Abstract
Study Objectives:
(1) To determine the sensitivity and specificity of the home sleep apnea test (HSAT) performed in typically developing children who were diagnosed with moderate to severe obstructive sleep apnea during overnight attended laboratory polysomnography (LPSG). (2) To determine the utility of a screening questionnaire to identify children at increased risk for obstructive sleep apnea.
Methods:
Participants completed 2 consecutive study nights, the first night with the HSAT followed by LPSG on the second night. The SHOOTS questionnaire, composed of 6 questions (snoring, hyperactivity, obesity, observed apnea, tonsillar hypertrophy, and sleepiness) concerning sleep-disordered breathing, was administered by the clinician before the first study night.
Results:
Thirty-eight participants completed both studies. The mean age was 13.8 ± 3.0 years. Twenty (53%) were male. Most participants were obese. The mean LPSG total sleep time was 7.34 ± 1.19 hours; the mean HSAT total recording time was 8.86 ± 1.73 hours (P < .001). The median obstructive apnea-hypopnea index for LPSG and HSAT was 6.6 and 0.8 events/h, respectively. For an obstructive apnea-hypopnea index ≥ 3.1 events/h by HSAT, the sensitivity was 71.43% (95% confidence interval, 41.9–91.6) and the specificity was 95.83% (95% confidence interval, 78.9–99.9) for identifying those with an LPSG obstructive apnea-hypopnea index of ≥ 10 events/h. For a SHOOTS score with ≥ 4 “yes” responses, the sensitivity and specificity were 85.7% (95% confidence interval, 57.2–98.2) and 54.2% (95% confidence interval, 32.8–74.4), respectively, for identifying those with an LPSG obstructive apnea-hypopnea index ≥ 10 events/h.
Conclusions:
Using HSAT, we clinically applied cutoff values to identify moderate to severe obstructive sleep apnea in typically developing children. The SHOOTS questionnaire may aid in identifying children at risk for obstructive sleep apnea and who are candidates for HSAT.
Citation:
Revana A, Vecchio J, Guffey D, Minard CG, Glaze DG. Clinical application of home sleep apnea testing in children: a prospective pilot study. J Clin Sleep Med. 2022;18(2):533–540.
Keywords: home sleep apnea test, HSAT, pediatrics, obstructive sleep apnea, OSA
BRIEF SUMMARY
Current Knowledge/Study Rationale: There is limited availability of in-laboratory polysomnography for children, resulting in delays in diagnosis and treatment. The purposes of this prospective study were (1) to determine the sensitivity and specificity of the home sleep apnea test to identify moderate to severe obstructive sleep apnea in typically developing children who were diagnosed with moderate to severe obstructive sleep apnea using laboratory polysomnography and (2) to determine the utility of a 6-item screening questionnaire to identify children at increased risk for obstructive sleep apnea.
Study Impact: Despite a small sample size, using home sleep apnea testing we were able to clinically apply cutoff values to identify moderate to severe obstructive sleep apnea in typically developing children. Home sleep apnea testing may improve access to care and thus earlier intervention.
INTRODUCTION
Pediatric obstructive sleep apnea (OSA) has been linked to several comorbidities, including behavioral/learning problems, metabolic dysfunction, and cardiopulmonary abnormalities.1 The prevalence of pediatric OSA has been reported to range from 1%–4%.1–3 An overnight attended laboratory polysomnography (LPSG) is the gold standard for the diagnosis of OSA because it provides a thorough evaluation of sleep architecture and arousals along with cardiorespiratory parameters. However, the LPSG is a labor-intensive and expensive test. The performance of LPSG in children may be challenging. The test involves multiple sensors and respiratory channels that may be poorly tolerated by infants and young children. The test requires the child and the caregiver to spend the night in the laboratory, which can be challenging if there are child-care and/or social barriers. There is limited availability and restricted access to pediatric LPSG, resulting in delays in diagnosis and treatment. Increasing evidence suggests that a home sleep apnea test (HSAT) can successfully be used to identify adults with moderate to severe OSA; however, there is limited evidence for the use of HSAT in children.4,5
HSAT performed in children may have advantages in comparison to LPSG, including better tolerability because fewer sensors are applied and it is performed in the home environment, less expense, and more readily available, avoiding a long delay in the diagnosis and management of sleep-disordered breathing. However, a recent position statement by the American Academy of Sleep Medicine (AASM) concluded that HSAT in children is not recommended because of insufficient evidence indicating its validity to identify OSA in children.6 A limited number of studies in pediatric patients have compared HSAT to LPSG, with the majority of studies involving small sample sizes and showing a wide range of specificities and sensitivities.5–9 In addition, the impact of the lack of transcutaneous carbon dioxide (TCO2) monitoring to aid in identifying hypoventilation and electroencephalogram monitoring to aid in sleep staging during HSAT has not been studied in children. Because HSAT has limited channels in comparison to LPSG, HSAT may underestimate the severity of OSA in children and alter clinical management options such as watchful waiting, positive airway pressure therapy, or surgical intervention.9,10 A few studies have supported the feasibility of HSAT in children, particularly when applied during controlled settings (HSAT sensors are applied by a trained sleep technologist or clinician).11–13,15–17
The purposes of this study were (1) to determine the sensitivity and specificity of HSAT to identify moderate to severe OSA under controlled settings in typically developing children and (2) to determine the utility of SHOOTS, a 6-item screening questionnaire to identify children at increased risk for moderate to severe OSA.
METHODS
The study was approved by the Institutional Review Board for Baylor College of Medicine in Houston, Texas (protocol H-31153). Participants were children evaluated in the Children’s Sleep Center clinic who fulfilled the following criteria: ages 7–18 years, symptoms of OSA such as snoring or witnessed apneas, no complex medical history, and the ability to complete the 2 consecutive study nights. Exclusion criteria included the absence of OSA symptoms such as snoring, history of adenotonsillectomy, previous diagnosis of OSA, use of positive airway pressure therapy, and the presence of complex medical problems such as chronic lung disease, congenital heart disease, craniofacial disorders, neurologic disorders, and other sleep disorders. Participants and participants’ caregivers in the study completed written assent/consent, respectively, before scheduling the 2 nights.
On the first night, participants had an evaluation at home with a type 3 ambulatory device, the Carefusion NOX T3. The ambulatory device included a nasal pressure transducer (to detect air flow and snoring), pulse oximetry (to monitor SpO2 and heart rate), and respiratory impedance plethysmography belts (to measure thoracoabdominal effort). Participants arrived at the Children’s Sleep Center and had sensors placed by a registered sleep technologist. The HSAT recording start time was set 1 hour earlier than the participants’ reported habitual bedtime. The HSAT recording end time was 1 hour later than the participants’ reported habitual wake time. Both recording times were programmed on the portable monitoring device, and the sensors were applied by a registered sleep technologist. The participants then went home while wearing the portable monitoring device to complete the ambulatory portion of the study. They removed the portable monitoring device at home the next morning and returned the ambulatory device on the second consecutive night of the study protocol.
During the second night, participants were evaluated with a diagnostic sleep study (LPSG) without the use of positive airway pressure. LPSG was performed at the Texas Children’s Hospital Children’s Sleep Center using a NicoletOne EEG System with the continuous presence of a registered polysomnographic technologist.
Study setup followed AASM standards: Electroencephalography (C3-M2, C4-M1, O1-M2, O2-M1), left and right electrooculograms, submental and leg electromyograms, and electrocardiogram were recorded. Nasal pressure and oronasal airflow (nasal cannula and oronasal thermistor), thoracic and abdominal effort (respiratory inductance plethysmography belts), pulse oximetry and plethysmography, and TCO2 were recorded to identify respiratory events. The caregivers were allowed to stay with the participants during LPSG in a separate bed. The total sleep time was calculated based on the amount of time spent during sleep stages (N1, N2, N3, and rapid eye movement sleep).
Both HSAT and LPSG were manually scored by a board-certified sleep medicine physician and a registered sleep technologist, using the AASM pediatric scoring criteria.14 Both the sleep medicine physician and the sleep technologist scoring LPSG were blinded to the results of the HSAT. The same sleep medicine physician scored and interpreted both LPSG and HSAT. Interscorer reliability was completed for LPSG per AASM laboratory standards but not for HSAT because there are no standardized guidelines for interscorer reliability for HSAT. Notably, for HSAT scoring, the adult algorithm was modified to adjust for pediatric scoring (scoring events of 2 breaths or more) per AASM scoring rules.14 For HSAT, an obstructive hypopnea was scored using the following criteria: ≥ 30% decrease in airflow for 2 breaths or longer followed by a 3% oxygen desaturation. The HSAT obstructive apnea-hypopnea index (oAHI) was defined as the number of obstructive respiratory events per hour of recording time. Respiratory events that had a ≥ 30% decrease in airflow for 2 breaths but not associated with a 3% oxygen desaturation were not scored. The LPSG oAHI was defined as the number of respiratory events per hour of sleep and included obstructive respiratory events that were associated with a 3% oxygen desaturation or arousal. Because HSAT did not include electroencephalogram electrodes to aid with sleep staging, arousals were not scored. Therefore, for HSAT only, central apneas were scored only if the events were longer than 20 seconds in duration or 2 breaths or more in duration that were associated with a 3% oxygen desaturation. Central apneas according to LPSG were scored if they were ≥ 20 seconds in duration or ≥ 2 breaths and were associated with an arousal or 3% oxygen desaturation. An uninterpretable airflow signal according to HSAT was defined as no airflow for ≥ 30 seconds. Data were excluded from the analysis if > 50% of the airflow signal was uninterpretable or if there was < 4 hours (240 minutes) of either recording time according to HSAT or total sleep time according to LPSG. In addition, participants who had < 4 hours (240 minutes) of HSAT or LPSG recording time were excluded from the analysis.
SHOOTS questionnaire
We created a pediatric clinical sleep questionnaire, the SHOOTS questionnaire, for which we compared responses to HSAT results. The questionnaire consisted of 6 questions asked by a clinician during the sleep clinic evaluation, representing common symptoms and etiological factors associated with OSA in children:
Snoring
Hyperactivity (by parental report)
Obesity (body mass index either ≥ 30 kg/m2 or > 90th percentile)
Observed apnea reported by caregiver
Tonsillar hypertrophy (assessed via clinical exam of having tonsillar size of +2 or greater17,18)
Sleepiness (either by report or by a pediatric daytime sleepiness score ≥ 15)7
The SHOOTS questionnaire was scored in a yes or no, yes = 1 and no = 0 format with a maximum total score of 6 and a minimum score of 0. Higher values indicated more symptoms of OSA. SHOOTS scores were compared to HSAT oAHI, and we determined the predictive likelihood of the SHOOTS score to identify children with significant OSA (oAHI > 3.1 events/h according to HSAT).
Statistical analysis
Patient demographics, clinical characteristics, and sleep indices were summarized using frequency with percentage, mean with standard deviation, and median with 25th and 75th percentiles. Spearman correlations were presented between HSAT and LPSG sleep indices with 95% confidence intervals. The paired t test was used to determine statistical differences between LPSG and HSAT. Scatterplots were also presented between HSAT and LPSG with a linear regression curve. A receiver operating characteristic curve was used to assess for a cutoff value for the HSAT index, and SHOOTS score was used to predict moderate to severe obstructive sleep apnea using LPSG cutoff values of oAHI ≥ 10 events/h and HSAT cutoff values of oAHI ≥ 3.1 events/h. Predictive values and Bland-Altman plots were used to assess the agreement of LPSG and HSAT oAHI. Statistical analyses were performed using Stata v 12.1 (StataCorp LP, College Station, TX).
RESULTS
Participant characteristics
Fifty-three participants consented and enrolled in the study. Of these, 46 (87%) completed both consecutive nights. Of these 46 participants, 8 (17%) had < 4 hours recorded on the HSAT or no data recorded on the HSAT. The remaining participants (13%) did not complete both nights or canceled participation. Overall, there was a 17% (n = 8) failure rate for having adequate data using HSAT.
Table 1 summarizes participant demographics. The majority of the participants were male teenagers with a mean body mass index of 35.0 ± 10.8 kg/m2. Table 2 summarizes participant symptoms and clinical findings based on the SHOOTS questionnaire. The predominant reported symptom was snoring (89%).
Table 1.
Participant demographics.
| All Patients (n = 38) | |
|---|---|
| Male, n (%) | 20 (53) |
| Race/ethnicity, n (%) | |
| White | 8 (21) |
| Black | 9 (24) |
| Hispanic | 21 (55) |
| Age (y), mean (SD) | 13.8 (3.0) |
| BMI (kg/m2), mean (SD) | 35.0 (10.8) |
BMI = body mass index, SD = standard deviation.
Table 2.
Participant clinical symptoms and exam findings.
| All Patients (n = 38) | |
|---|---|
| Snoring, n (%) | 34 (89) |
| Tonsillar hypertrophy, n (%) | |
| Yes | 17 (45) |
| No | 21 (55) |
| Tonsillar grade, n (%) | |
| 0 | 18 (47) |
| 1 | 3 (8) |
| 2 | 12 (32) |
| 3 | 5 (13) |
| Hyperactive behavior, n (%) | 4 (11) |
| Observed apneas, n (%) | 19 (50) |
| Daytime sleepiness, n (%) | 27 (71) |
| Pediatric Daytime Sleepiness Scale | |
| Mean (SD) | 12.9 (7.0) |
| Range | 2–26 |
| SHOOTS questionnaire tally | |
| Mean (SD) | 3.6 (1.0) |
| Range | 1–5 |
SD = standard deviation. SHOOTS = snoring, hyperactivity, obesity, observed apnea, tonsillar hypertrophy, sleepiness.
Sleep characteristics
The mean LPSG total sleep time was 7.34 ± 1.19 hours, and the mean HSAT total recording time was 8.86 ± 1.73 hours (P < .001). The median (25th and 75th percentile) oAHI for LPSG was 7.8 events/h (3.6 and 17.8) and 1.1 events/h (0.4 and 4.8) for HSAT. The median (25th and 75th percentile) oAHI for LPSG and HSAT was 6.6 events/h (2.7 and 14.7) and 0.8 events/h (0.3 and 3.1), respectively. In regard to the central apnea index, the median (25th and 75th percentile) for LPSG and HSAT were 0.51 events/h (0.11 and 1.7) and 0.2 events/h (0 and 0.4), respectively. The median (25th and 75th percentile) percentage of total sleep time in which the oxygen saturation nadir was < 90% for LPSG was 0% (0 and 0.2). For HSAT, the median (25th and 75th percentile) percentage of the total recording time in which the oxygen saturation nadir was < 90% was 0% (0 and 0.5). The median (25th and 75th percentile) oxygen nadir for LPSG and HSAT was 91 (87 and 93) and 91 (84 and 93), respectively. TCO2 monitoring was only completed for LPSG. The median (25th and 75th percentile) TCO2 value was 46 mm Hg (44 and 49). TCO2 values were > 50 mm Hg for only 1 participant.
Respiratory characteristics
Table 3 summarizes the correlations between LPSG and HSAT for all enrolled patients combined (n = 38) and stratified by sex. There was moderate correlation for the apnea-hypopnea index (AHI; central and obstructive events combined), oAHI, and oxygen nadir. The percentage of total sleep time according to LPSG and the percentage of total recording time according to HSAT in which the oxygen saturation was < 90% was not significantly different (P = .6612). The oxygen nadir and percentage of total sleep time/total recording time with < 90% oxygen saturation was lower for females. There was an overall low positive correlation for the central apnea index. However, the central apnea index was higher in females than in males.
Table 3.
Correlations with 95% CI, all patients and by sex.
| Correlation (95% CI) | All Patients | Males | Females |
|---|---|---|---|
| LPSG AHI and HSAT AHI | 0.597 (0.342–0.770) | 0.602 (0.217–0.825) | 0.630 (0.231–0.847) |
| P < .001 | P = .005 | P = .005 | |
| LPSG OAHI and HSAT OAHI | 0.656 (0.426–0.807) | 0.675 (0.332–0.861) | 0.678 (0.310–0.870) |
| P < .001 | P = .001 | P = .002 | |
| LPSG O2N and HSAT O2N | 0.570 (0.306–0.752) | 0.648 (0.289–0.848) | 0.394 (–0.089 to 0.727) |
| P < .001 | P = .002 | P = .105 | |
| LPSG 90 and HSAT 90 | 0.582 (0.322–0.760) | 0.777 (0.510–0.908) | 0.293 (–0.202 to 0.668) |
| P < .001 | P < .001 | P = .238 | |
| LPSG CAI and HSAT CAI | 0.302 (–0.020 to 0.567) | 0.123 (–0.338 to 0.537) | 0.507 (0.053–0.787) |
| P = .066 | P = .604 | P = .032 |
AHI = apnea-hypopnea index, CAI = central apnea index, CI = confidence interval, HSAT = home sleep apnea test, HSAT 90 = percentage of total recording time below oxygen saturation of 90%, LPSG = laboratory polysomnography, LPSG 90 = percentage of total sleep time below oxygen saturation of 90%, OAHI = obstructive apnea-hypopnea index, O2N = oxygen nadir.
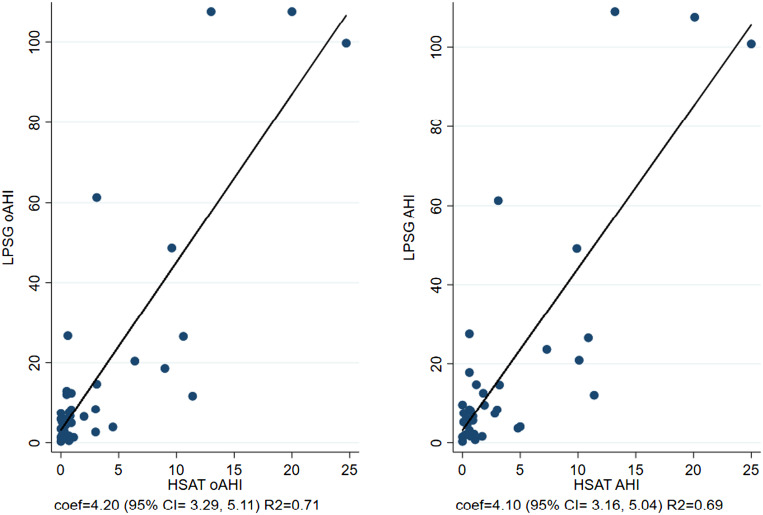
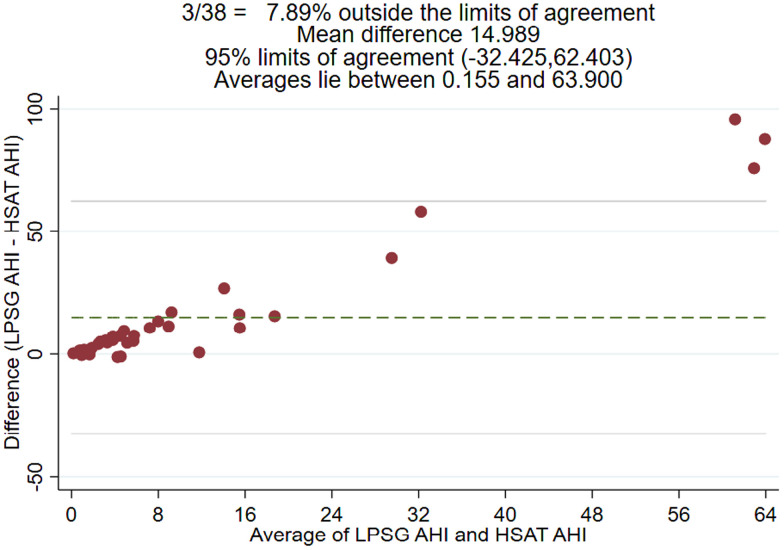
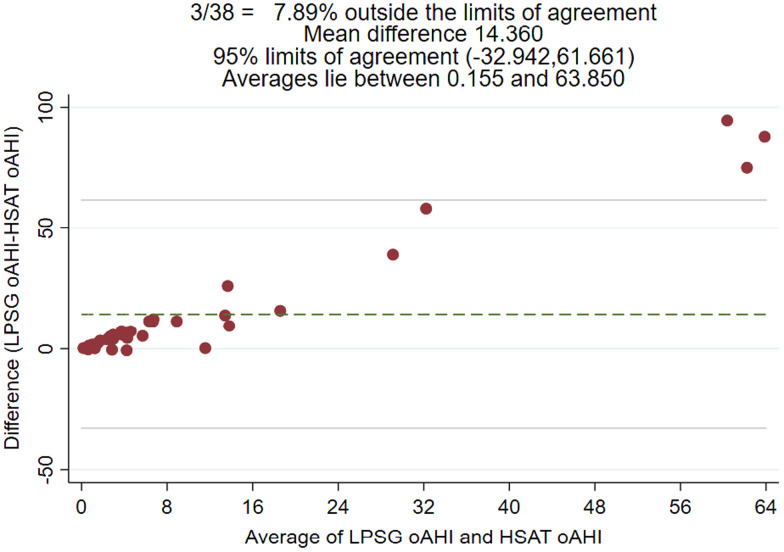
Figure 1 shows the association of sleep characteristics between LPSG and HSAT. Notably, LPSG used total sleep time whereas HSAT used total recording time to calculate sleep indices such as AHI and oAHI. Figure 2 and Figure 3 are Bland-Altman plots for LPSG and HSAT AHI and oAHI, respectively. The 95% confidence interval (CI) varied from –32.425 to 62.403 for AHI. The Bland-Altman analysis suggested disagreement between HSAT and LPSG AHI and oAHI scores but seemed to show a dependent relationship. There were 3 (8%) observations notably outside of the 95% limits of agreement, and the difference between the scores increased as the mean of the scores increased for oAHI and AHI.
Figure 1. Scatterplots.
Left: Scatterplot of oAHI for both LPSG and HSAT (regression coefficient = 4.20; 95% CI, 3.29–5.11; R2 = 0.71). Right: Scatterplot of AHI for LPSG and HSAT (regression coefficient = 4.10; 95% CI, 3.16–5.04; R2 = 0.69). AHI = apnea-hypopnea index, CI = confidence interval, coef = coefficient, HSAT = home sleep apnea test, LPSG = laboratory polysomnography, oAHI = obstructive apnea-hypopnea index.
Figure 2. Bland-Altman plot for LPSG and HSAT AHI.
AHI = apnea-hypopnea index, HSAT = home sleep apnea test, LPSG = laboratory polysomnography.
Figure 3. Bland-Altman plot for LPSG and HSAT oAHI.
HSAT = home sleep apnea test, LPSG = laboratory polysomnography, oAHI = obstructive apnea-hypopnea index.
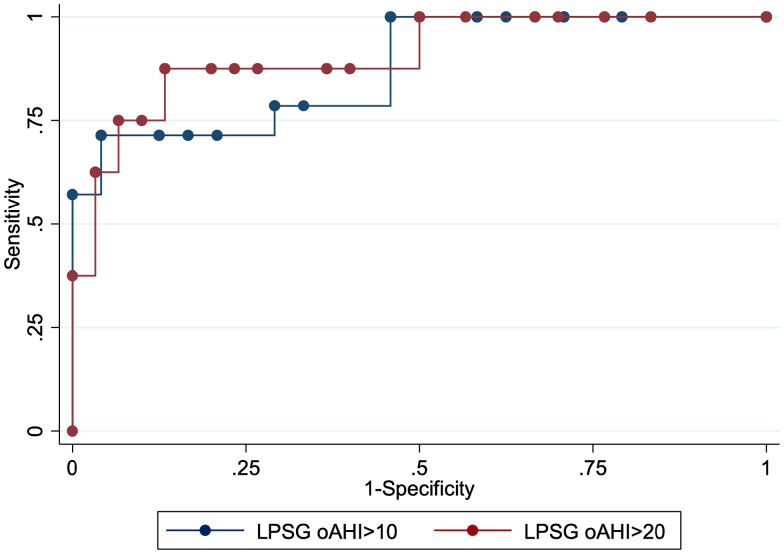
For a cutoff value for an HSAT oAHI of ≥ 3.1 events/h, the sensitivity was 71.43% (95% CI, 41.9–91.6), and the specificity was 95.83% (95% CI, 78.9–99.9) for identifying those with an LPSG oAHI of ≥ 10 events/h. There were 14 (37%) participants who were positive for the defined clinical cutoff of an LPSG oAHI ≥ 10 events/h, which corresponded to an area under the curve of 0.88 (95% CI, 0.76–0.99; Figure 4).
Figure 4. ROC curve for HSAT and LPSG for oAHI ≥ 10 events/h and LPSG oAHI ≥ 20 events/h.
HSAT = home sleep apnea test, LPSG = laboratory polysomnography, oAHI = obstructive apnea-hypopnea index, ROC = receiver operating characteristic.
For an HSAT oAHI ≥ 3.1 events/h, the sensitivity was 87.50% (95% CI, 47.3–99.7), and specificity was 86.67% (95% CI, 69.3–96.2) for identifying those with an LPSG oAHI of ≥ 20 events/h. There were 8 (21%) participants who were positive for the defined clinical cutoff of LPSG oAHI ≥ 20 events/h, which corresponded to an area under the curve of 0.91 (95% CI, 0.78–1.00; Figure 4).
SHOOTS questionnaire
The range of the SHOOTS total “yes” responses per patient was 1–5, with a mean score of 3.6 ± (1.0). All of the participants enrolled had symptoms of OSA per the inclusion criteria. For a SHOOTS score of ≥ 4 “yes” responses, the sensitivity was 72.73% (95% CI, 39–94) and specificity was 44.44% (95% CI, 25.5–64.7), with a positive predictive value of 34.8% (95% CI, 16.4–57.3) and a negative predictive value of 80% (95% CI, 51.5–95.7) for identifying those with an HSAT oAHI of ≥ 3.1 events/h. The sensitivity was 85.71% (95% CI, 57.2–98.2), the specificity was 54.17% (95% CI, 32.8–74.4), the positive predictive value was 52.2% (95% CI, 30.6–73.2), and the negative predictive value was 86.7% (95% CI, 59.5–98.3) for identifying those with an LPSG oAHI > 10 events/h.
An HSAT oAHI identifying an LPSG oAHI ≥ 10 events/h had an area under the curve of 0.88 (95% CI, 0.76–0.99), whereas a SHOOTS score identifying an LPSG oAHI ≥ 10 events/h had an area under the curve of 0.69 (95% CI, 0.53–0.86). The curves were not statistically different from each other (P = .0728; Figure 4).
DISCUSSION
During this prospective study, we evaluated the feasibility of HSAT in typically developing children with OSA as determined by LPSG. In 2017, an AASM task force recognized that there was insufficient data available to support the use of HSAT in children to diagnose OSA.6 However, the AASM position paper identified that HSAT is feasible in children with limited or no comorbidities under controlled conditions.2,6 When HSAT was applied by caregivers unlike our study, the amount of data collected was limited. We showed an 85% success rate of recording adequate data when the HSAT was applied under controlled settings. During this study, the HSAT device was applied in the laboratory setting by the sleep technologist to ensure the adequate placement of sensors in an effort to maximize the total recording time.
When comparing the results of LPSG to those of HSAT, we found a high specificity in identifying children with an oAHI > 10 events/h, which is suggestive of a low false-positive rate. In addition, there was moderate correlation for AHI (central and obstructive events combined), oAHI, and the oxygen nadir. Despite this moderate correlation, the values between LPSG and HSAT were similar in direction but not in range. Low values according to HSAT seemed to reflect low values according to LPSG. This discrepancy likely results from the determination of indices based on total recording time for HSAT and total sleep time for LSPG.
The Bland-Altman plots for oAHI indicated that higher values were obtained during LPSG in addition to wide confidence intervals. The wide confidence intervals resulted from the relatively small sample size and the very large variation in the differences. As the mean oAHI value increased, the difference between LPSG and HSAT increased. Clinically, this finding made the HSAT less reliable for higher values of oAHI. This development may not affect the clinical approach to a child with symptoms of OSA and thus may not be clinically significant. For example, a clinician may approach a child with an oAHI of 40 vs 25 events/h similarly. However, the oAHI may be clinically significant at lower values and thus direct the management of OSA more conservatively. HSAT does not replace the gold standard, LPSG; however, HSAT could be used as a tool to aid in identifying those children at high risk for severe OSA, particularly when there is limited access to LPSG.
Furthermore, we developed a screening questionnaire to be used to identify those children at risk for OSA and who might be candidates for HSAT. As in adults, screening is important to identify appropriate candidates for HSAT. HSAT is not indicated for individuals with complex medical problems. During this study, we only included typically developing children and eliminated those with complex medical problems. Although our questionnaire is not validated, we attempted to evaluate the utility of this screening tool by comparing it to the gold standard, LPSG. There is no validated questionnaire that can be used to screen children who are at high risk for moderate to severe OSA in conjunction with the use of HSAT. The availability of using such a tool to screen children who have several risk factors for OSA may prevent negative results according to HSAT. Our study showed that having a positive response to 4 or more questions resulted in a 72.7% sensitivity and a negative predictive value of 80%. The negative predictive value suggests that this questionnaire could be a good screening test to identify a patient with a low risk of moderate to severe OSA according to HSAT and who thus may not be an ideal candidate for HSAT. Adults and children should not be treated as equivalents, and thus screening for OSA in both populations may differ.
No formal sample size or power calculations were completed a priori for this pilot study. All patients who were eligible for the study were invited to participate based on resources and time available to complete the study. There are several other studies with smaller sample sizes that have shown that the HSAT can be utilized in children.6,13,16,17 Marcus et al17 showed that the HSAT in children was feasible under controlled had a sample size of 4 patients. In contrast, our study included 38 patients, our study included only 38 patients which is still considered a small sample size. Despite this limitation, we were able to estimate the sensitivity of the test and clinically apply cutoff values to identify moderate to severe OSA in typically developing children.
The participants were selected from those evaluated in a sleep clinic and who had symptoms suggesting OSA. Most were obese. Although the participants may not be reflective of the general population of children or children with developmental problems, they are typical of children evaluated in sleep clinics for suspected OSA. Many of these children are potential candidates for HSAT. Patients with minimal or no symptoms of OSA were not included. To establish the utility of HSAT in these children will require further studies.
The order of the 2 studies was not randomized. Night-to-night variability and adaptation to the recording situation may have contributed to an increased amount of sleep during LPSG and variations in the severity of OSA during the 2 nights. An AASM task force has noted that the impact of diagnostic accuracy on clinical outcomes is complicated by a number of factors that can cause discordance between tests, including night-to-night variability and inconsistent definitions of respiratory events (eg, hypopneas) between HSAT and PSG.4 Therefore, it is possible that multiple nights with LPSG and HSAT could produce more accurate results and possibly improve the correlation of the findings of these studies.
HSAT does not include the recording of CO2. The AASM position paper6 identified a lack of CO2 monitoring as a limitation to the HSAT. Our study identified only 1 participant in whom the TCO2 values were elevated above 50 mm Hg on the LPSG. This particular participant had a maximum TCO2 value of 51 mm Hg, an oAHI of 1.34 events/h according to LPSG, and an oAHI of 1.1 evens/h according to HSAT. The selection of patients who may undergo HSAT should consider eliminating those children with chronic lung disease or who are at high risk for hypoventilation. This population can be identified via history and physical examination. We suggest that if sleep-related hypoventilation is suspected, then LPSG is recommended.
In general, most type 3 and 4 devices do not include electroencephalography, which is required to score arousals. Therefore, the severity of sleep-disordered breathing may be underestimated and could produce a false-negative result when in fact the patient may have significant OSA. To account for this concern, if the HSAT does not indicate moderate to severe OSA in adults, then patients are recommended to have a confirmatory LPSG. At this time, there is no validated algorithm similar to that used for adults for the pediatric population. Therefore, excluding arousals from the scoring of respiratory events may result in underestimating the severity of OSA. The lack of sleep staging and reliance on total recording time according to HSAT may raise a significant question in regard to comparing the severity of OSA between LPSG and HSAT. However, with these data, the authors hope to contribute to further studies that may develop and validate such an algorithm for clinical practice.
We do realize that HSAT does not replace the gold standard, LPSG. However, there may be a subset of patients who may benefit from the availability of HSAT especially in situations in which there is limited access to care. HSAT may expedite treatment for OSA when the findings indicate the likelihood of moderate to severe sleep-disordered breathing. In contrast, if the HSAT results suggest minimal to mild sleep-disordered breathing, then the health care provider may consider a conservative approach of watchful waiting, including repeating the HSAT as a screening tool and/or performing an LPSG. The utility of HSAT when applied in the laboratory setting may expedite the institution of therapy for OSA in locations where access to health care is limited; it may also help reduce waiting times in completing an LPSG and may have a potential for cost reduction. In addition, HSAT may be useful in situations in which children are not able to tolerate an in-laboratory study. We propose that the SHOOTS questionnaire may have utility in identifying those children with a high probability of having a positive test for moderate to severe OSA according to HSAT and in eliminating those children who are unlikely to have significant sleep-disordered breathing.
HSAT is feasible in children, but may not be applicable to all children. Although our study suggests that typically developing children including those with obesity may be studied with HSAT, careful screening for complex medical problems and hypoventilation should be conducted. Future directions may involve performing a prospective study that includes a larger sample size and broader population, including children with neurodevelopmental and neuromuscular disorders, and the validation of the 6-item screening questionnaire. In addition, consideration of multiple nights of HSAT recording and, for children, the addition of a CO2 sensor may warrant evaluation to improve the determination of the severity of sleep-disordered breathing in the pediatric population.
DISCLOSURE STATEMENT
All authors on this manuscript have reviewed, edited, and approved the manuscript. Work for this study was performed at Texas Children’s Hospital, Houston, Texas/Baylor College of Medicine, Houston, Texas. The authors report no conflicts of interest.
ACKNOWLEDGMENTS
The authors thank the Texas Children’s Hospital Children’s Sleep Center; Binal Kancherla, MD; and Wes Moulden, MBA, RPSGT, for their support for this project.
ABBREVIATIONS
- AASM
American Academy of Sleep Medicine
- AHI
apnea-hypopnea index
- CI
confidence interval
- HSAT
home sleep apnea test/testing
- LPSG
laboratory polysomnography
- oAHI
obstructive apnea-hypopnea index
- OSA
obstructive sleep apnea
- TCO2
transcutaneous carbon dioxide
- SHOOTS
snoring, hyperactivity, obesity, observed apnea, tonsillar hypertrophy, sleepiness
REFERENCES
- 1. Capdevila OS , Kheirandish-Gozal L , Dayyat E , Gozal D . Pediatric obstructive sleep apnea: complications, management, and long-term outcomes . Proc Am Thorac Soc. 2008. ; 5 ( 2 ): 274 – 282 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Gozal D . Obstructive sleep apnea in children: implications for the developing central nervous system . Semin Pediatr Neurol. 2008. ; 15 ( 2 ): 100 – 106 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Greene MG , Carroll JL . Consequences of sleep-disordered breathing in childhood . Curr Opin Pulm Med. 1997. ; 3 ( 6 ): 456 – 463 . [DOI] [PubMed] [Google Scholar]
- 4. Kapur VK , Auckley DH , Chowdhuri S , et al . Clinical practice guideline for diagnostic testing for adult obstructive sleep apnea: an American Academy of Sleep Medicine clinical practice guideline . J Clin Sleep Med. 2017. ; 13 ( 3 ): 479 – 504 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Schechter MS ; Section on Pediatric Pulmonology, Subcommittee on Obstructive Sleep Apnea Syndrome . Technical report: diagnosis and management of childhood obstructive sleep apnea syndrome . Pediatrics. 2002. ; 109 ( 4 ): e69 . [DOI] [PubMed] [Google Scholar]
- 6. Kirk V , Baughn J , D’Andrea L , et al . American Academy of Sleep Medicine position paper for the use of a home sleep apnea test for the diagnosis of OSA in children . J Clin Sleep Med. 2017. ; 13 ( 10 ): 1199 – 1203 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Drake C , Nickel C , Burduvali E , Roth T , Jefferson C , Pietro B . The Pediatric Daytime Sleepiness Scale (PDSS): sleep habits and school outcomes in middle-school children . Sleep. 2003. ; 26 ( 4 ): 455 – 458 . [PubMed] [Google Scholar]
- 8. Chervin RD , Hedger K , Dillon JE , Pituch KJ . Pediatric Sleep Questionnaire (PSQ): validity and reliability of scales for sleep-disordered breathing, snoring, sleepiness, and behavioral problems . Sleep Med. 2000. ; 1 ( 1 ): 21 – 32 . [DOI] [PubMed] [Google Scholar]
- 9. Kaditis A , Kheirandish-Gozal L , Gozal D . Algorithm for the diagnosis and treatment of pediatric OSA: a proposal of two pediatric sleep centers . Sleep Med. 2012. ; 13 ( 3 ): 217 – 227 . [DOI] [PubMed] [Google Scholar]
- 10. Collop NA , Anderson WM , Boehlecke B , et al. Portable Monitoring Task Force of the American Academy of Sleep Medicine . Clinical guidelines for the use of unattended portable monitors in the diagnosis of obstructive sleep apnea in adult patients . J Clin Sleep Med. 2007. ; 3 ( 7 ): 737 – 747 . [PMC free article] [PubMed] [Google Scholar]
- 11. Skomro RP , Gjevre J , Reid J , et al . Outcomes of home-based diagnosis and treatment of obstructive sleep apnea . Chest. 2010. ; 138 ( 2 ): 257 – 263 . [DOI] [PubMed] [Google Scholar]
- 12. Whitelaw WA , Brant RF , Flemons WW . Clinical usefulness of home oximetry compared with polysomnography for assessment of sleep apnea . Am J Respir Crit Care Med. 2005. ; 171 ( 2 ): 188 – 193 . [DOI] [PubMed] [Google Scholar]
- 13. Kuna ST , Gurubhagavatula I , Maislin G , et al . Noninferiority of functional outcome in ambulatory management of obstructive sleep apnea . Am J Respir Crit Care Med. 2011. ; 183 ( 9 ): 1238 – 1244 . [DOI] [PubMed] [Google Scholar]
- 14.Berry RB, Brooks R, Gamaldo CE, et al; for American Academy of Sleep Medicine. The AASM Manual for the Scoring of Sleep and Associated Events: Rules, Terminology and Technical Specifications. Version 2.2. Darien, IL: American Academy of Sleep Medicine; 2015. [Google Scholar]
- 15. Kirk VG , Flemons WW , Adams C , Rimmer KP , Montgomery MD . Sleep-disordered breathing in Duchenne muscular dystrophy: a preliminary study of the role of portable monitoring . Pediatr Pulmonol. 2000. ; 29 ( 2 ): 135 – 140 . [DOI] [PubMed] [Google Scholar]
- 16.Kirjavainen J, Lehtonen L, Kirjavainen T, Kero P; 24-Hour Ambulatory Sleep Polygraphy study. Sleep of excessively crying infants: a 24-Hour Ambulatory Sleep Polygraphy study. Pediatrics. 2004;114(3):592–600. [DOI] [PubMed] [Google Scholar]
- 17. Marcus CL , Traylor J , Biggs SN , et al . Feasibility of comprehensive, unattended ambulatory polysomnography in school-aged children . J Clin Sleep Med. 2014. ; 10 ( 8 ): 913 – 918 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kumar HV , Schroeder JW , Gang Z , Sheldon SH . Mallampati score and pediatric obstructive sleep apnea . J Clin Sleep Med. 2014. ; 10 ( 9 ): 985 – 990 . [DOI] [PMC free article] [PubMed] [Google Scholar]