Abstract
Background
Antibody response following SARS-CoV-2 vaccination is somewhat defective in chronic lymphocytic leukemia (CLL). Moreover, the correlation between serologic response and status of cellular immunity has been poorly studied.
Objective
This study was undertaken to assess humoral immune and cellular responses to the BNT162b2 messenger RNA (mRNA) COVID-19 vaccination in CLL.
Methods
The presence of the spike antibodies was assessed at a median time of 14 days from the second vaccine dose of SARS-CoV-2 in 70 CLL patients followed up at a single institution.
Results
The antibody response rate (RR) in CLL patients was 58.5%, compared to 100% of 57 healthy controls of the same sex and age (p < 0.0001). Treatment-naïve patients and those in sustained clinical remission after therapy had the highest RR (87.0% and 87.7%, respectively). In contrast, patients on therapy with a pathway inhibitor as monotherapy and those treated with an association of anti-CD20 antibody were unlikely to respond to the SARS-CoV-2 vaccine (52% and 10%, respectively). In multivariate analysis, early Rai stage (OR, 0.19 [0.05–0.79]; p = 0.02) and no previous therapy (OR, 0.06 [0.02–0.27]; p < 0.0001) were found to be independent predictors of vaccination response. An increase in absolute NK cells (i.e., CD16/CD56 positive cells) in patients with a serological response was found following the second dose of vaccine (p = 0.02).
Conclusions
These results confirm that serological response to the BNT162b2 vaccine in patients with CLL is impaired. A third boosting vaccine dosage should be considered for these patients.
Keywords: Chronic lymphocytic leukemia, Severe acute respiratory syndrome coronavirus 2 mRNA vaccination, Serologic response, T-cell assessment
Introduction
Vaccination against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) demonstrated efficacy in about 95% of the general population enrolled in a pivotal efficacy trial [1, 2]. However, immunocompromised individuals were primarily excluded from early trials of SARS-CoV-2 mRNA immunization. Since immune system disturbance is a peculiar characteristic of chronic lymphocytic leukemia (CLL), assessing the extent of serologic response to the SARS-CoV-2 mRNA vaccination is an area of scientific interest [3].
In a prospective study conducted in the framework of the European Research Initiative on CLL (ERIC) and including 167 CLL patients, response rates (RRs) were 55.2% in treatment-naïve (TN) patients but only 16.0% in patients undergoing active treatment [4]. These findings were confirmed in a multicentric analysis that enrolled 373 CLL patients across 9 Israeli medical institutions. Serological response to the vaccine was 61% in TN patients and between 23% and 24% in those treated with Bruton kinase (BTK) and BCL2 inhibitor agents. Of note, the RR to vaccine dropped to 5% in patients given an anti-CD20 antibody during the year that preceded vaccination [5]. Overall, these results are similar to those observed in a smaller single-institution CLL series of patients who underwent vaccination with BNT162b2 mRNA-1273 vaccines at the Memorial Sloan Kettering Cancer Center, NY, USA [6].
The level of antibody response following the first or second vaccination dose is somewhat unknown in CLL [6]. In a study conducted in the United Kingdom, investigators assessed the spike-specific antibody responses after the first and second COVID-19 vaccination doses in 299 CLL patients (154 with BNT162b2 mRNA and 145 with ChAdOx1) [7]. Patients showed 34% spike-specific antibody responses after the first vaccination dose, compared to 94% healthy donors. However, antibody responses increased to 75% following the second dose in patients with CLL, compared to 100% in healthy donors [7].
With this background, we investigated the efficacy, safety, and impact of targeted therapy on the serologic response to the BNT162b2 mRNA COVID-19 vaccine in 70 CLL patients followed up at a single institution. In this patient cohort, we also analyzed the correlation between serologic response and status of cellular immunity before and after the vaccination. The study also aimed to understand the clinical impact of vaccination in different CLL patient subgroups and identify possible predictors of the antibody response to the SARS-CoV-2 vaccine.
Patients and Methods
From March 2021 through May 2021, 2 doses of BNT162b2 mRNA COVID-19 vaccine (21 days apart) were given to 70 CLL patients followed up at the Hematological Department of Azienda Ospedaliera Pugliese-Ciaccio, Catanzaro, Italy. Diagnosis of CLL was established according to the IWCLL criteria [8].
In these patients, the presence of the spike antibodies was tested at a median time of 14 days (range, 14–28) from the second vaccine dose. Serologic testing for SARS-CoV-2 IgG was performed using the LIAISON® SARS-CoV-2 S1/S2 IgG test (DiaSorin, Saluggia, Italy), a chemiluminescence immunoassay for the quantitative determination of anti-S1 and anti-S2 specific IgG antibodies to SARS-CoV-2. The sensitivity and specificity of the assay were 98.7% and 99.5%, respectively. Samples were considered negative for antibody titers below 13 AU/mL. Results were compared with those of an age-matched group of subjects with no hematological malignancy (n = 57). All patients were tested for the presence of antibodies to SARS-CoV-2 nucleocapsid with the Elecsys Anti-SARS-CoV-2 S assay using the Cobas e 601 (Roche Diagnostics) analyzer. Of note, anti-SARS-CoV-2 nucleocapsid antibodies were never detected, thus suggesting that no patient had been recently exposed to SARS-CoV-2.
The institutional review board approved the study. All patients provided informed consent.
The Mann-Whitney U test and Kruskal-Wallis H test were used for comparing medians. Correlation between CLL features and positive/negative serology testing was estimated through an unconditional logistic regression model.
Results
The median age of CLL patients was 72 years (range, 63–88), and 71.4% were males. The median time from CLL diagnosis to vaccination was 82.5 months (range, 1–280). Twenty-three patients (32.9%) were TN, 36 (51.4%) on active therapy (i.e., BTK inhibitors, 22 [ibrutinib, 21; acalabrutinib, 1]; anti-BCL2 [venetoclax], 12; phosphatidyl inositol 3-kinase inhibitor plus rituximab, 1; cyclophosphamide, 1), and 11 (15.7%) off-therapy (i.e., 8 in complete or partial remission and 3 in CLL relapse). Of note, 10 (28.5%) of 35 patients on therapy with a pathway inhibitor (PI) at the time of vaccination had been given an anti-CD20 antibody (i.e., 5 in association with ibrutinib, 4 with venetoclax, and 1 with idelalisib) (online suppl. Table 1; for all online suppl. material, see www.karger.com/doi/10.1159/000521229). Five patients were vaccinated within 12 months from the last anti-CD20 infusion (range, 1–7 months), while the remaining 5 after 12 months (range, 54–70 months).
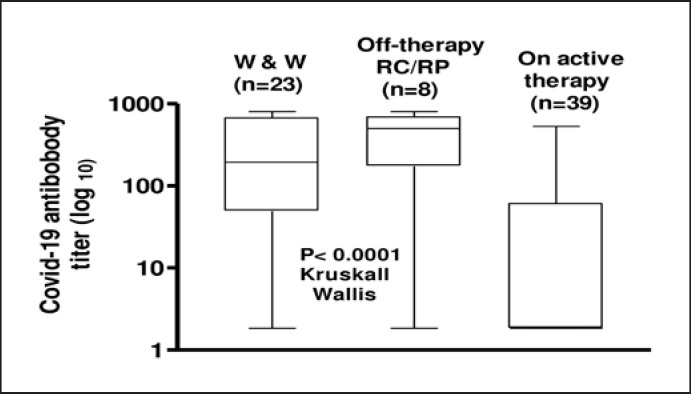
The vaccine elicited an antibody-mediated response in 41 (58.5%) of the 70 CLL patients (online suppl. Fig. 1). An inferior RR (58.5% vs. 100%, OR, 0.012 [0.0007–0.206]; p = 0.02) (online suppl. Fig. 2) and a lower SARS-CoV-2 antibody titer (median, 58 AU/mL; range, 1.8–800 vs. 284 AU/mL; range, 14–800; p < 0.0001) were observed in CLL patients in comparison with age-matched subjects with nonhematological malignancies. The RR was higher in TN (87%) or off-therapy patients with sustained clinical response (87.5%) in comparison with patients on therapy at the time of vaccination (41.7%) (p < 0.0001). Similar results were obtained when SARS-CoV-2 antibody titers of these different subgroups were compared (p = 0.02; Kruskal-Wallis test; Fig. 1).
Fig. 1.
Anti-SARS-CoV-2 antibody levels in patients with CLL according to disease status. Results are expressed as log value.
In comparison with patients treated with a PI as monotherapy, those treated with an association of anti-CD20 antibody were unlikely to respond to the SARS-CoV-2 vaccine (52% vs. 10%; OR, 0.107 [0.011–0.984]; p < 0.01). In univariate analysis, the following variables were significantly associated with serological response to SARS-CoV-2 vaccination: early Rai stage (i.e., Rai stage 0–I) (OR, 0.36 [0.13–0.97]; p = 0.04), mutated IGHV status (OR, 0.30 [0.10–0.88]; p = 0.02), lack of active therapy − which included patients TN and those off-therapy with sustained response − (OR, 0.09 [0.03–0.32]; p < 0.0001), and no anti-CD20 antibody exposure preceding vaccination (OR, 013 [0.01–1.23]; p = 0.04) (online suppl. Table 2). Of note, serum level of immunoglobulins (i.e., IgG, IgA, and IgM) did not predict for response to SARS-CoV-2 vaccine (online suppl. Table 2).
We also provide longitudinal information on T-cell subsets and NK cells at the baseline and at the time of assessment of serological response, respectively. No difference of absolute values of CD3, CD4, CD8, and CD16/CD56 cells was found when comparing SARS-CoV-2 responders and nonresponder patients at the baseline. The same analysis repeated after patients had received the second dose of vaccine confirmed a lack of significant difference between responders and nonresponders to vaccination only concerning T-cell subsets (online suppl. Table 3). Of note, in patients who experienced a serological response, an increase of the absolute NK cells (i.e., CD16/CD56 positive cells) was observed after the second dose of vaccine (p = 0.02) (online suppl. Table 4). Finally, Rai stage (OR, 0.19 [0.05–0.79]; p = 0.02) and therapy status (OR, 0.06 [0.02–0.27]; p < 0.0001) were independent predictors of response in multivariate analysis. We used these 2 variables to identify patients with a different response pattern to the vaccine. Serologic response to SARS-CoV-2 vaccination was 100% in patients with no factor (n = 21), 45% in patients with one factor (n = 38), and 36% in patients with 2 factors (n = 11) (p < 0.0001).
Overall, 8 (11.4%) and 9 (12.8%) patients reported systemic adverse events after the first and second vaccine dose, respectively. More frequently, patients reported headache (n = 5 [7.1%]), fever (n = 4 [5.7%]), and muscle pain (n = 3 [4.2%]).
Discussion
Although based on a small patient cohort, our findings indicate that patients with CLL have a suboptimal response to SARS-CoV-2 immunization, consistent with earlier studies [4, 5, 6, 7, 9]. On the other hand, the response pattern was heterogeneous, owing primarily to disease activity and treatment status. The highest immunological RR was observed in TN patients or those with sustained clinical response after therapy discontinuation. In contrast, in patients treated with a BTK or BCL2 inhibitor, the benefit of SARS-CoV-2 immunization was limited. These findings are in keeping with studies on the efficacy of adjuvanted recombinant hepatitis B and zoster vaccines [10].
While de novo immune response to hepatitis B vaccine was nearly absent in CLL patients on BTKis, recall immune response to zoster vaccine was not significantly different between CLL patients on BTKi and TN patients [10]. Furthermore, Parry et al. [7] recently reported that BTK inhibitor therapy was a strong and independent predictor of negative antibody response after the second SARS-CoV-2 vaccine dose. Collectively, these data suggest that BTK inhibitors strongly affect response to vaccines for pathogens in which pre-existing immunity is not present. In contrast, only limited data are available on the vaccine response in CLL patients treated with BCL2 inhibitors.
Our results also support prior findings showing that B-cell reconstitution is almost absent in CLL patients treated with an anti-CD20 antibody [11]. This means that SARS-CoV-2 vaccination should be done before starting anti-CD20-based therapies [12].
Possible strategies to be offered to CLL patients include an early third vaccine dose, a heterologous vaccination combination, or, maybe, a double vaccine dose at the first injection to optimize the immunological response. More realistically, a booster third dose of vaccine is currently given to CLL patients whatever the treatment status. Of note, with anti-influenza vaccination, a higher vaccine dose resulted in a greater immunological response in patients with multiple myeloma [13]. This option could be possibly explored with SARS-CoV-2 vaccine in CLL patients.
We also performed a T- and NK-cell assessment at baseline and following vaccination. Recent reports show that 2 doses of 1–50 μg of BNT162b1 can elicit robust CD4+ and CD8+ T-cell responses [14]. In our series, postvaccine absolute values of CD4 and CD8 were similar in patients who responded and patients who did not respond to mRNA SARS-CoV-2 vaccination. Compared to those reported in healthy populations, these different results might be due, at least in part, to impaired cellular immunity of CLL patients [15]. However, we have found a significant increase in the absolute number of NK cells in patients who achieved a humoral response. This observation is in keeping with recent evidence that NK cells are robustly activated in SARS-CoV-2 infection [16].
Although there are recommendations for anti-COVID vaccination in CLL [17], based on an individual case-by-case decision, our results provide a clue to select better CLL patients who are expected to achieve optimal response to COVID-19 vaccine similarly to age- and sex-matched controls. Nonetheless, until more data on clinical efficacy are available, individuals with CLL should continue to exercise extreme caution after vaccination.
Finally, while an antibody decay 6–12 months after vaccination has been demonstrated in in healthy population [1, 2], information on the decline of antibody titer in CLL is limited. In a single study, it was shown that CLL patients who respond to the vaccine can maintain their immune response [18].
In conclusion, our study provides a real-world experience on the humoral response to the SARS-CoV-2 vaccine in CLL patients. In addition, for the first time, T and NK response, following vaccination, was evaluated in CLL patients. Some limitations, including the relatively small patient sample, the short follow-up, and the absence of accurate cytofluorimetric analysis of T cells, permit only to generate hypotheses.
Statement of Ethics
Patients underwent vaccination in agreement with Italy's Strategic Plan for anti-SARS-CoV-2/COVID-19 vaccination. The study was also approved by the Comitato Etico Regione Calabria − Sezione Area Centro (Approval No. 2021/00151/88). All patients provided written informed consent.
Conflict of Interest Statement
The authors do not have anything to disclose.
Funding Sources
The authors did not receive any funding relevant for this research.
Author Contributions
S.M. initiated the trial, designed the study, analyzed data, and wrote the manuscript; P.M. and G.P. performed the serologic testing; M.L. and D.Z. collected the data; A.M. and D.L., performed cytofluorimetric studies; L.L. and V.G. followed up patients; F.T. initiated the trial and designed the study.
Data Availability Statement
All data generated or analyzed during this study are included in this article. Further enquiries can be directed to the corresponding author.
References
- 1.Thomas SJ, Moreira ED, Jr, Kitchin N, Absalon J, Gurtman A, Lockhart S, et al. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N Engl J Med. 2020;383:2603–15. doi: 10.1056/NEJMoa2034577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Polack FP, Thomas SJ, Kitchin N, Absalon J, Gurtman A, Lockhart S, et al. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N Engl J Med. 2020;383:2603–15. doi: 10.1056/NEJMoa2034577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tadmor T, Welslau M, Hus I. A review of the infection pathogenesis and prophylaxis recommendations in patients with chronic lymphocytic leukemia. Expert Rev Hematol. 2018;11((1)):57–70. doi: 10.1080/17474086.2018.1407645. [DOI] [PubMed] [Google Scholar]
- 4.Herishanu Y, Avivi I, Aharon A, Shefer G, Levi S, Bronstein Y, et al. Efficacy of the BNT162b2 mRNA COVID-19 vaccine in patients with chronic lymphocytic leukemia. Blood. 2021;137((23)):3165–73. doi: 10.1182/blood.2021011568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Benjamini O, Rokach L, Itchaki G, Braester A, Shvidel L, Goldschmidtet N, et al. Safety and efficeffacy of BNT162b mRNA Covid19 Vaccine in patients with chronic lymphocytic leukemia. Haematologica. 2021 Jul 29; doi: 10.3324/haematol.2021.279196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Roeker LE, Knorr DA, Thompson MC, Nivar M, Lebowitz S, Peters N, et al. COVID-19 vaccine efficacy in patients with chronic lymphocytic leukemia. Leukemia. 2021;35((9)):2703–5. doi: 10.1038/s41375-021-01270-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Parry H, McIlroy G, Bruton R, Ali M, Stephens C, Damery S, et al. Antibody responses after first and second Covid-19 vaccination in patients with chronic lymphocytic leukaemia. Blood Cancer J. 2021 Jul;11((7)):136. doi: 10.1038/s41408-021-00528-x. Published online 2021 Jul 30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hallek M, Cheson BD, Catovsky D, Caligaris-Cappio F, Dighiero G, Döhner H, et al. iwCLL guidelines for diagnosis, indications for treatment, response assessment, and supportive management of CLL. Blood. 2018;131((25)):2745–60. doi: 10.1182/blood-2017-09-806398. [DOI] [PubMed] [Google Scholar]
- 9.Greenberger LM, Saltzman LA, Senefeld JW, Johnson PW, DeGennaro LJ, Nichols GL. Antibody response to SARS-CoV-2 vaccines in patients with hematologic malignancies. Cancer Cell. 2021 Aug 9;39((8)):1031–3. doi: 10.1016/j.ccell.2021.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pleyer C, Ali MA, Cohen JI, Tian X, Soto S, Ahn IE, et al. Effect of Bruton tyrosine kinase inhibitor on efficacy of adjuvanted recombinant hepatitis B and zoster vaccines. Blood. 2021;137:185–9. doi: 10.1182/blood.2020008758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Eisenberg RA, Jawad AF, Boyer J, Maurer K, McDonald K, Prak ETL, et al. Rituximab-treated patients have a poor response to influenza vaccination. J Clin Immunol. 2013;33:388–96. doi: 10.1007/s10875-012-9813-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Besson C. It is time to adapt anti-CD20 administration schedule to allow efficient anti-SARS-Cov-2 vaccination in patients with lymphoid malignancies. Haematologica. 2021 Jul 29; doi: 10.3324/haematol.2021.279457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Branagan AR, Duffy E, Gan G, Li F, Foster C, Verma R, et al. Tandem high-dose influenza vaccination is associated with more durable serologic immunity in patients with plasma cell dyscrasias. Blood Adv. 2021 Mar 9;5((5)):1535–9. doi: 10.1182/bloodadvances.2020003880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sahin U, Muik A, Vogler I, Derhovanessian E, Kranz LM, Vormehr M, et al. COVID-19 vaccine BNT162b1 elicits human antibody and TH1 T cell responses. Nature. 2020;586((7830)):594–9. doi: 10.1038/s41586-020-2814-7. [DOI] [PubMed] [Google Scholar]
- 15.Forconi F, Moss P. Perturbation of the normal immune system in patients with CLL. Blood. 2015;126((5)):573–81. doi: 10.1182/blood-2015-03-567388. [DOI] [PubMed] [Google Scholar]
- 16.Björkström NK, Ponzetta A. Natural killer cells and unconventional T cells in COVID-19. Curr Opin Virol. 2021 Aug;49:176–82. doi: 10.1016/j.coviro.2021.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. https://www.hematology.org/covid-19/covid-19-and-cll .
- 18.Tadmor T, Benjamini O, Braester A, Rahav G, Rokach L. Antibody persistence 100 days following the second dose of BNT162b mRNA Covid19 vaccine in patients with chronic lymphocytic leukemia. Leukemia. 2021;35((9)):2727–30. doi: 10.1038/s41375-021-01380-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analyzed during this study are included in this article. Further enquiries can be directed to the corresponding author.