those having a true-positive designation (short sleep duration by self-report and actigraphy; n = 421). aSee footnotes to Table 2 regarding sample sizes for clinical characteristics. bFrom a multivariable model, including all demographic and clinical characteristics listed in Table 2, that was derived using backward selection with P ≤ .20 to remain in the model. The model maxRSQCv_PCT was 6.42, explaining 6% of total variance in the outcome. CI = confidence interval, ESS = Epworth Sleepiness Scale, OR = odds ratio, RLS = restless legs syndrome, SOF = Study of Osteoporotic Fractures.
DISCUSSION
We have described the agreement between self-reported and actigraphic short sleep duration (≤ 6 hours) in two large cohorts of community-dwelling older men (MrOS) and women (SOF). Our results show that there was minimal agreement for short sleep duration between self-report and actigraphy in both older men and women, yielding high false-negative and false-positive rates for self-report when compared with the more objective measure of actigraphy.
The frequent disagreement between self-reported and actigraphy-measured sleep duration in older men and women, whether in the direction of false negatives or false positives, has important clinical and research implications. False negatives represent a missed opportunity to intervene on modifiable risk factors and minimize adverse health outcomes. False positives, on the other hand, may lead to unnecessary or potentially harmful interventions (eg, hypnotic use to increase sleep time).39 With respect to research, these results suggest that reliance on self-reported sleep duration may result in misclassification, biasing toward null results. However, it is also possible that the perception of short sleep is the more potent correlate of adverse outcomes, or that self-reported and actigraphy-measured sleep represent different, albeit equally important, constructs that predict distinct adverse outcomes. These concerns underscore the need to confirm self-reported sleep duration with objective measures when evaluating the effect of short sleep on adverse health outcomes. Additionally, whether misclassification of short sleep by self-report leads to adverse health outcomes should be determined.
False-negative and false-positive rates were high in both sexes. Differences between the two cohorts should not be attributed solely to sex since substantial differences in clinical characteristics were present in the two cohorts (see Table S1). It is possible that rates may have been inflated in men due to systematic bias (ie, systematic overestimation of sleep duration by approximately 30 minutes in men by self-report compared to actigraphy). However, agreement among men remained poor, even after accounting for bias. False-positive rates were also high in both sexes. The opposing effect of certain characteristics (eg, obesity, daily napping, and SDB) with respect to false-negative vs false-positive designations suggests there may be a threshold effect, whereby persons with these conditions are aware of short sleep only when sleep duration is severely limited or when deeper stages of sleep are disrupted.
Several potential mechanisms may explain the disagreement between self-reported and objective measures of sleep duration. Previous studies among patients with CHF, SDB, and obesity have reported discrepancies between self-reported and objective sleep measures.40–42 Among patients with SDB and heart failure, Mehra et al found high levels of disagreement between self-reported and objective measures of daytime sleepiness.40 The authors noted an upregulation of inflammatory factors (tumor necrosis factor alpha and interleukin 6) in objective, but not self-reported, sleepiness, and postulated this upregulation may mediate the dissociation between self-reported and objective sleepiness.40 High levels of tumor necrosis factor alpha and interleukin 6 were also documented in a study by Vgontzas et al among persons with SDB, with the highest levels occurring in obese patients.41 In a subsequent study Vgontzas et al found that obesity alone (without SDB) was linked to underestimation of objective daytime sleepiness.42 Similarly, our results from multivariable models in men support the possibility that obesity may be linked to discrepancies between self-reported and objective measures, independent of the effect of SDB. Vgontzas et al invoked central nervous system effects of inflammatory cytokines as potential mediators of objective daytime sleepiness in obese patients and also postulated that a circadian shift may be responsible for more fragmented sleep in obese participants during the night and more consolidated sleep (higher sleep efficiency) during the day when compared to healthy, nonobese controls.42 While it is possible that persons with excessive daytime sleepiness or daily napping might overestimate overnight sleep duration as a result of daytime sleep, a circadian mechanism, similar to the shift postulated by Vgontzas et al among obese persons, may contribute to discrepancies between self-reported and objective sleep measures. Taken together, this body of work suggests disease-mediated inflammation or a circadian shift may contribute to the dissociation between self-reported and objective sleep measures. Interleukin 6 is of particular interest as levels of this cytokine are known to increase with obesity and advancing age, with evidence of sex-based differences in serum levels.43,44 Other potential mechanisms include lifestyle factors (eg, greater flexibility in sleep–wake scheduling)45,46 and neuropsychiatric mechanisms (eg, reduced symptom awareness).7,47
The strengths of the current study include analysis of two large and well-characterized cohorts with availability of validated sleep measures, a focus on vulnerable groups of older men and women at high risk for adverse outcomes, and examination of a broad range of aging-related factors in participants who were not selected for inclusion based on sleep-related criteria. We acknowledge, however, several limitations. First, this was a cross-sectional analysis, precluding evaluation of causal mechanisms or mediation by inflammatory factors. Second, actigraphy is an objective estimate rather than a direct measure of sleep and may estimate sleep poorly, especially in persons with poor sleep quality or highly sedentary status.32,33,48 Nonetheless, actigraphic measures are highly correlated with PSG measures, especially total sleep duration, and actigraphy represents a more feasible methodology in older populations.31 Third, PSG-confirmed SDB was not examined in the full SOF cohort and was not included in multivariable analyses. Fourth, the self-reported and actigraphy measures of sleep duration were not simultaneous and other differences in the methods (eg, the retrospective assessment of self-reported sleep over the past month vs prospective assessment of sleep by actigraphy over 5 nights and the requirement for participants to provide an estimate of sleep duration to the nearest integer) may have contributed to differences in the 2 measures. It is possible that direct comparisons between sleep diary and actigraphy measures of sleep duration may have better agreement. However, in clinical practice sleep duration is most often assessed through a retrospective estimate rather than through use of sleep diaries. In addition, it seems unlikely that variations in methodology alone could explain the large discrepancies between the two measures. Finally, a type 1 error may have occurred in 1 out of 20 comparisons in the multivariable models, which means that between 2 and 3 of the statistically significant results may have been false positives. Given these limitations, future work should confirm the associations demonstrated here. In addition, comparisons of self-reported, actigraphy-measured, and electroencephalography-measured sleep should examine which classification of short sleep is more strongly associated with adverse health outcomes using a longitudinal design that includes PSG measures and a broader array of mediating or moderating factors.
In conclusion, using data from 2 large cohorts of community-dwelling older persons, we have shown that there was minimal agreement between self-reported and actigraphic measures of sleep duration in older men and women, yielding high false-negative and false-positive rates for self-report when compared with objective measures of actigraphy. Our results highlight subgroups (eg, persons with obesity, daytime sleepiness, daily napping, or SDB) in whom an objective measure of sleep duration should be considered. These results have clinical implications, since false-negative designations may represent a missed opportunity to intervene on modifiable risk factors and minimize adverse health outcomes, while false-positive designations may lead to unnecessary or potentially harmful interventions.
DISCLOSURE STATEMENT
All authors have read and approved the manuscript. Dr. Miner is supported by the Claude D. Pepper Older Americans Independence Center at Yale School of Medicine (P30AG021342), the American Academy of Sleep Medicine Foundation, a foundation of the American Academy of Sleep Medicine, and the National Institute on Aging (R03AG073991). Dr. Gill was a recipient of an Academic Leadership Award (K07AG043587) from the National Institute on Aging. Dr. Yaggi is supported by a Midcareer Investigator Award in Patient Oriented Research by the National Heart, Lung, and Blood Institute (K24HL132093). The Study of Osteoporotic Fractures (SOF) is supported by National Institutes of Health funding. The National Institute on Aging (NIA) provides support under the following grant numbers: R01 AG005407, R01 AR35582, R01 AR35583, R01 AR35584, R01 AG005394, R01 AG027574, R01 AG027576, and R01 AG026720. The Osteoporotic Fractures in Men Study (MrOS) is supported by National Institutes of Health (NIH) funding. The following institutes provide support: the National Institute on Aging (NIA), the National Institute of Arthritis and Musculoskeletal and Skin Diseases (NIAMS), the National Center for Advancing Translational Sciences (NCATS), and NIH Roadmap for Medical Research under the following grant numbers: U01 AG027810, U01 AG042124, U01 AG042139, U01 AG042140, U01 AG042143, U01 AG042145, U01 AG042168, U01 AR066160, and UL1 TR000128. The National Heart, Lung, and Blood Institute (NHLBI) provides funding for the MrOS Sleep ancillary study “Outcomes of Sleep Disorders in Older Men” under the following grant numbers: R01 HL071194, R01 HL070848, R01 HL070847, R01 HL070842, R01 HL070841, R01 HL070837, R01 HL070838, and R01 HL070839. The authors report no conflicts of interest.
ABBREVIATIONS
- adjOR
adjusted odds ratio
- CHF
congestive heart failure
- ESS
Epworth Sleepiness Scale
- MrOS
Osteoporotic Fractures in Men Sleep Study
- OR
odds ratio
- PSG
polysomnography
- PSQI
Pittsburgh Sleep Quality Index
- SDB
sleep-disordered breathing
- SOF
Study of Osteoporotic Fractures
REFERENCES
- 1. Watson NF . Sleep duration: a consensus conference . J Clin Sleep Med. 2015. ; 11 ( 1 ): 7 – 8 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Aurora RN , Kim JS , Crainiceanu C , O’Hearn D , Punjabi NM . Habitual sleep duration and all-cause mortality in a general community sample . Sleep. 2016. ; 39 ( 11 ): 1903 – 1909 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Brimah P , Oulds F , Olafiranye O , et al . Sleep duration and reported functional capacity among black and white US adults . J Clin Sleep Med. 2013. ; 9 ( 6 ): 605 – 609 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Devore EE , Grodstein F , Duffy JF , Stampfer MJ , Czeisler CA , Schernhammer ES . Sleep duration in midlife and later life in relation to cognition . J Am Geriatr Soc. 2014. ; 62 ( 6 ): 1073 – 1081 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. World Health Organization . Global Action Plan on the Public Health Response to Dementia 2017-2025. Geneva, Switzerland: : World Health Organization; ; 2017. . [Google Scholar]
- 6. Newman AB , Simonsick EM , Naydeck BL , et al . Association of long-distance corridor walk performance with mortality, cardiovascular disease, mobility limitation, and disability . JAMA. 2006. ; 295 ( 17 ): 2018 – 2026 . [DOI] [PubMed] [Google Scholar]
- 7. Miner B , Gill TM , Yaggi HK , et al . The epidemiology of patient-reported hypersomnia in persons with advanced age . J Am Geriatr Soc. 2019. ; 67 ( 12 ): 2545 – 2552 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Vaz Fragoso CA , Van Ness PH , Araujo KL , Iannone LP , Klar Yaggi H . Age-related differences in sleep-wake symptoms of adults undergoing polysomnography . J Am Geriatr Soc. 2015. ; 63 ( 9 ): 1845 – 1851 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Jackson CL , Patel SR , Jackson WB 2nd , Lutsey PL , Redline S . Agreement between self-reported and objectively measured sleep duration among white, black, Hispanic, and Chinese adults in the United States: Multi-Ethnic Study of Atherosclerosis . Sleep. 2018. ; 41 ( 6 ): 41 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Jackson CL , Ward JB , Johnson DA , Sims M , Wilson J , Redline S . Concordance between self-reported and actigraphy-assessed sleep duration among African-American adults: findings from the Jackson Heart Sleep Study . Sleep. 2020. ; 43 ( 3 ): 43 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Van Den Berg JF , Van Rooij FJ , Vos H , et al . Disagreement between subjective and actigraphic measures of sleep duration in a population-based study of elderly persons . J Sleep Res. 2008. ; 17 ( 3 ): 295 – 302 . [DOI] [PubMed] [Google Scholar]
- 12. Blank JB , Cawthon PM , Carrion-Petersen ML , et al . Overview of recruitment for the osteoporotic fractures in men study (MrOS) . Contemp Clin Trials. 2005. ; 26 ( 5 ): 557 – 568 . [DOI] [PubMed] [Google Scholar]
- 13. Orwoll E , Blank JB , Barrett-Connor E , et al . Design and baseline characteristics of the osteoporotic fractures in men (MrOS) study--a large observational study of the determinants of fracture in older men . Contemp Clin Trials. 2005. ; 26 ( 5 ): 569 – 585 . [DOI] [PubMed] [Google Scholar]
- 14. Cummings SR , Black DM , Nevitt MC , et al .; Study of Osteoporotic Fractures Research Group . Bone density at various sites for prediction of hip fractures . Lancet. 1993. ; 341 ( 8837 ): 72 – 75 . [DOI] [PubMed] [Google Scholar]
- 15. Salive ME . Multimorbidity in older adults . Epidemiol Rev. 2013. ; 35 ( 1 ): 75 – 83 . [DOI] [PubMed] [Google Scholar]
- 16. Studenski S , Perera S , Patel K , et al . Gait speed and survival in older adults . JAMA. 2011. ; 305 ( 1 ): 50 – 58 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Lin MY , Gutierrez PR , Stone KL , et al. ; Study of Osteoporotic Fractures Research Group . Vision impairment and combined vision and hearing impairment predict cognitive and functional decline in older women . J Am Geriatr Soc. 2004. ; 52 ( 12 ): 1996 – 2002 . [DOI] [PubMed] [Google Scholar]
- 18. Matthews CE , Chen KY , Freedson PS , et al . Amount of time spent in sedentary behaviors in the United States, 2003-2004 . Am J Epidemiol. 2008. ; 167 ( 7 ): 875 – 881 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Yesavage JA , Sheikh JI . 9/Geriatric Depression Scale (GDS) . Clin Gerontol. 1986. ; 5 ( 1-2 ): 165 – 173 . [Google Scholar]
- 20. Goldberg D , Bridges K , Duncan-Jones P , Grayson D . Detecting anxiety and depression in general medical settings . BMJ. 1988. ; 297 ( 6653 ): 897 – 899 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Folstein MF , Folstein SE , McHugh PR . “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician . J Psychiatr Res. 1975. ; 12 ( 3 ): 189 – 198 . [DOI] [PubMed] [Google Scholar]
- 22. Spira AP , Blackwell T , Stone KL , et al . Sleep-disordered breathing and cognition in older women . J Am Geriatr Soc. 2008. ; 56 ( 1 ): 45 – 50 . [DOI] [PubMed] [Google Scholar]
- 23. Pahor M , Chrischilles EA , Guralnik JM , Brown SL , Wallace RB , Carbonin P . Drug data coding and analysis in epidemiologic studies . Eur J Epidemiol. 1994. ; 10 ( 4 ): 405 – 411 . [DOI] [PubMed] [Google Scholar]
- 24. Ensrud KE , Blackwell TL , Mangione CM , et al .; Study of Osteoporotic Fractures Research Group . Central nervous system-active medications and risk for falls in older women . J Am Geriatr Soc. 2002. ; 50 ( 10 ): 1629 – 1637 . [DOI] [PubMed] [Google Scholar]
- 25. Cummings SR , Nevitt MC , Browner WS , et al .; Study of Osteoporotic Fractures Research Group . Risk factors for hip fracture in white women . N Engl J Med. 1995. ; 332 ( 12 ): 767 – 773 . [DOI] [PubMed] [Google Scholar]
- 26. Buysse DJ , Reynolds CF 3rd , Monk TH , Berman SR , Kupfer DJ . The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research . Psychiatry Res. 1989. ; 28 ( 2 ): 193 – 213 . [DOI] [PubMed] [Google Scholar]
- 27. Johns MW . Sleepiness in different situations measured by the Epworth Sleepiness Scale . Sleep. 1994. ; 17 ( 8 ): 703 – 710 . [DOI] [PubMed] [Google Scholar]
- 28. Khan A , Harrison SL , Kezirian EJ , et al .; Osteoporotic Fractures in Men (MrOS) Study Research Group . Obstructive sleep apnea during rapid eye movement sleep, daytime sleepiness, and quality of life in older men in Osteoporotic Fractures in Men (MrOS) Sleep Study . J Clin Sleep Med. 2013. ; 9 ( 3 ): 191 – 198 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Watson NF , Badr MS , Belenky G , et al. ; Consensus Conference Panel . Joint consensus statement of the American Academy of Sleep Medicine and Sleep Research Society on the recommended amount of sleep for a healthy adult: methodology and discussion . J Clin Sleep Med. 2015. ; 11 ( 8 ): 931 – 952 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Gildner TE , Liebert MA , Kowal P , Chatterji S , Snodgrass JJ . Associations between sleep duration, sleep quality, and cognitive test performance among older adults from six middle income countries: results from the Study on Global Ageing and Adult Health (SAGE) . J Clin Sleep Med. 2014. ; 10 ( 6 ): 613 – 621 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Smith MT , McCrae CS , Cheung J , et al . Use of actigraphy for the evaluation of sleep disorders and circadian rhythm sleep-wake disorders: an American Academy of Sleep Medicine systematic review, meta-analysis, and GRADE assessment . J Clin Sleep Med. 2018. ; 14 ( 7 ): 1209 – 1230 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Blackwell T , Ancoli-Israel S , Redline S , Stone KL ; Osteoporotic Fractures in Men (MrOS) Study Group . Factors that may influence the classification of sleep-wake by wrist actigraphy: the MrOS Sleep Study . J Clin Sleep Med. 2011. ; 7 ( 4 ): 357 – 367 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Blackwell T , Redline S , Ancoli-Israel S , et al. ; Study of Osteoporotic Fractures Research Group . Comparison of sleep parameters from actigraphy and polysomnography in older women: the SOF study . Sleep. 2008. ; 31 ( 2 ): 283 – 291 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Jean-Louis G , Kripke DF , Mason WJ , Elliott JA , Youngstedt SD . Sleep estimation from wrist movement quantified by different actigraphic modalities . J Neurosci Methods. 2001. ; 105 ( 2 ): 185 – 191 . [DOI] [PubMed] [Google Scholar]
- 35. Bland JM , Altman DG . Statistical methods for assessing agreement between two methods of clinical measurement . Lancet. 1986. ; 1 ( 8476 ): 307 – 310 . [PubMed] [Google Scholar]
- 36. Hopkins WG . Measures of reliability in sports medicine and science . Sports Med. 2000. ; 30 ( 1 ): 1 – 15 . [DOI] [PubMed] [Google Scholar]
- 37. Ohayon MM . Epidemiology of insomnia: what we know and what we still need to learn . Sleep Med Rev. 2002. ; 6 ( 2 ): 97 – 111 . [DOI] [PubMed] [Google Scholar]
- 38. Hosmer DW , Lemeshow S . Assessing the Fit of the Model . In: Applied Logistic Regression. 2nd ed. New York: : John Wiley & Sons; ; 2000. : 143 – 202. [Google Scholar]
- 39. Kripke DF , Langer RD , Kline LE . Hypnotics’ association with mortality or cancer: a matched cohort study . BMJ Open. 2012. ; 2 ( 1 ): e000850 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Mehra R , Wang L , Andrews N , et al . Dissociation of objective and subjective daytime sleepiness and biomarkers of systemic inflammation in sleep-disordered breathing and systolic heart failure . J Clin Sleep Med. 2017. ; 13 ( 12 ): 1411 – 1422 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Vgontzas AN , Papanicolaou DA , Bixler EO , Kales A , Tyson K , Chrousos GP . Elevation of plasma cytokines in disorders of excessive daytime sleepiness: role of sleep disturbance and obesity . J Clin Endocrinol Metab. 1997. ; 82 ( 5 ): 1313 – 1316 . [DOI] [PubMed] [Google Scholar]
- 42. Vgontzas AN , Bixler EO , Tan T-L , Kantner D , Martin LF , Kales A . Obesity without sleep apnea is associated with daytime sleepiness . Arch Intern Med. 1998. ; 158 ( 12 ): 1333 – 1337 . [DOI] [PubMed] [Google Scholar]
- 43. Wellen KE , Hotamisligil GS . Inflammation, stress, and diabetes . J Clin Invest. 2005. ; 115 ( 5 ): 1111 – 1119 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Bonafè M , Olivieri F , Cavallone L , et al . A gender--dependent genetic predisposition to produce high levels of IL-6 is detrimental for longevity . Eur J Immunol. 2001. ; 31 ( 8 ): 2357 – 2361 . [DOI] [PubMed] [Google Scholar]
- 45. Roth T , Coulouvrat C , Hajak G , et al . Prevalence and perceived health associated with insomnia based on DSM-IV-TR; International Statistical Classification of Diseases and Related Health Problems, Tenth Revision; and Research Diagnostic Criteria/International Classification of Sleep Disorders, Second Edition criteria: results from the America Insomnia Survey . Biol Psychiatry. 2011. ; 69 ( 6 ): 592 – 600 . [DOI] [PubMed] [Google Scholar]
- 46. Myllyntausta S , Salo P , Kronholm E , et al . Changes in sleep difficulties during the transition to statutory retirement . Sleep. 2018. ; 41 ( 1 ): 1 – 14 . [DOI] [PubMed] [Google Scholar]
- 47. Miner B , Gill TM , Yaggi HK , et al . Insomnia in community-living persons with advanced age . J Am Geriatr Soc. 2018. ; 66 ( 8 ): 1592 – 1597 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Conley S , Knies A , Batten J , et al . Agreement between actigraphic and polysomnographic measures of sleep in adults with and without chronic conditions: a systematic review and meta-analysis . Sleep Med Rev. 2019. ; 46 : 151 – 160 . [DOI] [PMC free article] [PubMed] [Google Scholar]