Abstract
Study Objectives:
To determine whether there was evidence of circadian or sleep-regulatory dysfunction in sighted individuals with non–24-hour sleep-wake rhythm disorder.
Methods:
Three sighted individuals with signs and/or symptoms of non–24-hour sleep-wake rhythm disorder were studied. Thirty-five- to 332-day laboratory and home-based assessments of sleep-wake and circadian timing, endogenous circadian period, photic input to the circadian pacemaker, and/or circadian and sleep-wake–dependent regulation of sleep were conducted.
Results:
No evidence of circadian dysfunction was found in these individuals. Instead, sleep-wake timing appeared to dissociate from the circadian timing system, and/or self-selected sleep-wake and associated light/dark timing shifted the circadian pacemaker later, rather than the circadian pacemaker determining sleep-wake timing.
Conclusions:
These findings suggest that the etiology of this disorder may be light- and/or behaviorally induced in some sighted people, which has implications for the successful treatment of this disorder.
Citation:
Emens JS, St Hilaire MA, Klerman EB, et al. Behaviorally and environmentally induced non–24-hour sleep-wake rhythm disorder in sighted patients. J Clin Sleep Med. 2022;18(2):453–459.
Keywords: circadian rhythm, circadian rhythm sleep disorders, non–24-hour sleep-wake rhythm disorder, light, melatonin
BRIEF SUMMARY
Current Knowledge/Study Rationale: The etiology of non–24-hour sleep-wake rhythm disorder in sighted patients is unknown. However, similar patterns of sleep-wake timing are observed in healthy sighted individuals living under conditions of self-selected light/dark timing without knowledge of time of day.
Study Impact: Non–24-hour sleep-wake rhythm disorder may be behaviorally and environmentally induced in some sighted patients. This disorder therefore has a different etiology in sighted and blind individuals, which has nosological and treatment implications.
INTRODUCTION
Non–24-hour sleep-wake rhythm disorder (N24SWD)1 is defined by sleep-wake times that move to a later (or rarely, earlier) clock hour each day,2 similar to the sleep-wake timing of sighted humans living without knowledge of time in self-selected light/dark conditions.3–5 N24SWD in sighted individuals is assumed to result from an inability of the circadian pacemaker to synchronize (entrain) to the 24-hour day, as occurs in blind patients with N24SWD in whom light information is no longer conveyed from photosensitive retinal ganglion cells, rods, and cones6 to the circadian pacemaker located in the suprachiasmatic nuclei of the hypothalamus.2,7,8 Because the endogenous period of the circadian pacemaker is usually longer than 24 hours, inability of the pacemaker to entrain to the external 24-hour day results in delays of the circadian rhythm in sleep-wake propensity to a later time each day. Rarely, when the circadian period is less than 24 hours, sleep-wake propensity advances to an earlier time each day in N24SWD. Unlike sighted patients with N24SWD,2,9 the vast majority of blind patients attempt to maintain a 24-hour sleep-wake schedule10 and therefore present with a relapsing and remitting pattern of nighttime insomnia and daytime hypersomnolence as the circadian rhythm in sleep propensity delays (or less commonly, advances) in accordance with the non–24-hour endogenous period of the pacemaker.1,7,11 This difference in the clinical presentation of N24SWD in blind and sighted individuals is striking7,9 and it raises the possibility that the pathophysiology of the disorder differs in the 2 populations.
There are at least 6 possible etiologies for N24SWD in sighted individuals that are not mutually exclusive. First, there could be a selective loss of circadian photoreception sufficient to preclude entrainment in individuals whose vision is otherwise preserved; this has yet to be observed.8,12–14 Second, patients may have a circadian period that is too different from 24 hours to entrain to the 24-hour day, consistent with observed sleep-wake cycles in most patients with N24SWD that are generally much longer than the average ∼24.15-hour intrinsic period of the pacemaker.5,15–17 Third, patients may have altered sensitivity to the resetting effects of light (eg, decreased sensitivity to phase-advancing morning light and/or increased sensitivity to phase-delaying evening light). Fourth, the coupling between the circadian system and sleep timing may be weakened, due to biological and/or social factors, such that the 2 cycles are no longer synchronized. Fifth, there may be slower accumulation and/or dissipation of homeostatic sleep drive, with consequently longer bouts of wake and sleep. Sixth, patients’ self-selected patterns of light exposure may shift circadian phase to a later hour in the absence of any circadian or homeostatic dysfunction.2,5
We studied 3 sighted individuals with N24SWD symptoms by evaluating endogenous circadian period, circadian photoreception, circadian timing, and/or circadian and sleep-wake–dependent regulation of sleep; documenting self-selected sleep-wake schedules; and modeling the effects of self-selected light/dark timing on the circadian system.
METHODS
Participants
Participant 1 was a 31-year-old male with a history of childhood asthma, depressed mood in adolescence, and sleep-onset insomnia (Table 1). In his early 20s, he decided to sleep and wake whenever he wished and adopted a non–24-hour sleep-wake schedule with a day length of ∼25 hours, which he reported improved his mood. He was not taking any medications. He was irregularly employed as a computer programmer and lived with 2 other individuals. Study length was 84 days.
Table 1.
Participant study details.
| Participant | Study Length (days) | Ambulatory Assessment | Laboratory Assessment | ssessment of Circadian Phase | Overall Observed Sleep-Wake Period (h) | Maximum Weekly Sleep-Wake Period (h) | Intrinsic Period (h) |
|---|---|---|---|---|---|---|---|
| 1 | 84 | Sleep diary, actigraphy, CBT, and heart rate | Yes | DLMO and CBTmin | 24.8 and 25.2 | 26.4 | 24.5 |
| 2 | 332 | Sleep diary and actigraphy | Yes | DLMO | 24.7 | 39.8 | Not assessed |
| 3 | 35 | Sleep diary and actigraphy | No | DLMO | 24.9 | 31.0 | Not assessed |
CBTmin = fitted minimum core body temperature, DLMO = dim light melatonin onset.
Participant 2 was a 39-year-old female with a history of irregular and delayed sleep-wake timing dating to preadolescence and non–24-hour sleep-wake schedules with day lengths of up to ∼25 hours. She reported a history of cervical cancer (status post total abdominal hysterectomy with bilateral salpingo-oophorectomy), depression, hypothyroidism, and asthma. Medications included levothyroxine, estradiol, extended-release bupropion, and methylphenidate. She was unemployed and lived alone. Study length was 332 days.
Participant 3 was a 20-year-old male who was a full-time college student. He was part of a larger observational nonclinical study of college undergraduates; demographic and medical details were limited.18 Nightshift work or greater than 1 time zone of travel in the prior 3 months were exclusionary for that study. He reported delayed sleep-wake timing (Owl-Lark score = 31; moderate evening type) but did not report significant sleep-wake complaints (Global Pittsburgh Sleep Quality Index Score = 5), and was not recruited for that study on this basis. He had a body mass index of 29.4 kg/m2 and scored as high risk for sleep apnea on the Berlin Questionnaire but did not have a sleep apnea diagnosis. He denied selective serotonin reuptake inhibitor, monoamine oxidase inhibitor, steroid, beta-blocker, melatonin, and diuretic use; no other medical history was available. Study length was 35 days.
Assessments
All participants had outpatient assessments that included continuous wrist actigraphy, core body temperature (CBT) via rectal thermistor, heart rate monitoring, and sleep diaries (Participant 1, PMS-8 Recorder using a 1-minute sampling interval; Vitalog Corp., Redwood City, CA) or wrist actigraphy and sleep diaries (for Participants 2 and 3, respectively, both using 1-minute sampling intervals: Actiwatch-64 [Mini Mitter Co., Bend, OR] and MotionLogger [Ambulatory Monitoring, Ardsley, NY]). Participants 1 and 2 also had inpatient assessments.
Participant 1 (Figure 1A) was admitted to the Brigham and Women’s Hospital research unit and lived in an environment free of external time cues on 3 occasions. The first visit lasted 25 days (days 25–49 of study). All activities, including sleep episodes, were scheduled. Polysomnography was conducted during all sleep episodes.19 Light levels were ≤ 15 lux during wakefulness and 0 lux during sleep episodes. CBT was collected every minute via rectal thermistor and blood samples were collected every 10–60 minutes via intravenous catheter. Visits 2 and 3 were 24-hour assessments with an ad libitum sleep-wake schedule while in a constant, semirecumbent posture.
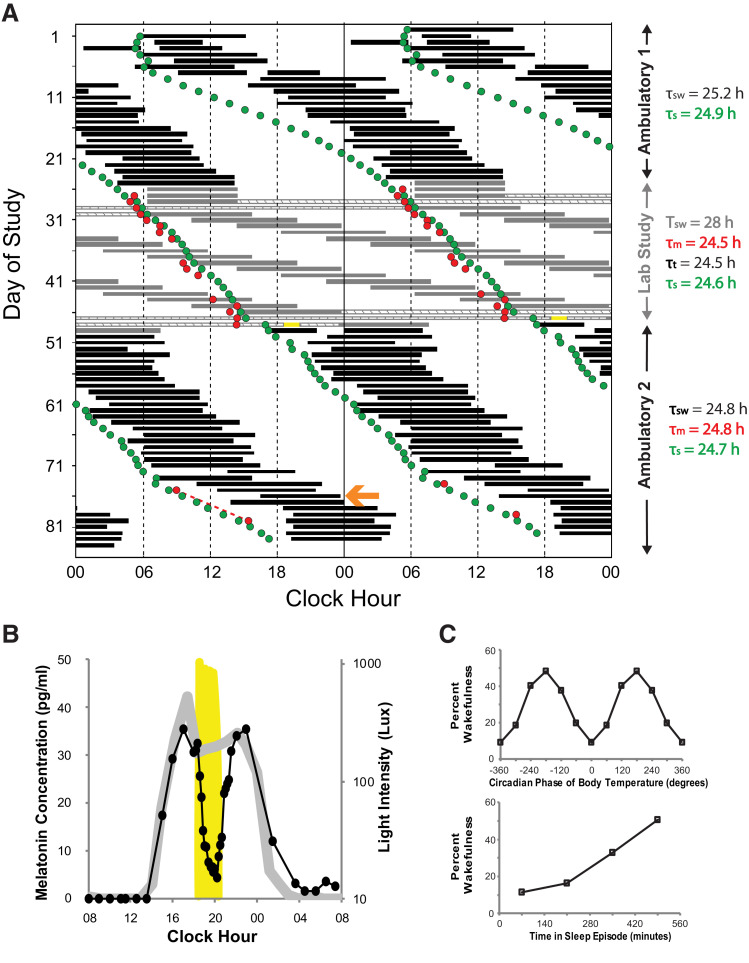
Figure 1. Participant 1.
(A) Double-raster plot of the experimental protocol. Hours are along the horizontal axis and days down the vertical axis; 2 days are plotted across each horizontal line. Sleep timing is represented by the black (self-selected) and gray (scheduled) bars. Hatched bars show the constant routines and yellow bar the timing of the bright light for the melatonin suppression test. Red circles indicate the timing of the dim light melatonin onsets and the green circles are the model simulated time of the onset of melatonin synthesis. The calculated period (denoted by the Greek letter τ) for each segment using different metrics is shown to the right using the same color as in the raster plot (sw: sleep-wake; s: simulation; m: melatonin; t: temperature). Note instances (eg, day 76, orange arrow) where sleep is initiated late relative to the DLMO and might therefore be expected to increase exposure to phase-delaying light and minimize exposure to phase-advancing light. (B) Results of the melatonin suppression test; melatonin levels on the night of the test are represented by the black closed circles and line while melatonin levels from 24-hours prior are represented by the gray line. Light levels are plotted on the secondary y-axis on a log scale; yellow shading plots the timing and intensity of the bright light. (C) Percent wakefulness during forced desynchrony as a function of circadian phase (0° defined as the core body temperature minimum) and time-into-sleep episodes.
Participant 2 (Figure 2) was admitted to the Oregon Health & Science University research center for 6–24 hours on 8 occasions. No limitations were placed on activity except that no exercise was permitted. During wakefulness, light levels were ≤ 10 lux and saliva samples were collected every 30 minutes.
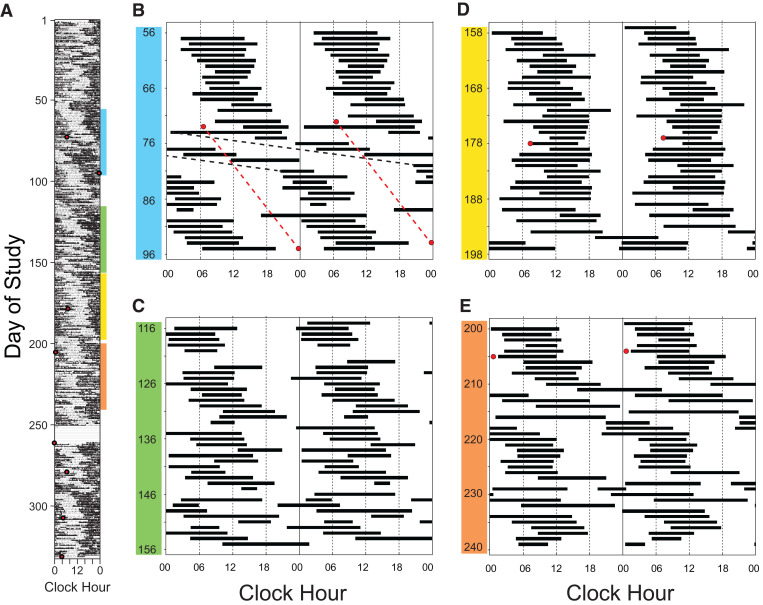
Figure 2. Participant 2.
(A) Single-raster plot of wrist actigraphy data from all 332 days of study (black denotes movement); hours are along the horizontal axis and days down the vertical axis. Red circles represent the timing of the salivary dim light melatonin onsets (DLMOs). (B–E) Double-raster plots (black bars denote sleep periods) of sleep diary data from selected days of study and highlight the different sleep-wake patterns observed. In (B) there is a non–24-hour sleep-wake schedule with an overall observed sleep-wake period of 24.7 hours (days 56–96). Note however that there was a week (days 74–81) when the observed sleep-wake period was 32.0 hours (black dashed line). The overall sleep-wake period and observed melatonin periods (red dashed line) were both 24.8 hours from days 73 to 95. (C) is an example of an irregular sleep-wake pattern, (D) shows a predominantly 24-hour sleep-wake pattern, and (E) shows again weeks where the observed sleep-wake period exceeded 30 hours (eg, 35.0 hours, days 210–217 and 39.8 hours, days 228–235).
Assessment of circadian timing
Inpatient plasma (participant 1), inpatient saliva (participant 2), or at-home saliva (participant 3) samples were collected and assayed for melatonin by radioimmunoassay.7,8 Circadian phase was estimated using the dim light melatonin onset (DLMO): the interpolated time when levels crossed the equivalent thresholds of 10 (plasma) and 3 (saliva) pg/ml.20
In participant 1, days 28–30 and 46–48 of visit 1 included a constant routine procedure.8 Circadian phase was estimated using the fitted minimum of CBT during those days.5
Assessment of intrinsic circadian period and circadian and homeostatic sleep regulation
Participant 1 completed a 17-day forced desynchrony (FD) protocol with a 28-hour cycle length during days 30–46 of visit 1 to estimate the intrinsic period of the central circadian pacemaker.5,15 Hourly sampling of plasma occurred during 242 of the 392 hours of FD. Endogenous circadian period was determined by both (1) nonorthogonal spectral analysis5 of CBT and plasma melatonin data during FD and (2) comparison of CBT and melatonin phase assessments during constant routines before and after FD.5 Circadian and sleep-dependent contributions to sleep timing were quantified by averaging relative to circadian phase and time within each sleep episode, respectively.21
Assessment of outpatient period length
Periods of the ambulatory activity, CBT, heart rate, and sleep diary data were calculated by chi squared periodogram using a 1-minute block size, test periods ranging from 8 to 40 hours, and a 0.001 confidence level (ClockLab 6.1.02; Actimetrics, Wilmette, IL).7,22 Observed melatonin period was determined by linear regression or interpolation between DLMOs.
Assessment of circadian photoreception with the melatonin-suppression test
Circadian photoreception was assessed utilizing the melatonin-suppression test.8,23 Participant 1 was exposed to 90 minutes of bright light (846 ± 92 lux [average ± SD]) (Figure 1B). A test was defined as positive when the average plasma melatonin concentration during the final 60 minutes of bright light exposure was ≥ 33% below that of the corresponding 60-minute time period 24 hours prior.8
Simulation of circadian timing
Sleep diary data of participant 1 were used to estimate circadian phase using a mathematical model of both photic and nonphotic effects on the circadian pacemaker (coded and run in MATLAB version 2015b; The MathWorks, Natick, MA).24 The inputs to the model were the participant’s reported sleep-wake times and either known inpatient light levels or 100 and 0 lux during self-reported outpatient wake and sleep episodes, respectively (when light levels were not recorded). No changes to the model equations or parameters were made from the published version, except we used the participant’s FD-assessed intrinsic period of 24.5 hours instead of the model’s default intrinsic period of 24.2. The primary model output was melatonin synthesis onset as defined in St. Hilaire et al.25
All 3 studies were approved by the appropriate institutional review board and each participant provided written informed consent.
RESULTS
Participant 1
Ambulatory data (days 1–25) demonstrated 25.1-hour period lengths in activity, CBT, and heart rate, and 25.2 hours from sleep diary (including a week when the average period was 26.4, days 5–12). In contrast, the average FD-assessed circadian period was ∼24.5 hours: 24.5 hours using constant routine phase assessment differences for both CBT (minimum of CBT at 11:13 and 20:19 on days 29 and 47, respectively) and melatonin data (DLMO at 05:25 and 14:26 on days 29 and 47, respectively), and 24.5 and 24.6 hours using non-orthogonal spectral analysis of DLMO and CBT data, respectively. Following the inpatient study (days 49–84), the period was 24.8 hours from sleep diary.
The melatonin suppression test was positive with a 78% decrease (Figure 1B). Percent wakefulness during sleep opportunities measured as a function of both circadian phase and time within the sleep episode was similar to that seen in individuals without N24SWD (Figure 1C).21
Participant 2
Sleep diary and activity data over all 332 days each showed both a non–24-hour period of 24.7 hours and a 24-hour rhythm, with the latter predominating (Figure 2A). A variety of sleep-wake patterns were observed: non–24-hour (Figure 2B), including weeks when the average period exceeded 30 hours (eg, Figure 2B and Figure 2E, days 74–81 and 210–217, respectively), irregular (Figure 2C), and 24-hour (Figure 2D). Her written logs noted several days when sleep timing was planned to meet social/work obligations (eg, Figure 2A, days 50–51).
Participant 3
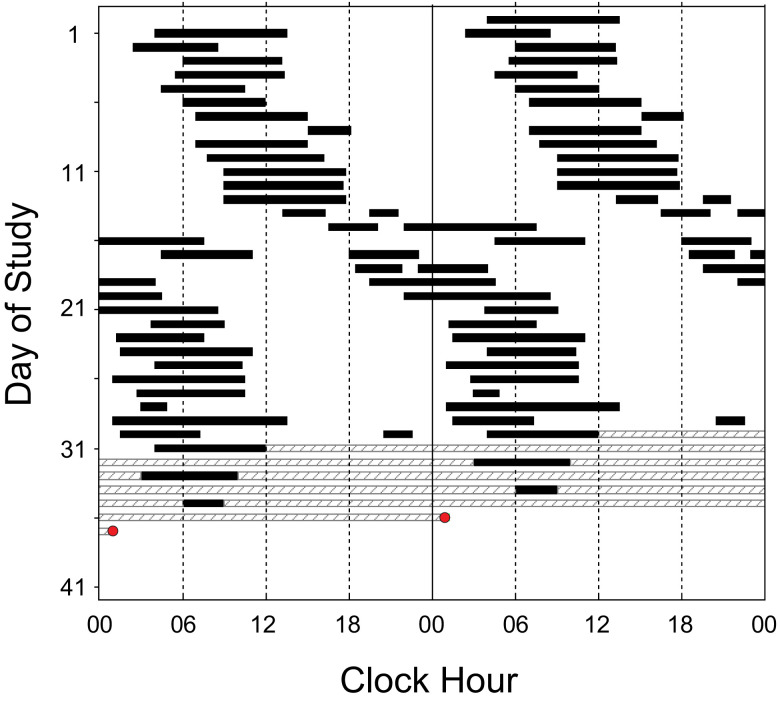
Sleep diary data across 31 days of study revealed a period of 24.9 hours with 1 week when the average period was 31.0 hours (Figure 3, days 13–20).
Figure 3. Participant 3.
Double-raster plot of sleep diary data. Hours are along the horizontal axis and days down the vertical axis; 2 days are shown on each horizontal line. Self-selected sleep timing is represented by the black bars and hatched bars denote missing data. The red circle indicates the timing of the dim light melatonin onset.
DISCUSSION
In contrast to N24SWD in blind individuals, non–24-hour sleep-wake cycles in these 3 sighted people were not primarily driven by the central circadian pacemaker. Instead, in these individuals the daily pattern of light exposure and associated sleep-wake timing appears to be recurrently driving the circadian pacemaker to a later hour for extended intervals, often interrupted by occasions when sleep-wake timing becomes desynchronized from the circadian pacemaker.
The most striking evidence of recurrent circadian phase delays driven by the self-selected sleep-wake and light/dark cycles was seen in participant 1, whose non–24-hour rest-activity patterns, CBT and heart rate rhythms, and sleep-wake schedules all had observed periods significantly longer than his intrinsic circadian period. Notably, his intrinsic circadian period was not abnormally long, eliminating this as an explanation for his non–24-hour sleep-wake timing. While intrinsic circadian period was not determined in participants 2 or 3, their observed non–24-hour sleep-wake patterns had segments with cycles that averaged ≥ 30 hours, far outside the range of any intrinsic circadian period measured in humans,5,15 revealing that the pacemaker was not driving the timing of their sleep-wake schedule.
In participants 1 and 2, the model-simulated and/or measured melatonin phase data demonstrated that sleep-wake timing sometimes delayed at a rate faster than the circadian system, resulting in sleep-wake timing that would be expected to induce recurrent circadian phase delays by increasing exposure to phase delaying light and minimizing exposure to phase advancing light5 (eg, Figure 1A, day 76 and Figure 2B, day 73) and/or desynchronization of pacemaker output from sleep-wake timing (eg, Figure 2B, days 74–81).5
The melatonin suppression test in participant 1 demonstrated intact circadian photoreception, indicating no detectable disruption to light input. Participant 1 also demonstrated circadian and sleep-wake–dependent patterns of wake propensity (Figure 1C) comparable to those of entrained individuals,21 suggesting that circadian and homeostatic sleep-wake regulatory mechanisms were intact. These mechanisms are therefore not necessary to explain N24SWD in this sighted individual.
Participant 3 demonstrated that non–24-hour sleep-wake patterns may be observed in the absence of sleep-wake complaints. The fact that non–24-hour sleep-wake schedules are recapitulated in healthy individuals studied in environments free of time cues further indicates that circadian dysfunction is not necessary to exhibit a non–24-hour sleep-wake behavioral pattern.5 Indeed, we previously reported a healthy individual who spontaneously adopted a 27.1-hour sleep-wake cycle in such an environment despite having an intrinsic circadian period of 24.3 hours (see figure 1 of Czeisler et al).5 These individuals highlight the fact that the behaviorally and environmentally induced influences on, and dissociation of sleep-wake timing from, the circadian timing system described above are not unique to the N24SWD patient population. Both of these routinely occur when healthy people self-select their light/dark schedule while living in an environment free of time cues during experimental protocols.3–5 Moreover, in the general population, phase-delay shifts of the circadian pacemaker may occur on weekends or work-free days (ie, social jet-lag) as a result of self-selected sleep-wake timing and associated phase-delaying evening light exposure,26,27 and both nightshift workers and transmeridian travelers routinely dissociate sleep-wake timing from the circadian rhythm of sleep propensity. Nonetheless, it is probable that certain properties of the circadian system (discussed above) predispose an individual to N24SWD or perpetuate the condition.14,17
In conclusion, the non–24-hour sleep-wake patterns observed in sighted individuals with N24SWD need not be initiated by circadian dysfunction. Rather than representing a failure of circadian entrainment, this disorder demonstrates the circadian system’s expected response to increased exposure to light at night and decreased exposure to light during the daytime.18,27 This was a limited case-series and further research is needed to understand the factors that predispose some individuals to go to bed and wake up later each day, even while exposed to synchronizers from the 24-hour day, and to promote the development of etiology-informed treatments, such as appropriately timed bright light and low-dose melatonin,11,28–30 that take into consideration the effects of even dim evening light exposure.27 When considering the diagnostic implications of these findings it must be noted that many of the variables we evaluated (eg, circadian phase and period) cannot be assessed in the clinical setting; this is also true in other circadian rhythm sleep disorders such as delayed sleep-wake phase disorder.31,32 Despite these diagnostic challenges and the small number of participants in the current case series, we contend that the current data and previous findings discussed above2–5 require a revision of the nosology of N24SWD. We therefore propose a new pathophysiologically based classification for some sighted patients with N24SWD: behaviorally and environmentally induced N24SWD, which has an etiology different from N24SWD in totally blind individuals.
DISCLOSURE STATEMENT
The final manuscript has been read and approved by all authors. Work for this study was performed at Brigham and Women’s Hospital and Oregon Health & Science University (OHSU). This work was supported by a NARSAD Young Investigator Award to J.S.E., NIH K24-HL105664 to E.B.K. PO1-AG09975 to C.A.C. and E.B.K., NIH MO1-RR02635 to the General Clinical Research Center at Brigham and Women’s Hospital and NIH UL1-RR024140 to the Oregon Clinical and Translational Research Institute at OHSU. None of the funders had any role in this work. Dr. Emens is an expert witness in legal cases, including those involving Teva Pharmaceuticals USA, Inc., Apotex Inc., Apotex Corp., MSN Pharmaceuticals Inc. and MSN Laboratories Pvt. Ltd. Dr. St. Hilaire has provided paid limited consulting for The MathWorks, Inc. Dr. St. Hilaire has received honoraria and travel funds as an invited speaker from the Providence Sleep Research Interest Group and the Mayo Clinic Metabolomics Resource Core. Dr. Klerman has received travel support from the Sleep Research Society, Santa Fe Institute, DGSM (German Sleep Society); Consulting with Puerto Rico Science, Technology and Research Trust, The National Sleep Foundation, Sanofi-Genzyme, and Circadian Therapeutics; partner owns Chronsulting. Dr. Czeisler reports grants to BWH from FAA, NHLBI, NIA, NIOSH, NASA, and DOD; is/was a paid consultant to AARP, American Academy of Dental Sleep Medicine, Eisenhower Medical Center, Emory University, Inselspital Bern, Institute of Digital Media and Child Development, Klarman Family Foundation, M. Davis and Co, Physician's Seal, Sleep Research Society Foundation, State of Washington Board of Pilotage Commissioners, Tencent Holdings Ltd, Teva Pharma Australia, UC San Diego, University of Washington, and Vanda Pharmaceuticals Inc, in which Dr. Czeisler also holds an equity interest; received travel support from Annenberg Center for Health Sciences at Eisenhower, Aspen Brain Institute, Bloomage International Investment Group, Inc., UK Biotechnology and Biological Sciences Research Council, Bouley Botanical, Dr. Stanley Ho Medical Development Foundation, European Biological Rhythms Society, German National Academy of Sciences (Leopoldina), Illuminating Engineering Society, National Safety Council, National Sleep Foundation, Society for Research on Biological Rhythms, Sleep Research Society Foundation, Stanford Medical School Alumni Association, Tencent Holdings Ltd, University of Zurich, and Vanda Pharmaceuticals Inc, Ludwig-Maximilians-Universität München, National Highway Transportation Safety Administration, Office of Naval Research, Salk Institute for Biological Studies/Fondation Ipsen; receives research/education support through BWH from Cephalon, Mary Ann & Stanley Snider via Combined Jewish Philanthropies, Harmony Biosciences LLC, Jazz Pharmaceuticals PLC Inc, Johnson & Johnson, NeuroCare, Inc., Philips Respironics Inc/Philips Homecare Solutions, Regeneron Pharmaceuticals, Regional Home Care, Teva Pharmaceuticals Industries Ltd, Sanofi SA, Optum, ResMed, San Francisco Bar Pilots, Sanofi, Schneider, Simmons, Sysco, Philips, Vanda Pharmaceuticals; is/was an expert witness in legal cases, including those involving Advanced Power Technologies, Aegis Chemical Solutions LLC, Amtrak; Casper Sleep Inc, C&J Energy Services, Catapult Energy Services Group, LLC, Covenant Testing Technologies, LLC, Dallas Police Association, Enterprise Rent-A-Car, Espinal Trucking/Eagle Transport Group LLC/Steel Warehouse Inc, FedEx, Greyhound Lines Inc/Motor Coach Industries/FirstGroup America, Pomerado Hospital/Palomar Health District, PAR Electrical Contractors Inc, Product & Logistics Services LLC/Schlumberger Technology Corp/Gelco Fleet Trust, Puckett Emergency Medical Services LLC, South Carolina Central Railroad Company LLC, Union Pacific Railroad, United Parcel Service/UPS Ground Freight Inc, and Vanda Pharmaceuticals; serves as the incumbent of an endowed professorship provided to Harvard University by Cephalon, Inc.; and receives royalties from McGraw Hill, and Philips Respironics for the Actiwatch-2 and Actiwatch Spectrum devices. Dr. Czeisler’s interests were reviewed and are managed by the Brigham and Women’s Hospital and Mass General Brigham in accordance with their conflict of interest policies. The other authors do not report any conflicts of interest.
ACKNOWLEDGMENTS
The authors thank the funding agencies, the study participants, and the staff of the General Clinical Research Center at Brigham and Women’s Hospital and the Oregon Clinical and Translational Research Institute at Oregon Health & Science University for their support in conducting these studies.
ABBREVIATIONS
- CBT
core body temperature
- DLMO
dim light melatonin onset
- FD
forced desynchrony
- N24SWD
non-24-hour sleep-wake rhythm disorder
REFERENCES
- 1. American Academy of Sleep Medicine . International Classification of Sleep Disorders. 3rd ed. Darien, IL: : American Academy of Sleep Medicine; ; 2014. . [Google Scholar]
- 2. Uchiyama M , Lockley SW . Non-24-hour sleep-wake syndrome in sighted and blind patients . Sleep Med Clin. 2009. ; 4 ( 2 ): 195 – 211 . [DOI] [PubMed] [Google Scholar]
- 3. Wever R . The circadian system of man: Results of experiments under temporal isolation. New York, NY: : Springer-Verlag; ; 1979. . [Google Scholar]
- 4. Czeisler CA , Weitzman E , Moore-Ede MC , Zimmerman JC , Knauer RS . Human sleep: its duration and organization depend on its circadian phase . Science. 1980. ; 210 ( 4475 ): 1264 – 1267 . [DOI] [PubMed] [Google Scholar]
- 5. Czeisler CA , Duffy JF , Shanahan TL , et al . Stability, precision, and near-24-hour period of the human circadian pacemaker . Science. 1999. ; 284 ( 5423 ): 2177 – 2181 . [DOI] [PubMed] [Google Scholar]
- 6. Lucas RJ , Lall GS , Allen AE , Brown TM . How rod, cone, and melanopsin photoreceptors come together to enlighten the mammalian circadian clock . Prog Brain Res. 2012. ; 199 : 1 – 18 . [DOI] [PubMed] [Google Scholar]
- 7. Emens JS , Laurie AL , Songer JB , Lewy AJ . Non-24-hour disorder in blind individuals revisited: variability and the influence of environmental time cues . Sleep. 2013. ; 36 ( 7 ): 1091 – 1100 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Czeisler CA , Shanahan TL , Klerman EB , et al . Suppression of melatonin secretion in some blind patients by exposure to bright light . N Engl J Med. 1995. ; 332 ( 1 ): 6 – 11 . [DOI] [PubMed] [Google Scholar]
- 9. Hayakawa T , Uchiyama M , Kamei Y , et al . Clinical analyses of sighted patients with non-24-hour sleep-wake syndrome: a study of 57 consecutively diagnosed cases . Sleep. 2005. ; 28 ( 8 ): 945 – 952 . [DOI] [PubMed] [Google Scholar]
- 10. Licamele L , Dressman MM , Feeney J , Polymeropoulos MH . Pleiomorphic expression of N24HSWD in the totally blind . Sleep. 2012. ; 35S : A206 – A207. [Google Scholar]
- 11. Sack RL , Brandes RW , Kendall AR , Lewy AJ . Entrainment of free-running circadian rhythms by melatonin in blind people . N Engl J Med. 2000. ; 343 ( 15 ): 1070 – 1077 . [DOI] [PubMed] [Google Scholar]
- 12. Hull JT , Czeisler CA , Lockley SW . Suppression of melatonin secretion in totally visually blind people by ocular exposure to white light . Ophthalmology. 2018. : 125 ( 8 ): 1160 – 1171 . [DOI] [PubMed] [Google Scholar]
- 13. Klerman EB , Shanahan TL , Brotman DJ , et al . Photic resetting of the human circadian pacemaker in the absence of conscious vision . J Biol Rhythms. 2002. ; 17 ( 6 ): 548 – 555 . [DOI] [PubMed] [Google Scholar]
- 14. Abbott SM , Choi J , Wilson J , Zee PC . Melanopsin-dependent phototransduction is impaired in delayed sleep-wake phase disorder and sighted non-24-hour sleep-wake rhythm disorder . Sleep. 2021. ; 44 ( 2 ):zsaa184. [DOI] [PubMed] [Google Scholar]
- 15. Duffy JF , Cain SW , Chang AM , et al . Sex difference in the near-24-hour intrinsic period of the human circadian timing system . Proc Natl Acad Sci USA. 2011. ; 108 ( Suppl 3 ): 15602 – 15608 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kitamura S , Hida A , Enomoto M , et al . Intrinsic circadian period of sighted patients with circadian rhythm sleep disorder, free-running type . Biol Psychiatry. 2013. ; 73 ( 1 ): 63 – 69 . [DOI] [PubMed] [Google Scholar]
- 17. Micic G , Lovato N , Ferguson SA , Burgess HJ , Lack L . Circadian tau differences and rhythm associations in delayed sleep-wake phase disorder and sighted non-24-hour sleep-wake rhythm disorder . Sleep. 2021. ; 44 ( 1 ): zsaa132 . [DOI] [PubMed] [Google Scholar]
- 18. Phillips AJK , Clerx WM , O’Brien CS , et al . Irregular sleep/wake patterns are associated with poorer academic performance and delayed circadian and sleep/wake timing . Sci Rep. 2017. ; 7 ( 1 ): 3216 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Rechtschaffen A , Kales A . A Manual of Standardized Terminology, Techniques and Scoring System for Sleep Stages of Human Subjects. Bethesda, MD: : US Department of Health, Education and Welfare, Public Health Service; ; 1968. . [Google Scholar]
- 20. Lewy AJ , Cutler NL , Sack RL . The endogenous melatonin profile as a marker for circadian phase position . J Biol Rhythms. 1999. ; 14 ( 3 ): 227 – 236 . [DOI] [PubMed] [Google Scholar]
- 21. Dijk DJ , Duffy JF , Riel E , Shanahan TL , Czeisler CA . Ageing and the circadian and homeostatic regulation of human sleep during forced desynchrony of rest, melatonin and temperature rhythms . J Physiol. 1999. ; 516 ( 2 ): 611 – 627 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Emens J , Lewy AJ , Laurie AL , Songer JB . Rest-activity cycle and melatonin rhythm in blind free-runners have similar periods . J Biol Rhythms. 2010. ; 25 ( 5 ): 381 – 384 . [DOI] [PubMed] [Google Scholar]
- 23. Lewy AJ , Wehr TA , Goodwin FK , Newsome DA , Markey SP . Light suppresses melatonin secretion in humans . Science. 1980. ; 210 ( 4475 ): 1267 – 1269 . [DOI] [PubMed] [Google Scholar]
- 24. St Hilaire MA , Klerman EB , Khalsa SBS , Wright KP Jr , Czeisler CA , Kronauer RE . Addition of a non-photic component to a light-based mathematical model of the human circadian pacemaker . J Theor Biol. 2007. ; 247 ( 4 ): 583 – 599 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. St Hilaire MA , Gronfier C , Zeitzer JM , Klerman EB . A physiologically based mathematical model of melatonin including ocular light suppression and interactions with the circadian pacemaker . J Pineal Res. 2007. ; 43 ( 3 ): 294 – 304 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Wittmann M , Dinich J , Merrow M , Roenneberg T . Social jetlag: misalignment of biological and social time . Chronobiol Int. 2006. ; 23 ( 1-2 ): 497 – 509 . [DOI] [PubMed] [Google Scholar]
- 27. Stothard ER , McHill AW , Depner CM , et al . Circadian entrainment to the natural light-dark cycle across seasons and the weekend . Curr Biol. 2017. ; 27 ( 4 ): 508 – 513 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Hoban TM , Sack RL , Lewy AJ , Miller LS , Singer CM . Entrainment of a free-running human with bright light? Chronobiol Int. 1989. ; 6 ( 4 ): 347 – 353 . [DOI] [PubMed] [Google Scholar]
- 29. McArthur AJ , Lewy AJ , Sack RL . Non-24-hour sleep-wake syndrome in a sighted man: circadian rhythm studies and efficacy of melatonin treatment . Sleep. 1996. ; 19 ( 7 ): 544 – 553 . [DOI] [PubMed] [Google Scholar]
- 30. Malkani RG , Abbott SM , Reid KJ , Zee PC . Diagnostic and treatment challenges of sighted non-24-hour sleep-wake disorder . J Clin Sleep Med. 2018. ; 14 ( 4 ): 603 – 613 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Murray JM , Sletten TL , Magee M , et al .; Delayed Sleep on Melatonin (DelSoM) Study Group . Prevalence of circadian misalignment and its association with depressive symptoms in delayed sleep phase disorder . Sleep. 2017. ; 40 ( 1 ): 1 – 10 . [DOI] [PubMed] [Google Scholar]
- 32. Rahman SA , Kayumov L , Shapiro CM . Antidepressant action of melatonin in the treatment of Delayed Sleep Phase Syndrome . Sleep Med. 2010. ; 11 ( 2 ): 131 – 136 . [DOI] [PubMed] [Google Scholar]