Abstract
Study Objectives:
To identify the association between insomnia symptoms and signs of prodromal neurodegeneration, including an analysis of potential differences between sleep-onset and sleep-maintenance insomnia.
Methods:
We included those aged 45–85 years, living in 1 of 10 Canadian provinces between 2012 and 2015 (at the baseline), recruited via 3 population-based sampling methods. Insomnia symptoms were assessed using questions adapted/modified from the Pittsburgh Sleep Quality Index. A panel of potential prodromal neurodegenerative markers including self-reported symptoms and objective gait motor, cognitive, and autonomic variables were assessed cross sectionally. We compared those who endorsed insomnia symptoms ≥ 3 times per week to controls, adjusting for age, sex, and education via logistic regression.
Results:
Overall, 2,051/30,097 people screened positive for sleep-onset insomnia alone and 4,333 for sleep-maintenance insomnia alone, while 2,371 endorsed both subtypes. On objective gait tests, participants with sleep-onset insomnia, but not sleep-maintenance insomnia, had worse balance (odds ratio [OR] = 1.33, 95% confidence interval = [1.16, 1.52]) and slower gait speed (OR = 1.52 [1.34, 1.73]). Although participants with any insomnia subtype endorsed more motor symptoms, these were more severe in those with sleep-onset insomnia (OR onset vs maintenance = 1.13 [1.07, 1.18]). On objective cognitive tests, those with sleep-maintenance insomnia scored normally. However, participants with sleep-onset insomnia performed worse on tests of verbal fluency (OR = 1.24 [1.06, 1.43]), immediate memory (OR = 1.23 [1.08, 1.41]), and prospective memory task (OR = 1.29 [1.11, 1.50]). The sleep-onset insomnia group also had lower heart rate variability (OR = 1.23 [1.07, 1.43]). Secondary analyses found generally similar results in young vs older age of insomnia development.
Conclusions:
Compared to maintenance insomnia, those with sleep-onset insomnia have more motor, cognitive, and autonomic signs/symptoms. When evaluating neurodegenerative risk, differentiating insomnia subtypes may increase precision.
Citation:
Yao CW, Pelletier A, Fereshtehnejad S-M, Cross N, Dang-Vu T, Postuma RB. Insomnia symptom subtypes and manifestations of prodromal neurodegeneration: a population-based study in the Canadian Longitudinal Study on Aging. J Clin Sleep Med. 2022;18(2):345–359.
Keywords: insomnia, neurodegeneration, dementia, movement disorder
BRIEF SUMMARY
Current Knowledge/Study Rationale: Insomnia is one of the most common sleep-related disorders affecting 16–21% of the population worldwide. Among studies to date, most have focused on neurodegeneration associated with primary insomnia, as a whole entity, but on its subtypes.
Study Impact: In this nation-wide population-based study, we found several objective motor, cognitive and autonomic abnormalities in those with symptoms of insomnia. These appear to be primarily driven by sleep-onset difficulties rather than sleep-maintenance insomnia.
INTRODUCTION
Insomnia is one of the most common sleep-related disorders. It is estimated that 16–21% of the general population experiences some difficulty sleeping at least 3 nights per week, and prevalence rises with age worldwide.1 Chronic insomnia has been associated with several major health events, such as cardiovascular diseases, diabetes, mental illnesses, and neurodegenerative diseases.2–5
Symptoms of insomnia are common in neurological diseases of aging, including parkinsonism, dementia, and cerebral vascular events.5,6 They are common early in the disease course, suggesting that they may be present before diagnosis. A recent meta-analysis suggested that insomnia symptoms may increase risk of dementia (including Alzheimer disease) in later life.5 Another recent study from our study group found that insomnia disorder was associated with lower cognitive performance on objective neuropsychological tests compared to individuals without insomnia.7 Insomnia was also found to be more prevalent in probable prodromal parkinsonism in 2 studies.8,9
Among studies to date, most have focused primarily on the association of primary insomnia, as a whole entity, but not the subtypes within.5 This leaves an important gap, as not all insomnia is the same; for example, Parkinson disease is more commonly associated with sleep-maintenance insomnia (MI)10 (falling asleep easily and early but then waking too early), but not difficulty falling asleep (sleep-onset insomnia [OI]), whereas Alzheimer disease is associated with a general circadian rhythm disruption.11 Combining insomnia subtypes together may then mask important differences in neurodegenerative associations.
In this study, we used the baseline data from the Canadian Longitudinal Study on Aging (CLSA), a cohort focused on detecting early signs of aging, recruiting 51,338 participants, aged 45 to 85 years randomly sampled from 10 Canadian provinces, stratified by age.12 The primary focus of the study was to examine to what degree insomnia and its subtypes were associated with objective prodromal motor, cognitive, and autonomic markers of neurodegeneration.
METHODS
Canadian Longitudinal Study on Aging cohort
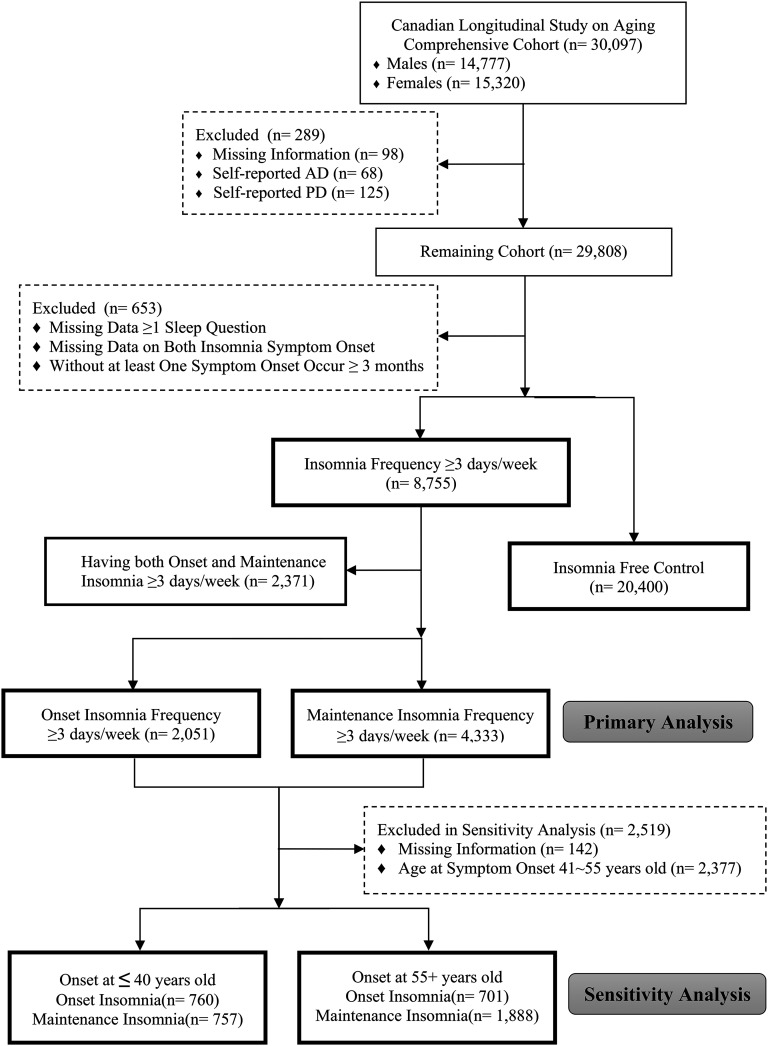
This study was performed using the 30,097-person comprehensive cohort, at the baseline, aged 45-85 years, recruited from the 51,000-person CLSA population-based cohort, as described previously.12 Since the purpose of the study was to assess the associations between insomnia symptoms and risk of parkinsonism and dementia, any participants reporting a diagnosis of dementia/Alzheimer disease or parkinsonism/Parkinson disease (PD) were excluded (details regarding the questionnaires are listed in methods in the supplemental material) (Figure 1).
Figure 1. STROBE flow diagram.
AD = Alzheimer disease, PD = Parkinson disease, STROBE = Strengthening the Reporting of Observational Studies in Epidemiology.
Case definition
Participants were screened for insomnia symptoms with 2 questions during the initial interview, adapted from the Pittsburgh Sleep Quality Index,13 namely:
“Over the last month, how often did it take you more than 30 minutes to fall asleep?” and
“Over the last month, how often did you wake in the middle of the night or too early in the morning and found it difficult to fall asleep again?”
Those who answered “yes” to either of the 2 questions, with symptom frequency of at least 3 nights per week were considered as having insomnia symptoms. Note that a clinical diagnosis of insomnia disorder requires that symptoms also have a detrimental impact on quality of life.14,15 Since our primary research question was centered around the relationship of sleep symptoms per se to neurodegenerative markers, we did not require symptoms to be distressing to participants or to impact their daily function. On secondary analysis, we also assessed relationships between full insomnia disorder and the same neurodegenerative markers, according to International Classification of Sleep Disorders, third edition.16 To avoid measuring acute transient insomnia symptoms, those with symptom onset within 3 months (n = 197) were excluded from the analysis.
Neurodegenerative signs and symptoms
A comprehensive list of self-reported symptoms and functional measures, associated with prodromal parkinsonism or dementia, were preselected to assess the risk of neurodegeneration. All degenerative signs/symptoms were assessed at baseline, expect for the incidence of falls, for which data were acquired approximately 1 year after the baseline visit. The primary variables of interest were objective markers of potential neurodegeneration (ie, dementia and gait/parkinsonism signs). These included:
Quantitative Motor Tasks: standing balance task (amount of time one can retain balanced while standing on 1 leg), timed Sit-to-Stand task (in absence of additional support, the total time needed to rise from sitting on a chair, with both feet on the ground, repeated 5 times), timed 4-m walk task (the amount of time to walk 4 m), timed Up-and-Go task (time needed to rise up from a chair, walk for 3 m, then return to the chair and sit back down),17 hand grip strength test (the strength exerted to squeeze a dynamometer with the dominant hand)18
Cognitive Assessments: verbal fluency ((F-A-S) task), recall task (immediate and delayed modules), Miami Prospective Memory Test19
- Autonomic Neurological Assessments: heart-rate variability (HRV). This was defined as the variation in heart rate between 5 different 1-minute measures separated by 1 minute. HRV was calculated based on the equation for calculating the root mean square of successive RR interval differences.20 This serves as an indirect index of the root mean square standard deviation – a time-domain index that is believed to reflect parasympathetic activities21
(Equation 1)
In addition, we assessed several potential symptoms of neurodegenerative disease. These included:
Motor Symptoms: Tanner’s PD questionnaire (a screening questionnaire for PD that queries 9 different potential symptoms),22 plus the incidence of falls
Other Sleep symptoms: Total hours of sleep < 6 (based on self-reported average hours of sleep), RBD-1Q (a single question screen for rapid eye movement sleep behavior disorder),23 daytime somnolence (a question adapted from Pittsburgh Sleep Quality Index)24
Psychological and Psychiatric Batteries: physician diagnosis of memory problem, physician diagnosis of depressive or anxiety disorder, prior and current use of antidepressants
Somatosensory Symptoms: self-reported chronic pain, assessed via a single question: “Are you usually free of pain or discomfort?”
To assess the associations with potential clinical neurodegenerative signs/symptoms, a cut-off of the bottom 15th percentile of performance was set based on the ranking of raw values. Sociodemographic variables were assessed as previously defined.25
Statistical analyses
Associations between insomnia (as a dependent variable) and assessed variables were estimated using logistic regression analysis adjusting for age and sex. For cognitive variables, we also adjusted for years of education. Any responses labeled as uncertain or “refused to answer” were omitted in all analyses. For heart rate variability analysis, outliers (defined as 1.5 interquartile range below the first quartile or above the third quartile) within each group were excluded. Statistical analysis was performed using R version 3.5.1.
Sensitivity analyses
Because of the differences in age, sex, education, and body mass index, we also conducted a sensitivity analysis using propensity score matching for these four using the nearest neighbor matching method in MatchIt, using a ratio of 1:1.26 Since restless legs syndrome (RLS) may trigger symptoms of insomnia and may be an independent risk factor for disease, we reassessed the associations by 1) adjusting for presence of RLS symptoms and 2) excluding participants with insomnia with RLS symptoms. To address potential confounding by other health conditions, we also conducted analyses adjusting for potential confounders that could also cause positive clinical abnormalities. These included:
Motor Signs: adjusted for arthritis, swelling joints, injuries or surgeries in lower extremity, polio, stroke, transient ischemic attack, diabetes, multiple sclerosis, body mass index, age and sex
Psychiatric and Cognitive Symptoms: stroke, transient ischemic attack, diabetes, diagnosis of depression or anxiety (not used when assessing depression/anxiety), age, and sex
Possible rapid eye movement sleep behavior disorder (RBD): use of antidepressant, posttraumatic stress disorder, age, and sex
Low HRV: any pre-existing cardiological condition, age, and sex
Total Number of Abnormal Items: all of the selected confounding variables listed above.
To address potential associations of insomnia between men and women, we conducted a secondary analyses stratified to sex. Finally, since late-onset insomnia symptoms may be more likely to reflect a recent-onset prodromal neurodegenerative symptom (ie, a prodromal sign rather than a risk factor), we conducted a secondary analysis stratifying participants with insomnia to older age of insomnia onset (> 55 years) vs those with young onset (before age 40 years). The 2 diverging cut-off points for age were to explore differences between insomnia as a lifelong/longstanding “risk factor” vs a recent-onset prodromal disease marker (ie, prodromal neurodegeneration causes insomnia). We chose the cut-offs to allow clear distinction (most neurodegenerative diseases do not start before age 40 years) and to avoid confounds of the perimenopausal state in women.27
Consent data availability
Written consent was obtained from all participants (or guardians of participants) in the study. Data access for the use of this study was reviewed and granted by the CLSA Data and Sample Access Committee. Applicants with a CLSA-approved project and the members of the project teams, with a signature on Schedule F of the CLSA Access Agreement form, are allowed to have direct access to the raw data.
RESULTS
Characteristics of study population
Of 30,097 cohort participants, 289 were excluded due to possible dementia/parkinsonism, and 199 were excluded for missing information on 1 of the insomnia questions. Among the remaining 29,155 participants, 8,755 (29.6%) endorsed symptoms of insomnia starting at least 3 months ago. Two thousand three hundred seventy-one (8.0%) had both OI and MI, 2,051 (6.9%) had isolated sleep-onset symptoms (OI) and 4,333 (14.6%) had isolated sleep-maintenance symptoms (MI) (Figure 1). Fewer than 5% of participants have at least 1 missing item of information on any of the sleep variables assessed in this study.
Sociodemographic features
Regarding all subtypes of insomnia, women were more likely to endorse insomnia symptoms (58.4% with insomnia were female vs 47.6% without) (Table 1). Although both OI and MI symptoms were more common among women, women were more likely to endorse sleep-onset difficulties (adjusted odds ratio [OR] onset vs maintenance = 1.51; 95% confidence interval: 1.35, 1.68). Age was similar between those with insomnia and those without (63.0 ± 10.2 vs 62.6 ± 10.2). No obvious difference in age was found between OI and MI subtypes (OI: 62.6 ± 10.2 vs MI: 62.5 ± 10.2); all subsequent estimates were adjusted by age and sex.
Table 1.
Sociodemographic status.
| No Insomnia (n = 20,400) |
All Insomnia (n = 8,755) |
Onset Insomnia (n = 2,051) |
Maintenance Insomnia (n = 4,333) |
All Insomnia vs. Ctrl |
Onset vs Ctrl | Maintenance vs Ctrl | Onset vs Maintenance | |
|---|---|---|---|---|---|---|---|---|
| n (%) or Mean ± SD | ORAge&Sex_Adjusted [95% CI] | |||||||
| Sex, female | 9,708 (47.6) | 5,109 (58.4) | 1,280 (62.4) | 2,273 (52.5) | 1.54 [1.46, 1.62] | 1.83 [1.66, 2.01] | 1.21 [1.14, 1.29] | 1.51 [1.35, 1.68] |
| Age, y | 63.0 ± 10.2 | 62.6 ± 10.2 | 62.6 ± 10.3 | 62.5 ± 10.2 | 1.00 [0.99, 1.00] | 1.00 [0.99, 1.00] | 1.00 [0.99, 1.00] | 1.00 [1.00, 1.01] |
| Body mass index, kg/m2 | 28.0 ± 5.3 | 28.2 ± 5.7 | 28.5 ± 5.7 | 27.9 ± 5.5 | 1.008 [1.004, 1.012] | 1.02 [1.01, 1.03] | 1.00 [0.99, 1.01] | 1.02 [1.01, 1.03] |
| Ethnicity, Caucasian | 19,486 (96.1) | 8,399 (96.7) | 1,946 (95.7) | 4,188 (97.3) | 1.16 [1.01, 1.33] | 0.86 [0.69, 1.09] | 1.43 [1.18, 1.75] | 0.61 [0.46, 0.81] |
| Married/common law | 15,317 (79.3) | 6,284 (76.9) | 1,405 (74.0) | 3,296 (80.6) | 0.92 [0.87, 0.98] | 0.81 [0.72, 0.90] | 1.13 [1.04, 1.23] | 0.71 [0.62, 0.80] |
| Annual income (per 1,000 CAD) | 59.2 ± 35.7 | 53.3 ± 35.3 | 48.5 ± 33.5 | 58.9 ± 36.3 | 0.9963 [0.9955, 0.9971] | 0.992 [0.991, 0.994] | 1.000 [0.999, 1.001] | 0.992 [0.991, 0.994] |
| Years of education | 13.7 ± 2.3 | 13.4 ± 2.3 | 13.2 ± 2.3 | 13.6 ± 2.3 | 0.94 [0.93, 0.95] | 0.92 [0.90, 0.94] | 0.98 [0.97, 0.99] | 0.94 [0.92, 0.96] |
| Employment status and work schedule | ||||||||
| Retired | 11,239 (55.3) | 4,855 (55.6) | 1,182 (57.9) | 2,333 (54.0) | 1.11 [1.04, 1.19] | 1.30 [1.14, 1.47] | 1.03 [0.94, 1.12] | 1.26 [1.09, 1.45] |
| Daytime job | 18,758 (92.6) | 8,005 (92.2) | 1,848 (91.5) | 4,021 (93.2) | - | - | - | - |
| Non-daytime job | 1,494 (7.4) | 680 (7.8) | 171 (8.5) | 292 (6.8) | 1.06 [0.96, 1.16] | 1.16 [0.98, 1.37] | 0.89 [0.77, 1.01] | 1.31 [1.07, 1.61] |
CAD = Canadian dollars, CI = confidence interval, Ctrl = control, OR = odds ratio, SD = standard deviation.
Participants with OI were less likely to have been married or in a common law relationship (OR to controls = 0.81 [0.72, 0.90]), whereas the opposite relationship to controls was seen among those with MI (OR = 1.13 [1.04, 1.23]). Compared to both controls and those with MI, those with OI had slightly increased weight (0.2 kg difference on average), had slightly fewer total years of education, lower annual income, and were more likely to have retired. Participants with OI were likely to report having a non-daytime work shift (8.5%) than MI participants (6.8%).
Prodromal neurodegenerative signs
Objective motor signs
Overall, those with any type of insomnia symptom were slower at numerous quantitative gait tests, including Timed-Up-and-Go (9.7 vs 9.5 s), 4-m walking speed (4.34 vs 4.25 s), and Sit-to-Stand task speed (2.70 vs 2.66 s) (Table 2 and Table 3). However, when divided according to subtypes, only those with OI demonstrated gait abnormalities. No significant differences in motor performance were observed between controls and those with MI. All motor tests were significantly worse in participants with OI vs those MI (balance time in OI = 36.6 s vs MI = 40.3 s, Timed-Up-and-Go = 9.9 s vs 9.5 s, 4-m walk = 4.34 s vs 4.26 s).
Table 2.
Prodromal neurodegenerative signs in all insomnia combined.
| No Insomnia (n = 20,400) |
All Insomnia (n = 8,755) |
All Insomnia vs Ctrl | |
|---|---|---|---|
| n (%) or Mean ± SD | ORAge&Sex(+Education)_Adjusted [95% CI] | ||
| Motor signs | |||
| Balance task | |||
| Best performance (seconds) | 39.7 ± 23.3 | 38.51 ± 23.48 | - |
| <15th percentile | 2,799 (14.5) | 1,271 (15.6) | 1.16 [1.07, 1.25] |
| Timed-Up-and-Go | |||
| Total time | 9.51 ± 2.39 | 9.69 ± 2.89 | - |
| <15th percentile | 2,862 (14.2) | 1,384 (16.1) | 1.23 [1.14, 1.32] |
| 4-m walk task | 4.25 ± 1.04 | 4.34 ± 1.17 | 1.08 [1.06, 1.11] |
| Sit-to-stand | |||
| Average time/trial | 2.66 ± 0.75 | 2.70 ± 0.86 | 1.07 [1.04, 1.11] |
| Neuropsychiatric signs | |||
| F-A-S verbal fluency task | |||
| Total score | 39.3 ± 12.7 | 39.08 ± 12.83 | - |
| <15th percentile | 3,090 (15.7) | 1,412 (16.7) | 1.06 [0.99, 1.14] |
| Recall task | |||
| Immediate recall | 5.85 ± 1.91 | 5.90 ± 1.89 | - |
| <15th percentile | 4,705 (23.8) | 1,928 (22.8) | 1.01 [0.94,1.07] |
| Delayed recall | 4.04 ± 2.17 | 4.11 ± 2.14 | - |
| <15th percentile | 4,628 (23.5) | 1,834 (21.7) | 0.96 [0.90, 1.03] |
| Miami prospective memory task | |||
| Time-based score | 8.67 ± 0.94 | 8.65 ± 0.95 | 0.97 [0.94, 0.99] |
| Event-based score | 8.45 ± 1.39 | 8.43 ± 1.39 | 0.98 [0.97, 1.00] |
| Accuracy | 11.2 ± 1.7 | 11.2 ± 1.7 | 0.98 [0.96, 0.99] |
| <15th percentile | 3,025 (15.1) | 1,394 (16.2) | 1.13 [1.05, 1.21] |
| Psychomotor speed task and task switching | |||
| Stroop interference error | 0.68 ± 2.02 | 0.71 ± 2.01 | - |
| >85th percentile | 5,180 (25.83) | 2,347 (27.32) | 1.07 [1.01, 1.13] |
| Mental Alternation Task (MMSE) | 26.68 ± 8.78 | 26.42 ± 8.57 | - |
| <15th percentile | 3,009 (15.5) | 1,340 (16.08) | 0.998 [0.927, 1.074] |
| Choice reaction task | |||
| Accuracy | 98.86 ± 3.03 | 98.88 ± 3.03 | - |
| <15th percentile | 5,359 (26.63) | 2,288 (26.53) | 1.04 [0.98, 1.10] |
| Nonmotor signs | |||
| Autonomic abnormality | |||
| Heart rate variability | 29.4 ± 28.6 | 28.5 ± 28.3 | - |
| <15th percentile | 2,924 (14.7) | 1,331 (15.6) | 1.08 [1.01, 1.16] |
CI = confidence interval, Ctrl = control, F-A-S = verbal fluency task, MMSE = Mini–Mental State Examination, OR = odds ratio, SD = standard deviation.
Table 3.
Prodromal neurodegenerative signs among sleep maintenance vs sleep onset insomnia subtypes.
| Onset Insomnia (n = 2,051) |
Maintenance Insomnia (n = 4,333) |
Onset vs Ctrl | Maintenance vs Ctrl | Onset vs Maintenance | |
|---|---|---|---|---|---|
| n (%) or Mean ± SD | ORAge&Sex(+Education)_Adjusted [95% CI] | ||||
| Motor signs | |||||
| Balance task | |||||
| Best performance (seconds) | 36.6 ± 23.7 | 40.3 ± 23.0 | - | - | - |
| <15th percentile | 330 (17.4) | 592 (14.4) | 1.33 [1.16, 1.52] | 1.05 [0.94, 1.16] | 1.28 [1.09, 1.50] |
| Timed-Up-and-Go | |||||
| Total time | 9.90 ± 3.64 | 9.50 ± 2.66 | - | - | - |
| <15th percentile | 378 (18.8) | 576 (13.5) | 1.52 [1.34, 1.73] | 0.98 [0.89, 1.09] | 1.55 [1.33, 1.80] |
| 4-m walk task | 4.34 ± 1.17 | 4.26 ± 1.17 | 1.13 [1.08, 1.17] | 1.01 [0.98, 1.04] | 1.11 [1.06, 1.17] |
| Sit-to-stand | |||||
| Average time/trial | 2.79 ± 1.06 | 2.63 ± 072 | 1.21 [1.14, 1.28] | 0.95 [0.91, 1.00] | 1.27 [1.18, 1.36] |
| Neuropsychiatric signs | |||||
| F-A-S verbal fluency task | |||||
| Total score | 38.4 ± 12.9 | 39.8 ± 12.8 | - | - | - |
| <15th percentile | 364 (18.3) | 630 (15.0) | 1.15 [1.01, 1.30] | 0.97 [0.88, 1.07] | 1.18 [1.01, 1.37] |
| Recall task | |||||
| Immediate recall | 5.83 ± 1.94 | 5.95 ± 1.88 | - | - | - |
| <15th percentile | 496 (25.0) | 918 (21.9) | 1.127 [1.004, 1.264] | 0.96 [0.88, 1.04] | 1.19 [1.04, 1.37] |
| Delayed recall | 4.06 ± 2.18 | 4.14 ± 2.15 | - | - | - |
| <15th percentile | 461 (23.2) | 897 (21.4) | 1.07 [0.95, 1.20] | 0.93 [0.86, 1.02] | 1.17 [1.02, 1.35] |
| Miami prospective memory task | |||||
| Time-based score | 8.63 ± 0.98 | 8.69 ± 0.88 | 0.95 [0.91, 1.00] | 1.01 [0.97, 1.05] | 0.94 [0.89, 1.00] |
| Event-based score | 8.37 ± 1.50 | 8.48 ± 1.32 | 0.96 [0.93, 0.99] | 1.01 [0.98, 1.04] | 0.95 [0.91, 0.99] |
| Accuracy | 11.1 ± 1.8 | 11.3 ± 1.6 | 0.96 [0.94, 0.99] | 1.00 [0.98, 1.02] | 0.96 [0.93, 0.99] |
| <15th percentile | 359 (17.8) | 614 (14.4) | 1.24 [1.09, 1.40] | 0.98 [0.88, 1.07] | 1.27 [1.09, 1.47] |
| Psychomotor speed task and task switching | |||||
| Stroop interference error | 0.77 ± 1.98 | 0.63 ± 1.96 | - | - | - |
| >85th percentile | 578 (28.73) | 1,050 (24.59) | 1.13 [1.01, 1.25] | 0.95 [0.88, 1.03] | 1.19 [1.05, 1.35] |
| Mental alternation task | 25.83 ± 8.9 | 27.16 ± 8.35 | - | - | - |
| <15th percentile | 367 (18.82) | 561 (13.55) | 1.16 [1.02, 1.32] | 0.86 [0.78, 0.95] | 1.38 [1.18, 1.60] |
| Choice reaction task | |||||
| Accuracy | 98.72 ± 3.28 | 98.97 ± 2.93 | - | - | - |
| <15th percentile | 595 (29.4) | 1,056 (24.71) | 1.19 [1.07, 1.32] | 0.94 [0.87, 1.02] | 1.27 [1.13, 1.44] |
| Nonmotor signs | |||||
| Autonomic abnormality | |||||
| Heart-rate variability | 27.6 ± 24.0 | 29.7 ± 30.5 | - | - | - |
| <15th percentile | 340 (17.1) | 605 (14.3) | 1.20 [1.06, 1.36] | 0.98 [0.89, 1.08] | 1.24 [1.07, 1.43] |
CI = confidence interval, Ctrl = control, F-A-S = verbal fluency task, OR = odds ratio, SD = standard deviation.
Neuropsychiatric assessments
The combined insomnia group did not, on average, differ from controls on cognitive performance except for reduced performance in task switching (Stroop ORadj = 1.07 [1.01, 1.13]) and prospective memory task (ORadj = 1.13 [1.05, 1.21]). However, when differentiated by subtype, participants with OI were more likely to score > 1 standard deviation below mean on numerous measures than both controls and those with MI. These included verbal fluency (adjusted OR of lower 15th percentile = 1.15 [1.01, 1.30] to controls), memory (immediate recall ORadj = 1.127 [1.004, 1.264], prospective memory ORadj = 1.24 [1.09,1.40]), task switching (Stroop ORadj = 1.13 [1.02, 1.26], mental alternation ORadj = 1.16[1.03,1.32]), and psychomotor speed (ORadj = 1.19 [1.07, 1.32]). There was no difference in any neuropsychological measure between patients with MI and controls.
Heart-rate variability
On the index of HRV, participants with insomnia overall had less fluctuation in between heartbeats (index = 29.4 vs 28.5) than controls, indicating possible sympathetic autonomic denervation (Table 2). When differentiated by subtype, sleep-onset participants had lower HRV (27.6) and were more likely to fall within the lowest 15th percentile of HRV (17.1 vs14.7% in control; ORadj = 1.20[1.06,1.36]). However, neither measure of HRV in the MI group was significantly different from controls.
Subjective measures
Motor symptoms
In terms of motor symptoms, the combined insomnia group reported more motor symptoms than controls (0.7 vs 0.5 symptoms on the 9-item Tanner Parkinson screening questionnaire) (Table 4). When differentiated by subtype, participants with OI reported more motor symptoms than those with MI (0.7 vs 0.6, OR = 1.13 [1.07,1.18]); 8.7% of participants with OI endorsed ≥ 3 symptoms (the threshold for a positive parkinsonism screen), compared to 4.5% of controls and 5.4% of those with MI) (Table 5). Among the individual motor symptoms assessed in Tanner’s questionnaire, most symptoms were associated with both insomnia subtypes (Table S2 in the supplemental material), except that gait freezing and festination were similar between those with MI and controls. Other than gait freezing, all symptoms related to poor function in lower extremities were more common in OI than MI groups. OI participants were more likely to report having a fall in the following year after the initial interview (13.2%) than controls (10.0%) or MI participants (11.2%).
Table 4.
Prodromal neurodegenerative symptoms in all insomnia combined.
| No Insomnia (n = 20,400) |
All Insomnia (n = 8,755) |
All Insomnia vs Ctrl | |
|---|---|---|---|
| n (%) or Mean ± SD | ORAge&Sex(+Education)_Adjusted [95% CI] | ||
| Motor symptoms | |||
| Tanner questionnaire | |||
| Overall score | 0.46 ± 0.92 | 0.67 ± 1.14 | 1.22 [1.19, 1.25] |
| Score ≥ 3 | 909 (4.46) | 669 (7.64) | 1.86 [1.68, 2.07] |
| Fall (last year) | |||
| At least 1 fall | 1,959 (10.0) | 1,047 (12.6) | 1.26 [1.16, 1.36] |
| Number of falls | 1.40 ± 1.57 | 0.20 ± 0.90 | 1.11 [1.07, 1.15] |
| Neuropsychiatric symptoms | |||
| Psychiatric and cognitive symptoms | |||
| Self-reported memory problem | 270 (1.33) | 171 (1.95) | 1.52 [1.25, 1.84] |
| Depression/anxiety | 3,618 (17.8) | 2,271 (26.0) | 1.53 [1.44, 1.62] |
| Prescribed antidepressant | 1,474 (7.24) | 868 (9.96) | 1.31 [1.20, 1.43] |
| Nonmotor symptoms | |||
| Sleep | |||
| Sleep hours | 7.14 ± 1.06 | 6.03 ± 1.30 | 0.41 [0.40, 0.42] |
| Poor sleep quality | 2,239 (11.0) | 5,104 (58.3) | 11.3 [10.6, 12.0] |
| Daytime sleepinessa | 1,292 (6.34) | 1,253 (14.3) | 2.55 [2.35, 2.77] |
| Possible RLS symptoms | 2,796 (13.84) | 1,924 (22.33) | 1.71 [1.60, 1.82] |
| Possible RBD | 517 (3.07) | 279 (4.08) | 1.41 [1.21, 1.64] |
| Pain | |||
| Chronic pain (most days) | 6,432 (32.9) | 3,798 (45.7) | 1.68 [1.59, 1.77] |
aDaytime sleepiness was defined as participants experiencing trouble staying awake during daytime at least 6 days per week for minimum of 3 month. Participants who slept less than 6 hours on average per night or self-reported endorsing narcolepsy were excluded. CI = confidence interval, Ctrl = control, OR = odds ratio, RBD = rapid eye movement sleep behavior disorder, RLS = restless legs syndrome, SD = standard deviation.
Table 5.
Prodromal neurodegenerative symptoms among sleep maintenance vs sleep onset insomnia subtypes.
| Onset Insomnia (n = 2,051) | Maintenance Insomnia (n = 4,333) |
Onset vs Ctrl | Maintenance vs Ctrl | Onset vs Maintenance | |
|---|---|---|---|---|---|
| n (%) or Mean ± SD | ORAge&Sex(+Education)_Adjusted [95% CI] | ||||
| Motor symptoms | |||||
| Tanner questionnaire | |||||
| Overall score | 0.70 ± 1.19 | 0.57 ± 1.00 | 1.26 [1.21, 1.31] | 1.14 [1.10, 1.18] | 1.13 [1.07, 1.18] |
| Score ≥3 | 179 (8.73) | 235 (5.42) | 2.17 [1.83, 2.58] | 1.29 [1.11, 1.49] | 1.68 [1.37, 2.07] |
| Fall (last year) | |||||
| At least 1 fall | 255 (13.2) | 463 (11.2) | 1.30 [1.13, 1.50] | 1.12 [1.00, 1.24] | 1.17 [1.00, 1.38] |
| Number of falls | 1.65 ± 2.60 | 1.40 ± 1.05 | 1.06 [1.00, 1.12] | 1.00 [0.93, 1.07] | 1.09 [1.00, 1.21] |
| Neuropsychiatric symptoms | |||||
| Psychiatric and cognitive symptoms | |||||
| Self-reported memory problem | 43 (2.10) | 69 (1.59) | 1.65 [1.17, 2.26] | 1.23 [0.94, 1.60] | 1.40 [0.94, 2.05] |
| Depression/anxiety | 666 (32.6) | 889 (20.6) | 2.06 [1.86, 2.28] | 1.15 [1.06, 1.25] | 1.81 [1.61, 2.05] |
| Prescribed antidepressant | 311 (15.3) | 251 (5.82) | 2.09 [1.82, 2.38] | 0.76 [0.66, 0.87] | 2.82 [2.36, 3.36] |
| Nonmotor symptoms | |||||
| Sleep | |||||
| Sleep hours | 6.5 ± 1.3 | 6.1 ± 1.2 | 0.57 [0.55, 0.59] | 0.40 [0.39, 0.42] | 1.30 [1.24, 1.35] |
| Poor sleep quality | 901 (44.0) | 2,446 (56.5) | 6.27 [5.68, 6.92] | 10.5 [9.8, 11.3] | 0.59 [0.53, 0.66] |
| Daytime sleepinessa | 216 (10.6) | 571 (13.2) | 1.82 [1.56, 2.11] | 2.30 [2.07, 2.55] | 0.80 [0.67, 0.94] |
| Possible RLS symptoms | 494 (24.56) | 824 (19.27) | 1.90 [1.70, 2.12] | 1.46 [1.34, 1.59] | 1.31 [1.15, 1.49] |
| Possible RBD | 67 (4.29) | 128 (3.65) | 1.54 [1.17, 1.98] | 1.22 [1.00, 1.48] | 1.23 [0.91, 1.67] |
| Pain | |||||
| Chronic pain (most days) | 912 (47.1) | 1,713 (41.3) | 1.75 [1.59, 1.92] | 1.43 [1.33, 1.53] | 1.23 [1.10, 1.37] |
Daytime sleepiness was defined as participants experiencing trouble staying awake during daytime at least 6 days per week for minimum of 3 month. Participants who slept less than 6 hours on average per night or self-reported endorsing narcolepsy were excluded. CI = confidence interval, Ctrl = control, OR = odds ratio, RBD = rapid eye movement sleep behavior disorder, RLS = restless legs syndrome, SD = standard deviation.
Other nonmotor symptoms
With regard to other sleep problems, insomnia participants overall slept less than controls (6.0 vs 7.1 hours) and were more likely to report poor sleep quality (58.3% vs 11.0% in controls), daytime sleepiness (14.4% vs 6.3%), and possible RBD-related dream enactment behavior (7.8% vs 4.5%). Divided according to subtype, both OI and MI subtypes had fewer hours of sleep than controls, with participants with MI having less sleep than those with OI (controls = 7.1, OI = 6.5 hours; MI = 6.1 hours). Although both insomnia subgroups endorsed daytime somnolence, those with MI reported this more often (OI = 10.6%; MI = 13.2%). On the contrary, the prevalence of possible RBD-related symptoms, although higher in both groups (OI = 7.2%, MI = 5.7%, controls = 4.5%), was highest among participants with OI (ORadj = 1.82 [1.44, 2.29] to controls).
Whereas insomnia combined was associated with increase in self-reported memory troubles compared to controls (2.0% vs 1.3%), this difference was driven mainly by participants with OI (OI = 2.1%, MI = 1.6%) (Table 3). Although clinically diagnosed depression and/or anxiety were more prevalent in both insomnia groups than controls, the prevalence was much larger in OI (OI = 32.6%, MI = 20.6%, controls = 17.8%). Use of antidepressants was the most common among OI participants (OI = 15.3%, controls = 7.2%) but was less common in sleep-maintenance group (5.8%). Both groups of participants with insomnia were more likely to report frequent pain than controls, again with participants with OI more likely to report having pain than those with MI (controls = 32.9%, OI = 47.1%, MI = 41.3%).
Sensitivity analyses
Modeling and adjustment
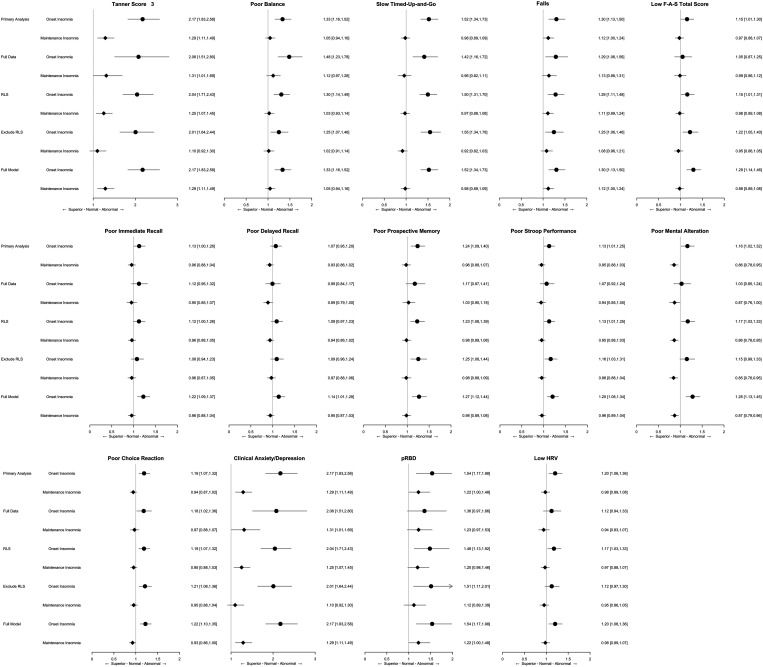
Since RLS may induce insomnia, we reassessed after adjusting for the presence of RLS symptoms and performed a subgroup analysis of those without RLS symptoms. After adjusting for RLS, most associations remained similar except for an attenuation of relationships with verbal fluency, memory tasks (immediate and delay recall), and task switching (Figure 2). Results remained similar after excluding those with RLS symptoms among participants with insomnia. To address potential confounding between insomnia and other health events, we also assessed the relationship between insomnia and several preselected disease comorbidities and conducted regression analyses with these additional covariates. Participants with MI, but not those with OI, were more likely to have history of both cerebral vascular attack (1.89 vs 1.50%; ORadj = 1.33 [1.03, 1.69]) and/or transient ischemic attack (3.41 vs 2.91%; ORadj = 1.24 [1.03, 1.49]) compared to controls (Table S4 in the supplemental material). Diabetes was more common in participants with OI than the controls (21.2% vs 16.8%, OR = 1.43 [1.26, 1.59]) and participants with MI (17.90%; ORadj = 1.29 [1.13, 1.47]). Adding these variables to the model had modest effects, again with attenuation of some cognitive measures (Figure 2).
Figure 2. Associations between neurodegenerative signs/symptoms and insomnia subtypes.
Primary analysis: age and sex (+ education and language). Full data: age and sex (+ education and language) among participants with complete information of the assessed neurodegenerative sign/symptoms. RLS: primary analysis + RLS. Exclude RLS: age and sex (+ education and language) with insomnia positive participants without RLS. Full model: adjusted with RLS, and the following classified according to the variable categories: aMotor sign: arthritis, injuries or surgeries, swelling joins in lower extremity, polio, stroke, transient ischemic attack, diabetes, multiple sclerosis, age, and sex. bPsychiatric and psychological symptoms: stroke, transient ischemic attack, diabetes, depression/anxiety, age, sex, and total years of education. cPossible RBD: use of antidepressant, posttraumatic stress disorder, age, and sex. dLow HRV: any pre-existing cardiological condition, age, and sex. eNumbers of abnormal items: all of the selected confounding variables listed above. F-A-S = verbal fluency task, HRV = heart-rate variability, pRBD = possible rapid eye movement sleep behavior disorder, RBD = rapid eye movement sleep behavior disorder, RLS = restless legs syndrome.
Demographic matching
Due to the differences in demographic characteristics among the 3 study groups, we performed a sensitivity analysis on a subsample that was closely matched for age, sex, body mass index, and years of education (n = 2,041 participants for each group). Point estimates of all OR were broadly similar to that of the primary analysis (Table S3 in the supplemental material), although in some cases, the previous observed associations within this smaller subgroup became nonsignificant (eg, diagnosis of poor cognition and average low HRV were significantly more common in OI than MI, but not more when comparing to controls).
Insomnia disorder
We reassessed all the associations in a subgroup of individuals who fulfilled the clinical diagnosis of insomnia disorder (ie, adding a requirement for impact of sleep symptoms on function). Overall, patterns of effects were similar to the broader group. As in the broader group, insomnia disorders were more common in female participants and associated with fewer total years of education and younger age. Participants with onset insomnia disorder were also slightly heavier than the controls. (Table 6). Isolated OI disorder was associated with poorer motor function than those without insomnia and those with maintenance insomnia disorder. Of the cognitive tasks, poor verbal fluency and choice reaction retained their associations with isolated OI disorder, with no associations found in those with the MI. Anxiety/depression were strongly associated with both insomnia disorders. Possible RBD and low HRV were associated with isolated OI disorders but not the MI. Results were similar after adjustment for the presence of RLS symptoms.
Table 6.
Prodromal neurodegenerative signs and symptoms according to diagnosis of possible insomnia disorder.
| Isolated Onset Insomnia (n = 473) |
Isolated Maintenance Insomnia Disorder (n = 1,044) |
Onset vs Ctrl | Maintenance vs Ctrl | Onset vs Maintenance | |
|---|---|---|---|---|---|
| n (%) or Mean ± SD | ORAge&Sex(+Education)_Adjusted [95% CI] | ||||
| Demography | |||||
| Sex, female | 317 (67.0) | 584 (55.9) | 2.21 [1.83,2.69] | 1.38 [1.22,1.57] | 1.60 [1.28,2.01] |
| Age, y | 60.4 ± 9.8 | 60.1 ± 9.7 | 0.97 [0.96,0.98] | 0.97 [0.97,0.98] | 1.00 [0.99,1.01] |
| Body mass index, kg/m2 | 27.8 ± 5.6 | 29.3 ± 6.4 | 1.04 [1.02,1.05] | 0.99 [0.98,1.01] | 1.05 [1.03,1.06] |
| Years of education | 13.7 ± 2.2 | 13.3 ± 2.2 | 0.91 [0.88,0.95] | 0.98 [0.95,1.01] | 0.93 [0.88,0.98] |
| Clinical signs/symptoms | |||||
| Motor sign | |||||
| Tanner score >3 | 56 (11.8) | 66 (6.32) | 3.81 [2.80,5.09] | 1.82 [1.38,2.35] | 2.17 [1.47,3.20] |
| Poor balance | 72 (16.5) | 115 (11.6) | 1.62 [1.22,2.12] | 1.00 [0.81,1.23] | 1.66 [1.17,2.35] |
| Slow Timed-Up-and-Go | 110 (23.8) | 135 (13.1) | 2.81 [2.21,3.55] | 1.17 [0.96,1.42] | 2.30 [1.70,3.11] |
| Fall (in last year) | 68 (15.3) | 122 (12.2) | 1.57 [1.19,2.02] | 1.25 [1.02,1.51] | 1.27 [0.91,1.74] |
| Psychiatric and psychological symptoms | |||||
| Low F-A-S total score | 84 (18.5) | 134 (13.2) | 1.33 [1.03,1.70] | 0.92 [0.76,1.12] | 1.44 [1.04,1.97] |
| Poor immediate recall | 98 (21.4) | 184 (18.3) | 1.14 [0.89,1.44] | 0.89 [0.75,1.05] | 1.32 [0.97,1.78] |
| Poor delayed recall | 88 (19.1) | 183 (18.1) | 1.00 [0.78,1.28] | 0.87 [0.73,1.04] | 1.17 [0.86,1.59] |
| Poor prospective memory | 118 (25.4) | 250 (24.2) | 1.08 [0.87,1.34] | 1.04 [0.89,1.21] | 1.06 [0.81,1.37] |
| Poor Stroop performance | 79 (17.5) | 137 (13.7) | 1.24 [0.95,1.59] | 0.98 [0.81,1.18] | 1.23 [0.89,1.69] |
| Poor mental alteration | 132 (28.1) | 254 (24.6) | 1.21 [0.98,1.48] | 0.98 [0.84,1.13] | 1.27 [0.99,1.63] |
| Poor choice reaction | 95 (20.4) | 144 (14.1) | 1.80 [1.41,2.28] | 1.10 [0.91,1.32] | 1.61 [1.18,2.17] |
| Clinical anxiety/depression | 230 (48.9) | 290 (27.8) | 3.85 [3.19,4.64] | 1.63 [1.41,1.88] | 2.40 [1.91,3.02] |
| Nonmotor signs | |||||
| pRBD | 18 (5.08) | 29 (3.58) | 1.86 [1.11,2.93] | 1.22 [0.82,1.76] | 1.52 [0.81,2.76] |
| Low HRV | 84 (18.3) | 143 (14) | 1.43 [1.12,1.82] | 1.03 [0.85,1.23] | 1.42 [1.05,1.91] |
| Other sleep symptoms | |||||
| Sleep | |||||
| Poor sleep quality | 267 (56.57) | 689 (66) | 9.99 [8.28,12.1] | 15.2 [13.3,17.4] | 0.67 [0.54,0.84] |
| Possible RLS symptoms | 131 (28.7) | 215 (20.9) | 2.34 [1.89,2.87] | 1.62 [1.38,1.89] | 1.46 [1.13,1.89] |
CI = confidence interval, Ctrl = control, F-A-S = verbal fluency task, HRV = heart-rate variability, OR = odds ratio, pRBD = possible rapid eye movement sleep behavior disorder, RBD = rapid eye movement sleep behavior disorder, RLS = restless legs syndrome, SD = standard deviation.
Secondary analyses
Men vs women
The relationship between prodromal markers and insomnia was present in both sexes (Table S5 in the supplemental material). However, associations were generally more robust in men than in women. Except for HRV, the point estimate of each marker’s OR was higher in men. In the case of verbal fluency and possible RBD, the 95% confidence interval between men and women did not overlap, and the strength of association was stronger in men.
Age of onset
To address the potential differences between lifelong insomnia (as a potential risk factor for disease) and recent-onset insomnia (as a possible prodromal marker of disease), we stratified groups into young-onset (≤ 40 years old) and older-onset (≥ 55 years old) insomnia (Table S6 and Table S7 in the supplemental material) The older-onset group was generally more likely to endorse isolated sleep-maintenance rather than OI. Self-reported motor symptoms were more common in young-onset MI than those with older-onset (eg, OR falls = 1.30 [1.03, 1.63] for young-onset maintenance group vs 1.01 [0.86, 1.18] for the older-onset maintenance group). However, for all objective neurodegenerative markers, no clear differences between early and late onset were seen.
Other confounders
We conducted additional analysis adjusting for additional confounders, including numerous major health events, smoking, and heavy drinking. Results were generally similar to models without these variables (eg, see Table S4; other data not shown).
DISCUSSION
Capitalizing upon a large population-based cohort, in which an array of objective neurological markers were assessed, we were able to explore the relationship between symptoms/subtypes of insomnia and signs of potential prodromal neurodegeneration. On numerous objective measures, participants with insomnia overall had worse gait function (balance and transfer/gait speed/turning), cognition (prospective memory and choice reaction task), and lower heart rate variability than controls. This was in addition to numerous self-reported motor/cognitive symptoms. However, when divided according to subtype, most of these differences were seen specifically in those with OI, with few differences between participants with MI and controls. Stratifying the cohort by sex or age-at-symptom-onset produced similar results.
Motor dysfunction
Our study found that those with insomnia symptoms, particularly OI, were more likely to endorse motor symptoms and have motor slowing on objective gait tests. Our findings are consistent with prior studies. In a study using the Taiwan National Health Insurance program, insomnia (as a global symptom) marked an increased risk of developing parkinsonism.28 In the United Kingdom primary care database,9 patients with PD were 1.4 times more likely to have visited a health care professional for insomnia 0–2 years before PD diagnosis (no significant relationship was seen at longer prediagnostic intervals). In a Taiwanese retrospective study, insomnia was associated with a 2-fold increased risk of PD, from as long as 7 to 10 years from baseline evaluation.28 Neither of these studies assessed sleep-onset vs MI subtypes. A study of patients with idiopathic rapid eye movement sleep behavior disorder (the strongest known prodromal marker of PD) found higher prevalence of insomnia compared to controls. However, insomnia symptoms in patients with RBD at baseline did not increase the risk of phenoconversion later on.8 This study did compare insomnia subtypes, finding that MI was more common among idiopathic RBD (iRBD) patients at baseline, but resolved over time (perhaps reflecting either progressive somnolence/sleep drive or treatment-related reduction in arousals caused by directly by RBD).
The fact that OI and MI were associated with motor deficits is somewhat surprising, considering that MI is the most common subtype observed in PD. Of note, the objective measures in this study were gait measures; therefore, gait problems unrelated to PD may underlie the effect; these might include consequences of cerebrovascular lesions, prodromal Alzheimer dementia symptoms (Alzheimer disease is much more common than PD, so even smaller prodromal motor changes could drive differences29,30), or other unrecognized confounds/conditions. Falling and other gait dysfunction may predate diagnosis of “vascular parkinsonism”, which is consistent with the findings in a recent meta-analysis suggesting that insomnia disorder is a risk factor for stroke.31 Several previous meta-analysis studies have identified that insomnia symptoms as a whole are associated with new incidence of stroke and cerebral vascular events but do not contribute to mortality.32,33 Prospective follow-up will be able to address whether OI is a risk factor for (vascular) parkinsonism in our population.
Cognition and nonmotor symptoms
Overall, we found a modest association between insomnia symptoms and poor cognitive performance on certain tasks, which was evident only in those with OI, even after adjusting for depression/anxiety, stroke, transient ischemic attack, diabetes, pain, apnea, RLS, and possible comorbid conditions (Figure 2 and Table S4, Table S9, and Table S10 in the supplemental material). These results are broadly similar to a recent study using the same cohort, in which patients with full defined insomnia disorder had increased cognitive impairment, without any clear differences between MI and OI (although power was insufficient for direct comparison).7 Note that the case definition of insomnia was not the same as the current study; here we were interested in studying whether changes in sleep per se are associated with neurodegenerative markers (ie, irrespective of a perceived negative impact upon quality of life, which is required for an insomnia clinical diagnosis). Our results are consistent with other prospective studies suggesting that insomnia may be a prodromal dementia symptom. These include 2 Taiwanese population-based studies that found that insomnia increases patients’ risk of developing dementia after adjusting for vascular- events and other related confounders.34,35 Hoile et al36 noted an increase in risk of developing dementia retrospectively among those with prior diagnosis of insomnia up to a decade. Similarly, Osorio et al37 also reported a 2.39-fold increased dementia risk among 655 New Yorkers with insomnia. In a retrospective all-male veteran US study (aged 55 years and above), insomnia at midlife was associated with 27% increased risk of developing various dementia subtypes, except vascular and Lewy body dementia.38 A Swedish study also found an increased risk in the occurrence of dementia among those with long-term insomnia.39 By contrast, the Honolulu-Asia Aging Study reported that insomnia was not able to predict the occurrence of cognitive decline or dementia among Asian men.40 Moreover, the prospective French Three-City Study found no association between insomnia and cognitive decline over an 8-year follow-up period41; this study also found that those endorsing MI were less likely to experience cognitive decline. Therefore, these findings suggest that any future study measuring insomnia as a risk factor for dementia should carefully delineate onset vs maintenance subtypes (Table S2).
Among the specific cognitive assessments, poor performance in prospective memory and choice reaction task were persistently associated with OI, even after adjusting for cerebral vascular events and related-health events (Figure 2). Since no difference was found when assessing the time needed to complete the tasks, the observed pattern may be associated with attention deficit and poor execution. Abnormalities on these tests can be associated with Alzheimer disease, vascular dementia, and Lewy body dementia, even during their prodromal phases.42–45 However, 2 recent UK Biobank Mendelian randomized studies found no association between overall insomnia and Alzheimer disease-related genetic risks.46,47 In our study, when assessing the cardinal signs of Alzheimer dementia, using the recall tasks, we found no association when pooling all insomnia symptoms together either (Table 2). Although we did observe a mild association between onset-insomnia symptom and poor immediate recall, the associations were stronger with non-Alzheimer dementia specific signs (such as poor choice reaction and mental alternation tests). Prospective follow-up will help to determine whether these tests can identify specific subtypes of dementia for which OI is a risk factor.
In addition to the motor signs, we also observed a relatively persistent association between OI and nonmotor signs/symptoms even after adjusting for multiple comorbidities. Depression and anxiety were associated with insomnia; these are well-established but nonspecific risk factors for many diseases and health event and may predate the phenoconversion up to 2 decades.48–51 There is also growing evidence showing that cardiac autonomic dysfunction manifested as loss of the normal HRV during midlife is a risk factor for developing cognitive impairment and dementia later in life.52,53 Interestingly, in our study, participants with OI had lower HRV and endorsed poorer performance in certain cognitive batteries.
Limitations and strengths
Some limitations of this study should be noted. Since insomnia diagnosis is primarily based on self-report, it is, by definition, subject to recall/reporting bias. This may manifest itself as differences in recognition of symptoms (eg, poor insight into insomnia may be more common in those with memory impairment), inaccurate recall of time of onset, or potential overreporting of symptoms among patients with anxiety or depressive disorders (although we saw no clear differences in effect when adjusting for mental illness) (Figure 2). The absence of information on sleep medications is another unmeasured confounder in this study, as long-term usage of certain medications (eg, long-acting benzodiazepines) may impair cognition (note that this should not clearly account for the observed differences in sleep onset vs maintenance insomnia). Although we were able to adjust for the potential confounding effects of comorbid sleep symptoms, comorbid medical conditions, and smoking/drinking in the sensitivity analyses, additional unmeasured confounds may still exist. Motor assessments were limited to gait measures; studies in other populations have suggested that upper limb tests (eg, Purdue Peg Board or Alternative Finger Tap Test) may be more sensitive for detecting early prodromal parkinsonism.50 Assessment of HRV used only the variation in pulse between 5 consecutive measures and should be considered exploratory. Because of the nature of the pulse data (5 independent pulse rates), we were unable to examine specific patterns of abnormality such as high-frequency, low-frequency, and very-low-frequency alterations. The recruitment procedures of the CLSA exclude those with baseline dementia from assessment; thus, we are unable to assess links between insomnia and dementia in this cross-sectional study. It is notable that participants with OI seem to be worse on numerous measures of health, depression, etc; this might suggest that unmeasured confounds could underlie the association between sleep and neurodegenerative markers. Finally, this is a cross-sectional study; prospective follow-up (ongoing) will allow direct assessment of whether insomnia predicts dementia or (vascular) parkinsonism.
On the other hand, this study has some important advantages. Because the study has a large sample size that used population-based sampling, the association between OI and neurodegeneration signs/symptoms were likely to be representative to the true population. With detailed assessment of general health events, we were able to adjust for multiple potential confounders. The fact that gait and cognition were assessed with standardized objective measures minimizes effects of response bias. Because we also screened for RLS, we were able to address confounding by potential RLS symptoms, finding results that were relatively similar (noting, however, that RLS screen does not include clinician interview to rule out mimics). Similarly, none of the potential interaction terms between other sleep disorders and the use of antidepressant significantly alter the associations (among the nonpsychiatric variables) found in the primary analyses.
In summary, our study found several objective motor and cognitive abnormalities in those with symptoms of insomnia. These appear to be largely driven by abnormalities in sleep onset, rather than MI. Future prospective studies will help confirm to what degree insomnia subtypes predict neurodegenerative disorders (eg, dementia, vascular diseases, and parkinsonism).
DISCLOSURE STATEMENT
All authors have seen and approved this manuscript. This study was funded by the Canadian Institutes of Health Research (CIHR) and le Fonds de la Recherche du Quebéc en Santé (FRQS). Dr. Thanh Dang-Vu is supported by the Canadian Institutes of Health Research (MOP 142191, PJT 153115, PJT 156125 and PJT 166167), the Fonds de Recherche du Québec–Santé, the Natural Sciences and Engineering Research Council of Canada, and the Canada Foundation for Innovation. T.D.V. was a consultant for Eisai. Dr. Ronald B. Postuma reports grants and personal fees from Fonds de la Recherche en Sante, grants from Canadian Institutes of Health Research, grants from The Parkinson Society of Canada, grants from Weston-Garfield Foundation, grants from Michael J. Fox Foundation, grants from Webster Foundation, personal fees from Biotie, personal fees from Roche/Prothena, personal fees from Teva Neurosciences, personal fees from Novartis Canada, personal fees from Biogen, personal fees from Boehringer Ingelheim, personal fees from Theranexus, and personal fees from GE HealthCare, outside the submitted work. The other authors report no conflicts of interest.
ACKNOWLEDGMENTS
This research was made possible using the data/biospecimens collected by the Canadian Longitudinal Study on Aging (CLSA). Funding for the CLSA was provided by the Government of Canada through the Canadian Institutes of Health Research (CIHR) under grant reference: LSA 94473 and the Canada Foundation for Innovation and by CIHR operating grant ACD 151284 (E.F.). This research has been conducted using the CLSA Baseline Comprehensive Dataset version 3.1, under Application Number 160607. The CLSA is led by Drs. Parminder Raina, Christina Wolfson, and Susan Kirkland. We are grateful for all participants’ contribution to the study and the opportunity provided by CLSA. Author contributions: Chun Yao: research project: conception; statistical analysis: design, execution, review and critique; manuscript preparation: writing of the first draft, review and critique. Amélie Pelletier: research project: organization, execution; statistical analysis: review and critique; manuscript preparation: review and critique. Seyed-Mohammad Fereshtehnejad: research project: organization, execution; statistical analysis: review and critique; manuscript preparation: review and critique. Nathan Cross: research project: conception, critique; statistical analysis: review and critique; manuscript preparation: review and critique. Thanh Dang-Vu: research project: organization, conception, critique; statistical analysis: review and critique; manuscript preparation: review and critique. Ronald b. Postuma: research project: conception, organization, execution, critique; statistical analysis: design, review and critique; manuscript preparation: writing of the first draft, review and critique.
ABBREVIATIONS
- CLSA
Canadian Longitudinal Study on Aging
- HRV
heart-rate variability
- MI
sleep-maintenance insomnia
- OI
sleep-onset insomnia
- OR
odds ratio
- PD
Parkinson disease
- RBD
rapid eye movement sleep behavior disorder
- RLS
restless legs syndrome
REFERENCES
- 1. Ohayon MM . Epidemiology of insomnia: what we know and what we still need to learn . Sleep Med Rev. 2002. ; 6 ( 2 ): 97 – 111 . [DOI] [PubMed] [Google Scholar]
- 2. Javaheri S , Redline S . Insomnia and risk of cardiovascular disease . Chest. 2017. ; 152 ( 2 ): 435 – 444 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Hertenstein E , Feige B , Gmeiner T , et al . Insomnia as a predictor of mental disorders: a systematic review and meta-analysis . Sleep Med Rev. 2019. ; 43 : 96 – 105 . [DOI] [PubMed] [Google Scholar]
- 4. Tholfsen LK , Larsen JP , Schulz J , Tysnes O-B , Gjerstad MD . Changes in insomnia subtypes in early Parkinson disease . Neurology. 2017. ; 88 ( 4 ): 352 – 358 . [DOI] [PubMed] [Google Scholar]
- 5. Shi L , Chen S-J , Ma M-Y , et al . Sleep disturbances increase the risk of dementia: A systematic review and meta-analysis . Sleep Med Rev. 2018. ; 40 : 4 – 16 . [DOI] [PubMed] [Google Scholar]
- 6. Ju YS , Videnovic A , Vaughn BV . Comorbid Sleep Disturbances in Neurologic Disorders . Continuum (Minneap Minn). 2017. ; 23 ( 4, Sleep Neurology ): 1117 – 1131 . [DOI] [PubMed] [Google Scholar]
- 7. Cross NE , Carrier J , Postuma RB , et al . Association between insomnia disorder and cognitive function in middle-aged and older adults: a cross-sectional analysis of the Canadian Longitudinal Study on Aging . Sleep. 2019. ; 42 ( 8 ): zsz114 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Postuma RB , Gagnon JF , Pelletier A , Montplaisir JY . Insomnia and somnolence in idiopathic RBD: a prospective cohort study . NPJ Parkinsons Dis. 2017. ; 3 ( 1 ): 9 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Schrag A , Horsfall L , Walters K , Noyce A , Petersen I . Prediagnostic presentations of Parkinson’s disease in primary care: a case-control study . Lancet Neurol. 2015. ; 14 ( 1 ): 57 – 64 . [DOI] [PubMed] [Google Scholar]
- 10. Loddo G , Calandra-Buonaura G , Sambati L , et al . The treatment of sleep disorders in Parkinson’s disease: from research to clinical practice . Front Neurol. 2017. ; 8 : 42 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Homolak J , Mudrovčić M , Vukić B , Toljan K . Circadian rhythm and Alzheimer’s Disease . Med Sci (Basel). 2018. ; 6 ( 3 ): 52 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Raina PS , Wolfson C , Kirkland SA , et al . The Canadian longitudinal study on aging (CLSA) . Can J Aging. 2009. ; 28 ( 3 ): 221 – 229 . [DOI] [PubMed] [Google Scholar]
- 13. Buysse DJ , Reynolds CF 3rd , Monk TH , Berman SR , Kupfer DJ . The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research . Psychiatry Res. 1989. ; 28 ( 2 ): 193 – 213 . [DOI] [PubMed] [Google Scholar]
- 14. American Psychiatric Association . Diagnostic and Statistical Manual of Mental Disorders. 5th ed. Arlington, VA: : American Psychiatric Association; ; 2013. . [Google Scholar]
- 15. American Academy of Sleep Medicine . International Classification of Sleep Disorders. 3rd ed. Darien, IL: : American Academy of Sleep Medicine; ; 2014. . [Google Scholar]
- 16. Ito E , Inoue Y . [The International Classification of Sleep Disorders, third edition. American Academy of Sleep Medicine. Includes bibliographies and index] . Nihon Rinsho. 2015. ; 73 ( 6 ): 916 – 923 . [PubMed] [Google Scholar]
- 17. Avila-Funes JA , Gray-Donald K , Payette H . Association of nutritional risk and depressive symptoms with physical performance in the elderly: the Quebec longitudinal study of nutrition as a determinant of successful aging (NuAge) . J Am Coll Nutr. 2008. ; 27 ( 4 ): 492 – 498 . [DOI] [PubMed] [Google Scholar]
- 18. Ashton LA , Myers S . Serial grip strength testing—its role in assessment of wrist and hand disability . Internet J Surg. 2004. ; 5 . 10.5580/834. [Google Scholar]
- 19. Capp K , Diaz-Santos A , Raffo A , et al . A-23 The Miami Prospective Memory Test (MPMT) in discriminating community-dwelling older adults with amnestic MCI from cognitively normal elders . Arch Clin Neuropsychol. 2016. ; 31 ( 6 ): 592 . [Google Scholar]
- 20. Shaffer F , Ginsberg JP . An overview of heart rate variability metrics and norms . Front Public Health. 2017. ; 5 : 258 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Zygmunt A , Stanczyk J . Methods of evaluation of autonomic nervous system function . Arch Med Sci. 2010. ; 6 ( 1 ): 11 – 18 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Tanner CM , Gilley DW , Goetz CG . A brief screening questionnaire for parkinsonism . Ann Neurol. 1990. ; 28 : 267 – 268 . [Google Scholar]
- 23. Postuma RB , Arnulf I , Hogl B , et al . A single-question screen for rapid eye movement sleep behavior disorder: a multicenter validation study . Mov Disord. 2012. ; 27 ( 7 ): 913 – 916 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Chaudhuri KR , Pal S , DiMarco A , et al . The Parkinson’s disease sleep scale: a new instrument for assessing sleep and nocturnal disability in Parkinson’s disease . J Neurol Neurosurg Psychiatry. 2002. ; 73 ( 6 ): 629 – 635 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Yao C , Fereshtehnejad SM , Keezer MR , Wolfson C , Pelletier A , Postuma RB . Risk factors for possible REM sleep behavior disorder: a CLSA population-based cohort study . Neurology. 2019. ; 92 ( 5 ): e475 – e485 . 10.1212/WNL.0000000000006849 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Ho D , Imai K , King G , Stuart EA . MatchIt: nonparametric preprocessing for parametric causal inference . J Statist Software. 2011. ; 42 ( 8 ): 1 – 28. [Google Scholar]
- 27. Zolfaghari S , Yao C , Thompson C , et al . Effects of menopause on sleep quality and sleep disorders: Canadian Longitudinal Study on Aging . Menopause. 2020. ; 27 ( 3 ): 295 – 304 . [DOI] [PubMed] [Google Scholar]
- 28. Hsiao YH , Chen YT , Tseng CM , et al . Sleep disorders and an increased risk of Parkinson’s disease in individuals with non-apnea sleep disorders: a population-based cohort study . J Sleep Res. 2017. ; 26 ( 5 ): 623 – 628 . [DOI] [PubMed] [Google Scholar]
- 29. Buchman AS , Bennett DA . Loss of motor function in preclinical Alzheimer’s disease . Expert Rev Neurother. 2011. ; 11 ( 5 ): 665 – 676 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Aggarwal NT , Wilson RS , Beck TL , Bienias JL , Bennett DA . Motor dysfunction in mild cognitive impairment and the risk of incident Alzheimer disease . Arch Neurol. 2006. ; 63 ( 12 ): 1763 – 1769 . [DOI] [PubMed] [Google Scholar]
- 31. Koo DL , Nam H , Thomas RJ , Yun CH . Sleep disturbances as a risk factor for stroke . J Stroke. 2018. ; 20 ( 1 ): 12 – 32 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Sofi F , Cesari F , Casini A , Macchi C , Abbate R , Gensini GF . Insomnia and risk of cardiovascular disease: a meta-analysis . Eur J Prev Cardiol. 2014. ; 21 ( 1 ): 57 – 64 . [DOI] [PubMed] [Google Scholar]
- 33. Ge L , Guyatt G , Tian J , et al . Insomnia and risk of mortality from all-cause, cardiovascular disease, and cancer: Systematic review and meta-analysis of prospective cohort studies . Sleep Med Rev. 2019. ; 48 : 101215 . [DOI] [PubMed] [Google Scholar]
- 34. Hung C-M , Li Y-C , Chen H-J , et al . Risk of dementia in patients with primary insomnia: a nationwide population-based case-control study . BMC Psychiatry. 2018. ; 18 ( 1 ): 38 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Chen PL , Lee WJ , Sun WZ , Oyang YJ , Fuh JL . Risk of dementia in patients with insomnia and long-term use of hypnotics: a population-based retrospective cohort study . PLoS One. 2012. ; 7 ( 11 ): e49113 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Hoile R , Tabet N , Smith H , Bremner S , Cassell J , Ford E . Are symptoms of insomnia in primary care associated with subsequent onset of dementia? A matched retrospective case-control study . Aging Ment Health. 2020. ; 24 ( 9 ): 1466 – 1471 . [DOI] [PubMed] [Google Scholar]
- 37. Osorio RS , Pirraglia E , Agüera-Ortiz LF , et al . Greater risk of Alzheimer’s disease in older adults with insomnia . J Am Geriatr Soc. 2011. ; 59 ( 3 ): 559 – 562 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Yaffe K , Nettiksimmons J , Yesavage J , Byers A . Sleep quality and risk of dementia among older male veterans . Am J Geriatr Psychiatry. 2015. ; 23 ( 6 ): 651 – 654 . [DOI] [PubMed] [Google Scholar]
- 39. Benedict C , Byberg L , Cedernaes J , et al . Self-reported sleep disturbance is associated with Alzheimer’s disease risk in men . Alzheimers Dement. 2015. ; 11 ( 9 ): 1090 – 1097 . [DOI] [PubMed] [Google Scholar]
- 40. Foley D , Monjan A , Masaki K , et al . Daytime sleepiness is associated with 3-year incident dementia and cognitive decline in older Japanese-American men . J Am Geriatr Soc. 2001. ; 49 ( 12 ): 1628 – 1632 . [DOI] [PubMed] [Google Scholar]
- 41. Jaussent I , Bouyer J , Ancelin ML , et al . Excessive sleepiness is predictive of cognitive decline in the elderly . Sleep. 2012. ; 35 ( 9 ): 1201 – 1207 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Crossley M , D’Arcy C , Rawson NSB . Letter and category fluency in community-dwelling Canadian seniors: a comparison of normal participants to those with dementia of the Alzheimer or vascular type . J Clin Exp Neuropsychol. 1997. ; 19 ( 1 ): 52 – 62 . [DOI] [PubMed] [Google Scholar]
- 43. Jones S , Laukka EJ , Bäckman L . Differential verbal fluency deficits in the preclinical stages of Alzheimer’s disease and vascular dementia . Cortex. 2006. ; 42 ( 3 ): 347 – 355 . [DOI] [PubMed] [Google Scholar]
- 44. Williams VG , Bruce JM , Westervelt HJ , et al . Boston naming performance distinguishes between Lewy body and Alzheimer’s dementias . Arch Clin Neuropsychol. 2007. ; 22 ( 8 ): 925 – 931 . [DOI] [PubMed] [Google Scholar]
- 45. Génier Marchand D , Montplaisir J , Postuma RB , Rahayel S , Gagnon JF . Detecting the cognitive prodrome of dementia with Lewy bodies: a prospective study of REM sleep behavior disorder . Sleep. 2017. ; 40 ( 1 ): zsw014 . [DOI] [PubMed] [Google Scholar]
- 46. Anderson EL , Richmond RC , Jones SE , et al . Is disrupted sleep a risk factor for Alzheimer’s disease? Evidence from a two-sample Mendelian randomization analysis . Int J Epidemiol. 2021. ; 50 ( 3 ): 817 – 828 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Huang J , Zuber V , Matthews PM , Elliott P , Tzoulaki J , Dehghan A . Sleep, major depressive disorder, and Alzheimer disease: A Mendelian randomization study . Neurology. 2020. ; 95 ( 14 ): e1963 – e1970 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Ismail Z , Gatchel J , Bateman DR , et al . Affective and emotional dysregulation as pre-dementia risk markers: exploring the mild behavioral impairment symptoms of depression, anxiety, irritability, and euphoria . Int Psychogeriatr. 2018. ; 30 ( 2 ): 185 – 196 . [DOI] [PubMed] [Google Scholar]
- 49. Dong JY , Zhang YH , Tong J , Qin LQ . Depression and risk of stroke: a meta-analysis of prospective studies . Stroke. 2012. ; 43 ( 1 ): 32 – 37 . [DOI] [PubMed] [Google Scholar]
- 50. Fereshtehnejad SM , Yao C , Pelletier A , Montplaisir JY , Gagnon JF , Postuma RB . Evolution of prodromal Parkinson’s disease and dementia with Lewy bodies: a prospective study . Brain. 2019. ; 142 ( 7 ): 2051 – 2067 . [DOI] [PubMed] [Google Scholar]
- 51. Emdin CA , Odutayo A , Wong CX , Tran J , Hsiao AJ , Hunn BH . Meta-analysis of anxiety as a risk factor for cardiovascular disease . Am J Cardiol. 2016. ; 118 ( 4 ): 511 – 519 . [DOI] [PubMed] [Google Scholar]
- 52. da Silva VP , Ramalho Oliveira BR , et al . Heart rate variability indexes in dementia: a systematic review with a quantitative analysis . Curr Alzheimer Res. 2018. ; 15 : 80 – 88 . [DOI] [PubMed] [Google Scholar]
- 53. Forte G , Favieri F , Casagrande M . Heart rate variability and cognitive function: a systematic review . Front Neurosci. 2019. ; 13 : 710 . [DOI] [PMC free article] [PubMed] [Google Scholar]