Abstract
Study Objectives:
Individuals with opioid use disorder (OUD) may experience worsening sleep quality over time, and a subset of individuals may have sleep disturbances that precede opioid use and do not resolve following abstinence. The purpose of the present study was to (1) collect retrospective reports of sleep across the lifespan and (2) identify characteristics associated with persistent sleep disturbance and changes in sleep quality in persons with OUD.
Methods:
Adults with OUD (n = 154) completed a cross-sectional study assessing current and past sleep disturbance, opioid use history, and chronic pain. Repeated-measures analysis of variance was used to examine changes in retrospectively reported sleep quality, and whether changes varied by screening positive for insomnia and/or chronic pain. Multivariate linear regression analyses were used to identify additional correlates of persistent sleep disturbance.
Results:
Participants reported that their sleep quality declined over their lifespan. Changes in reported sleep over time varied based on whether the individual screened positive for co-occurring insomnia and/or chronic pain. In regression analyses, female sex (β = 0.16, P = .042), a greater number of treatment episodes (β = 0.20, P = .024), and positive screens for chronic pain (β = 0.19, P = .018) and insomnia (β=0.22, P = .013) were associated with self-reported persistent sleep disturbance. Only a portion of participants who screened positive for sleep disorders had received a formal diagnosis.
Conclusions:
OUD treatment providers should routinely screen for co-occurring sleep disturbance and chronic pain. Interventions that treat co-occurring OUD, sleep disturbance, and chronic pain are needed.
Citation:
Ellis JD, Mayo JL, Gamaldo CE, Finan PH, Huhn AS. Worsening sleep quality across the lifespan and persistent sleep disturbances in persons with opioid use disorder. J Clin Sleep Med. 2022;18(2):587–595.
Keywords: opioid use disorder, treatment, sleep disturbance, insomnia, chronic pain
BRIEF SUMMARY
Current Knowledge/Study Rationale: Co-occurring sleep disorders are common among individuals with opioid use disorder (OUD), and opioid use can lead to changes in sleep architecture. However, very little is known about the onset or correlates of sleep pathology in OUD (ie, whether a subset of individuals with OUD have sleep disturbance that precedes opioid use).
Study Impact: The present study is among the first to collect retrospective reports of sleep quality across the lifespan among individuals with OUD. Results suggest that a subset of individuals with OUD report chronic sleep disturbance, and worsening sleep over time.
INTRODUCTION
Sleep disturbances are common among persons with opioid use disorder (OUD), as well as in those in opioid recovery (recovery is defined here as individuals are in treatment or remission from OUD). Individuals who are physically dependent on opioids have reduced sleep-onset latency, shorter sleep duration, reduced sleep efficiency (time asleep/time in bed × 100), and altered sleep architecture including reduced rapid eye movement sleep relative to healthy controls.1–3 Over half of persons in OUD treatment report clinically significant sleep disturbances4–7 and/or symptoms of insomnia.8 These sleep difficulties can contribute to continued drug use or return to use following a period of abstinence, and several studies have cited the desire to improve sleep as direct motivation for opioid use9,10 and an antecedent to opioid craving11 in persons with OUD. Characterizing associations of sleep and OUD recovery is important in improving comprehensive care for OUD.
Numerous preclinical studies have demonstrated that chronic opioid exposure leads to disturbances in both sleep architecture and continuity,12–15 and that cessation of opioid use restores these markers of sleep health,15 although it should be noted that these relationships are complex and heavily influenced by relevant comorbidities (eg, a recent meta-analysis suggested that, among individuals with chronic pain, use of opioids as prescribed may be associated with better self-reported sleep quality16). Additionally, 1 study in humans demonstrated that cessation of opioid use and/or stabilization in opioid medication treatment produces at least modest improvements in sleep, but other evidence suggests that a subset of individuals continue to experience sleep disturbance even after they have stopped using opioids.17 One potential explanation for this finding is that opioids have a sustainable, negative impact on sleep even after cessation of use. Alternatively, a subset of individuals in OUD treatment or recovery may have had sleep disturbance that preceded opioid use; that is, some individuals may have pre-OUD sleep disturbance that is further exacerbated by opioid use but does not naturally resolve during opioid abstinence.
Currently, very little is known about how self-reported sleep quality changes across the lifespan among patients in OUD recovery. Cross-sectional research has linked sleep problems with prescription opioid use in adolescents.18–20 However, none of these studies assessed participants’ perception of sleep quality prior to initiation of opioid use, leaving open the question of whether the sleep disturbance was a motive for, or a consequence of, nonmedical opioid use, or if the 2 phenomena emerged separately or converged together. Consequently, further investigation may improve our understanding of the antecedents of problematic opioid use and inform OUD treatment development. Retrospective self-reports, although not without limitations, can be an important tool in assessing for sleep disturbance, and are often used in informing sleep disorder diagnoses.21
Collecting lifespan retrospective reports may help identify subgroups of individuals who warrant special attention as being at high risk for persistent sleep disturbance and/or worsening sleep across the lifespan in future work. For example, clinical insomnia can be chronic,22 and may precede opioid use in some individuals in OUD recovery. Chronic pain commonly co-occurs with both insomnia23 and OUD,24,25 and insomnia can occur secondary to chronic pain.26 Thus, individuals with chronic pain may also represent a group at high risk of persistent and/or worsening sleep disturbance. Additionally, characteristics of an individual’s opioid use history (ie, age of onset, severity of opioid use) may be related to co-occurring sleep pathology.
The primary aim of the present study was to better understand sleep across the lifespan and self-reports of persistent sleep disturbance among individuals who reported OUD symptoms and who identified being in OUD treatment or recovery. Individuals who used prescription opioids nonmedically were eligible to participate if they reported being in OUD treatment or recovery and endorsed OUD symptoms; however, individuals who were prescribed opioids but did not report symptoms consistent with OUD and did not endorse being in OUD treatment or recovery were not eligible. We further sought to examine how sleep across the lifespan varied among individuals screening positive for insomnia and/or chronic pain during OUD recovery. It was hypothesized that individuals who screened positive for insomnia and chronic pain would be more likely to report (1) persistent sleep disturbance and (2) that their sleep had worsened across childhood, adolescence, and adulthood. A secondary aim of the study was to conduct an exploratory analysis to identify demographic and opioid use variables potentially associated with persistent sleep disturbance that may be relevant to explore in future work.
METHODS
Participants
Participants were sourced from Amazon Mechanical Turk from May 22, 2020, to August 8, 2020. Amazon Mechanical Turk is an online crowd-sourcing marketplace that is regularly used in biomedical survey research of difficult-to-reach populations,27 including substance use disorders.28 Amazon Mechanical Turk requestors develop human intelligence tasks (HITs) in the form of surveys or other remote assignments, which offer compensation to workers that register to complete them. Following submission of a HIT, requestors indicate quality and completion of the task by approving or rejecting a HIT. Each participant who responded to the screener received a $0.10 payment, and participants who completed the entire survey received $3.50 and a $0.50 bonus for answering an open-ended question. Attention check items (eg, “Are you from Mars?” and “What does 2 + 2 equal”) were included in the survey, and participants were asked to report their age and sex twice during the survey. Respondents who failed attention checks or demonstrated inconsistent responding were removed. Additionally, duplicate ID addresses, sequential responses from similar IP addresses, and nonsensical text responses were removed.
The current study was advertised on Amazon Mechanical Turk as a brief health survey consisting of several questionnaires (described below), as well as questionnaires related to a separate research question among individuals with OUD, selected by the investigators. On average, participants spent 31.25 minutes (standard deviation = 19.02 minutes) completing the survey. Survey access was limited to workers located in the United States, with an approval rating ≥ 90%, and who had not previously completed the survey. Persons meeting those criteria completed a screening survey that collected information regarding demographics and treatment/recovery status of several health conditions. Eligibility criteria were blinded via distractor questions and access to the main survey was reserved to persons aged ≥ 18 years living in the United States, who endorsed being currently in treatment or recovery from OUD. The standard Diagnostic and Statistical Manual for Mental Disorders, fifth edition (DSM-5), self-report checklist for OUD was used to assess whether participants endorsed symptoms consistent with at least mild OUD leading up to their most recent treatment episode (ie, endorsed at least 2 DSM-5 symptoms).
The survey was hosted on Qualtrics (Provo, UT). Participants were informed that participation was voluntary and anonymous. Due to the nature of data collection, the study was submitted to the Johns Hopkins School of Medicine Institutional Review Board as exempt from Institutional Review Board review and was acknowledged by the Institutional Review Board.
Measures
Potential correlates of retrospective reports of sleep disturbance
Demographic characteristics:
Information was collected regarding age, sex, race, marital status, and current household income. Participants also responded to questions about lifetime use of various substances in addition to opioids.
Opioid use history:
The 16-item Subjective Opioid Withdrawal Scale (SOWS)29 was adapted to measure the severity of withdrawal that an individual experienced when abruptly stopping opioid use in the past. Participants also reported their age of first opioid use and how many times they had been in substance use treatment. Participants were able to select from a scale ranging from 0 to 6 or more times; however, categories of 0 and 1 were collapsed into a single category, as were individuals reporting 4 or more treatment episodes due to small cell sizes. Participants also indicated whether they had overdosed, defined in the present study as loss of consciousness, overdose requiring hospitalization, and/or needing to be revived via Narcan (Emergent Devices Inc. Plymouth Meeting, PA).
Insomnia screen:
Symptoms of insomnia over the past 2 weeks were measured using the 7-item Insomnia Severity Index (ISI),30 using a 0–4-point Likert scale. Participants were dichotomized into individuals who screened positive for clinical insomnia (ie, those who reported scores of 15 or higher on the ISI) and individuals who screened negative.
Chronic pain screen:
The 6-item Graded Chronic Pain Scale, Revised (GCPS-R),31 was used to index the presence of chronic pain over the past 3 months. Participants who screened positive for grade 1 chronic pain or higher were compared against individuals who screened negative.
Outcome measures
Sleep across the lifespan:
Participants were asked to report on their sleep quality at 3 time points during their life (childhood, adolescence, and adulthood) on 3 visual analog scales (VASs) ranging from 0 (“Bad sleep—never get a full night’s rest”) to 100 (“Great sleep—always get a full night’s rest”).
Persistent sleep disturbance:
To assess persistent sleep disturbance, participants answered 6 questions on a 5-point Likert scale ranging from strongly agree to strongly disagree: (1) I feel that I have experienced sleep problems throughout my life, (2) I feel that it has been hard for me to maintain regular sleep patterns throughout my life, (3) Sleep problems have interfered with my daily activities such as work and school throughout my life, (4) Sleep problems have interfered with my mood and behavior throughout my life, (5) Sleep problems have interfered with motivation throughout my life, and (6) I have used opioids to improve my sleep problems. Items were developed for the purposes of the present study but demonstrated acceptable reliability (α = .88).
Descriptive information to characterize sleep in the present sample
Sleep quality:
The 19-item Pittsburgh Sleep Quality Index (PSQI)32 was administered to determine atypical sleep patterns and sleep disturbance over the past month. Individuals with a score > 5 were classified as poor sleepers.
Sleep apnea risk:
To index the risk of obstructive sleep apnea, the 7-item snoring, tiredness, observed apnea, high blood pressure, body mass index, age, and male gender (STOP-BAG)33 questionnaire was administered. This questionnaire is a modified version of the STOP-Bang questionnaire,34 in which the neck circumference item is omitted. The total score was calculated based on summing the items and participants were classified as low risk (0–2), moderate risk (3–4), or severe risk (5–7).
Sleep disturbance checklist:
Participants indicated whether they had, at any point in their life, experienced a number of disturbances related to sleep. Participants were also asked to indicate if they had ever been formally diagnosed a sleep disorder by a professional. Diagnoses were based on participant report and were not verified by clinic records. The checklist consisted of 30 items. See Table 2 for items included in the checklist.
Table 2.
Rates of lifetime sleep disturbance.
| Variable | Values |
|---|---|
| Lifetime sleep disturbances | |
| Night sweats | 106 (68.8%) |
| Nightmares | 103 (67.3%) |
| Difficulty falling or staying asleep | 102 (66.7%) |
| Vivid dreams | 99 (64.3%) |
| Severe restlessness | 97 (63.0%) |
| Strong urge to nap during the day | 94 (61.0%) |
| Racing thoughts or worries | 87 (56.5%) |
| Easily disturbed by light | 83 (53.9%) |
| Easily disturbed by sounds | 82 (53.2%) |
| Sleep talking | 82 (53.2%) |
| Waking up unable to move | 75 (48.7%) |
| Teeth grinding/jaw clenching | 75 (48.7%) |
| Irresistible urge to move legs | 71 (46.1%) |
| Rapid heartbeat, fluttering heartbeat, or irregular heartbeat | 69 (44.8%) |
| Frequent nighttime urination | 66 (42.9%) |
| Suddenly gasping for breath | 61 (39.6%) |
| Groaning | 61 (39.6%) |
| Waking up screaming or with violent outbursts | 55 (35.7%) |
| Kicking | 52 (33.8%) |
| Sleep walking | 44 (28.6%) |
| Wake up with convulsions/seizures | 40 (26.0%) |
| Bedwetting (after age 7) | 37 (24.0%) |
| Formal diagnosis—sleep disorders | |
| Insomnia | 46 (29.9%) |
| Sleep apnea | 41 (26.6%) |
| Sleep paralysis | 37 (24.0%) |
| Restless legs syndrome | 22 (14.3%) |
| Parasomnias (sleep talking/sleep walking) | 14 (9.1%) |
| Night terrors | 14 (9.1%) |
| Circadian disorders | 10 (6.5%) |
| Narcolepsy | 9 (5.8%) |
Data are presented as n (%).
Data analysis
A between-group, repeated-measures analysis of variance (ANOVA) was used to examine reported changes in sleep quality across the lifespan (as measured by VAS) for childhood, adolescence, and the past 30 days, as a function of insomnia and chronic pain. Mauchly’s test of sphericity was used to test whether variances in the difference scores were equal and Levene’s test was used to test assumptions related to homogeneity of variance; neither assumption was determined to be violated. The outcome measures were roughly normally distributed and did not contain outliers. Follow-up Bonferroni-corrected post hoc tests were used to further explore significant interaction effects by examining whether changes across time among the 4 groups were significant.
Next, bivariate correlations, t tests, and 1-way analysis of variance were used, as appropriate, to explore potential correlates of self-reported persistent sleep disturbance (a total score of the 6 Likert scales described above). Demographic characteristics (ie, sex and age), opioid use history (ie, age of first opioid use, number of treatment episodes for substance use, overdose history, history of opioid withdrawal severity), and physical health variables (ie, positive screen for insomnia, positive screen for chronic pain) were considered as potential correlates in bivariate analyses. Variables related to self-reported persistent sleep disturbance at the P < .2 threshold35 were entered into a multivariate linear regression analysis. Assumptions related to multivariate linear regression (ie, absence of multicollinearity, linearity, independence, normality, homoscedasticity) were checked and determined to be met. The ratio of cases to independent variables was determined to be appropriate.36 For all analyses, alpha levels were set at .05 and analyses were performed using SPSS (IBM Corporation, Armonk, NY).
RESULTS
Participant characteristics
We screened 5,632 potentially interested respondents. Following exclusion of individuals who did not meet eligibility criteria and individuals with duplicate IP addresses, sequential responses from similar IP addresses, or nonsensical text responses, 196 individuals were determined to be eligible. Forty-one individuals were excluded for inconsistent responding or for failing attention check items, and 1 individual was removed for screening negative for OUD on the DSM-5 checklist; thus, n = 154 participants were included in the final sample.
Descriptive demographic information is presented in Table 1. In the present sample, 52.6% (n = 81) of participants screened positive for at least moderate insomnia and 46.1% (n = 71) screened positive for being at moderate or high risk for sleep apnea. Consistent with rates observed in some other work,37 the majority of participants exceeded the cut score of 5 for poor sleep quality on the PSQI (n = 131, 85.1%), although scores ranged from 1 to 20. The median bedtime was 10:30 pm and the median wake time was 7:00 am. Lifetime use of other substances was common in the present sample. Around two-thirds of the sample reported receiving a medication for OUD, but medication use was unrelated to past 30-day sleep quality on the VAS (P = .596). Insomnia positive-screen rates were 41.8% of those on no medications, 42.9% of those on buprenorphine or extended-release buprenorphine (Suboxone; Indivior, Richmond, VA), 60.5% of those on methadone (Mallinckrodt, Staines, United Kingdom), and 65.0% of those on naltrexone (Barr Pharmaceuticals, Montvale, NJ) or extended-release naltrexone (Alkermes, Waltham, MA) (Barr Pharmaceuticals, Montvale, NJ); while these differences were not statistically significant (P = .079), there may be clinically meaningful differences in the propensity for insomnia as a function of medication for opioid use disorder (MOUD) status.
Table 1.
Demographic characteristics of the sample.
| Variable | Values |
|---|---|
| Sex (female) | 61 (39.6%) |
| Employment (% employed full or part time) | 135 (87.7%) |
| Hispanic ethnicity | 31 (20.1%) |
| Race | |
| White/Caucasian | 109 (70.8%) |
| Black/African American | 19 (12.3%) |
| American Indian | 7 (4.5%) |
| Asian | 9 (5.8%) |
| Native Hawaiian/Pacific Islander | 5 (3.2%) |
| More than 1 race | 5 (3.2%) |
| Marital status | |
| Married or remarried | 98 (63.6%) |
| Never married | 46 (29.9%) |
| Divorced/separated | 9 (5.8%) |
| Widowed | 1 (0.6%) |
| Income | |
| $0–$30,000 | 54 (35.1%) |
| $30,001–$60,000 | 55 (35.7%) |
| $60,001–$90,000 | 23 (14.9%) |
| $90,001+ | 22 (14.3%) |
| Age (years) | 35.13 (8.42) |
| Current medication for OUD treatment | |
| None | 55 (35.7%) |
| Methadone | 38 (24.7%) |
| Buprenorphine/Suboxone | 18 (11.7%) |
| Extended-release buprenorphine | 3 (1.9%) |
| Naltrexone | 18 (11.7%) |
| Extended-release naltrexone/Vivitrol | 22 (14.3%) |
| Lifetime substance use | |
| Nicotine | 94 (61.0%) |
| Alcohol | 139 (90.3%) |
| Cannabis/marijuana | 101 (65.6%) |
| Synthetic marijuana | 35 (22.7%) |
| Cocaine | 103 (66.9%) |
| Methamphetamines | 61 (39.6%) |
| Hallucinogens | 52 (33.8%) |
| Dissociative hallucinogens | 29 (18.8%) |
| Inhalants | 35 (22.7%) |
| Prescription stimulants (nonmedical use) | 60 (39.0%) |
| Prescription sedative/sleeping medication (nonmedical use) | 63 (40.9%) |
Data are presented as n (%) except for age, which is presented as mean (standard deviation). OUD = opioid use disorder.
Rates of specific sleep disturbances, and rates of self-reported diagnoses by a medical professional, are presented in Table 2. Night sweats, nightmares, and difficulty falling asleep were the most common lifetime sleep disturbance symptoms reported in the present sample. Insomnia, sleep apnea, and sleep paralysis were among the most commonly reported medical diagnoses. However, only a portion of individuals who screened positive for insomnia on the ISI (n = 34, 42.0%) and as presenting as moderate or high risk for sleep apnea on the STOP-BAG questionnaire (n = 25, 35.2%) reported being formally diagnosed for each condition by a professional.
Retrospective reports of changes in sleep quality across the lifespan
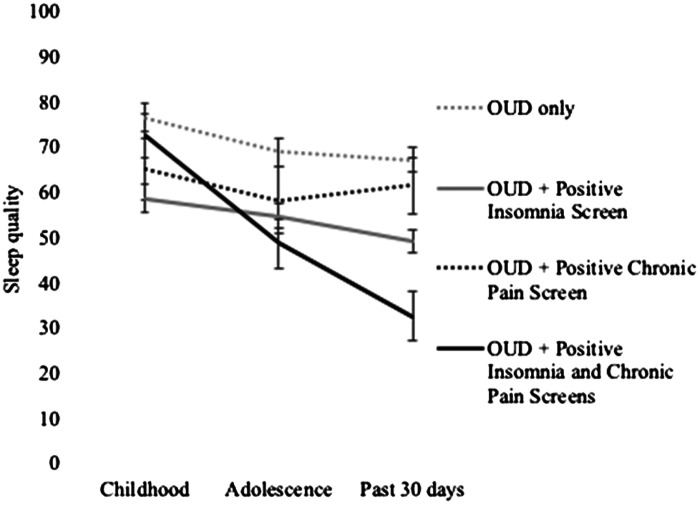
Self-reported VAS sleep quality in the past 30 days was associated with VAS sleep quality in both childhood (r = .37, P < .001) and adolescence (r = .51, P < .001). However, as shown in Figure 1, sleep changed significantly across the 3 time points [F(2, 300) = 25.57, P < .001]. Post hoc pairwise comparisons suggested that participants reported that their sleep worsened over time; self-reported past-30-day sleep quality was lower than in adolescence (mean difference = −4.97, P = .027) and childhood (mean difference = −16.52, P < .001), and self-reported sleep quality in adolescence was lower than in childhood (mean difference = 10.64, P < .001).
Figure 1. Retrospective report of self-reported sleep quality across the lifespan.
Analysis was based on retrospective reporting of sleep at each of the 3 time points on a 0–100 visual analog scale. OUD = opioid use disorder.
Reported changes in sleep quality also interacted with screening positive for chronic pain and insomnia. There was a significant time point × positive chronic pain screen interaction [F(2, 300) = 4.18, P = .016], as well as a significant time point × positive insomnia screen interaction [F(2, 300) = 8.52, P < .001] and a significant time point × positive chronic pain screen × positive insomnia screen interaction [F(2, 300) = 8.15, P < .001]. These interactions revealed that, relative to OUD alone, having 1 or more comorbidities was associated with different trajectories of self-reported sleep quality over time.
Follow-up Bonferroni-corrected post hoc tests, presented in Table 3, suggested that the sharpest declines in sleep quality occurred among individuals screening positive for co-occurring chronic pain and insomnia. Sleep quality during childhood was significantly better than in adolescence (mean difference = 23.86, P < .001) and adulthood (mean difference = 39.86, P < .001), and sleep quality in adolescence was significantly better than sleep quality in adulthood (mean difference = 16.00, P = .007). Individuals who screened positive for insomnia, but negative for chronic pain, had better sleep quality in childhood relative to adulthood (mean difference = 9.58, P = .012). Post hoc tests comparing changes in sleep quality in childhood, adolescence, and adulthood among individuals scoring positive for chronic pain but negative for insomnia were not significant. Self-reported sleep quality worsened among individuals with OUD only, although the magnitude of change was less than among individuals with co-occurring chronic pain and insomnia (childhood vs adolescence: mean difference = 7.77, P = .028; childhood vs adulthood: mean difference = 9.34, P = .02), and sleep quality in adolescence did not differ from sleep quality in adulthood in this group (mean difference = 1.57, P = 1.00).
Table 3.
Bonferroni-corrected post hoc tests.
| Mean Difference (95% CI) | SE | Bonferroni Adjusted P | |
|---|---|---|---|
| OUD only | |||
| Childhood vs adolescence | 7.77 (0.64, 14.90) | 2.95 | .028* |
| Childhood vs adulthood | 9.34 (1.11, 17.57) | 3.40 | .020* |
| Adolescence vs adulthood | 1.57 (−6.11, 9.25) | 3.17 | .999 |
| OUD + pos. chronic pain screen (neg. insomnia screen) | |||
| Childhood vs adolescence | 6.88 (−6.06, 19.83) | 5.35 | .600 |
| Childhood vs adulthood | 3.71 (−11.23, 18.64) | 6.17 | .999 |
| Adolescence vs adulthood | −3.18 (−17.11, 10.76) | 5.76 | .999 |
| OUD + pos. insomnia screen (neg. chronic pain screen) | |||
| Childhood vs adolescence | 4.03 (−2.86, 10.93) | 2.85 | .476 |
| Childhood vs adulthood | 9.58 (1.63, 17.53) | 3.28 | .012* |
| Adolescence vs adulthood | 5.55 (−1.87, 12.97) | 3.06 | .216 |
| OUD + pos. screens for insomnia and chronic pain | |||
| Childhood vs adolescence | 23.86 (12.21, 35.51) | 4.81 | < .001* |
| Childhood vs adulthood | 39.86 (26.42, 53.29) | 5.55 | < .001* |
| Adolescence vs adulthood | 16.00 (3.46, 28.54) | 5.18 | .007* |
*Denotes a P value < .05. CI = confidence interval, neg. = negative, OUD = opioid use disorder, pos. = positive, SE = standard error.
Correlates of persistent sleep disturbance
Reports of persistent sleep disturbance were common in the present sample, with 48.1% of respondents (n = 74) somewhat or strongly agreeing with statements that they experienced sleep problems throughout their life and 50.6% (n = 78) had difficulties maintaining regular sleep patterns. Reports of sleep problems affecting daily activities, behavior and mood, and motivation across the lifespan were also common, with 44.8% (n = 69), 48.1% (n = 74), and 46.1% (n = 71) endorsing each statement, respectively. Sixty-nine participants (44.8%) reported that they had used opioids to improve sleep problems. Persistent sleep disturbance was related to lower self-reported sleep quality in both adolescence (r = −.302, P < .001) and in the past 30 days (r = −.299, P < .001), but not to lower self-reported sleep quality in childhood (r = −.114, P = .159).
Bivariate analyses suggested that persistent sleep disturbance was associated with more severe opioid withdrawal when abruptly stopping opioid use (r = .29, P < .001), a greater number of treatment episodes for substance use [F(3,150) = 3.62, P = .015], with a positive insomnia screen [t(152) = 2.94, P = .005], and with a positive chronic pain screen [t(152) = 3.01, P = .003]. Sex, age, income, history of overdose, and age at first opioid use were unrelated to reports of persistent sleep disturbance at the bivariate level. A multivariate linear regression analysis controlling for all variables associated with the outcome at the P < .2 level, presented in Table 4, suggested that female sex (β = 0.16, P = .042), a positive insomnia screen (β = 0.22, P = .013), a positive chronic pain screen (β = .19, P = .018), and being in treatment 4 or more times relative to being in treatment 0 or 1 time (β = .20, P = .024) were significantly related to self-reported persistent sleep disturbance. Overdose, income, and severe withdrawal history were unrelated to persistent sleep disturbance in the multivariate model.
Table 4.
Correlates of persistent sleep disturbance.
| Variable | B | SE | P | Beta | 95% CI |
|---|---|---|---|---|---|
| Female sexa | 1.88 | 0.91 | .042* | 0.16 | 0.07, 3.68 |
| Incomeb | |||||
| $30,000–$60,000 | −1.90 | 1.10 | .088 | −0.15 | −4.08, 0.28 |
| $60,000–$90,000 | 0.93 | 1.43 | .517 | 0.06 | −1.90, 3.76 |
| $90,000+ | −1.17 | 1.43 | .415 | −0.07 | −4.01, 1.66 |
| Insomnia—positive screen | 2.53 | 1.01 | .013* | 0.22 | 0.54, 4.52 |
| Chronic pain—positive screen | 2.53 | 1.05 | .018* | 0.19 | 0.45, 4.61 |
| Treatment episodesc | |||||
| 2 | 1.21 | 1.17 | .306 | 0.10 | −1.11, 3.53 |
| 3 | 0.58 | 1.45 | .690 | 0.04 | −2.29, 3.45 |
| 4+ | 3.51 | 1.54 | .024* | 0.20 | 0.47, 6.54 |
| Overdose history | −0.57 | 1.01 | .575 | −0.05 | −2.57, 1.43 |
| Severe withdrawal history | 0.07 | 0.04 | .089 | 0.15 | −0.01, 0.15 |
Exploratory analysis examining correlates of persistent sleep disturbance on a 6-item scale. *Denotes a P value < .05. aRef = Male. bRef = $0–$30,000. cRef = 0–1 treatment episode. CI = confidence interval, Ref = reference, SE = standard error.
DISCUSSION
Understanding sleep difficulties across the lifespan and during OUD recovery is important in establishing the role of sleep in disease progression and whether sleep improvement should be included as a core feature of comprehensive care for OUD. The results of this study suggest that persistent sleep disturbance is associated with clinical insomnia and/or chronic pain in OUD recovery. Consistent with results in the general population,38 self-reported sleep quality was highest in childhood before declining in adolescence and adulthood; however, different trajectories were observed for different subgroups of individual with OUD. Individuals with OUD and insomnia retrospectively reported chronic low sleep quality relative to those with OUD only, and individuals with co-occurring OUD, insomnia, and chronic pain reported more dramatic declines in sleep quality across their life than other participants. Thus, the etiology of sleep disturbance and the onset of sleep difficulties among individuals with OUD may be influenced by a number of factors, including relevant comorbidities.
Individuals with persistent sleep disturbance may benefit from adjunctive sleep interventions in addition to standard OUD treatment, in order to improve overall well-being and address co-occurring symptoms that may have clinical relevance. For example, adjunctive interventions addressing sleep, such as pharmacotherapies, cognitive behavioral therapy for insomnia (CBT-I), or referral to a sleep specialist may be beneficial. Some participants in the present sample may have experienced sleep disorders that were not formally diagnosed by professionals; although over half of participants screened positive for at least moderate insomnia on the ISI and nearly half screened positive for moderate to high risk of sleep apnea on the STOP-BAG questionnaire, less than one-third of patients had received a formal diagnosis of insomnia from a health professional and only around one-quarter had received a formal diagnosis of sleep apnea. There are a number of brief tools that can be used to assess sleep quality and sleep disorders, including measures used in the present study.30,32 It is recommended that opioid treatment providers regularly assess sleep in order to maximize the likelihood that sleep disorders are recognized and appropriately treated, especially because poor sleep has been associated with premature treatment discontinuation.39
Although many of the relationships in the present study are complex and may be bidirectional, adequately addressing symptoms of chronic pain among the subset of individuals with co-occurring OUD, chronic pain, and insomnia may provide additional beneficial effects on sleep, particularly if there are a subset of patients who experience sleep disturbance secondary to chronic pain. For example, combined cognitive behavioral therapy for chronic pain and insomnia has also shown positive effects on pain, sleep, and mood symptoms among patients with co-occurring chronic pain and insomnia.40–42 Additionally, routinely assessing for untreated pain and helping patients with OUD access medical care and attain insurance may also be beneficial, as lack of assessment of chronic pain by OUD treatment providers,43 low rates of insurance coverage among individuals with OUD,44 and negative attitudes toward individuals with OUD among physicians45 may all increase the likelihood that co-occurring chronic pain is inadequately managed in populations with OUD.
Future work should aim to understand the directionality of these relationships, including the specific ages at which chronic pain, insomnia, and OUD emerge. Gathering participant reports about whether these phenomena emerged together, sequentially, or several years apart may be useful. In addition, it would be useful to gather information about participant-reported directionality and motives for use (ie, nonmedical opioid use following emergence of a diagnosis of insomnia or chronic pain), as this may also help identify specific subtypes of patients presenting with co-occurring symptoms, as well as treatment efforts. Finally, it should be noted that women in the present study were more likely to report persistent sleep disturbance than men. This finding is consistent with results from other studies that have also found sex differences in insomnia.46 Future work should further probe interactions between sex, sleep disturbance, and chronic pain. Recent work suggests that experimental sleep disruption may have sex-dependent effects on pain processing.47 Thus, future work should explore whether OUD, sleep disturbance, and chronic pain emerge through sex-specific pathways.
Exploratory analyses indicated that a greater number of treatment episodes was also associated with self-reported persistent sleep disturbance, providing some evidence that more severe OUD may influence persistent sleep disturbance and/or that persistent sleep disturbance could hamper recovery attempts. Future work regarding how sleep disturbance influences the course of opioid use treatment is warranted. This finding also reflects that individuals with co-occurring OUD and sleep disturbance are more likely to have contacts with clinical providers, which may represent opportunities to be assessed for relevant co-occurring symptoms.
The retrospective nature of the present study may lead to recall bias that could lead to inaccurate appraisals of past sleep quality. Thus, longitudinal studies are needed in order to examine whether individuals with sleep disorders who develop OUD are more likely to have persistent sleep disturbance that continues even after stopping opioid use. However, although longitudinal studies examining sleep and OUD beginning in adolescence and extending into adulthood represent the ideal methodology to answer these questions, such studies are costly and time intensive. Longitudinal studies in this area would also require recruitment of a large sample to ensure that the study is statistically powered to recruit a subset of patients who will develop OUD. Additionally, retrospective self-reports are commonly used in clinical diagnosis of sleep disorder.21 Thus, this initial study collecting retrospective reports of sleep quality presents a beneficial initial step in informing future work.
In addition to the cross-sectional nature of the study, other limitations should be considered. First, the present study utilized a convenience sample of individuals in OUD recovery, and future studies could examine retrospective reports of sleep in distinct clinical populations such as persons with varying severities of OUD or persons in specific MOUD treatments. Although 1 significant strength of the present study was that the race and average age of the present sample were comparable to estimates from the 2019 census, the present study included a smaller proportion of women and a larger proportion of individuals who reported working full or part time relative to the Census. Thus, the results should be replicated in samples with a higher proportion of female and unemployed respondents. Contrary to expectations, we did not find significant differences in insomnia based on use of MOUDs, and we did not exclude participants based on factors such as time in treatment or other substance use, both of which may have influenced the finding that rates of insomnia did not significantly differ by type of medication for OUD. Future studies should further explore the role that medications play in resolution of sleep disturbance in OUD. Many of the self-reported parasomnia and diagnoses of sleep disorders were within range or slightly higher than prevalence estimates in the general population;48–50 however, it is possible that some participants incorrectly remembered their diagnoses or were unaware of a formal diagnosis. Further, although we selected well-validated measures for the present study, some items (eg, those assessing persistent sleep disturbance) were developed for the purposes of the present study and should undergo further validation. Future work will benefit from utilizing multi-method approaches to examine the etiology of OUD and sleep.
In conclusion, the present study is among the first to examine retrospective reports of persistent sleep disturbance and sleep across the lifespan among individuals in OUD recovery. Results suggest that a subset of individuals may be more likely to report persistent sleep disturbance across the lifespan, especially women, individuals screening positive for chronic pain, individuals screening positive for insomnia, and individuals with more treatment episodes. Further, individuals who have symptoms of insomnia, particularly those with co-occurring insomnia and chronic pain, may be particularly likely to report that their sleep worsened over time. Longitudinal studies that explore changes in sleep quality, chronic pain, and OUD symptoms over time and intervention studies that explore the best way to treat co-occurring symptoms would be beneficial.
DISCLOSURE STATEMENT
All authors have seen and approved the final manuscript. Work for this study was performed virtually at the Johns Hopkins School of Medicine, Department of Psychiatry and Behavioral Sciences. This study was funded by the National Institute on Drug Abuse T32 DA007209 (Bigelow) and the National Heart, Lung, and Blood Institute (U01 HL150835 01; Huhn/Finan). A.S.H. receives research funding from Ashley Addiction Treatment through his university. The other authors report no conflicts of interest.
ACKNOWLEDGMENTS
The authors acknowledge and thank the participants in this study. Author contributions: J.D.E. wrote the original draft of the Introduction, Results, and Discussion sections of the manuscript and conducted data analyses; J.L.M. wrote the original draft of the Methods section of the manuscript and was involved in study conceptualization, data collection, and data curation; C.E.G. reviewed and edited the manuscript and was involved in study conceptualization; P.H.F. reviewed and edited the manuscript; A.S.H. conceptualized the study, reviewed and edited the manuscript, and supervised data collection, data curation, data analysis, and writing of the original draft.
ABBREVIATIONS
- OUD
opioid use disorder
- VAS
visual analog scale
REFERENCES
- 1. Asaad TA , Ghanem MH , Abdel Samee AM , et al . Sleep profile in patients with chronic opioid abuse: a polysomnographic evaluation in an Egyptian sample . Addict Disord Their Treat. 2011. ; 10 ( 1 ): 21 – 28 . [Google Scholar]
- 2. Kay DC , Pickworth WB , Neider GL . Morphine-like insomnia from heroin in nondependent human addicts . Br J Clin Pharmacol. 1981. ; 11 ( 2 ): 159 – 169 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Xiao L , Tang YL , Smith AK , et al . Nocturnal sleep architecture disturbances in early methadone treatment patients . Psychiatry Res. 2010. ; 179 ( 1 ): 91 – 95 . [DOI] [PubMed] [Google Scholar]
- 4. Dunn KE , Finan PH , Andrew Tompkins D , Strain EC . Frequency and correlates of sleep disturbance in methadone and buprenorphine-maintained patients . Addict Behav. 2018. ; 76 : 8 – 14 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Chen VC , Ting H , Wu MH , Lin TY , Gossop M . Sleep disturbance and its associations with severity of dependence, depression and quality of life among heroin-dependent patients: a cross-sectional descriptive study . Subst Abuse Treat Prev Policy. 2017. ; 12 ( 1 ): 16 – 23 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Hsu WY , Chiu NY , Liu JT , et al . Sleep quality in heroin addicts under methadone maintenance treatment . Acta Neuropsychiatr. 2012. ; 24 ( 6 ): 356 – 360 . [DOI] [PubMed] [Google Scholar]
- 7. Tripathi R , Dhawan A , Rao R , Mishra AK , Jain R , Sinha S . Assessment of subjective sleep problems in men With opioid dependence maintained on buprenorphine . J Addict Med. 2020. ; 14 ( 2 ): 132 – 138 . [DOI] [PubMed] [Google Scholar]
- 8. Hallinan R , Elsayed M , Espinoza D , et al . Insomnia and excessive daytime sleepiness in women and men receiving methadone and buprenorphine maintenance treatment . Subst Use Misuse. 2019. ; 54 ( 10 ): 1589 – 1598 . [DOI] [PubMed] [Google Scholar]
- 9. Rigg KK , Ibañez GE . Motivations for non-medical prescription drug use: a mixed methods analysis . J Subst Abuse Treat. 2010. ; 39 ( 3 ): 236 – 247 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Barth KS , Maria MM , Lawson K , Shaftman S , Brady KT , Back SE . Pain and motives for use among non-treatment seeking individuals with prescription opioid dependence . Am J Addict. 2013. ; 22 ( 5 ): 486 – 491 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Lydon-Staley DM , Cleveland HH , Huhn AS , et al . Daily sleep quality affects drug craving, partially through indirect associations with positive affect, in patients in treatment for nonmedical use of prescription drugs . Addict Behav. 2017. ; 65 : 275 – 282 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Snyder EW , Dustman RE , Beck EC . Sustained ingestion of methadone and the sleep of monkeys . Psychopharmacology (Berl). 1978. ; 60 ( 1 ): 29 – 34 . [DOI] [PubMed] [Google Scholar]
- 13. Gauthier EA , Guzick SE , Brummett CM , Baghdoyan HA , Lydic R . Buprenorphine disrupts sleep and decreases adenosine concentrations in sleep-regulating brain regions of Sprague Dawley rat . Anesthesiology. 2011. ; 115 ( 4 ): 743 – 753 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Wang Q , Yue XF , Qu WM , et al . Morphine inhibits sleep-promoting neurons in the ventrolateral preoptic area via mu receptors and induces wakefulness in rats . Neuropsychopharmacology. 2013. ; 38 ( 5 ): 791 – 801 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Coffey AA , Guan Z , Grigson PS , Fang J . Reversal of the sleep-wake cycle by heroin self-administration in rats . Brain Res Bull. 2016. ; 123 : 33 – 46 . [DOI] [PubMed] [Google Scholar]
- 16. Tang NKY , Stella MT , Banks PDW , Sandhu HK , Berna C . The effect of opioid therapy on sleep quality in patients with chronic non-malignant pain: a systematic review and exploratory meta-analysis . Sleep Med Rev. 2019. ; 45 : 105 – 126 . [DOI] [PubMed] [Google Scholar]
- 17. Chrobok AI , Krause D , Winter C , et al . Sleeping patterns in patients with opioid use disorder: Effects of opioid maintenance treatment and detoxification . J Psychoactive Drugs. 2020. ; 52 ( 3 ): 203 – 210 . [DOI] [PubMed] [Google Scholar]
- 18. Guo L , Luo M , Wang W , Huang G , Zhang WH , Lu C . Association between weekday sleep duration and nonmedical use of prescription drug among adolescents: the role of academic performance . Eur Child Adolesc Psychiatry. 2019. ; 28 ( 9 ): 1265 – 1275 . [DOI] [PubMed] [Google Scholar]
- 19. Tang D , Li P , Guo L , et al . The prevalences of and association between nonmedical prescription opioid use and poor sleep among Chinese high school students . Sci Rep. 2016. ; 6 ( 1 ): 30411 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Zhabenko O , Austic E , Conroy DA , et al . Substance use as a risk factor for sleep problems among adolescents presenting to the emergency department . J Addict Med. 2016. ; 10 ( 5 ): 331 – 338 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Bathgate CJ , Edinger JD . Diagnostic criteria and assessment of sleep disorders. In: Handbook of Sleep Disorders in Medical Conditions. Cambridge, MA: Academic Press; 2019. :3–25.
- 22. Morin CM , Benca R . Chronic insomnia . Lancet. 2012. ; 379 ( 9821 ): 1129 – 1141 . [DOI] [PubMed] [Google Scholar]
- 23. Finan PH , Smith MT . The comorbidity of insomnia, chronic pain, and depression: dopamine as a putative mechanism . Sleep Med Rev. 2013. ; 17 ( 3 ): 173 – 183 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Boscarino JA , Rukstalis MR , Hoffman SN , et al . Prevalence of prescription opioid-use disorder among chronic pain patients: comparison of the DSM-5 vs. DSM-4 diagnostic criteria . J Addict Dis. 2011. ; 30 ( 3 ): 185 – 194 . [DOI] [PubMed] [Google Scholar]
- 25. Hser YI , Huang D , Saxon AJ , et al . Distinctive trajectories of opioid use over an extended follow-up of patients in a multisite trial on buprenorphine + naloxone and methadone . J Addict Med. 2017. ; 11 ( 1 ): 63 – 69 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Currie SR , Wilson KG , Pontefract AJ , deLaplante L . Cognitive-behavioral treatment of insomnia secondary to chronic pain . J Consult Clin Psychol. 2000. ; 68 ( 3 ): 407 – 416 . [DOI] [PubMed] [Google Scholar]
- 27. Mason W , Suri S . Conducting behavioral research on Amazon’s Mechanical Turk . Behav Res Methods. 2012. ; 44 ( 1 ): 1 – 23 . [DOI] [PubMed] [Google Scholar]
- 28. Strickland JC , Stoops WW . The use of crowdsourcing in addiction science research: Amazon Mechanical Turk . Exp Clin Psychopharmacol. 2019. ; 27 ( 1 ): 1 – 18 . [DOI] [PubMed] [Google Scholar]
- 29. Gossop M . The development of a Short Opiate Withdrawal Scale (SOWS) . Addict Behav. 1990. ; 15 ( 5 ): 487 – 490 . [DOI] [PubMed] [Google Scholar]
- 30. Bastien CH , Vallières A , Morin CM . Validation of the Insomnia Severity Index as an outcome measure for insomnia research . Sleep Med. 2001. ; 2 ( 4 ): 297 – 307 . [DOI] [PubMed] [Google Scholar]
- 31. Von Korff M , DeBar LL , Krebs EE , Kerns RD , Deyo RA , Keefe FJ . Graded chronic pain scale revised: mild, bothersome, and high-impact chronic pain . Pain. 2020. ; 161 ( 3 ): 651 – 661 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Buysse DJ , Reynolds CF III , Monk TH , Berman SR , Kupfer DJ . The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research . Psychiatry Res. 1989. ; 28 ( 2 ): 193 – 213 . [DOI] [PubMed] [Google Scholar]
- 33. Strange C , Richard CL , Shan S , et al . A population-based estimate of the health care burden of obstructive sleep apnea using a STOP-BAG questionnaire in South Carolina . J Clin Sleep Med. 2021. ; 17 ( 3 ): 367 – 374 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Nagappa M , Liao P , Wong J , et al . Validation of the STOP-Bang questionnaire as a screening tool for obstructive sleep apnea among different populations: a systematic review and meta-analysis . PLoS One. 2015. ; 10 ( 12 ): e0143697 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Hosmer DW Jr , Lemeshow S , Sturdivant RX . Applied Logistic Regression. Hoboken, NJ: : Wiley; ; 2013. . [Google Scholar]
- 36. Green SB . How many subjects does it take to do a regression analysis . Multivariate Behav Res. 1991. ; 26 ( 3 ): 499 – 510 . [DOI] [PubMed] [Google Scholar]
- 37. Baldassarri SR , Beitel M , Zinchuk A , et al . Correlates of sleep quality and excessive daytime sleepiness in people with opioid use disorder receiving methadone treatment . Sleep Breath. 2020. ; 24 ( 4 ): 1729 – 1737 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Kocevska D , Lysen TS , Dotinga A , et al . Sleep characteristics across the lifespan in 1.1 million people from the Netherlands, United Kingdom and United States: a systematic review and meta-analysis . Nat Hum Behav. 2021. ; 5 ( 1 ): 113 – 122 . [DOI] [PubMed] [Google Scholar]
- 39. Carroll KM , Nich C , Frankforter TL , et al . Accounting for the uncounted: Physical and affective distress in individuals dropping out of oral naltrexone treatment for opioid use disorder . Drug Alcohol Depend. 2018. ; 192 : 264 – 270 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Finan PH , Buenaver LF , Coryell VT , Smith MT . Cognitive-behavioral therapy for comorbid insomnia and chronic pain . Sleep Med Clin. 2014. ; 9 ( 2 ): 261 – 274 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Selvanathan J , Pham C , Nagappa M , et al . Cognitive behavioral therapy for insomnia in patients with chronic pain—a systematic review and meta-analysis of randomized controlled trials . Sleep Med Rev. 2021. ; 60 : 101460 . [DOI] [PubMed] [Google Scholar]
- 42. Tang NKY . Cognitive behavioural therapy in pain and psychological disorders: towards a hybrid future . Prog Neuropsychopharmacol Biol Psychiatry. 2018. ; 87 ( Pt B ): 281 – 289 . [DOI] [PubMed] [Google Scholar]
- 43. Ellis MS , Kasper Z , Cicero T . Assessment of chronic pain management in the treatment of opioid use disorder: gaps in care and implications for treatment outcomes . J Pain. 2021. ; 22 ( 4 ): 432 – 439 . [DOI] [PubMed] [Google Scholar]
- 44. Huhn AS , Tompkins DA , Dunn KE . The relationship between treatment accessibility and preference amongst out-of-treatment individuals who engage in non-medical prescription opioid use . Drug Alcohol Depend. 2017. ; 180 : 279 – 285 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Kennedy-Hendricks A , Busch SH , McGinty EE , et al . Primary care physicians’ perspectives on the prescription opioid epidemic . Drug Alcohol Depend. 2016. ; 165 : 61 – 70 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Zhang B , Wing YK . Sex differences in insomnia: a meta-analysis . Sleep. 2006. ; 29 ( 1 ): 85 – 93 . [DOI] [PubMed] [Google Scholar]
- 47. Smith MT Jr , Remeniuk B , Finan PH , et al . Sex differences in measures of central sensitization and pain sensitivity to experimental sleep disruption: implications for sex differences in chronic pain . Sleep. 2019. ; 42 ( 2 ): zsy209 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Bjorvatn B , Grønli J , Pallesen S . Prevalence of different parasomnias in the general population . Sleep Med. 2010. ; 11 ( 10 ): 1031 – 1034 . [DOI] [PubMed] [Google Scholar]
- 49. Innes KE , Selfe TK , Agarwal P . Prevalence of restless legs syndrome in North American and Western European populations: a systematic review . Sleep Med. 2011. ; 12 ( 7 ): 623 – 634 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Sharpless BA , Barber JP . Lifetime prevalence rates of sleep paralysis: a systematic review . Sleep Med Rev. 2011. ; 15 ( 5 ): 311 – 315 . [DOI] [PMC free article] [PubMed] [Google Scholar]