ABSTRACT
Excessive inflammation can cause tissue damage and autoimmunity, sometimes accompanied by severe morbidity or mortality. Numerous negative feedback mechanisms exist to prevent unchecked inflammation, but this restraint may come at the cost of suboptimal infection control. Regnase-1 (MCPIP1), a feedback regulator of IL-17 and LPS signaling, binds and degrades target mRNAs. Consequently, Reg1 deficiency exacerbates autoimmunity in multiple models. However, the role of Reg1 in bacterial immunity remains poorly defined. Here, we show that mice deficient in Reg1 are resistant to Klebsiella pneumoniae (KP). Reg1 deficiency did not accelerate bacterial eradication. Rather, Reg1-deficient alveolar macrophages had elevated Ifnb1 and enrichment of type I IFN genes. Blockade of IFNR during KP infection reversed disease improvement. Reg1 did not impact Ifnb1 stability directly, but Irf7 expression was affected. Thus, Reg1 suppresses type I IFN signaling restricting resistance to KP, suggesting that Reg1 could potentially be a target in severe bacterial infections.
KEYWORDS: Klebsiella, RNA binding proteins, Regnase
INTRODUCTION
Klebsiella pneumoniae (KP) is a constituent of the normal human microbiome and is detected in approximately 10% of the Human Microbiome Project samples (1, 2). KP is the third most common cause of ventilator-associated pneumonia (VAP) in the United States, and its presence correlates with prolonged duration of ventilation and ICU hospital stay (3–5). KP imposes a major infectious disease challenge due to increasing antimicrobial resistance combined with comparatively limited therapeutic options (6). Hence, an improved understanding of host-pathogen interactions is needed to elucidate, and ultimately exploit, the host mechanisms that control KP pneumonia.
The immune response to infectious pathogens is finely balanced to allow eradication of microbes yet still prevent excessive inflammatory pathology. Thus, numerous mechanisms are triggered during immune responses to counter-regulate deleterious responses and thereby minimize collateral damage to the host. The RNA-binding protein (RBP) Regnase-1 (Reg1), also known as MCP1-induced protein 1 (MCPIP1), is encoded by the ZC3H12A gene and has intrinsic endoribonuclease activity that keeps inflammatory responses in check (7). In this capacity, Reg1 binds and degrades many mRNA transcripts, including its own mRNA, through recognition of characteristic stem-loop secondary structures in the 3′ untranslated region (UTR) of target mRNAs (8). Reg1 is induced by many inflammatory stimuli, including IL-1, Toll-like-receptors (TLR), and IL-17 (9–12). In addition, TCR signaling leads to cleavage of Reg1, releasing T cells from Reg1-mediated suppression (13). Thus, Reg1 plays a central role in negative feedback control of pro-inflammatory cytokine gene expression through post-transcriptional control of mRNA expression.
Multiple mouse models support the vital role of Reg1 as a central negative regulator of pathogenic inflammation. Reg1 knockout mice (Zc3h12a−/−, here called Reg1−/−) have a life span only of 6 to 8 weeks. Reg1−/− mice display significant constitutive inflammation, manifesting as growth retardation, splenomegaly, lymphadenopathy, heightened systemic, and localized cytokine production, and ultimately multi-organ failure (9, 14). In order to avoid confounding issues with the complete Reg1 knockout mouse, we have previously taken advantage of Reg1 heterozygous mice (Reg1+/−), which have normal life spans and fertility, normal tonic expression of Reg1, and minimal peripheral organ inflammation at baseline (11, 15). Even so, cells from Reg1+/− mice display markedly reduced Reg1 expression after LPS or IL-17 stimulation, and tissues from Reg1+/− animals (lung, kidney, central nervous system) exhibit elevated cytokine responses (11). Consequently, Reg1+/− mice show exacerbated signs of autoimmunity in multiple IL-17-driven autoimmune conditions, including experimental autoimmune encephalomyelitis (EAE), imiquimod (IMQ)-induced dermatitis, and autoantibody-induced glomerulonephritis (AGN). In all these settings, IL-17R deficiency reversed the phenotype, demonstrating that Reg1 functions in these settings through restricting IL-17-driven inflammation (11, 15, 16).
In contrast to exacerbating pathology in autoimmune conditions, Reg1-deficiency leads to improved resistance to at least one IL-17-mediated host response, disseminated candidiasis, causing enhanced survival and concomitantly reduced fungal kidney burdens (11). Therefore, although restraint of Reg1 is clearly beneficial to limit autoimmune pathology, it is conceivable that temporary blockade of Reg1 could be exploited to improve inflammatory immune responses during certain infectious settings. However, the impact of Reg1 in infectious disease settings has not been extensively explored.
KP is sensed by pattern recognition receptors such as TLR4, which activates a myeloid differentiation primary response gene 88 (MyD88)-dependent pathway leading to induction of pro-inflammatory chemokines and cytokines. TLR4 signaling also induces the TIR-domain-containing adaptor-inducing interferon-β (TRIF) pathway, which induces the type I IFNs (IFN-α, IFN-β). Both MyD88 and TRIF signaling pathways are needed for defense against KP infection (17, 18). TLR4 is also upstream of IL-17 production from γδ T cells in response to KP (19), and Il17ra−/− mice have uncontrolled KP infection with associated decreased expression of CXC chemokines, G-CSF, and impaired neutrophil recruitment (20). Additionally, IL-17 synergizes with cytokines such as IL-22 to increase production of antimicrobial peptides in lung epithelium, such as LCN2, calprotectin (S100A8/9), and MUC1(21). Though produced by lymphocytes, IL-17 mediates downstream signals selectively on non-hematopoietic cells within the pulmonary epithelium during KP infection (22). Thus, the TLR4 and IL-17R pathways mediate an integrated inflammatory cascade that contains KP lung infection.
Given the documented role of Reg1 in restricting both TRL4- and IL-17-dependent signaling pathways, we hypothesized that Reg1-deficiency would be protective in the context of KP pneumonia, likely through decreased decay of pro-inflammatory mRNA transcripts. Indeed, we find here that Reg1-deficiency renders mice resistant to KP. However, bacterial burdens and typical pro-inflammatory cytokines known to be targeted by Reg1 were not altered in Reg1-deficent mice. Gene profiling of alveolar macrophages from KP-infected mice showed striking enrichment of type I IFN-associated gene pathways during Reg1 deficiency. Consistent with this, blockade of IFNR signaling reversed the protective effects of Reg1 deficiency. Mechanistically, Reg1 regulated the stability of mRNA encoding IRF7, an upstream regulator of type 1 IFN gene expression by TLRs.
RESULTS
Reg1-deficient mice are resistant to KP lung infection.
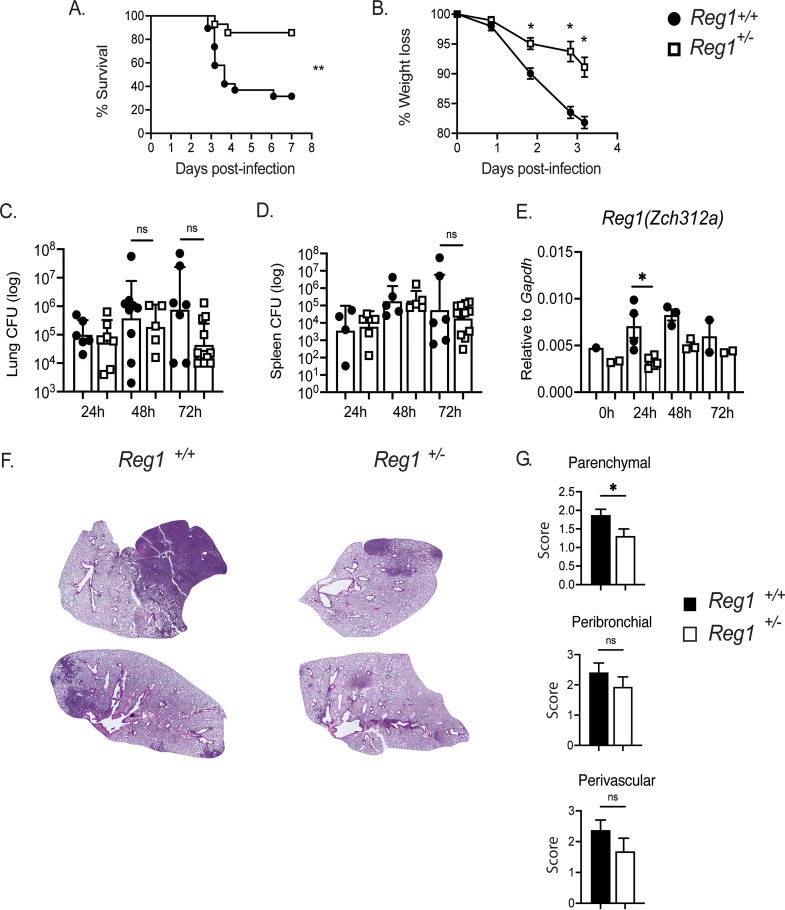
A complete absence of Reg1 is extremely deleterious, as Reg1−/− mice exhibit severe inflammation and severely shortened life spans (9). In contrast, Reg1+/− mice (sometimes termed here Reg1-deficient) have a normal life span without peripheral organ inflammation. Nonetheless, Reg1+/− mice show enhanced pro-inflammatory responses in multiple IL-17-driven autoinflammatory model settings (11, 15, 16). To determine whether Reg1 haploinsufficiency influences immunity to KP infection, Reg1+/− and Reg1+/+ littermate controls were infected with KP by oropharyngeal aspiration. Survival and parameters of health including weight loss were monitored over time. Indeed, infection-induced survival and weight loss were significantly ameliorated in Reg1-deficient mice compared with Reg1+/+ littermate controls (Fig. 1A and B). Unexpectedly, this improvement in survival was not accompanied by statistically significant differences in lung bacterial burdens (Fig. 1C). Moreover, there were no changes in bacterial dissemination into the spleen between groups at any measured time point, even though Reg1+/− mice had lower expression of Reg1 (Zc3h12a) than controls (Fig. 1D, E). Furthermore, histologic analysis of infected lung tissues between Reg1+/+ and Reg1+/− animals, showed that Reg1+/− mice developed smaller foci of pneumonia compared with controls, with particularly reduced levels of parenchymal inflammation (Fig. 1F, G). These data suggest that factors other than bacterial burden underlie the improved survival of Reg1-deficient animals.
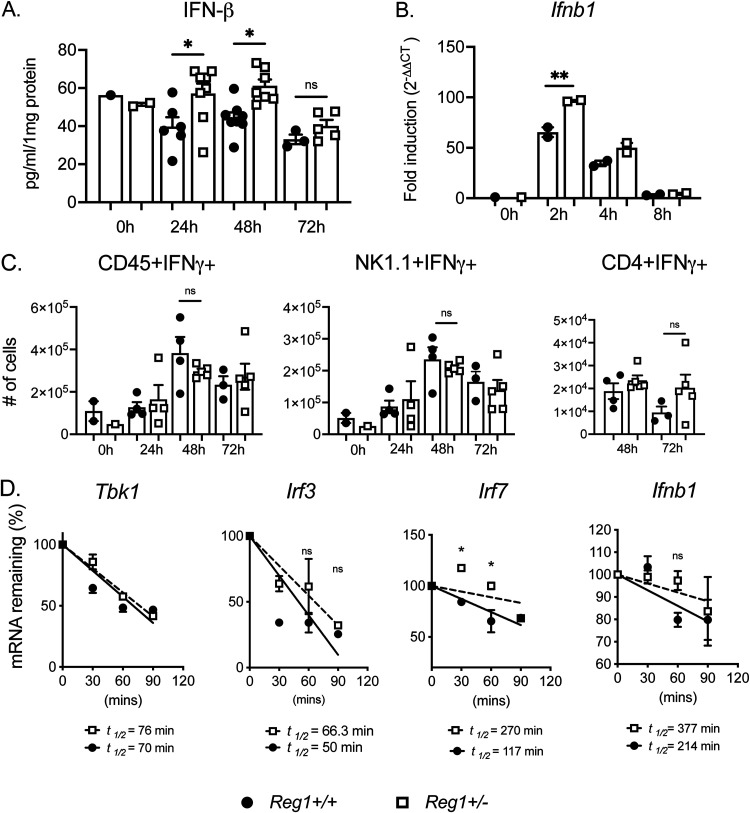
FIG 1.
Reg1-haploinsufficient mice are resistant to KP lung infection. (A) Lung and (B) spleen bacterial burdens were assessed by CFU assessment at the indicated time points. Each symbol represents one mouse. Filled circle = Reg1+/+ and open box= Reg1+/− mice. (C) Weight loss was assessed at the indicated time points (n = 15 mice/group). (D) Survival of Reg1+/− or Reg1+/+ littermates was assessed up to 8-days postinfection (n = 15 mice/group). (E) Reg1 gene expression (Zc3h12a) in lung homogenates of KP-infected mice. Data are pooled from two experiments. *, P < 0.05, **, P <0.01, Log-Rank test. (F) Representative images of H&E lung sections from Reg1+/+ and Reg1+/− mice at 48-h post-KP infection. (G) Scoring of parenchymal, peribronchial, and perivascular inflammation (Reg1+/+ n = 6, Reg1+/− n = 4). *, P < 0.05, unpaired-t test.
Reg1 functions in both hematopoietic and non-hematopoietic compartments during KP pneumonia.
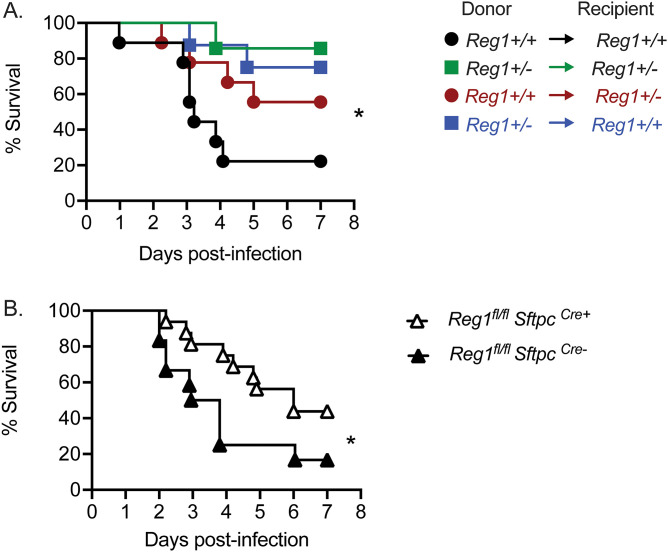
Reg1 has been shown to act in multiple cell types, including T cells, macrophages, and epithelial cells (7, 23). Of relevance to KP, Reg1 can restrict signaling by both TLR4 and IL-17. Whereas TLR4 acts predominantly on hematopoietic cells (especially macrophages), IL-17 predominantly mediates signaling in epithelial and/or mesenchymal target tissues, demonstrated in many settings including KP-infected lung (22). As a first step to define the essential compartments in which Reg1 functions to limit immunity to pulmonary KP infection, we used an adoptive transfer approach. Femoral bone marrow (BM) from Reg1+/− (CD45.2) or WT (CD45.1) mice were transferred into reciprocal irradiated Reg1+/− or WT recipients. After 8 weeks, engraftment was confirmed by flow cytometry (data not shown). Successfully reconstituted mice were infected with KP by oropharyngeal aspiration and followed up to 7 days. As expected, WT mice that received WT BM cells were susceptible to KP, whereas Reg1+/− mice receiving Reg1+/− BM were more resistant. As shown, Reg1+/− or WT mice that received Reg1+/− BM cells were resistant to KP, with almost 80% survival at day 7 postinfection. Additionally, Reg1+/− mice that received WT BM cells were resistant to KP, showing 60% survival at day 7 (Fig. 2A). Thus, Reg1 appears to act in multiple compartments.
FIG 2.
Reg1 deficiency in both hematopoietic and non-hematopoietic compartments contribute to prolonged survival upon KP lung infection. A) Reg1+/- and Reg1+/+ littermates were irradiated to ablate bone marrow (BM) and later reconstituted with reciprocal femoral BM cells. 8 weeks after BM transfer, mice were infected with KP and survival monitored over 7 days. B) Reg1fl/fl were crossed to SftpcCreERT2 infected with KP and monitored over 7 d. Data are pooled from two experiments. *, P < 0.05, **, P < 0.01, Log-Rank test.
Loss of IL-17RA in pulmonary epithelial cells renders mice highly susceptible to KP infection (22). Accordingly, given the potent impact of Reg1 on IL-17 signaling seen in prior studies (11, 12, 15, 24), we crossed Reg1fl/fl to surfactant protein C (Sftpc)Cre mice in order to delete Reg1 conditionally in distal pulmonary epithelial cells (distal bronchi and alveoli). As shown, mice were modestly more resistant to KP than controls, though the improvement survival was much less profound than seen in Reg1+/− mice (Fig. 2B), which is consistent with the BM chimera data showing contributions from both the hematopoietic and non-hematopoietic compartments contribute upon KP infection.
Reg1 deficiency does not influence immune cell recruitment or proliferation during KP infection.
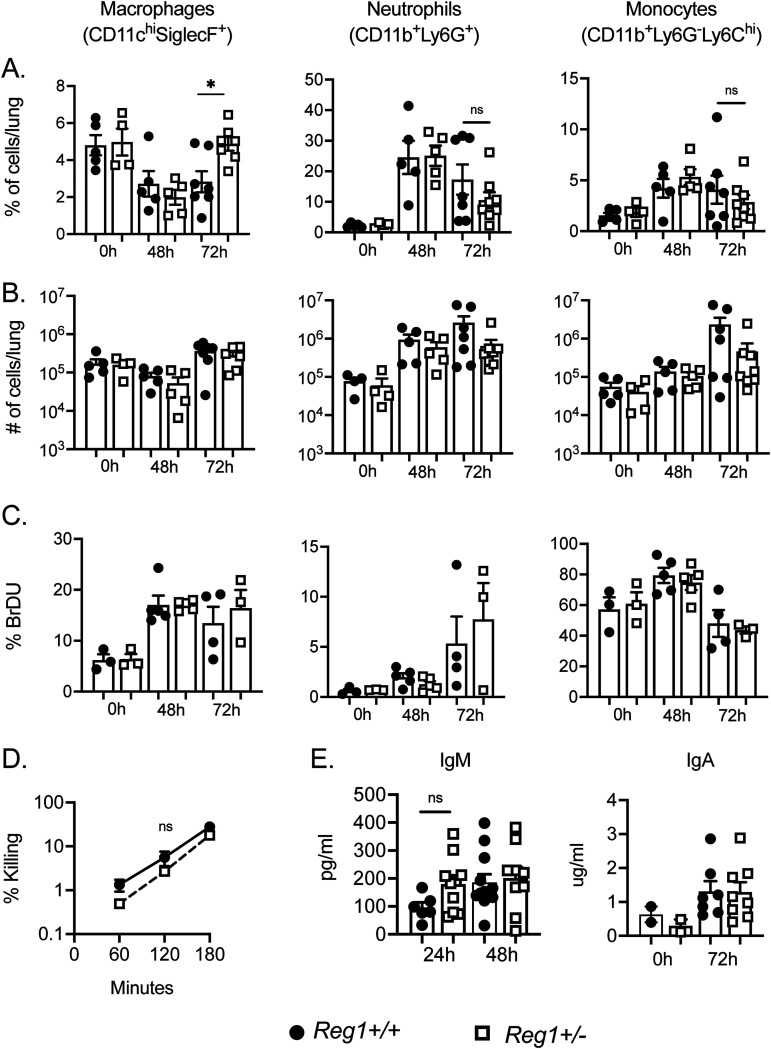
Based on these data, it is evident that a Reg1 deficiency in the hematopoietic compartment provides a survival advantage to the host. We saw increased percentage of alveolar macrophages at 72-h postinfection (Fig. 3A). However, there were no changes in the absolute numbers of recruited myeloid cells between Reg1+/− and control mice at 48-h and 72-h postinfection (Fig. 3B). Consistent with this, expression of myeloid-recruiting chemokines such as Cxcl1, Cxcl5, and Ccl2 were similar among groups, despite the fact that these are all known transcriptional targets of Reg1 in other settings (Fig. S1A) (8, 25). There were also no differences in cellular proliferation and macrophage bacterial killing between Reg1+/− and control mice (Fig. 3B and C). It is known that Reg1 controls B cell homeostasis and that complete Reg1 deletion in B cells increases antibody secretion (26). However, IgM and IgA levels in BALF and lung tissue were comparable between Reg1+/− and control mice (Fig. 3D). Taken together, these data suggest that differences in survival during KP infection in Reg1+/− mice is not explained by altered recruitment of myeloid cells, cellular proliferation, macrophage killing capacity, or a B cell antibody response.
FIG 3.
Immune cell recruitment, proliferation and antibody production are not affected by Reg1 deficiency during KP infection. (A, B) Percentage and absolute numbers of cellular recruitment to the lung of infected mice up to 72-h post- KP infection. (C) BrDU incorporation of lung myeloid cell populations were assessed by flow cytometry at baseline (0 h) or 48-h and 72-h postinfection. Data are pooled from two independent experiments (A to C). (D) Macrophage killing and Ab production in lung was assessed at the indicated time points. Data are pooled from two independent experiments. Data analyzed by one-way ANOVA *, P < 0.05; ns, not significant.
Expression of chemokines and mitochondrial respiration is similar between Reg1+/− and controls. Reg 1+/− and Reg1+/+ littermates were given PBS or KP by oropharyngeal aspiration and sacrificed at 24 h. (A) Cxcl1, Cxcl5, and Ccl2 expression was measured in lung tissue. Data analyzed by Student t-test and found nonsignificant (B) CD11c+ cells from PBS-treated or (C) KP- infected mice were sorted and evaluated for oxygen consumption rate (OCR) by Seahorse flux analyzer (n = 4 mice per group). Data analyzed by ANOVA and found to be nonsignificant. Download FIG S1, PDF file, 0.04 MB (41.5KB, pdf) .
Copyright © 2022 Trevejo-Nuñez et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Resistance to KP caused by Reg1 deficiency is linked to increased interferon signature.
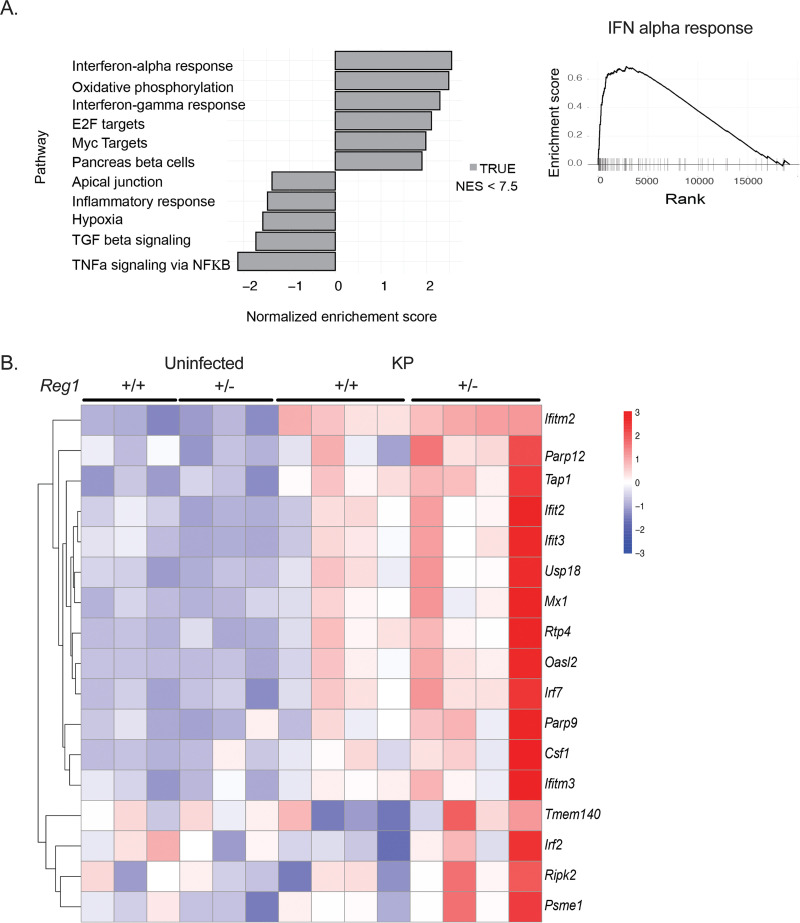
To understand the mechanisms by which Reg1 deficiency promotes survival, we performed transcriptomic profiling of purified alveolar macrophages from Reg1+/− and littermate control lungs at 24-h post-KP infection. This time point was chosen to define the early events that are operative during the initial stages of infection. There were 796 differentially expressed genes, of which 237 were upregulated and 559 were downregulated. Gene set enrichment analysis (GSEA) revealed major changes in type I IFN-related responses (IFN-α), oxidative phosphorylation, and IFN-γ response as the top three enriched gene sets, with a normalized enrichment score (NES) <2.5 for the type I IFN group (Fig. 4A and B).
FIG 4.
RNASeq of sorted alveolar macrophages reveals an enriched. Type 1 IFN gene signature. Reg1+/- and Reg1+/+ littermates were uninfected or infected with KP by oropharyngeal aspiration. Alveolar macrophages were sorted by flow cytometry at 24-h postinfection and subjected to RNASeq. (A) Gene set enrichment analysis (GSEA) of type I IFN gene sets. (B) Heatmap depicting differentially expressed genes in the type I IFN pathway (n = 3 or 4 mice/group).
We next validated these pathways by functional analysis. Consistent with the RNASeq data, type I IFN (IFN-β) expression was higher in lung homogenates of Reg1-deficient mice compared with controls at 24-h and 48-h postinfection (Fig. 5A). Because IFN-β can be produced by macrophages during Gram-negative pneumonia (27), we stimulated Reg1+/− or control BMDMs with heat-killed KP and assessed Ifnb1 mRNA. Indeed, Ifnb1 was elevated in Reg1-deficient macrophages compared to controls as early as 2-h postinfection (Fig. 5B). In contrast, IFN-γ expression was similarly expressed between Reg1+/− and Reg1+/+ mice after KP infection (Fig. 5C). Similarly, oxidative phosphorylation, of sorted alveolar macrophages assessed by mitochondrial respiration, was not different between Reg1+/− mice and controls (Fig. S1B,C). Collectively, these data indicate that Reg1 deficiency induces IFN-β upon KP infection.
FIG 5.
Increased IFN-β but not IFN-γ in Reg1 deficient mice during KP infection. Reg1+/- and Reg1+/+ littermates were infected with KP. (A) IFN-β was assessed in lung homogenates by ELISA. (B) Ifnb1 mRNA was assessed in cultured BMDMs infected with heat-killed KP for the indicated time points. (C) Levels of IFN-γ were assessed by intracellular staining in total CD45+, NK1.1+, and CD4+ cells. (B) Each symbol represents one mouse. *, P < 0.05 one-way ANOVA with post hoc Tukey’s test. (D) BMDMs were pretreated with LPS for 3 h and treated with Act D to stop new transcription. Expression of the indicated mRNAs were assessed for the indicated times by qPCR, normalized to Gapdh. Levels compared to time = 0 data are presented as means ± SEM, representative of two independent experiments. One-way ANOVA with post hoc Tukey’s test *, P < 0.05 by; RNA half-life (t½) was determined by linear regression as described (48).
Reg1 functions primarily by promoting endonucleolytic decay of target mRNA transcripts (28). Based on the observed increased Ifnb1 expression in Reg-deficient BMDMs, we hypothesized that Reg1 deficiency may result in prolonged stabilization of Ifnb1 mRNA or regulatory factors upstream of Ifnb1 regulation. To test this, Reg1+/− and control BMDMs were stimulated with LPS to prime expression of genes in the IFN pathway. Cells were treated with actinomycin D (Act D) to block further transcription, and the half-life of candidate target transcripts was assessed over a 2-h time course. Although the intrinsic mRNA stability of Ifnb1 or other mRNAs was not detectably altered in Reg1-deficient cells, the half-life of Irf7 mRNA considerably increased in the setting of Reg1 deficiency (Fig. 5D), potentially explaining the increased IFN-β seen in Reg-1 deficient mice upon KP pneumonia.
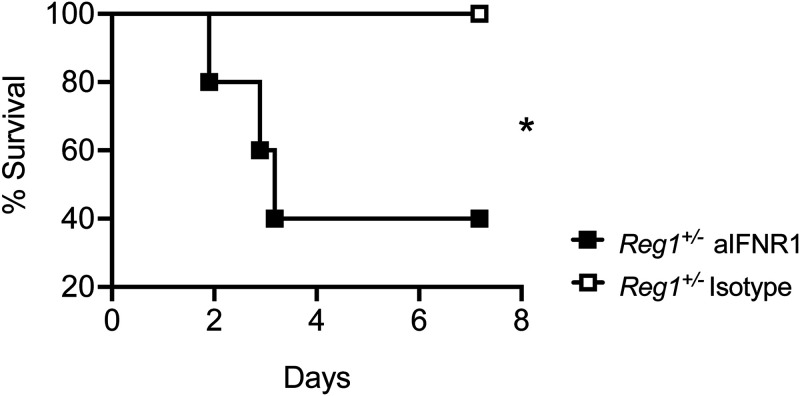
To determine whether type I IFN accounted for the prolonged survival in Reg1+/− mice, we administered anti-IFNR1 Abs or isotype controls at days 0 and 2 post-KP infection and followed mice for 7 days. Strikingly, 100% of Reg1+/− mice that received isotype control Abs survived, while 60% of Reg1+/− mice that received anti-IFNR1 succumbed (Fig. 6). Therefore, the protection afforded by Reg1-deficiency requires type I IFN signaling.
FIG 6.
IFNR1 blockade in Reg1-deficient mice enhances susceptibility to KP infection. Reg1+/− mice received anti-IFNR1 antibody or isotype control by i.p. injection on day 0 and day 2 post-KP infection. Mice were followed up to 7-days postinfection. (n = 5 mice/group). *, P < 0.05; Log-Rank test.
Increased Ifnb1 correlates with enhanced anti-inflammatory response in Reg1-deficient mice.
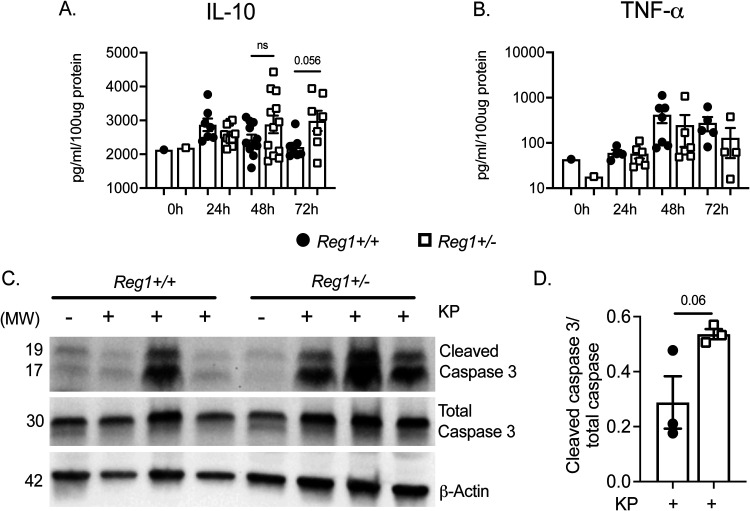
IFN-β has the capacity to exert anti-inflammatory effects in the host, reported in several disease models. This effect has been attributed in part to type I IFN induction of IL-10, which dampens expression of transcription of cytokines such as Tnfa, Cxcl1, and Il6 (29, 30). Based on this, we assessed IL-10 in lung homogenates of KP-infected mice. There was a trend to increased IL-10 at 72-h postinfection in Reg1+/− mice compared with control mice, though this did not reach statistical significance (Fig. 7A). TNF-α was not affected Reg1+/− compared with controls (Fig. 7B). We then asked whether apoptosis was affected in Reg1+/− mice compared with controls. As shown, cleaved caspase 3 expression in total lung homogenates was increased in Reg1 +/− mice compared with controls (Fig. 7C and D). These data suggest that increased IFN-β arising from Reg1 deficiency may confer an anti-inflammatory advantage to Reg1+/− mice sufficient to prolong survival upon KP infection.
FIG 7.
Reg1 deficiency enhances the anti-inflammatory response and increases apoptosis. (A, B) IL-10 and TFNα were assessed by ELISA in lung homogenates of the indicated mice infected with KP. Data are pooled from two independent experiments. (C) Reg1+/− and Reg1+/+ littermates were infected with KP. Mice were sacrificed at 48-h postinfection, and cleaved Caspase 3 and total Caspase were assessed in lung homogenates by immunoblotting. At right, quantified band intensity values ± SEM is shown. Data are representative of individual replicates.
DISCUSSION
Reg1 controls the inflammatory response by degrading many individual inflammatory cytokine mRNAs, including Il6, Il1b, and Il12b, thus tempering the overall inflammatory milieu (9). Perhaps even more significantly, Reg1 degrades transcripts encoding inflammatory transcription factors (TF) such as Nfkbiz, which encodes the non-canonical TF IκBξ, and therefore Reg1 can indirectly affect all IκBξ-inducible genes (11). Thus, it is not unexpected that a complete knockout of Reg1 gene (Zc3h12a) is fatal due to severe autoinflammation. Reg1+/− mice, on the other hand, appear to have a normal life span, fertility, and do not exhibit peripheral baseline inflammation. However, Reg1+/− mice still have an enhanced inflammatory response, particularly when the disease model relies heavily on the IL-17R pathway (11, 15, 24). In this KP infection model, we were surprised that levels of inflammatory cytokines and chemokines were similar between Reg1-deficient mice and controls. Because these mice are haploinsufficient for the Reg1 gene (Zc3h12a), and KP is sensed by TLR4, one potential explanation is the remaining Reg1 levels may be enough to compensate for the cytoplasmic MyD88-driven pathway, but not the endosomal TRIF-dependent pathway, thus leading to increased type I IFN expression and protection from KP.
Given that most pro-inflammatory genes known to be driven by Reg1 were unchanged in lung tissues regardless of Reg1 deficiency, and there was a clear increase in the percentage of alveolar macrophage (AM) recruitment, we performed RNAseq in AMs cells in order to evaluate macrophage-intrinsic activities underlying the Reg1-deficient phenotype. This approach has the caveat that contributions from other cells such as interstitial macrophages, inflammatory monocytes, or other immune cells may be missed. In this regard, CCR2+ inflammatory monocytes recruited during KP infection are known to enhance IL-17 production from ILC3 cells leading to KP eradication (31, 32). However, this mechanism has been shown to be important with clinical strains of KP and not with the serotype used here (ATCC 43816). Future studies will focus on additional cell types to determine more broadly where Reg1 is operative in this setting.
The role of type I interferons (IFN-α/IFN-β) in bacterial infections is not fully defined, especially when compared with its extensively-studied roles in viral infections. Even among bacterial pathogens, the response to type I IFN differs depending on site of infection and pathogen characteristics. For instance, type I IFN is detrimental in animal models of Staphylococcus aureus and Listeria monocytogenes (33, 34). On the other hand, there is a protective response in models of Legionella pneumophilia and Streptococcus pyogenes (35). In the case of Klebsiella pneumoniae, IFN-β signaling in NK cells enhances secretion of IFN-γ, decreasing bacterial burden (36). In this study, we did not see differences in recruitment of NK cells or enhanced IFN-γ secretion by NK cells in Reg1+/− mice compared with controls. However, the enhanced mortality in control WT mice is significantly decreased in Reg1+/− mice, which we propose is due to increased type I IFN that modulates the inflammatory response enough to facilitate bacterial eradication and pneumonia resolution, and this model is supported by histological evidence as well as the observation that anti-IFNR1 blockade reverses the protection provided by Reg1 deficiency.
IFN-β can lead to many complex events in bacterial infections, including increased apoptosis, macrophage efferocytosis and resolution of infection in models of Escherichia coli pneumonia and peritonitis, associated with enhanced IL-10 secretion (27). IL-10 mediates many anti-inflammatory effects and regulates metabolic reprograming of macrophages (37). Related to this, interstitial macrophages have immunoregulatory properties by secreting IL-10 upon LPS and CPG-DNA stimulation in models of asthma (38). Although there was only a modest trend of increased IL-10 in these settings, there was a clear increase in cleaved Caspase-3 in Reg1-deficient lungs, correlating with increased IFN-β and resistance to KP pneumonia. Concomitantly, transcriptomic profiles revealed several IFN-stimulated genes (ISGs) implicated in regulating apoptosis, including Ifit2, Ifit3, and Ifitm3. Intriguingly, IFIT2 is an RBP that enhances apoptosis (39, 40), though its precise role in the context of Reg1 immunoregulation is as yet unknown. Thus, we speculate one mechanism underlying these results is through actions of type I IFN mediating increases in apoptosis, an important step for resolution of pneumonia (41).
Because IFN-β levels were increased in Reg1-deficient mice upon KP infection, we initially expected that Reg1 deficiency would result in enhanced stability of the Ifnb1 transcript. However, our data instead indicate that the impact on Ifnb1 appears to be indirect via control of its upstream regulator Irf7 mRNA, opening a new facet of how Reg1 influences immune responses. A deficiency in a related RBP, Regnase-3 (Zc3h12c) also leads to increased IFN (type I and II) signaling, and IRF7 can transcriptionally control expression of Regnase-3 (42). However, Reg3 expression was not altered in AMs based on the RNAseq data or in total lung by qPCR, and thus we believe this axis is not a major driver of the effects seen here (Fig. S2D).
Expression of IL-17, IL-22, and IFN-λ are similar between Reg1+/− and controls. Reg 1+/− and Reg1+/+ littermates were infected with KP and sacrificed at the indicated time points. Lung tissues were assessed for protein and mRNA. (A) IL-17; (B) IL-22; (C) IFN-λ, expression in lung homogenates; (D) Regnase-3 (Zc3h12c) mRNA expression in total lung. Data are pooled from two independent experiments. Data analyzed by ANOVA and found to be nonsignificant. Download FIG S2, PDF file, 0.06 MB (57.3KB, pdf) .
Copyright © 2022 Trevejo-Nuñez et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
The type III interferons have emerged in recent years as key regulators of antiviral and antifungal immunity (43). It has been shown that IFN-lambda (L) increases lung epithelial permeability and facilitates bacterial transmigration in animal models infected with KP (KP ST258), an effect that is counteracted by IL-22 (44). In this model we did not see increases in IFN-L despite increase of type I IFN and in vitro stability of IRF-7, though determining whether this axis of interferon activity contributes in any way will require further study (Fig. S2C). Surprisingly, although IL-22 and IL-17 signaling are well described to play important roles in KP eradication (20–22), their expression was also not altered by Reg1 deficiency (Fig. S2A,B). This would be consistent with activities of Reg1 being downstream of these or other cytokines rather than upstream of their production, though further analyses will be required to prove that point definitively.
The immune system has evolved to balance the vital effects of anti-microbial effector functions with the potential for causing collateral tissue damage. In this regard, the activation of every immune signaling pathway is accompanied by negative feedback signaling events that restrain inflammation (45, 46). In selected conditions, however, it may be clinically beneficial to allow more fulminant inflammation in order to treat a life-threatening condition. Indeed, checkpoint inhibitor blockade for cancer therapy is built on exploiting this concept (47). By analogy, releasing inflammatory “brakes” may be useful in the context of severe infections such as bacterial pneumonia, and the present data suggest that Reg1 could, in principle at least, be one such target.
MATERIALS AND METHODS
Mice.
Reg1+/− and Reg1+/+ (Zc3h12a) littermates on the C57BL/6 background were cohoused and used for all experiments. Reg1(Zc3h12a)fl/fl mice are under material transfer agreement (MTA) from University of Central Florida (Orlando, FL, USA). SftpcCre mice and CD45.1 mice were from The Jackson Laboratory. Mice were 8 to 12-weeks old and both sexes were used. All animal studies were approved by the Institutional Animal Care and Use Committee (IACUC) of the University of Pittsburgh.
Bacterial infections and anti-IFNR1 treatment.
KP ATCC strain 43816 was grown in tryptic soy broth to reach early log phase. 1-2 × 103 CFU/mouse in PBS was administered by deep oropharyngeal aspiration. Where indicated, tamoxifen (TAM) was administered i.p. at 75 mg/kg dissolved in corn oil for 5 days and then rested for 5 days prior to induction of infection. Reg1+/− mice were treated with anti-IFNR1 antibodies or isotype control by i.p. injection (Bio-Xcell MAR1-5A3, 250 μg/mouse, administered on day 0 and day 2 p.i.). Mice were followed for 7 days.
Bone marrow chimeras.
WT (CD45.1) and Reg1+/− (CD45.2) mice were lethally irradiated (900 Gy). After 24 h, each mouse received 4 × 106 bone marrow cells by tail vein injection. Mice received antibiotics in drinking water starting 1 day before irradiation and continued for 10 days (trimethroprim/sulfametoxazole 200/40 mg). After 6 weeks, engraftment was confirmed by flow cytometry of blood for CD45.1 and CD45.2 markers.
Bacterial burden, mRNA, and protein analysis.
Tissues were homogenized in PBS and CFU levels were assessed by serial dilution plating. Lung tissues were homogenized in TRIzol (Invitrogen) and subjected to qPCR with SybrGreen probes. Threshold cycle (CT) values were normalized to Gapdh. ELISA kits were from eBioscience (Thermo Fisher Scientific) and R&D Systems. Abs used in Western blots were cleaved caspase-3, total caspase 3 (Cell Signaling) and beta-actin (Abcam).
Flow cytometry.
Lungs were digested with lung dissociation kits (Miltenyi). Lung cell suspensions were stained for CD45, Ly6G, CD11b, CD11c, SiglecF, Ly6C, CD3, CD4, NK1.1, and IFN-γ. Abs were from eBioscience, BD Biosciences, or Biolegend. Alveolar macrophages (CD45+, Ly6G- CD11b- CD11chi, SiglecF+) were isolated by FACS. Proliferation was assessed by BrDU incorporation (BD Biosciences); briefly, mice were injected i.p. with 1 mg of BrDU 1 day before sacrifice. Data were acquired on LSR Fortessa and analyzed with FlowJo software.
Bone marrow derived macrophages.
BM cells were extracted from Reg1+/− and Reg1+/+ mice femur and tibia and differentiated into bone marrow derived macrophages (BMDM) with L929 media (30%) for 5 to 6 days. Cultured BMDMs were stimulated with heat-killed KP (MOI = 10) up to 8 h or Klebsiella LPS (1 μg/mL) (Sigma-Aldrich).
RNA sequencing and analysis.
Samples from 12 individual mice (uninfected Reg1+/+ n = 3, uninfected Reg1+/− n = 3, KP-infected Reg1+/+ n = 4, KP-infected Reg1+/− n = 4) were used. Mice were infected with KP by oropharyngeal aspiration. After 24 h, alveolar macrophages were stained as described above and sorted by flow cytometry with a purity of 99%. Cells were placed into RLT-plus (Qiagen, Valencia, CA) and total RNA extracted using RNeasy MiniKits (Qiagen). RNA was quantitated using Nanodrop and integrity determined with a total RNA Nano Chip (Agilent Technologies). Single-stranded total RNA-seq libraries were sequenced with an Illumina Nextseq500 sequencer with a depth of 25 million reads per sample (75 bp single-end) at the University of Pittsburgh Health Sciences Sequencing Core. Fastq files with high quality reads (phred score >30) were uploaded to the CLC Genomics Workbench (Qiagen) and reads aligned to the mouse reference genome. Transcript counts and differential expression analyses were carried out using the CLC Genomics Workbench. RNAseq data were deposited to Sequence Read Archive (SRA), BioProject ID: PRJNA789160.
Lung histology.
Lungs from Reg1+/− and Reg1+/+ mice isolated at 48-h p.i. were fixed in 10% neutral buffered formalin for 24 h. Sections were stained with hematoxylin and eosin (H&E) by the University of Pittsburgh histology core. Slides were visualized on an EVOS microscope. Lung damage parameters measured were: (i) parenchymal inflammation and percentage of compromised tissue; (ii) peribronchial; and (iii) perivascular inflammation. The assigned score values were 0 = none; 1 = <25%; 2 = 25% to 50%; 3 = 50% to 75%; 4 = >75%.
Statistical analysis.
Data were analyzed on Prism (GraphPad). Data were analyzed by log-rank, one-way analysis of variance (ANOVA), Student's t test, and post hoc tests were used as indicated. Each symbol represents one mouse unless indicated.
ACKNOWLEDGMENTS
G.T.N. was supported by NIH grants HL135476, HL154231, AI153549. K.C. was supported by HL137709. S.L.G. was supported by AI147383. We thank B.M. Coleman for technical support and J.K. Kolls for helpful discussions. We thank the reviewers for excellent suggestions that significantly improved the manuscript.
AM, alveolar macrophage; BM, bone marrow; BMDM, bone marrow-derived macrophages; KP, Klebsiella pneumoniae; MCPIP1, MCP1-induced protein 1 (alternative name for Regnase-1); MyD88, myeloid differentiation primary response gene 88; RBP, RNA binding protein; Reg1, regnase-1; TAM, tamoxifen; TLR, Toll-like receptor; UTR, untranslated region; VAP, ventilator-associated pneumonia.
Conceptualization – G.T.N., P.S.B., S.L.G., K.C.; Investigation – G.T.N., B.L., L.F., F.E.Y.A., K.C.; Writing – G.T.N., S.L.G.; Reviewing and Editing – G.T.N., K.C., P.S.B., S.L.G.; Visualization – G.T.N.; Supervision – G.T.N., S.L.G., P.S.B.; Funding Acquisition – G.T.N., S.L.G., K.C., P.S.B.
Footnotes
This article is a direct contribution from Sarah L. Gaffen, a Fellow of the American Academy of Microbiology, who arranged for and secured reviews by George Deepe, University of Cincinnati, and Amariliz Rivera, New Jersey Medical School.
Contributor Information
Giraldina Trevejo-Nuñez, Email: giraldina.trevejo@pitt.edu.
Nancy C. Reich, Stony Brook University
REFERENCES
- 1.Conlan S, Kong HH, Segre JA. 2012. Species-level analysis of DNA sequence data from the NIH Human Microbiome Project. PLoS One 7:e47075. doi: 10.1371/journal.pone.0047075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Martin RM, Cao J, Brisse S, Passet V, Wu W, Zhao L, Malani PN, Rao K, Bachman MA. 2016. Molecular epidemiology of colonizing and infecting isolates of Klebsiella pneumoniae. mSphere 1:e00261-16. doi: 10.1128/mSphere.00261-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Papazian L, Klompas M, Luyt CE. 2020. Ventilator-associated pneumonia in adults: a narrative review. Intensive Care Med 46:888–906. doi: 10.1007/s00134-020-05980-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Weiner LM, Webb AK, Limbago B, Dudeck MA, Patel J, Kallen AJ, Edwards JR, Sievert DM. 2016. Antimicrobial-resistant pathogens associated with healthcare-associated infections: summary of data reported to the National Healthcare Safety Network at the Centers for Disease Control and Prevention, 2011–2014. Infect Control Hosp Epidemiol 37:1288–1301. doi: 10.1017/ice.2016.174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gorrie CL, Mirceta M, Wick RR, Edwards DJ, Thomson NR, Strugnell RA, Pratt NF, Garlick JS, Watson KM, Pilcher DV, McGloughlin SA, Spelman DW, Jenney AWJ, Holt KE. 2017. Gastrointestinal carriage is a major reservoir of Klebsiella pneumoniae infection in intensive care patients. Clin Infect Dis 65:208–215. doi: 10.1093/cid/cix270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Adler A, Katz DE, Marchaim D. 2020. The continuing plague of extended-spectrum β-lactamase producing enterbacterales infections: an update. Infect Dis Clin North Am 34:677–708. doi: 10.1016/j.idc.2020.06.003. [DOI] [PubMed] [Google Scholar]
- 7.Fu M, Blackshear PJ. 2017. RNA-binding proteins in immune regulation: a focus on CCCH zinc finger proteins. Nat Rev Immunol 17:130–143. doi: 10.1038/nri.2016.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mino T, Murakawa Y, Fukao A, Vandenbon A, Wessels HH, Ori D, Uehata T, Tartey S, Akira S, Suzuki Y, Vinuesa CG, Ohler U, Standley DM, Landthaler M, Fujiwara T, Takeuchi O. 2015. Regnase-1 and roquin regulate a common element in inflammatory mRNAs by spatiotemporally distinct mechanisms. Cell 161:1058–1073. doi: 10.1016/j.cell.2015.04.029. [DOI] [PubMed] [Google Scholar]
- 9.Matsushita K, Takeuchi O, Standley DM, Kumagai Y, Kawagoe T, Miyake T, Satoh T, Kato H, Tsujimura T, Nakamura H, Akira S. 2009. Zc3h12a is an RNase essential for controlling immune responses by regulating mRNA decay. Nature 458:1185–1190. doi: 10.1038/nature07924. [DOI] [PubMed] [Google Scholar]
- 10.Iwasaki H, Takeuchi O, Teraguchi S, Matsushita K, Uehata T, Kuniyoshi K, Satoh T, Saitoh T, Matsushita M, Standley DM, Akira S. 2011. The IκB kinase complex regulates the stability of cytokine-encoding mRNA induced by TLR-IL-1R by controlling degradation of regnase-1. Nat Immunol 12:1167–1175. doi: 10.1038/ni.2137. [DOI] [PubMed] [Google Scholar]
- 11.Garg AV, Amatya N, Chen K, Cruz JA, Grover P, Whibley N, Conti HR, Hernandez Mir G, Sirakova T, Childs EC, Smithgall TE, Biswas PS, Kolls JK, McGeachy MJ, Kolattukudy PE, Gaffen SL. 2015. MCPIP1 endoribonuclease activity negatively regulates interleukin-17-mediated signaling and inflammation. Immunity 43:475–487. doi: 10.1016/j.immuni.2015.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tanaka H, Arima Y, Kamimura D, Tanaka Y, Takahashi N, Uehata T, Maeda K, Satoh T, Murakami M, Akira S. 2019. Phosphorylation-dependent Regnase-1 release from endoplasmic reticulum is critical in IL-17 response. J Exp Med 216:1431–1449. doi: 10.1084/jem.20181078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Uehata T, Iwasaki H, Vandenbon A, Matsushita K, Hernandez-Cuellar E, Kuniyoshi K, Satoh T, Mino T, Suzuki Y, Standley DM, Tsujimura T, Rakugi H, Isaka Y, Takeuchi O, Akira S. 2013. Malt1-induced cleavage of Regnase-1 in CD4(+) helper T cells regulates immune activation. Cell 153:1036–1049. doi: 10.1016/j.cell.2013.04.034. [DOI] [PubMed] [Google Scholar]
- 14.Miao R, Huang S, Zhou Z, Quinn T, Van Treeck B, Nayyar T, Dim D, Jiang Z, Papasian CJ, Eugene Chen Y, Liu G, Fu M. 2013. Targeted disruption of MCPIP1/Zc3h12a results in fatal inflammatory disease. Immunol Cell Biol 91:368–376. doi: 10.1038/icb.2013.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Monin L, Gudjonsson JE, Childs EE, Amatya N, Xing X, Verma AH, Coleman BM, Garg AV, Killeen M, Mathers A, Ward NL, Gaffen SL. 2017. MCPIP1/Regnase-1 Restricts IL-17A- and IL-17C-dependent skin inflammation. J Immunol 198:767–775. doi: 10.4049/jimmunol.1601551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li D-D, Bechara R, Ramani K, Jawale CV, Li Y, Kolls JK, Gaffen SL, Biswas PS. 2021. RTEC-intrinsic IL-17–driven inflammatory circuit amplifies antibody-induced glomerulonephritis and is constrained by Regnase-1. JCI Insight 6:e147505. doi: 10.1172/jci.insight.147505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cai S, Batra S, Shen L, Wakamatsu N, Jeyaseelan S. 2009. Both TRIF- and MyD88-dependent signaling contribute to host defense against pulmonary Klebsiella infection. J Immunol 183:6629–6638. doi: 10.4049/jimmunol.0901033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.van Lieshout MH, Blok DC, Wieland CW, de Vos AF, van 't Veer C, van der Poll T. 2012. Differential roles of MyD88 and TRIF in hematopoietic and resident cells during murine gram-negative pneumonia. J Infect Dis 206:1415–1423. doi: 10.1093/infdis/jis505. [DOI] [PubMed] [Google Scholar]
- 19.Happel KI, Zheng M, Young E, Quinton LJ, Lockhart E, Ramsay AJ, Shellito JE, Schurr JR, Bagby GJ, Nelson S, Kolls JK. 2003. Cutting edge: roles of Toll-like receptor 4 and IL-23 in IL-17 expression in response to Klebsiella pneumoniae infection. J Immunol 170:4432–4436. doi: 10.4049/jimmunol.170.9.4432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ye P, Rodriguez FH, Kanaly S, Stocking KL, Schurr J, Schwarzenberger P, Oliver P, Huang W, Zhang P, Zhang J, Shellito JE, Bagby GJ, Nelson S, Charrier K, Peschon JJ, Kolls JK. 2001. Requirement of interleukin 17 receptor signaling for lung CXC chemokine and granulocyte colony-stimulating factor expression, neutrophil recruitment, and host defense. J Exp Med 194:519–527. doi: 10.1084/jem.194.4.519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Aujla SJ, Chan YR, Zheng M, Fei M, Askew DJ, Pociask DA, Reinhart TA, McAllister F, Edeal J, Gaus K, Husain S, Kreindler JL, Dubin PJ, Pilewski JM, Myerburg MM, Mason CA, Iwakura Y, Kolls JK. 2008. IL-22 mediates mucosal host defense against Gram-negative bacterial pneumonia. Nat Med 14:275–281. doi: 10.1038/nm1710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chen K, Eddens T, Trevejo-Nunez G, Way EE, Elsegeiny W, Ricks DM, Garg AV, Erb CJ, Bo M, Wang T, Chen W, Lee JS, Gaffen SL, Kolls JK. 2016. IL-17 receptor signaling in the lung epithelium is required for mucosal chemokine gradients and pulmonary host defense against K. pneumoniae. Cell Host Microbe 20:596–605. doi: 10.1016/j.chom.2016.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yang C, Huang S, Wang X, Gu Y. 2015. Emerging roles of CCCH-type zinc finger proteins in destabilizing mRNA encoding inflammatory factors and regulating immune responses. Crit Rev Eukaryot Gene Expr 25:77–89. doi: 10.1615/critreveukaryotgeneexpr.2015013022. [DOI] [PubMed] [Google Scholar]
- 24.Jeltsch KM, Hu D, Brenner S, Zoller J, Heinz GA, Nagel D, Vogel KU, Rehage N, Warth SC, Edelmann SL, Gloury R, Martin N, Lohs C, Lech M, Stehklein JE, Geerlof A, Kremmer E, Weber A, Anders HJ, Schmitz I, Schmidt-Supprian M, Fu M, Holtmann H, Krappmann D, Ruland J, Kallies A, Heikenwalder M, Heissmeyer V. 2014. Cleavage of roquin and regnase-1 by the paracaspase MALT1 releases their cooperatively repressed targets to promote T(H)17 differentiation. Nat Immunol 15:1079–1089. doi: 10.1038/ni.3008. [DOI] [PubMed] [Google Scholar]
- 25.Akaki K, Ogata K, Yamauchi Y, Iwai N, Tse KM, Hia F, Mochizuki A, Ishihama Y, Mino T, Takeuchi O. 2021. IRAK1-dependent Regnase-1–14-3–3 complex formation controls Regnase-1-mediated mRNA decay. Elife 10. doi: 10.7554/eLife.71966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bhat N, Virgen-Slane R, Ramezani-Rad P, Leung CR, Chen C, Balsells D, Shukla A, Kao E, Apgar JR, Fu M, Ware CF, Rickert RC. 2021. Regnase-1 is essential for B cell homeostasis to prevent immunopathology. J Exp Med 218:e20200971. doi: 10.1084/jem.20200971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kumaran Satyanarayanan S, El Kebir D, Soboh S, Butenko S, Sekheri M, Saadi J, Peled N, Assi S, Othman A, Schif-Zuck S, Feuermann Y, Barkan D, Sher N, Filep JG, Ariel A. 2019. IFN-β is a macrophage-derived effector cytokine facilitating the resolution of bacterial inflammation. Nat Commun 10:3471. doi: 10.1038/s41467-019-10903-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Akira S. 2013. Regnase-1, a Ribonuclease involved in the regulation of immune responses. Cold Spring Harbor Symp Quant Biol 78:51–60. doi: 10.1101/sqb.2013.78.019877. [DOI] [PubMed] [Google Scholar]
- 29.Katakura K, Lee J, Rachmilewitz D, Li G, Eckmann L, Raz E. 2005. Toll-like receptor 9-induced type I IFN protects mice from experimental colitis. J Clin Invest 115:695–702. doi: 10.1172/JCI22996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hamana A, Takahashi Y, Tanioka A, Nishikawa M, Takakura Y. 2018. Safe and effective interferon-beta gene therapy for the treatment of multiple sclerosis by regulating biological activity through the design of interferon-beta-galectin-9 fusion proteins. Int J Pharm 536:310–317. doi: 10.1016/j.ijpharm.2017.12.010. [DOI] [PubMed] [Google Scholar]
- 31.Xiong H, Carter RA, Leiner IM, Tang YW, Chen L, Kreiswirth BN, Pamer EG. 2015. Distinct contributions of neutrophils and CCR2+ monocytes to pulmonary clearance of different Klebsiella pneumoniae strains. Infect Immun 83:3418–3427. doi: 10.1128/IAI.00678-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Xiong H, Keith JW, Samilo DW, Carter RA, Leiner IM, Pamer EG. 2016. Innate lymphocyte/Ly6C(hi) monocyte crosstalk promotes Klebsiella pneumoniae clearance. Cell 165:679–689. doi: 10.1016/j.cell.2016.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Carrero JA, Calderon B, Unanue ER. 2004. Type I interferon sensitizes lymphocytes to apoptosis and reduces resistance to Listeria infection. J Exp Med 200:535–540. doi: 10.1084/jem.20040769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Martin FJ, Gomez MI, Wetzel DM, Memmi G, O’Seaghdha M, Soong G, Schindler C, Prince A. 2009. Staphylococcus aureus activates type I IFN signaling in mice and humans through the Xr repeated sequences of protein A. J Clin Invest 119:1931–1939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Boxx GM, Cheng G. 2016. The roles of type i interferon in bacterial infection. Cell Host Microbe 19:760–769. doi: 10.1016/j.chom.2016.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ivin M, Dumigan A, de Vasconcelos FN, Ebner F, Borroni M, Kavirayani A, Przybyszewska KN, Ingram RJ, Lienenklaus S, Kalinke U, Stoiber D, Bengoechea JA, Kovarik P. 2017. Natural killer cell-intrinsic type I IFN signaling controls Klebsiella pneumoniae growth during lung infection. PLoS Pathog 13:e1006696. doi: 10.1371/journal.ppat.1006696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ip WKE, Hoshi N, Shouval DS, Snapper S, Medzhitov R. 2017. Anti-inflammatory effect of IL-10 mediated by metabolic reprogramming of macrophages. Science 356:513–519. doi: 10.1126/science.aal3535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sabatel C, Radermecker C, Fievez L, Paulissen G, Chakarov S, Fernandes C, Olivier S, Toussaint M, Pirottin D, Xiao X, Quatresooz P, Sirard JC, Cataldo D, Gillet L, Bouabe H, Desmet CJ, Ginhoux F, Marichal T, Bureau F. 2017. Exposure to bacterial CpG DNA protects from airway allergic inflammation by expanding regulatory lung interstitial macrophages. Immunity 46:457–473. doi: 10.1016/j.immuni.2017.02.016. [DOI] [PubMed] [Google Scholar]
- 39.Reich NC. 2013. A death-promoting role for ISG54/IFIT2. J Interferon Cytokine Res 33:199–205. doi: 10.1089/jir.2012.0159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yang Z, Liang H, Zhou Q, Li Y, Chen H, Ye W, Chen D, Fleming J, Shu H, Liu Y. 2012. Crystal structure of ISG54 reveals a novel RNA binding structure and potential functional mechanisms. Cell Res 22:1328–1338. doi: 10.1038/cr.2012.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Voll RE, Herrmann M, Roth EA, Stach C, Kalden JR, Girkontaite I. 1997. Immunosuppressive effects of apoptotic cells. Nature 390:350–351. doi: 10.1038/37022. [DOI] [PubMed] [Google Scholar]
- 42.von Gamm M, Schaub A, Jones AN, Wolf C, Behrens G, Lichti J, Essig K, Macht A, Pircher J, Ehrlich A, Davari K, Chauhan D, Busch B, Wurst W, Feederle R, Feuchtinger A, Tschöp MH, Friedel CC, Hauck SM, Sattler M, Geerlof A, Hornung V, Heissmeyer V, Schulz C, Heikenwalder M, Glasmacher E. 2019. Immune homeostasis and regulation of the interferon pathway require myeloid-derived Regnase-3. J Exp Med 216:1700–1723. doi: 10.1084/jem.20181762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Espinosa V, Dutta O, McElrath C, Du P, Chang YJ, Cicciarelli B, Pitler A, Whitehead I, Obar JJ, Durbin JE, Kotenko SV, Rivera A. 2017. Type III interferon is a critical regulator of innate antifungal immunity. Sci Immunol 2. doi: 10.1126/sciimmunol.aan5357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ahn D, Wickersham M, Riquelme S, Prince A. 2019. The effects of IFN-λ on epithelial barrier function contribute to Klebsiella pneumoniae ST258 pneumonia. Am J Respir Cell Mol Biol 60:158–166. doi: 10.1165/rcmb.2018-0021OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Carpenter S, Ricci EP, Mercier BC, Moore MJ, Fitzgerald KA. 2014. Post-transcriptional regulation of gene expression in innate immunity. Nat Rev Immunol 14:361–376. doi: 10.1038/nri3682. [DOI] [PubMed] [Google Scholar]
- 46.Li X, Bechara R, Zhao J, McGeachy MJ, Gaffen SL. 2019. IL-17 receptor-based signaling and implications for disease. Nat Immunol 20:1594–1602. doi: 10.1038/s41590-019-0514-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sharma P, Allison JP. 2020. Dissecting the mechanisms of immune checkpoint therapy. Nat Rev Immunol 20:75–76. doi: 10.1038/s41577-020-0275-8. [DOI] [PubMed] [Google Scholar]
- 48.Amatya N, Childs EE, Cruz JA, Aggor FEY, Garg AV, Berman AJ, Gudjonsson JE, Atasoy U, Gaffen SL. 2018. IL-17 integrates multiple self-reinforcing, feed-forward mechanisms through the RNA-binding protein Arid5a. Sci Signal 11:eaat4617. doi: 10.1126/scisignal.aat4617. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Expression of chemokines and mitochondrial respiration is similar between Reg1+/− and controls. Reg 1+/− and Reg1+/+ littermates were given PBS or KP by oropharyngeal aspiration and sacrificed at 24 h. (A) Cxcl1, Cxcl5, and Ccl2 expression was measured in lung tissue. Data analyzed by Student t-test and found nonsignificant (B) CD11c+ cells from PBS-treated or (C) KP- infected mice were sorted and evaluated for oxygen consumption rate (OCR) by Seahorse flux analyzer (n = 4 mice per group). Data analyzed by ANOVA and found to be nonsignificant. Download FIG S1, PDF file, 0.04 MB (41.5KB, pdf) .
Copyright © 2022 Trevejo-Nuñez et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Expression of IL-17, IL-22, and IFN-λ are similar between Reg1+/− and controls. Reg 1+/− and Reg1+/+ littermates were infected with KP and sacrificed at the indicated time points. Lung tissues were assessed for protein and mRNA. (A) IL-17; (B) IL-22; (C) IFN-λ, expression in lung homogenates; (D) Regnase-3 (Zc3h12c) mRNA expression in total lung. Data are pooled from two independent experiments. Data analyzed by ANOVA and found to be nonsignificant. Download FIG S2, PDF file, 0.06 MB (57.3KB, pdf) .
Copyright © 2022 Trevejo-Nuñez et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.