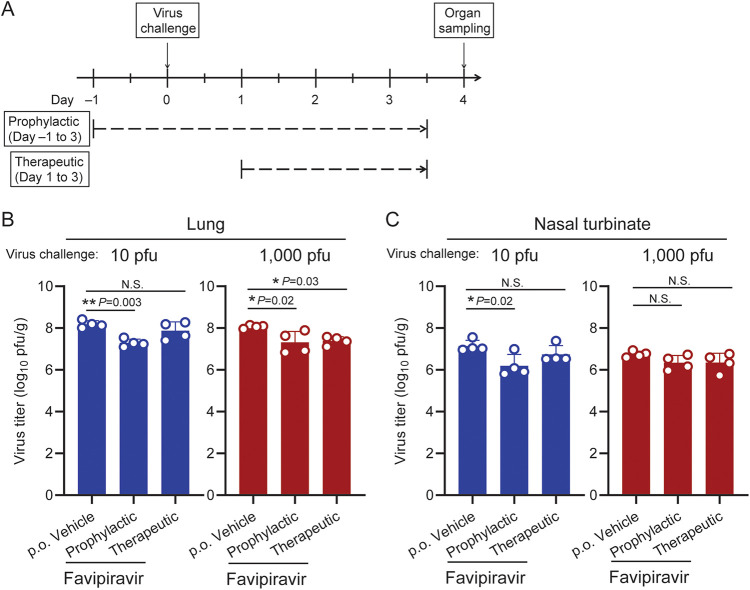
FIG 1.
Inhibitory effects of favipiravir on SARS-CoV-2 replication in the lungs and nasal turbinate of hamsters. (A) Timeline of favipiravir administration and virus challenge schedule in the hamster infection model. (B, C) Each group of hamsters (n = 4) was administered vehicle control or favipiravir (300 mg/kg) as a prophylactic (days –1 to 3; twice/day) or therapeutic regimen (days 1 to 3; twice/day). The hamsters were intranasally inoculated with 10 or 1,000 PFU of SARS-CoV-2 (day 0). Infectious virus titers (PFU/g) in the lungs (panel B) and nasal turbinate (panel C) were examined by performing plaque assays. Dots and bars show the value for each animal and the average in each group, respectively (n = 4, mean ± SD [standard deviation of the mean]). *, P < 0.05; NS, not significant; determined by a one-way analysis of variance (ANOVA) and corrected for multi-group comparison using Dunnett’s test.