Abstract
Opioids are widely prescribed for pain management, and it is estimated that 40% of adults in the United States use prescription opioids every year. Opioid misuse leads to high mortality, with respiratory depression as the main cause of death. Animal and human studies indicate that opioid use may lead to sleep-disordered breathing. Opioids affect control of breathing and impair upper airway function, causing central apneas, upper airway obstruction, and hypoxemia during sleep. The presence of obstructive sleep apnea (OSA) increases the risk of opioid-induced respiratory depression. However, even if the relationship between opioids and central sleep apnea is firmly established, the question of whether opioids can aggravate OSA remains unanswered. While several reports have shown a high prevalence of OSA and nocturnal hypoxemia in patients receiving a high dose of opioids, other studies did not find a correlation between opioid use and obstructive events. These differences can be attributed to considerable interindividual variability, divergent effects of opioids on different phenotypic traits of OSA, and wide-ranging methodology. This review will discuss mechanistic insights into the effects of opioids on the upper airway and hypoglossal motor activity and the association of opioid use and obstructive sleep apnea.
Citation:
Freire C, Sennes LU, Polotsky VY. Opioids and obstructive sleep apnea. J Clin Sleep Med. 2022;18(2):647–652.
Keywords: obstructive sleep apnea, opioids, hypoglossal nerve, respiratory physiology
INTRODUCTION
Opioids are frequently used to treat chronic and acute pain with a considerable increase in noncancer pain prescriptions in the last decades. Despite the efforts to reduce opioid prescriptions and minimize the effects of the opioid epidemic, the amount of prescribed opioids increased 3-fold since 1999, and 91.8 million Americans used prescription opioids in 2015.1,2 Mortality associated with opioids increased further during the coronavirus disease 2019 (COVID-19) pandemic.3 Respiratory depression is the main adverse effect of opioids, and comorbidities such as obesity and obstructive sleep apnea (OSA) increase the risk of opioid-induced respiratory depression (OIRD).4,5
Mu opioid receptors (MORs) play a pivotal role in OIRD. MORs are expressed in central and peripheral centers of the respiratory network, affecting rhythm generation and hypoxic and hypercapnic ventilatory responses. The ability to block pathways that are responsible for respiratory depression without affecting analgesia or inducing withdrawal symptoms is fundamental to mitigating morbidity and mortality related to opioids.6–8
Opioids are known to interfere with various aspects of sleep, which leads to altered sleep architecture and poor quality of sleep.9 Evidence from animal and human studies show that opioids are associated with central, obstructive, and hypoxemic events during sleep.9–13 Opioids may cause central sleep apnea (CSA) and ataxic breathing. CSA is characterized by impaired respiratory drive and breathing cessation without respiratory effort. CSA is present in approximately 20% of chronic opioid users; in individuals with chronic pain, CSA is more common in those who use opioids than in those who are not on opioid therapy.13–15
Opioids impair upper airway function. However, the mechanisms of this impairment and relationships between OSA and opioid use are unclear. This lack of clarity can be attributed to opposite effects of opioids on different phenotypic traits of OSA. Specifically, animal studies showed that opioids reduce upper airway muscle tone, which may increase pharyngeal collapsibility and exacerbate the disease.10,11,16–18 Opioids also decrease chemosensitivity, which may stabilize ventilation and alleviate OSA in patients with overly robust ventilatory responses to hypoxia/hypercapnia.19 An outcome of this tug of war will determine the effect of opioids on OSA in an individual patient.
TRANSLATIONAL SCIENCE AND MECHANISTIC INSIGHTS
The genioglossus muscle is critical to the maintenance of upper airway patency during sleep.20 Therefore, most efforts to study the pathophysiology of OSA have been focused on effects of opioids on genioglossus, the hypoglossal nerve, and hypoglossal motoneurons innervating this muscle. Several authors demonstrated that opioids suppress hypoglossal motoneuron activity in vitro and genioglossal muscle activity in vivo.10,11,16–18 However, it remains unclear if MORs are present in the hypoglossal nucleus (XIIN) and if opioids can act directly on hypoglossal motoneurons. Data on opioid receptor distribution in the brainstem is limited and methodology has been inconsistent. In murine models, most studies observed at least weak expression of MORs in the XIIN.21,22 A study in cats found expression of delta opioid receptors but not MORs,23 whereas in humans hypoglossal expression was not observed or could not be determined precisely.24,25
In vitro recordings demonstrated that local application of MOR agonist, the synthetic opioid peptide [d-Ala2, N-MePhe4, Gly-ol]-enkephalin (DAMGO), to the XIIN reduced burst amplitude, area, and duration of hypoglossal inspiratory activity and decreased the frequency of miniature excitatory postsynaptic currents of hypoglossal motoneurons.10,17 Microdialysis of fentanyl to the XIIN suppressed genioglossal activity in anesthetized rats. Interestingly, administration of a muscarinic receptor antagonist did not affect the response, suggesting that this effect was not mediated by acetylcholine.11 These data were also confirmed by in vitro studies where a MOR agonist DAMGO decreased XII nerve inspiratory burst amplitude despite the addition of atropine.17
Non–rapid eye movement sleep recording in unanesthetized goats treated with DAMGO dialyzed into the hypoglossal nucleus showed the reduction of genioglossal muscle activity, whereas sleep recording in mice treated with intraperitoneal morphine revealed inspiratory flow limitation indicating upper airway obstruction.10,18 These findings are consistent with earlier reports suggesting that endogenous opioids modulate ventilatory response and might play a role in the pathophysiology of OSA in humans.26 However, attempts to block endogenous opioids and treat sleep apnea with naloxone yielded inconsistent results.27,28
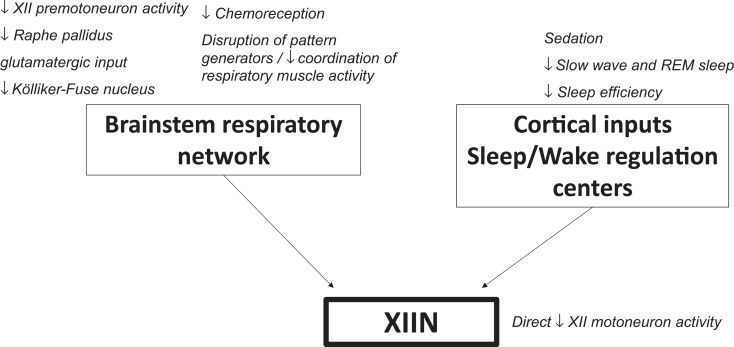
What are potential mechanisms by which opioids may modulate upper airway patency? Microcircuits in the respiratory network are interconnected to act on the respiratory rhythm, and pattern generation regulating respiratory muscles and suppression of any population of respiratory neurons may impair upper airway patency. Lorier et al demonstrated that MORs modulate the activity of hypoglossal motoneurons both directly and indirectly via inputs from premotoneurons in the pre-Bötzinger region.17 Opioids may affect hypoglossal motoneurons presynaptically by inhibiting MORs on the raphe pallidus neurons, thus diminishing the glutamatergic input to the XII nucleus.29 The Kölliker-Fuse nucleus, which projects to the hypoglossal nucleus and regulates upper airway patency, was identified as one of the key centers associated with OIRD,30,31 but the effect of opioids at this site on hypoglossal motoneuron activity has not been investigated. Opioid-induced hypoglossal motoneuron dysfunction and upper airway obstruction may be reversed by ampakines, modulators of 2-amino-3-(3-hydroxy-5-methyl-isoxazol-4-yl)propanoic acid (AMPA) glutamatergic receptors.17,32
In addition, opioids decrease afferent input to hypoglossal motoneurons via opioid-induced sedation and suppression of chemoreception, which may lead to upper airway dysfunction.33 Biologicals and pharmaceuticals augmenting hypercapnic and hypoxic sensitivity, such as the adipocyte-derived hormone leptin, reverse opioid-induced suppression of hypoglossal neuron activity and relieve upper airway obstruction acting presynaptically.10,17 Thus, emerging evidence from animal studies suggests that opioids affect the activity of hypoglossal motoneurons via multiple mechanisms, which may aggravate OSA. Figure 1 summarizes the potential mechanisms by which opioids modulate upper airway function.
Figure 1. Putative mechanisms by which opioids affect activity of the hypoglossal motoneurons.
REM = rapid eye movement, XIIN = hypoglossal nucleus.
HUMAN STUDIES AND CLINICAL CONSIDERATIONS
Acute effects
Acute effects of opioids on OSA are particularly relevant for perioperative management of pain. OSA increases risk of perioperative respiratory depression and postoperative cardiopulmonary complications.34,35 Case reports and medicolegal literature suggest that OSA is associated with a risk of respiratory arrest induced by opioids.36,37 In an attempt to minimize adverse effects and reduce the volume of opioid prescriptions, anesthesiologists have been studying the benefits and state-of-the art approaches to opioid free anesthesia.38 A systematic review determined that most anesthetic agents cause some degree of airway collapse and opioids are associated with upper airway obstruction and depression of upper airway reflexes.39 Children with history of hypoxemia due to OSA have increased sensitivity to opioids and increased risk of respiratory depression postoperatively.40
However, studies in larger cohorts failed to find an association between OSA and perioperative mortality.41 This lack of association could be attributed to a combination of factors. Anesthesia and surgery became extremely safe due to the advances of anesthetic drugs and noninvasive minimal surgery. Over the last decade anesthesiologists became increasingly aware of a potential risk imposed by OSA42 and employ relatively sensitive questionnaires, to detect undiagnosed OSA.43 Observations made in many patients with untreated OSA are required to establish an effect on mortality, and, fortunately, remarkable progress achieved by anesthesiologists and surgeons makes these observations impossible. Nevertheless, surrogate markers suggest a significant impact of severe OSA on surgical outcomes. A prospective study of 1,218 undergoing major surgery showed that severe untreated OSA was associated with a significant increase in rates of postoperative cardiovascular events, and post-hoc analyses established a strong association with cardiac death, with a hazard ratio of 13.66.44 In contrast, mild and moderate OSA did not have an effect.
The lack of an impact of mild-moderate OSA postoperatively was reported in a smaller prospective observational study. Polysomnography was performed before and after surgery in 38 patients with OSA (median apnea-hypopnea index of 18 events/h) and 20 patients without OSA who received either balanced anesthesia without opioids or postoperative narcotics. Both groups had an increase in apnea-hypopnea index, but there was no significant difference between the groups. Other factors such as disrupted sleep architecture, methodological differences between the sleep studies, oxygen therapy, surgical stress, and other medications might have confounded the results.45
There are only a few randomized controlled trials investigating the effects of opioids on patients with OSA. In patients with moderate apnea, remifentanil increased the number of central apneas and decreased the number of obstructive apneas, with significant reduction in oxygen saturation levels. The decrease in obstructive apneas was attributed to the disruption of sleep architecture with marked decrease in rapid eye movement sleep.46 Wang et al reported that the variability in ventilatory responses after a 30-mg dose of morphine is related to the variability in plasma morphine levels. Paradoxically, higher plasma morphine concentrations and a decrease in ventilatory chemosensitivity directly correlated with improvement of the time with oxygen saturation below 90% (T90).47 Rowsell et al treated patients with 40 mg of controlled release morphine and showed a large interindividual variability in levels of respiratory depression in the absence of change in mean apnea-hypopnea index and T90. The investigators reported that opioid receptor mu 1 (OPRM1) gene polymorphisms might influence the response to opioids during sleep. OSA worsened in patients with the A/A OPRM1 phenotype and improved with the A/G OPRM1 phenotype.48 Martins et al studied the effects of 40 mg of controlled release morphine on different OSA phenotypical traits. The investigators showed a reduction in hypercapnic ventilatory response but no change in arousal threshold or upper airway collapsibility. Morphine blunted the genioglossus response to hypercapnia.49
In patients with over robust chemoreflex, the low arousal threshold in response to stimulation of chemo- and mechanoreceptors leads to respiratory instability exacerbating OSA. Opioids blunt the hypercapnic and hypoxic ventilatory responses and, in this patient population, opioids stabilize breathing and increase the arousal threshold, which may alleviate OSA.50 Overall, acute effects of opioids on OSA are complex and may vary depending on the dose, and individual patient characteristics, especially chemoreflex and the arousal threshold.
Chronic effects
Increasing trends in prescription of high doses of opioids (>200 mg morphine equivalent daily doses [MEDD]) have been observed in Canada and United Kingdom.51,52 Between 2000 and 2010, more than 200-mg MEDD have been prescribed for noncancer pain in approximately 8% of primary care patients in the United Kingdom.52 Sleep-disordered breathing has been examined in patients with opioid use disorder on maintenance therapy with methadone or buprenorphine. However, these reports are inconsistent due to variability in medications and MEDD. In addition, sleep-disordered breathing is rarely considered as an adverse event.53 Patients treated for opioid dependency with buprenorphine and methadone develop central sleep apnea and blunted hypoxic and hypercapnic responses.9,54 A systematic review focused on central sleep apnea identified a relationship between MEDD and severity of sleep-disordered breathing.55 Discontinuation of opioids was related to a reversal of central sleep apnea.55
Evidence on the relationship between chronic opioid use and OSA is scarce. Recent systematic reviews and meta-analysis found fewer than 15 studies addressing relationships between OSA and opioid therapy, none of which was a randomized clinical trial with OSA as the main outcome.15,56 There was no significant association between opioid use and severity of OSA.56 Other investigators reported that OSA was present in more than one third of patients with chronic use of opioids,57,58 but there was no control group and the prevalence in the general population was extrapolated from a study with different methodology.59 Another study showed that OSA was twice as common as CSA in a subgroup of patients treated with methadone who had self-reported sleep complaints. It is not clear if the diagnosis of OSA was independently related to the use of methadone or if it was dependent on other factors, such as body mass index.58
Overall, given the high prevalence of opioid use disorder and basic research findings suggesting the direct effect of opioids on upper airway patency, future well-structured clinical studies are needed to evaluate causal relationships between chronic opioid use and OSA.
POSITIVE AIRWAY PRESSURE TREATMENT
In this section we will review current evidence on benefits of positive airway pressure (PAP) therapy in patients with OSA and comorbid opioid use disorder.
In an acute perioperative setting, surgical patients with OSA on continuous positive airway pressure therapy (CPAP) had better postoperative outcomes than untreated OSA patients. The use of CPAP also reduced postoperative respiratory complications in patients without OSA.35 Overall, studies suggest a positive effect of positive pressure, but since complications are rare, most studies have been underpowered. There are relative and absolute contraindications to PAP therapy depending on the surgical procedure, and with some procedures, patients have a high risk of developing OSA, such as bariatric surgery, transsphenoidal pituitary surgery in patients with Cushing and acromegaly, and upper airway surgeries to improve upper airway patency. However, initial clinical suspicion that PAP may induce anastomotic leaks post–bariatric surgery was not confirmed and current evidence suggests that PAP therapy is safe in these patients.35 If PAP is contraindicated, an individual approach is recommended, minimizing opioid prescription and considering respiratory monitoring and therapies such as oral devices, positional therapy, and even tracheostomy as alternatives.60
Current recommendations in opioid users suggest the use of the lowest possible dose, avoiding doses higher than 200 mg MEDD, and consideration of drug interactions that may suppress breathing during sleep. Guilleminault et al demonstrated that, in chronic opioid users, CPAP therapy resolved mixed and obstructive apneas but led to the emergence of central apneas. The use of bilevel PAP with back-up rate abolished central apneas, improved oxygen saturation, and resolved sleep-related symptoms.61 Shapiro et al compared CPAP vs adaptive servo-ventilation (ASV) therapy in patients with chronic pain prescribed more than 100 MEDD (average 390 ± 338 MEDD). CPAP improved OSA but did not normalize apnea-hypopnea index, central apneas, or hypoxia. ASV significantly improved respiratory variables compared to CPAP, and sustained results after 3 months of ASV home treatment have been observed.62 Thus, bilevel PAP and ASV treatment of OSA should be considered in patients on chronic opioid therapy or with opioid use disorder. Of note, ASV should be avoided in patients with concomitant heart failure.63 Although this treatments was proven effective, a recent study that evaluated a cohort of patients with chronic pain on opioid therapy demonstrated that over 50% of the patients diagnosed with OSA were not compliant, whereas those who were prescribed PAP therapy had a 55% adherence rate.64
CONCLUSIONS
Animal studies suggest that opioids inhibit hypoglossal motoneuron and genioglossal muscle activity and induce upper airway obstruction, ie, OSA, but mechanisms are insufficiently understood. While there is a consensus that opioids cause central sleep apnea, the effects of opioids on OSA in clinical studies are not uniform and depend on the disease phenotype. Large clinical studies addressing the role of phenotypic traits, such as upper airway muscle response to pharyngeal obstruction, chemoreflex, and the arousal threshold, may identify the patients with OSA at risk of OIRD.
DISCLOSURE STATEMENT
All authors have seen and approved the manuscript. Work for this study was supported by the US National Institutes of Health (grants: R01HL 128970, R01HL 138932, R61 HL156240, U18 DA052301) and the São Paulo Research Foundation (FAPESP; grant 2018/08758-3). C.F. and V.Y.P. have a patent pending (Leptin Prevents Opiate-induced Respiratory Depression). L.U.S. report no conflicts of interest.
ACKNOWLEDGMENTS
Author contributions: C.F., V.Y.P., and L.U.S. wrote the manuscript.
ABBREVIATIONS
- ASV
adaptive servo-ventilation
- CPAP
continuous positive airway pressure therapy
- CSA
central sleep apnea
- MEDD
morphine equivalent daily doses
- MOR
mu opioid receptor
- OIRD
opioid-induced respiratory depression
- OSA
obstructive sleep apnea
- PAP
positive airway pressure
- XIIN
hypoglossal nucleus
REFERENCES
- 1.Centers for Disease Control and Prevention. The Drug Overdose Epidemic . Behind the Numbers. Published March 19, 2020. . https://www.cdc.gov/drugoverdose/data/index.html . Accessed October 23, 2021.
- 2. Han B , Compton WM , Blanco C , Crane E , Lee J , Jones CM . Prescription opioid use, misuse, and use disorders in U.S. adults: 2015 National Survey on Drug Use and Health . Ann Intern Med. 2017. ; 167 ( 5 ): 293 – 301 . [DOI] [PubMed] [Google Scholar]
- 3. Ahmad F , Rossen L , Sutton P . Provisional Drug Overdose Death Counts. National Center for Health Statistics. Published online 2020. https://www.cdc.gov/nchs/nvss/vsrr/drug-overdose-data.htm
- 4. Lin L , Brummett CM , Waljee JF , et al . Association of opioid overdose risk factors and naloxone prescribing in US adults . J Gen Intern Med. 2020. ; 35 : 420 – 427 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Gupta K , Nagappa M , Prasad A , et al . Risk factors for opioid-induced respiratory depression in surgical patients: a systematic review and meta-analyses . BMJ Open. 2018. ; 8 ( 12 ): e024086 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Montandon G , Slutsky AS . Solving the opioid crisis: respiratory depression by opioids as critical end point . Chest. 2019. ; 156 ( 4 ): 653 – 658 . [DOI] [PubMed] [Google Scholar]
- 7. Pattinson KTS . Opioids and the control of respiration . Br J Anaesth. 2008. ; 100 ( 6 ): 747 – 758 . [DOI] [PubMed] [Google Scholar]
- 8. Montandon G , Qin W , Liu H , Ren J , Greer JJ , Horner RL . PreBotzinger complex neurokinin-1 receptor-expressing neurons mediate opioid-induced respiratory depression . J Neurosci. 2011. ; 31 ( 4 ): 1292 – 1301 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Rosen IM , Aurora RN , Kirsch DB , et al. American Academy of Sleep Medicine Board of Directors . Chronic opioid therapy and sleep: an American Academy of Sleep Medicine position statement . J Clin Sleep Med. 2019. ; 15 ( 11 ): 1671 – 1673 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Freire C , Pho H , Kim LJ , et al . Intranasal leptin prevents opioid-induced sleep-disordered breathing in obese mice . Am J Respir Cell Mol Biol. 2020. ; 63 ( 4 ): 502 – 509 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Hajiha M , DuBord M-A , Liu H , Horner RL . Opioid receptor mechanisms at the hypoglossal motor pool and effects on tongue muscle activity in vivo . J Physiol. 2009. ; 587 ( 11 ): 2677 – 2692 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Brown KA , Laferrière A , Lakheeram I , Moss IR . Recurrent hypoxemia in children is associated with increased analgesic sensitivity to opiates . Anesthesiology. 2006. ; 105 ( 4 ): 665 – 669 . [DOI] [PubMed] [Google Scholar]
- 13. Correa D , Farney RJ , Chung F , Prasad A , Lam D , Wong J . Chronic opioid use and central sleep apnea: a review of the prevalence, mechanisms, and perioperative considerations . Anesth Analg. 2015. ; 120 ( 6 ): 1273 – 1285 . [DOI] [PubMed] [Google Scholar]
- 14. Chung F , Wong J , Bellingham G , et al. Op-Safe Investigators . Predictive factors for sleep apnoea in patients on opioids for chronic pain . BMJ Open Respir Res. 2019. ; 6 ( 1 ): e000523 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Mubashir T , Nagappa M , Esfahanian N , et al . Prevalence of sleep-disordered breathing in opioid users with chronic pain: a systematic review and meta-analysis . J Clin Sleep Med. 2020. ; 16 ( 6 ): 961 – 969 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bateman JT, Saunders SE, Levitt ES. Understanding and countering opioid-induced respiratory depression. British Journal of Pharmacology. 2021; Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Lorier AR , Funk GD , Greer JJ . Opiate-induced suppression of rat hypoglossal motoneuron activity and its reversal by ampakine therapy . PLoS One. 2010. ; 5 ( 1 ): e8766 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Langer TM III , Neumueller SE , Crumley E , et al . Ventilation and neurochemical changes during µ-opioid receptor activation or blockade of excitatory receptors in the hypoglossal motor nucleus of goats . J Appl Physiol 1985. 2017. ; 123 ( 6 ): 1532 – 1544 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Wang D , Eckert DJ , Grunstein RR . Drug effects on ventilatory control and upper airway physiology related to sleep apnea . Respir Physiol Neurobiol. 2013. ; 188 ( 3 ): 257 – 266 . [DOI] [PubMed] [Google Scholar]
- 20. Remmers JE , deGroot WJ , Sauerland EK , Anch AM . Pathogenesis of upper airway occlusion during sleep . J Appl Physiol. 1978. ; 44 ( 6 ): 931 – 938 . [DOI] [PubMed] [Google Scholar]
- 21. Kivell BM , Day DJ , McDonald FJ , Miller JH . Developmental expression of μ and δ opioid receptors in the rat brainstem: evidence for a postnatal switch in μ isoform expression . Brain Res Dev Brain Res. 2004. ; 148 ( 2 ): 185 – 196 . [DOI] [PubMed] [Google Scholar]
- 22. Erbs E , Faget L , Scherrer G , et al . A mu-delta opioid receptor brain atlas reveals neuronal co-occurrence in subcortical networks . Brain Struct Funct. 2015. ; 220 ( 2 ): 677 – 702 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Richardson KA , Gatti PJ . Genioglossal hypoglossal muscle motoneurons are contacted by nerve terminals containing delta opioid receptor but not mu opioid receptor-like immunoreactivity in the cat: a dual labeling electron microscopic study . Brain Res. 2005. ; 1032 ( 1-2 ): 23 – 29 . [DOI] [PubMed] [Google Scholar]
- 24. Peckys D , Landwehrmeyer GB . Expression of mu, kappa, and delta opioid receptor messenger RNA in the human CNS: a 33P in situ hybridization study . Neuroscience. 1999. ; 88 ( 4 ): 1093 – 1135 . [DOI] [PubMed] [Google Scholar]
- 25. Pfeiffer A , Pasi A , Mehraein P , Herz A . Opiate receptor binding sites in human brain . Brain Res. 1982. ; 248 ( 1 ): 87 – 96 . [DOI] [PubMed] [Google Scholar]
- 26. Meurice JC , Marc I , Sériès F . Effects of naloxone on upper airway collapsibility in normal sleeping subjects . Thorax. 1996. ; 51 ( 8 ): 851 – 852 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Atkinson RL , Suratt PM , Wilhoit SC , Recant L . Naloxone improves sleep apnea in obese humans . Int J Obes. 1985. ; 9 ( 4 ): 233 – 239 . [PubMed] [Google Scholar]
- 28. Guilleminault C , Hayes B . Naloxone, theophylline, bromocriptine, and obstructive sleep apnea. Negative results . Bull Eur Physiopathol Respir. 1983. ; 19 ( 6 ): 632 – 634 . [PubMed] [Google Scholar]
- 29. Bouryi VA , Lewis DI . Enkephalinergic inhibition of raphe pallidus inputs to rat hypoglossal motoneurones in vitro . Neuroscience. 2004. ; 129 ( 1 ): 55 – 64 . [DOI] [PubMed] [Google Scholar]
- 30. Dutschmann M , Bautista TG , Trevizan-Baú P , Dhingra RR , Furuya WI . The pontine Kölliker-Fuse nucleus gates facial, hypoglossal, and vagal upper airway related motor activity . Respir Physiol Neurobiol. 2021. ; 284 : 103563 . [DOI] [PubMed] [Google Scholar]
- 31. Saunders SE , Levitt ES . Kölliker-Fuse/parabrachial complex mu opioid receptors contribute to fentanyl-induced apnea and respiratory rate depression . Respir Physiol Neurobiol. 2020. ; 275 : 103388 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Zaig S , da Silveira Scarpellini C , Montandon G . Respiratory depression and analgesia by opioid drugs in freely behaving larval zebrafish . eLife. 2021. ; 10 : e63407 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Smith JC , Abdala APL , Borgmann A , Rybak IA , Paton JFR . Brainstem respiratory networks: building blocks and microcircuits . Trends Neurosci. 2013. ; 36 ( 3 ): 152 – 162 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Hillman DR , Chung F . Anaesthetic management of sleep-disordered breathing in adults . Respirology. 2017. ; 22 ( 2 ): 230 – 239 . [DOI] [PubMed] [Google Scholar]
- 35. Chung F , Nagappa M , Singh M , Mokhlesi B . CPAP in the perioperative setting: evidence of support . Chest. 2016. ; 149 ( 2 ): 586 – 597 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Subramani Y , Nagappa M , Wong J , Patra J , Chung F . Death or near-death in patients with obstructive sleep apnoea: a compendium of case reports of critical complications . Br J Anaesth. 2017. ; 119 ( 5 ): 885 – 899 . [DOI] [PubMed] [Google Scholar]
- 37. Fouladpour N , Jesudoss R , Bolden N , Shaman Z , Auckley D . Perioperative complications in obstructive sleep apnea patients undergoing surgery: a review of the legal literature . Anesth Analg. 2016. ; 122 ( 1 ): 145 – 151 . [DOI] [PubMed] [Google Scholar]
- 38. Lavand’homme P , Estebe J-P . Opioid-free anesthesia: a different regard to anesthesia practice . Curr Opin Anaesthesiol. 2018. ; 31 ( 5 ): 556 – 561 . [DOI] [PubMed] [Google Scholar]
- 39. Ehsan Z , Mahmoud M , Shott SR , Amin RS , Ishman SL . The effects of anesthesia and opioids on the upper airway: a systematic review . Laryngoscope. 2016. ; 126 ( 1 ): 270 – 284 . [DOI] [PubMed] [Google Scholar]
- 40. Brown KA , Laferrière A , Moss IR . Recurrent hypoxemia in young children with obstructive sleep apnea is associated with reduced opioid requirement for analgesia . Anesthesiology. 2004. ; 100 ( 4 ): 806 – 810, discussion 5A . [DOI] [PubMed] [Google Scholar]
- 41. Opperer M , Cozowicz C , Bugada D , et al . Does obstructive sleep apnea influence perioperative outcome? A qualitative systematic review for the Society of Anesthesia and Sleep Medicine Task Force on preoperative preparation of patients with sleep-disordered breathing . Anesth Analg. 2016. ; 122 ( 5 ): 1321 – 1334 . [DOI] [PubMed] [Google Scholar]
- 42. Gross JB , Bachenberg KL , Benumof JL , et al. American Society of Anesthesiologists Task Force on Perioperative Management . Practice guidelines for the perioperative management of patients with obstructive sleep apnea: a report by the American Society of Anesthesiologists Task Force on Perioperative Management of patients with obstructive sleep apnea . Anesthesiology. 2006. ; 104 (5 ): 1081 – 1093, quiz 1117-1118 . [DOI] [PubMed] [Google Scholar]
- 43. Pivetta B , Chen L , Nagappa M , et al . Use and performance of the STOP-Bang questionnaire for obstructive sleep apnea screening across geographic regions: a systematic review and meta-analysis . JAMA Netw Open. 2021. ; 4 ( 3 ): e211009 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Chan MTV , Wang CY , Seet E , et al. Postoperative Vascular Complications in Unrecognized Obstructive Sleep Apnea (POSA) Study Investigators . Association of unrecognized obstructive sleep apnea with postoperative cardiovascular events in patients undergoing major noncardiac surgery . JAMA. 2019. ; 321 ( 18 ): 1788 – 1798 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Chung F , Liao P , Yegneswaran B , Shapiro CM , Kang W . Postoperative changes in sleep-disordered breathing and sleep architecture in patients with obstructive sleep apnea . Anesthesiology. 2014. ; 120 ( 2 ): 287 – 298 . [DOI] [PubMed] [Google Scholar]
- 46. Bernards CM , Knowlton SL , Schmidt DF , et al . Respiratory and sleep effects of remifentanil in volunteers with moderate obstructive sleep apnea . Anesthesiology. 2009. ; 110 ( 1 ): 41 – 49 . [DOI] [PubMed] [Google Scholar]
- 47. Wang D , Somogyi AA , Yee BJ , et al . The effects of a single mild dose of morphine on chemoreflexes and breathing in obstructive sleep apnea . Respir Physiol Neurobiol. 2013. ; 185 ( 3 ): 526 – 532 . [DOI] [PubMed] [Google Scholar]
- 48. Rowsell L , Wong KKH , Yee BJ , et al . The effect of acute morphine on obstructive sleep apnoea: a randomised double-blind placebo-controlled crossover trial . Thorax. 2019. ; 74 ( 2 ): 177 – 184 . [DOI] [PubMed] [Google Scholar]
- 49. Martins RT , Carberry JC , Wang D , Rowsell L , Grunstein RR , Eckert DJ . Morphine alters respiratory control but not other key obstructive sleep apnoea phenotypes: a randomised trial . Eur Respir J. 2020. ; 55 ( 6 ): 1901344 . [DOI] [PubMed] [Google Scholar]
- 50. Isono S . Instability of Upper Airway during Anesthesia and Sedation: How Is Upper Airway Unstable during Anesthesia and Sedation? In: Yamaguchi K, ed. Structure-Function Relationships in Various Respiratory Systems. Singapore: Springer Singapore; 2020. :67-91.
- 51. Gomes T , Mamdani MM , Paterson JM , Dhalla IA , Juurlink DN . Trends in high-dose opioid prescribing in Canada . Can Fam Physician. 2014. ; 60 ( 9 ): 826 – 832 . [PMC free article] [PubMed] [Google Scholar]
- 52. Zin CS , Chen L-C , Knaggs RD . Changes in trends and pattern of strong opioid prescribing in primary care . Eur J Pain. 2014. ; 18 ( 9 ): 1343 – 1351 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Els C , Jackson TD , Kunyk D , et al . Adverse events associated with medium- and long-term use of opioids for chronic non-cancer pain: an overview of Cochrane Reviews . Cochrane Database Syst Rev. 2017. ; 10 ( 10 ): CD012509 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Teichtahl H , Wang D , Cunnington D , et al . Ventilatory responses to hypoxia and hypercapnia in stable methadone maintenance treatment patients . Chest. 2005. ; 128 ( 3 ): 1339 – 1347 . [DOI] [PubMed] [Google Scholar]
- 55. Javaheri S , Patel S . Opioids cause central and complex sleep apnea in humans and reversal with discontinuation: a plea for detoxification . J Clin Sleep Med. 2017. ; 13 ( 6 ): 829 – 833 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Ahmad A , Ahmad R , Meteb M , et al . The relationship between opioid use and obstructive sleep apnea: a systematic review and meta-analysis . Sleep Med Rev. 2021. ; 58 : 101441 . [DOI] [PubMed] [Google Scholar]
- 57. Webster LR , Choi Y , Desai H , Webster L , Grant BJ . Sleep-disordered breathing and chronic opioid therapy . Pain Med. 2008. ; 9 ( 4 ): 425 – 432 . [DOI] [PubMed] [Google Scholar]
- 58. Sharkey KM , Kurth ME , Anderson BJ , Corso RP , Millman RP , Stein MD . Obstructive sleep apnea is more common than central sleep apnea in methadone maintenance patients with subjective sleep complaints . Drug Alcohol Depend. 2010. ; 108 ( 1-2 ): 77 – 83 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Young T , Palta M , Dempsey J , Skatrud J , Weber S , Badr S . The occurrence of sleep-disordered breathing among middle-aged adults . N Engl J Med. 1993. ; 328 (17 ): 1230 – 1235 . [DOI] [PubMed] [Google Scholar]
- 60. Ravesloot MJL , de Raaff CAL , van de Beek MJ , et al . Perioperative care of patients with obstructive sleep apnea undergoing upper airway surgery: a review and consensus recommendations . JAMA Otolaryngol Head Neck Surg. 2019. ; 145 ( 8 ): 751 – 760 . [DOI] [PubMed] [Google Scholar]
- 61. Guilleminault C , Cao M , Yue HJ , Chawla P . Obstructive sleep apnea and chronic opioid use . Lung. 2010. ; 188 ( 6 ): 459 – 468 . [DOI] [PubMed] [Google Scholar]
- 62. Shapiro CM , Chung SA , Wylie PE , et al . Home-use servo-ventilation therapy in chronic pain patients with central sleep apnea: initial and 3-month follow-up . Sleep Breath. 2015. ; 19 ( 4 ): 1285 – 1292 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Van Ryswyk E , Antic NA . Opioids and sleep-disordered breathing . Chest. 2016. ; 150 ( 4 ): 934 – 944 . [DOI] [PubMed] [Google Scholar]
- 64. Wasef S , Mir S , Ryan C , et al . Treatment for patients with sleep apnea on opioids for chronic pain: results of the OpSafe trial . J Clin Sleep Med. 2021. ; 17 ( 4 ): 819 – 824 . [DOI] [PMC free article] [PubMed] [Google Scholar]