Abstract
Background
Over recent decades, epidemiological studies have shown relationships between vitamins and Helicobacter pylori (H. pylori) infection and eradication, but the results are controversial.
Methods
A comprehensive meta-analysis and systematic review were conducted to clarify the relationships between common types of vitamins and H. pylori. We applied meta-regression, subgroup analysis and sensitivity analysis to obtain available evidence. Articles published from January 1991 to June 2021 in PubMed, EMBASE, and the Cochrane Library were searched.
Results
In total, we identified 48 studies. The results indicate that H. pylori -positive patients had lower serum vitamin B12 [standardized mean difference (SMD) = −0.30; 95% confidence interval (CI): −0.53 – −0.08], folate (SMD = −0.69; 95% CI: −1.34 – −0.04), vitamin C (SMD = −0.37; 95%CI: −0.57 – −0.18) and vitamin D (SMD = −0.34; 95% CI: −0.49 – −0.18) levels than H. pylori-negative patients. Patients in which H. pylori had been successfully eradicated had higher serum vitamin D levels (SMD = 1.37; 95% CI: 0.37–2.38) than in patients in which eradication had been unsuccessful. The serum vitamin B12 levels of H. pylori-positive patients improved after successful H. pylori eradication therapy (SMD = 1.85; 95% CI: 0.81–2.90), and antioxidant vitamin supplementation to an H. pylori eradication regimen improved the eradication rate (risk ratio = 1.22; 95% CI: 1.02–1.44 for per-protocol analysis; risk ratio = 1.25; 95% CI: 1.06–1.47 for intention-to-treat analysis).
Conclusions
H. pylori infections decrease the serum levels of several types of vitamins, eradication of H. pylori could rescue its adverse effects, and antioxidant vitamin supplementation may improve the H. pylori eradication rate.
Systematic Review Registration
identifier: CRD42021268127.
Keywords: vitamins, helicobacter pylori, meta-analysis, systematic review, relationship
Introduction
Helicobacter pylori (H. pylori) is a gastric gram-negative, spiral-shaped microaerophilic pathogen (1). About half of the global population is infected with H. pylori, and the infection rate in developing countries is higher than in developed countries (2, 3). H. pylori is the main risk for chronic gastritis, gastric ulcer, gastric cancer and mucosa-associated lymphoid tissue–associated lymphoma (4–6). It can damage the gastric mucosa and affect the absorption of trace elements, especially vitamins (7). Vitamin deficiency can upset the internal balance of the human body and cause a variety of diseases outside of the digestive system (8–10).
Vitamins are members of a huge family. At present, there are dozens of known vitamins that may be divided into fat-soluble and water-soluble categories. The relationships between H. pylori infection and various vitamins have attracted attention worldwide. Some studies found that H. pylori infections reduce serum vitamin levels (11, 12); several studies have revealed that after H. pylori eradication, the serum vitamin levels increase (13, 14). Some randomized controlled trials (RCTs) found that vitamin supplementation combined with standard anti-H. pylori therapy increase the H. pylori eradication rate (15, 16). However, the results have been inconsistent.
Meta-analyses on the relationships between vitamins and H. pylori have been published (17–19). These studies involved one vitamin or a certain aspect of the relationships between vitamins and H. pylori, or the number of included studies was limited. Yang et al. (17) found vitamin D could improve the success rate of H. pylori eradication. However, they only identified three relevant studies to support this conclusion. Afsar et al. (18) reported a relationship between H. pylori infection and micronutrient (vitamin B12 and folate) levels in pregnant women. Nevertheless, the effects of H. pylori on the population excluding pregnant women were not evaluated. Li et al. (19) assessed the effects of antioxidant vitamins supplementation on the rate of H. pylori eradication. However, only three supporting studies were referenced in that work. In recent years, many excellent articles have been published. To update the results and obtain more credible conclusions, we conducted this systematic review and meta-analysis to evaluate the relationships more comprehensively, thereby providing a theoretical basis for clinical practice and public health policy-making.
Methods
Data Sources and Search Strategy
This meta-analysis was registered on PROSPERP (No. CRD42021268127) (20) and compliant with the main PRISMA statement (21). A comprehensive and systematic search was carried out for relevant studies describing relationships between vitamins and H. pylori in biologic and medical databases (Medline, Web of Science, Embase, Chinese Biomedical Database and Cambridge Scientific Abstracts databases). We developed a search strategy using following keywords: “vitamins,” “vitamin A,” “vitamin B,” “vitamin C,” “vitamin D,” “vitamin E,” “β-Carotene,” “retinol,” “cobalamin” “folate,” “folic acid,” “tocopherol,” “antioxidants,” “micronutritent,” and “Helicobacter pylori” (as shown in Supplementary Table 1). Duplicate works were collapsed into a single entry. Additionally, we scanned the reference lists of all the relevant published studies and reviews. The two blinded reviewers (Xianlei Cai and Xueying Li) selected the studies and specified the exclusion criteria.
Inclusion and Exclusion Criteria
The inclusion criteria were as follows: (1) Observational or experimental research; (2) Comparisons of serum vitamin levels between H. pylori- positive and H. pylori- negative patients; (3) Comparisons of serum vitamin levels between successful and failed H. pylori eradication patients; (4) Comparisons of serum vitamin levels before and after successful H. pylori eradication therapy; (5) Comparisons of H. pylori eradication rate between antioxidant vitamin supplementation groups and controlled groups for H. pylori – positive patients; and (6) Original studies in English or Chinese indexed up to June 2021.
The exclusion criteria were as follows: (1) Original studies did not involve the relationships between vitamins and H. pylori; (3) Studies did not provide sufficient data for a meta-analysis; (4) reviews, comments, letters, and animal studies; and (5) low-quality studies [Newcastle-Ottawa scale and Cochrane collaboration's tool were used to assess the quality and bias risk for cross-sectional, cohort and random-controlled studies as described in a previous study (22)].
Data Extraction
All the data were extracted by three researchers independently (Xianlei Cai, Xueying Li and Yangli Jin) using standardized form. The characteristics of the identified relevant works were records as follows: name of first author, year of publication, country, study design (cross-sectional, case series, cohort and RCTs), age, number of subjects, gender, type of vitamins (vitamins A, B, C, D and E) and presentation of effect magnitude [mean ± standard deviation (SD), mean ± standard error of mean (SEM), median (interquartile range), median (range), odds risk (OR), risk ratio (RR), or hazard ratio (HR) with 95% confidence interval (CI)].
Statistical Analyses
Mean serum vitamin levels and the SDs were used in pooling analyses. If the original studies provided other estimates [mean ± SEM, median (interquartile range) or median (range)], we converted the data using a common method described by Hozo et al. (23). Because of the inconsistent units used in different studies, the pooled results were expressed in terms of standardized mean difference (SMD) with 95% CI. If the RCTs and cohort studies only provided 2 × 2 table data, we calculated the responding RRs. Additionally, RRs and 95% CIs were used to show the differences in the H. pylori eradication rates between vitamin supplementation and control groups.
A meta-regression was performed to examine the sources of heterogeneity from disparate types of vitamin supplementation (vitamin C or vitamin C plus vitamin E), and we identified influence factors having positive coefficients (p ≤ 0.05). Q-test and I2 were used to assess heterogeneity. If the results showed notable heterogeneity (p ≤ 0.05 and I2 > 50%), then pooled estimates were calculated using random-effects models (DerSimonian and Laird method) (24). Otherwise, fixed-effects models were used (Mantel-Haenszel method) (25). Subgroup analyses were performed to evaluate relationships between diverse types of vitamins and H. pylori. Forest and funnel plots were drawn and publication bias was tested by a weighted Egger and Begg's tests (26, 27). Sensitivity analyses were performed by omitting one estimate at a time to assess the relative influence of each work on pooled results. If the included estimates were less than four, then we did not carry out the meta-analysis and conducted systematic review instead. All the analyses were performed using STATA version 12.0 (StataCorp LP).
Results
Study Characteristics
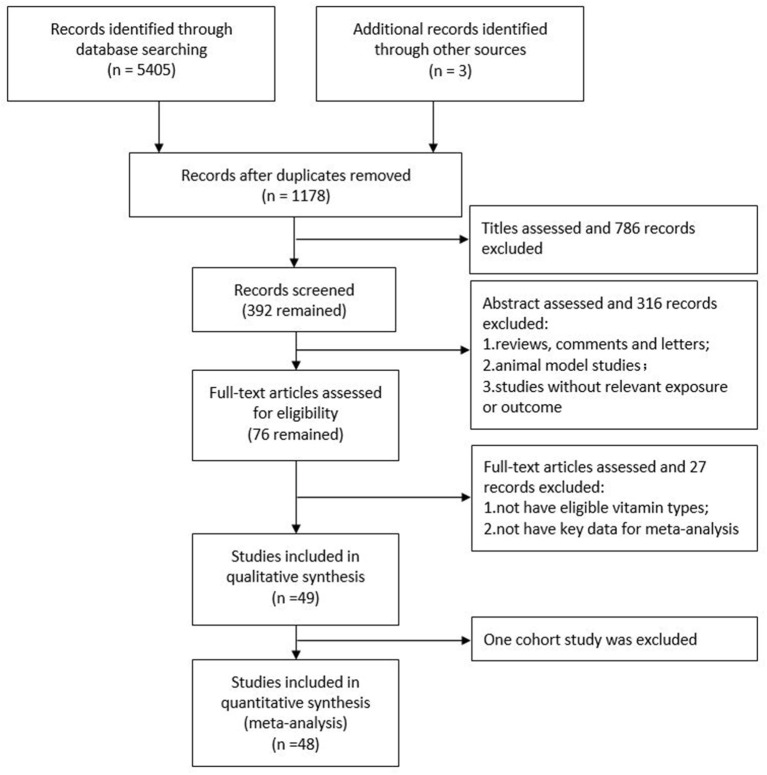
The flow-through of the study selection process is described using a modified PRISMA diagram (Figure 1). In total, forty-eight high quality studies (39 observational studies and 9 RCTs) with 73 independent estimates of relationships between vitamins and H. pylori in four fields were included in this meta-analysis and systematic review. In total, 31 studies compared serum vitamin levels between H. pylori- positive and - negative patients; 5 studies compared serum vitamin levels between successful and failed H. pylori eradication patients; 6 studies compared patient serum vitamin levels before and after successful H. pylori eradication therapy; and 10 studies focused on the effects of vitamin supplementation on the H. pylori eradication rate. Of these, three studies (28–30) considered more than one effect of H. pylori on vitamins. There were 173,013 participants from Turkey (12 studies), the United Kingdom (6 studies), China (4 studies), Italy (4 studies), Brazil (2 studies), Iran (2 studies), Israel (2 studies), Japan (2 studies), the USA (2 studies), and one each from Argentina, Australia, Egypt, Germany, Greece, India, Lebanon, Netherlands, Pakistan, Palestine, Poland, Saudi Arabia and Switzerland. Overall, Tables 1–4 present the summaries of all the studies included in the meta-analysis. The original estimates reported in the articles are summarized in Supplementary Tables 2–5.
Figure 1.
Flow diagram of the studies search process.
Table 1.
Basic characteristics of studies comparing serum vitamin levels between H. pylori + groups and H. pylori- negative groups.
| Study | Year | Area | Design | Age of HP + groups | No. of HP + groups (male/female) | Age of HP - groups | No. of HP - groups (male/female) | Quality |
|---|---|---|---|---|---|---|---|---|
| Vitamin A | ||||||||
| Phull et al. (31) | 1998 | UK | Cross-sectional | Mean 46.0 y | 25 (18/7) | Mean 54 y | 18 (7/11) | 7* |
| Zhang et al. (32) | 2000 | UK | Cross-sectional | 19–89 y | 41 (N/A) | 19-89 y | 27 (N/A) | 7* |
| Toyonaga et al. (33) | 2000 | Japan | Cross-sectional | Mean 47.0 y | 37 (13/24) | Mean 44.7 y | 40 (14/26) | 8* |
| Vitamin B 12 | ||||||||
| Tamura et al. (34) | 2002 | Japan | Cross-sectional | Mean 64 y | 57 (44/13) | Mean 63 y | 36 (25/11) | 8* |
| Cenerelli et al. (35) | 2002 | Italy | Cross-sectional | Mean 54.7 y | 31 (19/12) | Mean 50.9 y | 42 (23/19) | 8* |
| Shuval-Sudai et al. (36) | 2003 | Israel | Cross-sectional | Mean 52.8 y | 96 (N/A) | Mean 49.2 y | 37 (N/A) | 7* |
| Trimarchi et al. (37) | 2004 | Argentina | Cross-sectional | Mean 56.8 y | 8 (3/5) | Mean 62.4 y | 21 (9/12) | 7* |
| Oijen et al. (38) | 2004 | Netherlands | Cross-sectional | N/A | 29 (N/A) | N/A | 60 (N/A) | 7* |
| Sarari et al. (11) | 2008 | Palestine | Cross-sectional | Mean 43.4 y | 43 (24/19) | N/A | 17 (N/A) | 7* |
| Stettin et al. (39) | 2008 | Germany | Cross-sectional | Mean 50.8 y | 69 (27/42) | Mean 47.3 y | 21 (8/13) | 8* |
| Kakehasi et al. (40) | 2009 | Brazil | Cross-sectional | Mean 63.7 y | 34 (0/37) | Mean 62.5 y | 27 (0/27) | 8* |
| Gerig et al. (41) | 2013 | Switzerland | Cross-sectional | Mean 42.3 y | 85 (21/64) | Mean 40.9 y | 319 (85/234) | 8* |
| Ulasoglu et al. (42) | 2019 | Turkey | Cross-sectional | Mean 44.8 y | 213 (N/A) | Mean 44.8 y | 76 (N/A) | 7* |
| Surmeli et al. (43) | 2019 | Turkey | Cross-sectional | Mean 74.7 y | 43 (11/32) | Mean 78.2 y | 211 (91/120) | 9* |
| Soyocak et al. (44) | 2021 | Turkey | Cross-sectional | Mean 46.4 y | 31 (12/19) | Mean 45.2 y | 19 (8/11) | 8* |
| Folate | ||||||||
| Tamura et al. (34) | 2002 | Japan | Cross-sectional | Mean 64 y | 57 (44/13) | Mean 63 y | 36 (25/11) | 8* |
| Cenerelli et al. (35) | 2002 | Italy | Cross-sectional | Mean 54.7 y | 31 (19/12) | Mean 50.9 y | 42 (23/19) | 8* |
| Shuval-Sudai et al. (36) | 2003 | Israel | Cross-sectional | Mean 52.8 y | 96 (N/A) | Mean 49.2 y | 37 (N/A) | 7* |
| Stettin et al. (39) | 2008 | Germany | Cross-sectional | Mean 50.8 y | 69 (27/42) | Mean 47.3 y | 21 (8/13) | 8* |
| Gerig et al. (41) | 2013 | Switzerland | Cross-sectional | Mean 42.3 y | 85 (21/64) | Mean 40.9 y | 319 (85/234) | 8* |
| Ulasoglu et al. (42) | 2019 | Turkey | Cross-sectional | Mean 44.8 y | 213 (N/A) | Mean 44.8 y | 76 (N/A) | 7* |
| Surmeli et al. (43) | 2019 | Turkey | Cross-sectional | Mean 74.7 y | 43 (11/32) | Mean 78.2 y | 211 (91/120) | 9* |
| Soyocak et al. (44) | 2021 | Turkey | Cross-sectional | Mean 46.4 y | 31 (12/19) | Mean 45.2 y | 19 (8/11) | 8* |
| Vitamin C | ||||||||
| Banerjee et al. (28) | 1994 | UK | Cross-sectional | N/A | 19 (N/A) | N/A | 10 (N/A) | 7* |
| Rokka et al. (45) | 1995 | USA | Cross-sectional | Mean 43.5 y | 58 (30/28) | Mean 45.5 y | 30 (16/14) | 8* |
| Webb et al. (46) | 1997 | Australia | Cross-sectional | N/A | 666 (N/A) | N/A | 737 (N/A) | 7* |
| Phull et al. (31) | 1998 | UK | Cross-sectional | Mean 46 y | 25 (18/7) | Mean 54 y | 18 (7/11) | 7* |
| Rokkas et al. (47) | 1999 | Greece | Cross-sectional | Mean 42.0 y | 30 (17/13) | Mean 42.5 y | 10 (6/4) | 7* |
| Jarosz et al. (48) | 2000 | Poland | Cross-sectional | Mean 45.5 y/39.0 y | 21 (11/10) 32 (18/14) | Mean 37.5 y/41.5 y | 17 (10/7) 16 (9/7) | 8* |
| Toyonaga et al. (33) | 2000 | Japan | Cross-sectional | Mean 47.0 y | 37 (13/24) | Mean 44.7 y | 40 (14/26) | 8* |
| Woodward et al. (49) | 2001 | UK | Cross-sectional | 25–74 y | 765 (N/A) | 25–74 y | 403 (N/A) | 8* |
| Everett et al. (50) | 2001 | UK | Cross-sectional | Mean 51 y | 85 (47/38) | Mean 45 y | 39 (25/14) | 7* |
| Annibale et al. (29) | 2003 | Italy | Cross-sectional | Median 47 y | 30 (6/24) | Median 37 y | 13 (1/12) | 7* |
| Capurso et al. (51) | 2003 | Italy | Cross-sectional | Median 44 y | 32 (5/27) | Median 37 y | 13 (1/12) | 7* |
| Simon (52) | 2003 | USA | Cross-sectional | Mean 51 y | 2189 (1072/1117) | Mean 41 y | 4557 (2142/2415) | 9* |
| Khanzode et al. (30) | 2003 | India | Cross-sectional | Mean 45.4 y | 37 (15/22) | Mean 48.2 y | 40 (22/18) | 8* |
| Vitamin D | ||||||||
| Antico et al. (53) | 2012 | Italy | Cross-sectional | 20–80 y | 21 (N/A) | 20–80 y | 163 (N/A) | 7* |
| Gerig et al. (41) | 2013 | Switzerland | Cross-sectional | Mean 42.3 y | 85 (21/64) | Mean 40.9 y | 319 (85/234) | 8* |
| Han et al. (54) | 2019 | China | Cross-sectional | Mean 47.1 y | 496 (236/260) | Mean 48.1 y | 257 (127/300) | 8* |
| Surmeli et al. (43) | 2019 | Turkey | Cross-sectional | Mean 74.7 y | 43 (11/32) | Mean 78.2 y | 211 (91/120) | 9* |
| Assaad et al. (12) | 2019 | Lebanon | Cross-sectional | Mean 39.3 y | 225 (88/137) | Mean 41.9 y | 235 (88/137) | 8* |
| Gao et al. (55) | 2020 | China | Cross-sectional | Mean 12.1 m | 2113 (1202/911) | Mean 12.4 m | 4783 (2865/2098) | 9* |
| Shafrir et al. (56) | 2021 | Israel | Cross-sectional | Mean 41.0 | 75640 (38576/37064) | Mean 42.2 | 74843 (37421/37422) | 9* |
| Vitamin E | ||||||||
| Phull et al. (31) | 1998 | UK | Cross-sectional | Mean 46 y | 25 (18/7) | Mean 54 y | 18 (7/11) | 7* |
| Zhang et al. (32) | 2000 | UK | Cross-sectional | 19–89 y | 41 (N/A) | 19–89 y | 27 (N/A) | 7* |
| Toyonaga et al. (33) | 2000 | Japan | Cross-sectional | Mean 47.0 y | 37 (13/24) | Mean 44.7 y | 40 (14/26) | 8* |
UK, United Kingdom; N/A, not available; y, years; m, months;
The “star system” of the Newcastle–Ottawa scale.
Vitamin Levels Discrepancies Between H. pylori- Positive and - Negative Patients
The number of studies on the relationships between H. pylori and vitamin B12, folate, vitamin C and vitamin D was sufficient for a meta-analysis (Table 1, Supplementary Table 2).
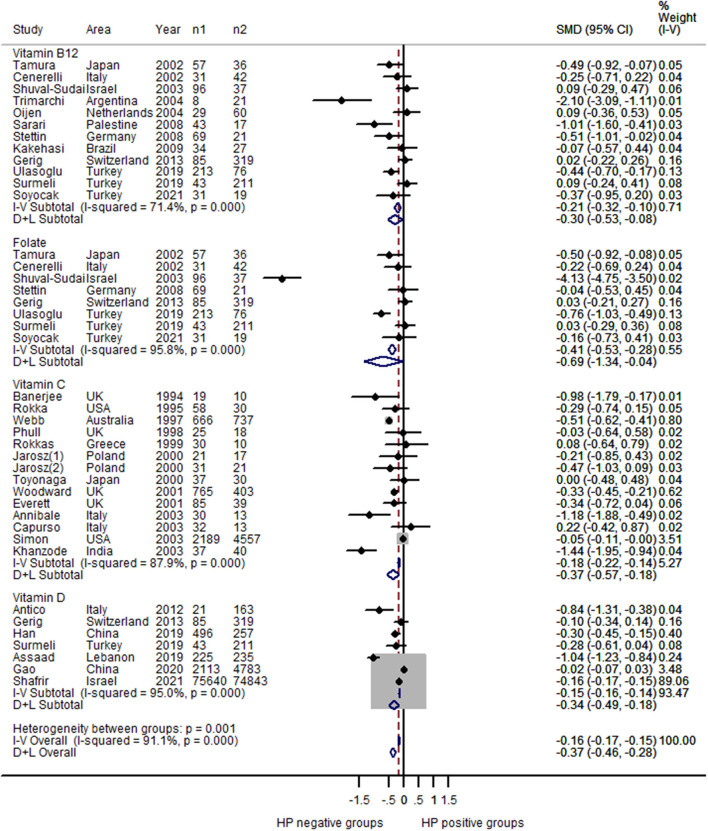
For vitamin B12, 12 estimates were included in the pooled analysis. The results indicated that H. pylori – positive patients had lower serum vitamin B12 levels than H. pylori – negative patients (SMD = −0.30; 95% CI: −0.53 – −0.08; Figure 2), with heterogeneity (P < 0.001; I2 = 71.4%) and publication bias (Begg's test zc = 2.26, P = 0.024; Egger's test t = 0.05; Figure 3A). The sensitivity analysis showed that the results were stable and reliable (Supplementary Figure 1A).
Figure 2.
Forest plot of the meta-analysis on comparison of serum vitamin levels between H. pylori - positive and H. pylori - negative patients (n1: number of H. pylori – positive patients; n2: number of H. pylori – negative patients); H. pylori – positive patients had lower serum vitamin B12 levels than H. pylori – negative patients (SMD = –0.30; 95% CI: −0.53 – −0.08; P < 0.001; I2 = 71.4%); H. pylori – positive patients had lower serum folate levels than H. pylori-negative patients (SMD = −0.69; 95% CI: −1.34 – −0.04; P < 0.001; I2 = 95.8%); H. pylori – positive patients had lower serum vitamin C levels than H. pylori-negative patients (SMD = −0.37; 95% CI: −0.57 – −0.18; P < 0.001; I2 = 87.9%); H. pylori – positive patients had lower serum vitamin D levels than H. pylori-negative patients (SMD = −0.34; 95% CI: −0.49 – −0.18; P < 0.001; I2 = 95.0%).
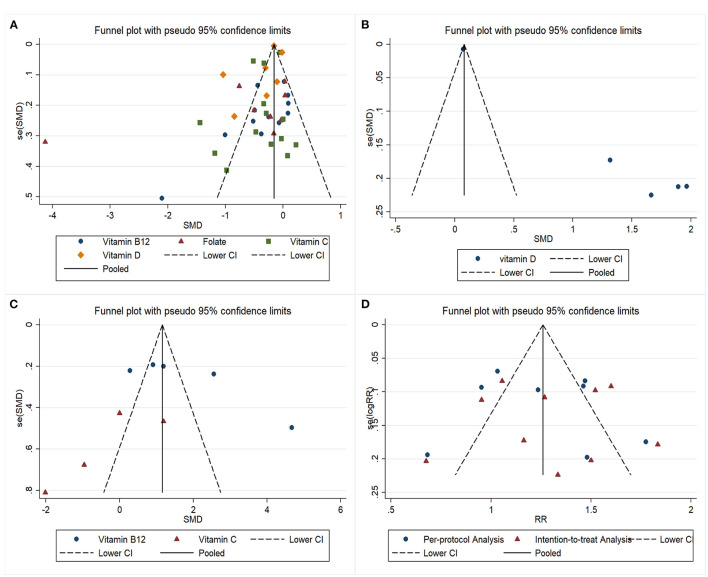
Figure 3.
Funnel plots of the meta-analysis on relationships between H. pylori and vitamins: (A) Comparison of serum vitamin levels between H. pylori - positive and H. pylori - negative patients; (B) Comparison of serum vitamin levels between H. pylori successful and failed eradication patients; (C) Comparison of serum vitamin levels before and after successful H. pylori eradication therapy; (D) Effect of vitamin supplements on the H. pylori eradication rate.
For folate, eight estimates were incorporated into the meta-analysis. Similarly, the results showed that H. pylori – positive patients had lower serum folate levels than H. pylori-negative patients (SMD = −0.69; 95% CI: −1.34 – −0.04; Figure 2), with obvious heterogeneity (P < 0.001; I2 = 95.8%). Publication bias was not found (Begg's test zc = 0.87, P = 0.386; Egger's test t = 0.254; Figure 3A). The sensitivity analysis showed that the results were influenced by some positive data (Supplementary Figure 1B).
For vitamin C, 14 estimates were incorporated into the pooled analysis. The results revealed that H. pylori – positive patients had lower serum vitamin C levels than H. pylori-negative patients (SMD = −0.37; 95% CI: −0.57 – −0.18; Figure 2), with obvious heterogeneity (P < 0.001; I2 = 87.9%). There was no publication bias (Begg's test zc = 0.44, P = 0.661; Egger's test t = 0.130; Figure 3A). The sensitivity analysis showed that the result was stable (Supplementary Figure 1C).
For vitamin D, seven estimates were included in the meta-analysis. The result also found that H. pylori – positive patients had lower serum vitamin D levels than H. pylori-negative patients (SMD = −0.34; 95% CI: −0.49 – −0.18; Figure 2), with heterogeneity (P < 0.001; I2 = 95.0%). Publication bias was not found (Begg's test zc = 0.60, P = 0.548; Egger's test t = 0.412; Figure 3A). The sensitivity analysis revealed that the results were robust (Supplementary Figure 1D).
For vitamins A and E, only three studies were identified; therefore, we did not conduct a pooled analysis. Among these three studies, Phull et al. (31) indicated that there was no relationship between H. pylori infection and serum vitamin A and E levels, and their conclusion were similar to those of Zhang et al. (32) and Toyonaga et al. (33).
Vitamin Levels Discrepancies Between Successful and Failed H. pylori Eradication Patients
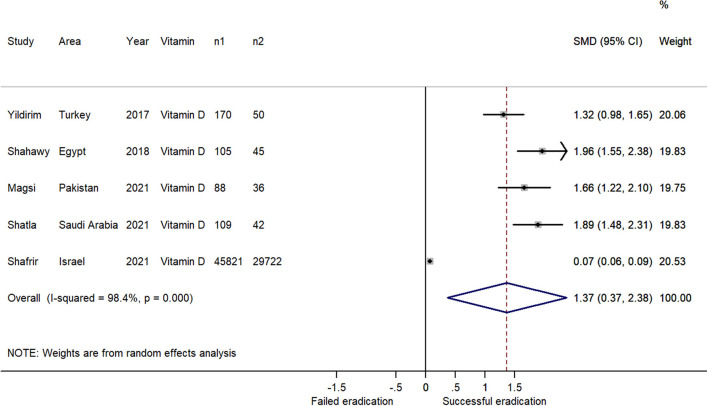
Overall, five studies focusing on vitamin D level discrepancies between successful and failed H. pylori eradication patients were included in the meta-analysis (Table 2, Supplementary Table 3). The results indicated that the patients with successful H. pylori eradication had higher serum vitamin D levels than the failed patients (SMD = 1.37; 95% CI: 0.37–2.38; Figure 4), with heterogeneity (P < 0.001; I2 = 98.4%). Because all five studies reported positive estimates, the funnel plot was asymmetric (Figure 3B). We did not assess publication bias using weighted Egger test and Begg's tests owing to the insufficient numbers of estimates. The sensitivity analysis revealed that the results were robust (Supplementary Figure 2).
Table 2.
Basic characteristics of studies comparing serum vitamin levels between the successful H. pylori eradication groups and the failed groups.
| Study | Year | Area | Design | Age of successful groups | No. of successful groups (male/female) | Age of failed groups | No. of failed groups (male/female) | Quality |
|---|---|---|---|---|---|---|---|---|
| Vitamin D | ||||||||
| Yildirim et al. (57) | 2017 | Turkey | Cross-sectional | N/A | 170 (N/A) | N/A | 50 (N/A) | 7* |
| Shahawy et al. (58) | 2018 | Egypt | Cross-sectional | 18–80 y | 105 (N/A) | 18–80 y | 45 N/A) | 7* |
| Magsi et al. (2) | 2021 | Pakistan | Cross-sectional | 18–60 y | 88 (42/46) | 18–60 y | 36 (18/18) | 8* |
| Shatla et al. (59) | 2021 | Saudi Arabia | Cross-sectional | N/A | 109 (N/A) | N/A | 42 (N/A) | 7* |
| Shafrir et al. (56) | 2021 | Israel | Cross-sectional | N/A | 45821 (N/A) | N/A | 29722 (N/A) | 9* |
N/A, not available; y, years;
The “star system” of the Newcastle–Ottawa scale.
Figure 4.
Forest plot of the meta-analysis on the comparison of serum vitamin levels between H. pylori successful and failed eradication patients (n1: number of successful eradication patients; n2: number of failed eradication patients); The patients with successful H. pylori eradication had higher serum vitamin D levels than the failed patients (SMD = 1.37; 95% CI: 0.37 – 2.38; P < 0.001; I2 = 98.4%).
There were no studies exploring other vitamin levels (vitamin A, B, C and E) discrepancies between successful and failed H. pylori eradication patients. More relevant research is recommended to clarify the relationships.
Vitamin Level Discrepancies Before and After Successful H. pylori Eradication Therapy
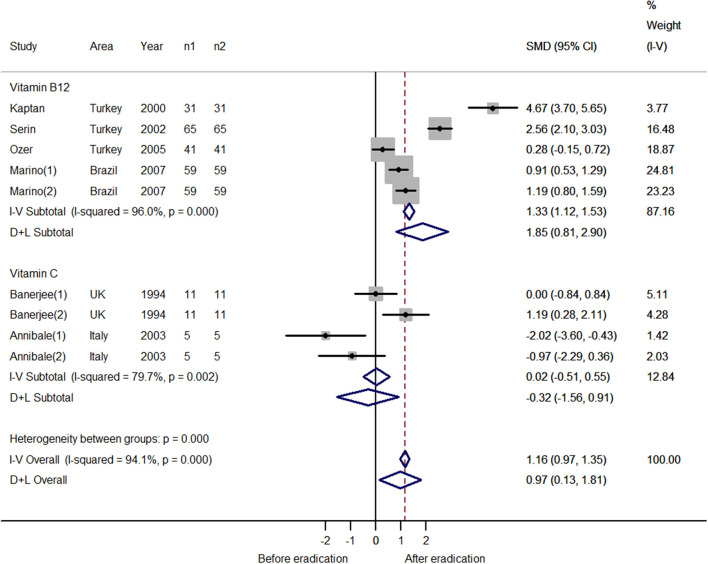
The numbers of studies for vitamins B12 and C were sufficient for a meta-analysis (Table 3, Supplementary Table 4). For vitamin B12, five estimates were incorporated into the pooled analysis. The result indicated that after successful H. pylori eradication, serum vitamin B12 increased (SMD = 1.85; 95% CI: 0.81–2.90; Figure 5), with heterogeneity (P < 0.001; I2 = 96.0%). The funnel plot was symmetric (Figure 3C). The sensitivity analysis showed that the results were stable and reliable (Supplementary Figure 3). For vitamin C, four estimates were included in the meta-analysis. The results showed that H. pylori eradication did not increase the serum vitamin C level (SMD = −0.32; 95% CI: −1.56–0.91; Figure 5), with heterogeneity (P = 0.002; I2 = 79.7%). The funnel plot was symmetric (Figure 3C). Because the four estimates were extracted from two studies, we did not conduct a sensitivity analysis and the results should be interpreted cautiously.
Table 3.
Basic characteristics of studies comparing serum vitamin levels before and after H. pylori eradication therapy.
| Study | Year | Area | Design | Age | No. before eradication (male/female) | No. after eradication (male/female) | Test time after eradication | Quality |
|---|---|---|---|---|---|---|---|---|
| Vitamin B 12 | ||||||||
| Kaptan et al. (13) | 2000 | Turkey | Case series | Mean 59.5 y | 31 (19/12) | 31 (19/12) | 3 or 6 m | 8* |
| Serin et al. (60) | 2002 | Turkey | Case series | Mean 43 y | 65 (N/A) | 65 (N/A) | 2–3 m | 7* |
| Ozer et al. (61) | 2005 | Turkey | Case series | Mean 41 y | 41 (N/A) | 41 (N/A) | 1 m | 7* |
| Marino et al. (14) | 2007 | Brazil | Case series | Mean 72.8 y | 59 (N/A) | 59 (N/A) | 6 m / 12 m | 7* |
| Folate | ||||||||
| Kaptan et al. (13) | 2000 | Turkey | Case series | Mean 59.5 y | 31 (19/12) | 31 (19/12) | 3 or 6 m | 8* |
| Ozer et al. (61) | 2005 | Turkey | Case series | Mean 41 y | 41 (N/A) | 41 (N/A) | 1 m | 7* |
| Vitamin C | ||||||||
| Banerjee et al. (28) | 1994 | UK | Case series | N/A | 11 (N/A) | 11 (N/A) | 1 m / 6 m | 7* |
| Annibale et al. (29) | 2003 | Italy | Case series | Median 47 y | 5 (N/A) | 5 (N/A) | 6 m | 7* |
N/A, not available; y, years; m, months;
The “star system” of the Newcastle–Ottawa scale.
Figure 5.
Forest plot of the meta-analysis on the comparison of serum vitamin levels before and after successful H. pylori eradication therapy (n1: number of patients after eradication therapy; n2: number of patients before eradication therapy); After successful H. pylori eradication, serum vitamin B12 increased (SMD = 1.85; 95% CI: 0.81–2.90; P < 0.001; I2 = 96.0%); H. pylori eradication did not increase the serum vitamin C level (SMD = −0.32; 95% CI: −1.56–0.91; P = 0.002; I2 = 79.7%).
For folate, we only identified two relevant studies; therefore, a pooled analysis was not performed. Both Kaptan et al. (13) et al. and Ozer et al. (61) concluded that successful H. pylori eradication was not associated with an increased serum folate level.
The Effects of Antioxidant Vitamin Supplementation on H. pylori Eradication
A total of 10 trials on the effects of antioxidant vitamin supplementation on H. pylori eradication were identified (Table 4 and Supplementary Table 4). Among them, nine studies were RCTs, and one study was cohort research. The risks of bias were summarized in Supplementary Table 6. To avoid heterogeneity derived from different study designs, we did not include the cohort research (69) in the meta-analysis. We calculated the pooled results of the per-protocol analysis and intention-to-treat analysis from RCTs.
Table 4.
Basic characteristics of studies focusing on the effect of vitamin supplementation on H. pylori eradication rate.
| Study | Year | Area | Design | Age of group 1 | No. of group 1 (male/female) | Age of group 2 | No. of group 2 (male/female) | Type of vitamin supplementation | Method of HP eradication |
|---|---|---|---|---|---|---|---|---|---|
| Chuang et al. (62) | 2002 | China | RCT | Mean 37.9 y | 55 (21/34) | Mean 35.6 y | 49 (19/30) | Vitamin C plus Vitamin E | Triple therapy |
| Everett et al. (63) | 2002 | UK | RCT | Mean 52 y | 29 (17/12) | Mean 49 y | 30 (16/14) | Vitamin C plus Vitamin E | Triple therapy |
| Sezikli et al. (15) | 2009 | Turkey | RCT | Mean 43 y | 80 (24/56) | Mean 44 y | 80 (28/52) | Vitamin C plus Vitamin E | Quadruple therapy |
| Sezikli et al. (64) | 2011 | Turkey | RCT | Mean 42 y | 80 (25/55) | Mean 43 y | 40 (15/25) | Vitamin C plus Vitamin E | Triple therapy |
| Sezikli et al. (65) | 2012 | Turkey | RCT | Mean 39.7 | 160 (53/107) | Mean 42.7 | 40 (13/27) | Vitamin C plus Vitamin E | Triple therapy |
| Demirci et al. (66) | 2015 | Turkey | RCT | Mean 40 y | 100 (59/41) | Mean 41 y | 100 (53/47) | Vitamin C plus Vitamin E | Triple therapy / Quadruple therapy |
| Kockar et al. (67) | 2001 | Turkey | RCT | Mean 40.0 y | 30 (N/A) | Mean 38.9 y | 30 (N/A) | Vitamin C / Vitamin A |
Triple therapy |
| Chuang et al. (68) | 2007 | China | RCT | Mean 53.2 y | 61 (N/A) | Mean 49.9 y | 55 (N/A) | Vitamin C | Triple therapy |
| Zojaji et al. (16) | 2009 | Iran | RCT | Mean 43 y | 150 (N/A) | Mean 45 y | 162 (N/A) | Vitamin C | Triple therapy |
| Kaboli et al. (69) | 2009 | Iran | Cohort | N/A | 114 (N/A) | N/A | 100 (N/A) | Vitamin C | Triple therapy |
Group 1: antibiotic + vitamin groups.
Group 2: antibiotic groups.
RCT, random controlled trial; N/A, not available; y, years; UK, United Kingdom.
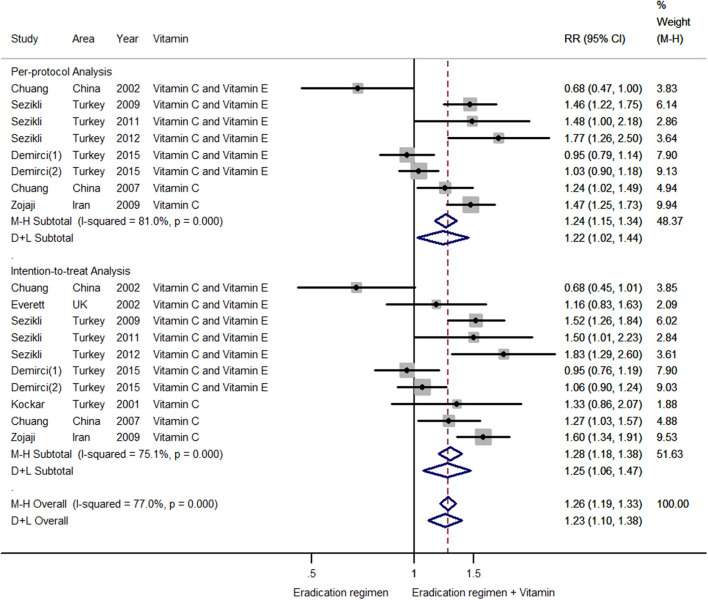
For the per-protocol analysis, eight estimates were included. A meta-regression was performed to assess potential sources of heterogeneity from eradication therapy (triple or quadruple therapy) and types of vitamin supplementation (vitamin C or vitamin C plus vitamin E). We found that eradication therapy and types of vitamin supplementation were not influencing factors (P = 0.441 for eradication therapy; P = 0.707 for types of vitamin supplementation). Therefore, all eight estimates were incorporated into the meta-analysis. The results indicated that combining antioxidant vitamin supplementation with standard therapy could increase the H. pylori eradication rate (RR = 1.22; 95% CI: 1.02–1.44; Figure 6), with heterogeneity (P < 0.001; I2 = 81.0%). There was no publication bias (Begg's test zc = 0.12, P = 0.902; Egger's test t = 0.662), and the funnel plot was symmetric (Figure 3D). The sensitivity analysis revealed that the results were robust (Supplementary Figure 4).
Figure 6.
Forest plot of the meta-analysis on the effects of vitamin supplements on H. pylori eradication rate (n1: number of eradication regimen plus vitamin supplement group; n2: number of eradication regimen group); For the per-protocol analysis, combining antioxidant vitamin supplementation with the standard therapy increased the H. pylori eradication rate (RR = 1.22; 95% CI: 1.02–1.44; P < 0.001; I2 = 81.0%); For the intention-to-treat analysis, antioxidant vitamins supplementation increased the H. pylori eradication rate (RR = 1.25; 95%CI: 1.06–1.47; P < 0.001; I2 = 75.1%).
For the intention-to-treat analysis, 10 estimates were included. A meta-regression was conducted, and the result revealed that eradication therapy and types of vitamin supplementation were not influencing factors (P = 0.832 for eradication therapy; P = 0.510 for types of vitamin supplementation). The results of the pooled analysis showed that antioxidant vitamins supplementation increased the H. pylori eradication rate (RR = 1.25; 95%CI: 1.06–1.47; Figure 6), with heterogeneity (P < 0.001; I2 = 75.1%). Publication bias was not found (Begg's test zc = 0.18, P = 0.858; Egger's test t = 0.973; Figure 3D). The sensitivity analysis showed that the results were stable.
Only the Kockar et al. study (67) explored the effects of vitamin A supplementation on H. pylori eradication. They determined that vitamin A was ineffective in H. pylori eradication. More relevant RCTs are recommended to clear the relationship.
Discussion
The debate over the H. pylori-vitamin association has been persistent. The controversy mainly focuses on the following four aspects: (1) vitamin level discrepancies between H. pylori-positive and -negative patients; (2) vitamin level discrepancies between successful and failed H. pylori eradication patients; (3) vitamin level discrepancies before and after successful H. pylori eradication therapy; and (4) the effects of vitamin supplementation on H. pylori eradication. Phull et al. (31), Zhang et al. (32) and Toyonaga et al. (33) found that H. pylori infection was not associated with serum vitamin A and E levels. The vitamin B12 results from Trimarchi et al. (37), Sarari et al. (11) and Ulasoglu et al. (42) indicated that H. pylori had an adverse effect on serum vitamin B12 levels, whereas other observational studies (35, 36, 40, 44) presented a null association between H. pylori and vitamin B12. The findings of Tamura et al. (34), Shuval-Sudai et al. (36) and Ulasoglu et al. (42) showed that H. pylori-negative patients had higher serum folate levels than H. pylori-positive patients; however, some studies (35, 39, 41, 43, 44) did not present similar results. A similar controversy exists regarding the associations between serum vitamin C or D levels and H. pylori. The effects of H. pylori eradication on serum vitamin levels have aroused extensive interest. Several studies (2, 57–59) found that patients that underwent successful H. pylori eradication had higher serum vitamin D levels than patients with in which eradication failed. Several studies (13, 14, 60, 61) described vitamin B12 level discrepancies before and after successful H. pylori eradication therapy; nevertheless, there were also inconsistent results. Vitamin supplementation was hypothesized to aid in H. pylori eradication. Some high-quality RCTs were performed to assess the assisting role of antioxidant vitamin supplementation on H. pylori eradication. Sezikli et al. (65) found that supplementation with vitamin C plus vitamin E increases the H. pylori eradication rate; whereas Chuang et al. (68) and Zojaji et al. (16) indicated that simple vitamin C supplementation increases the H. pylori eradication rate. In contrast, studies from other researchers (66, 67) showed that vitamin supplementation has no effects on the H. pylori eradication rate. Several meta-analyses had also been published. Yang et al. (17) found that 25-hydroxyvitamin D levels in H. pylori - negative patients were higher than in H. pylori – positive patients, and patients with vitamin D deficiency had lower eradication rates of H. pylori. Lahner et al. (70) reported a comprehensive meta-analysis in 2012 focused on the relationships between micronutrients and H. pylori. This study found that H. pylori was associated with ascorbic acid levels in gastric juice, which were increased by the eradication treatment. At present, many relevant articles have been published, and we have the opportunity to update the results and obtain reliable conclusions.
The results of the meta-analysis suggested negative effects of H. pylori on serum vitamin B12, folate, vitamin C and vitamin D levels. Helicobacter pylori-induced atrophic gastritis impairs stomach acidification and secretion functions and causes the malabsorption of nutrients. Vitamin B12 plays indispensable roles in promoting the development and maturation of red blood cells and in maintaining normal hematopoietic functions (71). A deficiency in an intrinsic factor caused by H. pylori infection would aggravate vitamin B12 absorption-related disorders. Folate participates in the metabolism of genetic materials and proteins, and it affects mammal reproduction (72). A folate deficiency may cause neural tube malformation, megaloblastic anemia, depression and malignant tumor formation (71, 73). A vitamin B12 deficiency may cause a folate metabolic disorder and aggravate the folate deficiency. Vitamin C and E, as vitamins with antioxidant effects, play roles in eradicating oxygen radicals and in maintaining the body's steady state (74, 75). Vitamin C may also promote the formation of tetrahydrofolic acid (75, 76). A shortage of vitamin C aggravates a folate deficiency. Therefore, the effects of H. pylori infections on human vitamin levels are not independent, and there are interactions among the various vitamins.
Our study found that patients that had undergone successful H. pylori eradication had higher serum vitamin D levels than patients in which H. pylori eradication failed. We speculated that vitamin D is a protective factor for H. pylori. In terms of mechanism, a combination of vitamin D and the vitamin D receptor may activate immune responses and participate in the anti-H. pylori process (77, 78). Yang et al. (17) also performed a related meta-analysis, but they only included three papers; consequently, the limited number of studies was not suitable for a pooled analysis. Our work included five relevant studies, including the study of Shafrir et al. (56) that covered data from more than 70,000 patients, which improved the reliability of the results. Apart from those on vitamin D, we did not find any studies that explored the relationships between other vitamins and the success of H. pylori eradication. Further research is needed to investigate the differences in other vitamin levels between patients with successful H. pylori eradication compared with those that failed.
This work revealed that serum vitamin B12 levels in patients after H. pylori eradication were significantly higher than those before H. pylori eradication. We reaffirmed the adverse effects of H. pylori on vitamin B12. For patients with a vitamin B12 deficiency, aggressive H. pylori eradication therapy and additional vitamin B12 supplementation are necessary. For vitamin C, this meta-analysis did not produce a statistically significant result. However, the four estimates included in this study were from only two reports. Thus, there were too few relevant studies included, and the results should be interpreted cautiously.
The pooled results of the RCTs showed that both the per-protocol analysis and intention-to-treat analysis suggested that antioxidant vitamin supplementation could improve the eradication rates of H. pylori of standard regimens. In the selection of standard eradication regimens, Kockar et al. (67), Chuang et al. (68) and Zojaji et al. (16) chose vitamin C for additional supplementation, whereas other studies used vitamin C combined with vitamin E for supplementation. Although both vitamin C and vitamin E have antioxidant effects, we were still concerned that inconsistent supplementation selection would introduce bias. Moreover, except the studies of Sezikli et al. (15) and Demirci et al. (66), which adopted the quadruple regimen, other studies adopted the triple regimen. The choices of eradication therapy (triple or quadruple therapy) also could introduce bias. We used a meta-regression to eliminate the possible bias caused by different kinds of vitamin supplementation and different eradication schemes from the statistical field, and then, we conducted pooled analyses to improve the reliability of the results. The meta-analysis of Li et al. (19) found that supplementation with antioxidant vitamins did not benefit the eradication rate. However, this analysis included only three studies. Although Ochoa et al. (79) also conducted a meta-analysis on a similar topic in 2018, there were errors and shortcomings in the data extraction and chart construction. Moreover, the above two articles only performed intention-to-treat analyses of the data. We believed that the per-protocol analysis should not be ignored in the RCTs. The meta-analysis of Yang-Ou et al. (80) focused on the effects of antioxidants on H. pylori eradication. However, this study combined antioxidant vitamins with other antioxidant (curcumin and cranberry). Our study incorporated intention-to-treat and per-protocol analyses and updated the results. Interestingly, the cohort study by Kaboli et al. (69) found that vitamin C supplementation reduces the dosage of clarithromycin. Li et al. (81) revealed that the H. pylori treatment and vitamin supplementation reduced the incidence of gastric cancer.
A complete and comprehensive search is necessary to find all the published data relevant to the meta-analysis. In addition to the usual English databases (Medline, Web of Science and Embase), we also searched for Chinese studies using the Chinese Biomedical Database and for conference papers using the Cambridge Scientific Abstracts databases to identify suitable studies. Although the search results did not ultimately expand the number of relevant articles, the processes remained essential. Unfortunately, it was hard for us to search manuscript with other languages, which might have resulted in the exclusion of some suitable articles. At last, we identified 48 relevant articles in this meta-analysis. We ran a better selection of studies and the number of included articles was larger than previous meta-analysis.
The heterogeneity of the selected studies cannot be ignored. The different methods of vitamin detection may result in variations in the results. The different areas, different numbers of patients enrolled in each study, some positive data and large amounts of included estimates may have increased the heterogeneity. Because the number of included studies was limited, it was hard for us to conduct subgroup analyses to reduce the influence of heterogeneity. Nevertheless, we performed meta-regression and sensitivity analyses to reduce the effects of heterogeneity on the credibility of the conclusions. The results of the meta-regression helped us eliminate the possibility that heterogeneity was a result of different eradication methods or vitamin supplementations. The sensitivity analyses helped us eliminate the influence of some positive data on the conclusions. In addition to the meta-regression and sensitivity analyses, we used random-effects models to establish relationships among the variables with high heterogeneity. Therefore, this work is appropriate to provide evidence, but the conclusions should be interpreted cautiously.
The study provides a comprehensive analysis of the interrelations between H. pylori and the most common vitamins from four aspects. Nevertheless, this work has some shortcomings. First, Turkish scholars have done a great deal of work on the effects of vitamin supplementation on H. pylori eradication. However, the contributions of researchers from other countries are limited. Different races and dietary habits may influence the results. It is difficult to conduct subgroup analyses to address this issue. Second, quadruple anti-H. pylori therapy is recommended currently. Some studies included in the work still used the triple approach. Moreover, the dose of vitamin supplementation is also controversial. Thus, the results should be interpreted cautiously. More RCTs with large samples focusing on the effects of vitamin supplementation on H. pylori eradication are needed to confirm our results and explore the appropriate dose.
Conclusions
In summary, this meta-analysis demonstrates that H. pylori infections can reduce the serum levels of several vitamins. The eradication of H. pylori rescues its adverse effects. Antioxidant vitamin supplementation may increase the rate of H. pylori eradication. Aggressive H. pylori eradication therapy is necessary, and the advantages of multivitamin supplementation for H. pylori-positive patients outweigh the disadvantages.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding authors.
Author Contributions
XC, WY, and XiL conceived and designed the study. XC and XuL acquired and analyzed the data. XC, YJ, MZ, YX, YW, and CL interpreted the data and drafted the manuscript. XiL and WY reviewed and corrected the manuscript. All authors approved the final version to be published.
Funding
This work was supported by the Soft Science Key Project of the Science and Technology Department of Zhejiang Province (2019C25009 and 2022C25040), the Key Project of Social Science Planning in Hangzhou City (hzjz20180110), the Soft Science Key Project of Hangzhou Municipal Science Committee (20160834M03), the Ningbo Clinical Medicine Research Center Project (2019A21003), Zhejiang Provincial Natural Science Foundation (LQ21H160013), and Zhejiang Provincial Medical and Health Science and Technology Project (2022493920). This study also received funding from Chiatai Qingchunbao Pharmaceutical Co. Ltd. The funder was not involved in the study design, collection, analysis, interpretation of data, the writing of this article or the decision to submit it for publication.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnut.2021.781333/full#supplementary-material
References
- 1.Hu Y, Zhu Y, Lu NH. Recent progress in Helicobacter pylori treatment. Chin Med J (Engl). (2020) 133:335–43. 10.1097/CM9.0000000000000618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Magsi I, Hussain Keerio S, Kumar C, Talpur AS, Shahzeen F, Mushtaq Abbasi Z, et al. Response of helicobacter pylori eradication treatment in patients with normal and below-normal serum vitamin D levels. Cureus. (2021) 13:e14777. 10.7759/cureus.14777 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bener A, Uduman SA, Ameen A, Alwash R, Pasha MA, Usmani MA, et al. Prevalence of Helicobacter pylori infection among low socio-economic workers. J Commun Dis. (2002) 34:179–84. [PubMed] [Google Scholar]
- 4.Xue Y, Zhou LY. Lu HP, Liu JZ. Recurrence of Helicobacter pylori infection: incidence and influential factors. Chin Med J (Engl). (2019) 132:765–71. 10.1097/CM9.0000000000000146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang L, Lin XC, Li HL, Yang XS, Zhang L, Li X, et al. Clinical significance and influencing factors of linked color imaging technique in real-time diagnosis of active Helicobacter pylori infection. Chin Med J (Engl). (2019) 132:2395–401. 10.1097/CM9.0000000000000486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kpoghomou MA, Wang J, Wang T, Jin G. Association of Helicobacter pylori babA2 gene and gastric cancer risk: a meta-analysis. BMC Cancer. (2020) 20:465. 10.1186/s12885-020-06962-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Franceschi F, Annalisa T, Teresa DR, Giovanna D, Ianiro G, Franco S et al. Role of Helicobacter pylori infection on nutrition and metabolism. World J Gastroenterol. (2014) 20:12809–17. 10.3748/wjg.v20.i36.12809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhang YY, Qiu HB, Tian JW. Association between vitamin D and hyperuricemia among adults in the United States. Front Nutr. (2020) 7:592777. 10.3389/fnut.2020.592777 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhang FF, Barr SI, McNulty H, Li D, Blumberg JB. Health effects of vitamin and mineral supplements. BMJ. (2020) 369:m2511. 10.1136/bmj.m2511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.de la Guía-Galipienso F, Martínez-Ferran M, Vallecillo N, Lavie CJ, Sanchis-Gomar F, Pareja-Galeano H. Vitamin D and cardiovascular health. Clin Nutr. (2021) 40:2946–57. 10.1016/j.clnu.2020.12.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sarari AS, Farraj MA, Hamoudi W, Essawi TA. Helicobacter pylori, a causative agent of vitamin B12 deficiency. J Infect Dev Ctries. (2008) 2:346–49. 10.3855/jidc.194 [DOI] [PubMed] [Google Scholar]
- 12.Assaad S, Costanian C, Jaffal L, Tannous F, Stathopoulou MG, El Shamieh S. Association of TLR4 Polymorphisms, Expression, and Vitamin D with Helicobacter pylori Infection. J Pers Med. (2019) 9:2. 10.3390/jpm9010002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kaptan K, Beyan C, Ural AU, Cetin T, Avcu F, Gülşen M, et al. Helicobacter pylori–is it a novel causative agent in Vitamin B12 deficiency? Arch Intern Med. (2000) 160:1349–53. 10.1001/archinte.160.9.1349 [DOI] [PubMed] [Google Scholar]
- 14.Marino MCA, de Oliveira CA, Rocha AMC, Rocha GA, Clementino NCD, Antunes LF, et al. Long-term effect of Helicobacter pylori eradication on plasma homocysteine in elderly patients with cobalamin deficiency. Gut. (2007) 56:469–74. 10.1136/gut.2006.095125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sezikli M, Cetinkaya ZA, Sezikli H, Güzelbulut F, Tiftikçi A, Ince AT, et al. Oxidative stress in Helicobacter pylori infection: does supplementation with vitamins C and E increase the eradication rate? Helicobacter. (2009) 14:280–85. 10.1111/j.1523-5378.2009.00686.x [DOI] [PubMed] [Google Scholar]
- 16.Zojaji H, Talaie R, Mirsattari D, Haghazali M, Molaei M, Mohsenian N, et al. The efficacy of Helicobacter pylori eradication regimen with and without vitamin C supplementation. Dig Liver Dis. (2009) 41:644–47. 10.1016/j.dld.2008.09.008 [DOI] [PubMed] [Google Scholar]
- 17.Yang L, He X, Li L, Lu C. Effect of vitamin D on Helicobacter pylori infection and eradication: A meta-analysis. Helicobacter. (2019) 24:e12655. 10.1111/hel.12655 [DOI] [PubMed] [Google Scholar]
- 18.Afsar MNA, Jhinu ZN, Bhuiyan MAI, Islam ZSiddiqua TJ. Infection and micronutrient deficiency in pregnant women: a systematic review and meta-analysis. BMJ Open Gastroenterol. (2020) 7:e000490. 10.1136/bmjgast-2020-000490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li G, Li L, Yu C, Chen L. Effect of vitamins C and E supplementation on Helicobacter pylori eradication: a meta-analysis. Br J Nutr. (2011) 106:1632–37. 10.1017/S0007114511003813 [DOI] [PubMed] [Google Scholar]
- 20.Booth A, Clarke M, Dooley G, Ghersi D, Moher D, Petticrew M, et al. The nuts and bolts of PROSPERO: an international prospective register of systematic reviews. Syst Rev. (2012) 1:2. 10.1186/2046-4053-1-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. (2009) 6:e1000097. 10.1371/journal.pmed.1000097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cai XL, Li XY, Liang C, Xu Y, Zhang MZ, Yu WM, et al. Endoscopic or laparoscopic resection for small gastrointestinal stromal tumors: a cumulative meta-analysis. Chin Med J (Engl). (2020) 133:2731–42. 10.1097/CM9.0000000000001069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hozo SP, Djulbegovic BHozo I. Estimating the mean and variance from the median, range, and the size of a sample. BMC Med Res Methodol. (2005) 5:13. 10.1186/1471-2288-5-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. (1986) 7:177–88. 10.1016/0197-2456(86)90046-2 [DOI] [PubMed] [Google Scholar]
- 25.Mantel N, Haenszel W. Statistical aspects of the analysis of data from retrospective studies of disease. J Natl Cancer Inst. (1959) 22:719–48. [PubMed] [Google Scholar]
- 26.Egger M, Smith GD. Bias in location and selection of studies. BMJ. (1998) 316:61–6. 10.1136/bmj.316.7124.61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. (1994) 50:1088–101. 10.2307/2533446 [DOI] [PubMed] [Google Scholar]
- 28.Banerjee S, Hawksby C, Miller S, Dahill S, Beattie ADMcColl KE. Effect of Helicobacter pylori and its eradication on gastric juice ascorbic acid. Gut. (1994) 35:317–22. 10.1136/gut.35.3.317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Annibale B, Capurso G, Lahner E, Passi S, Ricci R, Maggio F, et al. Concomitant alterations in intragastric pH and ascorbic acid concentration in patients with Helicobacter pylori gastritis and associated iron deficiency anaemia. Gut. (2003) 52:496–501. 10.1136/gut.52.4.496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Khanzode SS, Khanzode SD, Dakhale GN. Serum and plasma concentration of oxidant and antioxidants in patients of Helicobacter pylori gastritis and its correlation with gastric cancer. Cancer Lett. (2003) 195:27–31. 10.1016/S0304-3835(03)00147-2 [DOI] [PubMed] [Google Scholar]
- 31.Phull PS, Price AB, Thorniley MS, Green CJ, Jacyna MR. Plasma free radical activity and antioxidant vitamin levels in dyspeptic patients: correlation with smoking and Helicobacter pylori infection. Eur J Gastroenterol Hepatol. (1998) 10:573–78. 10.1097/00042737-199807000-00009 [DOI] [PubMed] [Google Scholar]
- 32.Zhang ZW, Patchett SE, Perrett D, Domizio P, Farthing MJ. Gastric alpha-tocopherol and beta-carotene concentrations in association with Helicobacter pylori infection. Eur J Gastroenterol Hepatol. (2000) 12:497–503. 10.1097/00042737-200012050-00004 [DOI] [PubMed] [Google Scholar]
- 33.Toyonaga A, Okamatsu H, Sasaki K, Kimura H, Saito T, Shimizu S, et al. Epidemiological study on food intake and Helicobacter pylori infection. Kurume Med J. (2000) 47:25–30. 10.2739/kurumemedj.47.25 [DOI] [PubMed] [Google Scholar]
- 34.Tamura A, Fujioka T, Nasu M. Relation of Helicobacter pylori infection to plasma vitamin B12, folic acid, and homocysteine levels in patients who underwent diagnostic coronary arteriography. Am J Gastroenterol. (2002) 97:861–66. 10.1111/j.1572-0241.2002.05601.x [DOI] [PubMed] [Google Scholar]
- 35.Cenerelli S, Bonazzi P, Galeazzi R, Testa I, Bonfigli AR, Sirolla C, et al. Helicobacter pylori masks differences in homocysteine plasma levels between controls and type 2 diabetic patients. Eur J Clin Invest. (2002) 32:158–62. 10.1046/j.1365-2362.2002.00962.x [DOI] [PubMed] [Google Scholar]
- 36.Shuval-Sudai O, Granot E. An association between Helicobacter pylori infection and serum vitamin B12 levels in healthy adults. J Clin Gastroenterol. (2003) 36:130–33. 10.1097/00004836-200302000-00008 [DOI] [PubMed] [Google Scholar]
- 37.Trimarchi H, Forrester M, Schropp J, Pereyra H, Freixas EA. Low initial vitamin B12 levels in Helicobacter pylori–positive patients on chronic hemodialysis. Nephron Clin Pract. (2004) 96:c28–32. 10.1159/000075569 [DOI] [PubMed] [Google Scholar]
- 38.van Oijen MGH, Laheij RJF, de Jong CAJ, Peters WHM, Jansen JBMJ. Vitamin B12 status and its association with Helicobacter pylori infection in alcohol dependent patients. J Nutr Sci Vitaminol (Tokyo). (2004) 50:305–08. 10.3177/jnsv.50.305 [DOI] [PubMed] [Google Scholar]
- 39.Stettin D, Waldmann A, Ströhle A, Hahn A. Association between Helicobacter pylori-infection, C-reactive protein and status of B vitamins. Adv Med Sci. (2008) 53:205–13. 10.2478/v10039-008-0050-8 [DOI] [PubMed] [Google Scholar]
- 40.Kakehasi AM, Rodrigues CB, Carvalho AV, Barbosa AJA. Chronic gastritis and bone mineral density in women. Dig Dis Sci. (2009) 54:819–24. 10.1007/s10620-008-0417-5 [DOI] [PubMed] [Google Scholar]
- 41.Gerig R, Ernst B, Wilms B, Thurnheer M, Schultes B. Preoperative nutritional deficiencies in severely obese bariatric candidates are not linked to gastric Helicobacter pylori infection. Obes Surg. (2013) 23:698–702. 10.1007/s11695-013-0878-2 [DOI] [PubMed] [Google Scholar]
- 42.Ulasoglu C, Temiz HE, Saglam ZA. The relation of cytotoxin-associated gene-A seropositivity with vitamin B12 deficiency in -positive patients. Biomed Res Int. (2019) 2019:1450536. 10.1155/2019/1450536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mut Surmeli D, Surmeli ZG, Bahsi R, Turgut T, Selvi Oztorun H, Atmis V, et al. Vitamin D deficiency and risk of Helicobacter pylori infection in older adults: a cross-sectional study. Aging Clin Exp Res. (2019) 31:985–91. 10.1007/s40520-018-1039-1 [DOI] [PubMed] [Google Scholar]
- 44.Soyocak A, Ergun DD, Koc G, Ergun S, Ozsobaci NP. Investigation of aryl hydrocarbon receptor, zinc, and vitamin B12 levels in chronic gastritis with Helicobacter pylori infection. Biol Trace Elem Res. (2021) 199:2431–37. 10.1007/s12011-021-02667-5 [DOI] [PubMed] [Google Scholar]
- 45.Rokkas T, Papatheodorou G, Karameris A, Mavrogeorgis A, Kalogeropoulos NGiannikos N. Helicobacter pylori infection and gastric juice vitamin C levels. Impact of eradication. Dig Dis Sci. (1995) 40:615–21. 10.1007/BF02064380 [DOI] [PubMed] [Google Scholar]
- 46.Webb PM, Bates CJ, Palli D, Forman D. Gastric cancer, gastritis and plasma vitamin C: results from an international correlation and cross-sectional study. The Eurogast Study Group. Int J Cancer. (1997) 73:684–89. [DOI] [PubMed] [Google Scholar]
- 47.Rokkas T, Liatsos C, Petridou E, Papatheodorou G, Karameris A, Ladas SD, et al. Relationship of Helicobacter pylori CagA(+) status to gastric juice vitamin C levels. Eur J Clin Invest. (1999) 29:56–62. 10.1046/j.1365-2362.1999.00432.x [DOI] [PubMed] [Google Scholar]
- 48.Jarosz M, Dzieniszewski J, Dabrowska-Ufniarz E, Wartanowicz MZiemlanski S. Tobacco smoking and vitamin C concentration in gastric juice in healthy subjects and patients with Helicobacter pylori infection. Eur J Cancer Prev. (2000) 9:423–28. 10.1097/00008469-200012000-00008 [DOI] [PubMed] [Google Scholar]
- 49.Woodward M, Tunstall-Pedoe H, McColl K. Helicobacter pylori infection reduces systemic availability of dietary vitamin C. Eur J Gastroenterol Hepatol. (2001) 13:233–37. 10.1097/00042737-200103000-00003 [DOI] [PubMed] [Google Scholar]
- 50.Everett SM, Singh R, Leuratti C, White KL, Neville P, Greenwood D, et al. Levels of malondialdehyde-deoxyguanosine in the gastric mucosa: relationship with lipid peroxidation, ascorbic acid, and Helicobacter pylori. Cancer Epidemiol Biomarkers Prev. (2001) 10:369–76. [PubMed] [Google Scholar]
- 51.Capurso G, Ricci R, Panzuto F, Baccini F, Passi S, Di Giulio E, et al. Intragastric ascorbic but not uric acid is depleted in relation with the increased pH in patients with atrophic body gastritis and H. pylori gastritis. Helicobacter. (2003) 8:300–06. 10.1046/j.1523-5378.2003.00157.x [DOI] [PubMed] [Google Scholar]
- 52.Simon JA, Hudes ES, Perez-Perez GI. Relation of serum ascorbic acid to Helicobacter pylori serology in US adults: the Third National Health and Nutrition Examination Survey. J Am Coll Nutr. (2003) 22:283–89. 10.1080/07315724.2003.10719305 [DOI] [PubMed] [Google Scholar]
- 53.Antico A, Tozzoli R, Giavarina D, Tonutti E, Bizzaro N. Hypovitaminosis D as predisposing factor for atrophic type A gastritis: a case-control study and review of the literature on the interaction of Vitamin D with the immune system. Clin Rev Allergy Immunol. (2012) 42:355–64. 10.1007/s12016-011-8255-1 [DOI] [PubMed] [Google Scholar]
- 54.Han C, Ni Z, Yuan T, Zhang J, Wang C, Wang X, et al. Influence of serum vitamin D level on Helicobacter pylori eradication: a multi-center, observational, prospective and cohort study. J Dig Dis. (2019) 20:421–26. 10.1111/1751-2980.12793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gao T, Zhao M, Zhang C, Wang P, Zhou W, Tan S, et al. Association of Infection with Vitamin D deficiency in infants and toddlers. Am J Trop Med Hyg. (2020) 102:541–46. 10.4269/ajtmh.19-0523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Shafrir A, Shauly-Aharonov M, Katz LH, Paltiel O, Pickman Y, Ackerman Z. The association between serum vitamin D levels and presence and eradication. Nutrients. (2021) 13:278. 10.3390/nu13010278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yildirim O, Yildirim T, Seckin Y, Osanmaz P, Bilgic Y, Mete R. The influence of vitamin D deficiency on eradication rates of Helicobacter pylori. Adv Clin Exp Med. (2017) 26:1377–81. 10.17219/acem/65430 [DOI] [PubMed] [Google Scholar]
- 58.El Shahawy MS, Hemida MH, El Metwaly IShady ZM. The effect of vitamin D deficiency on eradication rates of infection. JGH Open. (2018) 2:270–75. 10.1002/jgh3.12081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Shatla MM, Faisal AS, El-Readi MZ. Is vitamin D deficiency a risk factor for helicobacter pylori eradication failure? Clin Lab. (2021) 67:217–22. 10.7754/Clin.Lab.2020.200118 [DOI] [PubMed] [Google Scholar]
- 60.Serin E, Gümürdülü Y, Ozer B, Kayaselçuk F, Yilmaz U, Koçak R. Impact of Helicobacter pylori on the development of vitamin B12 deficiency in the absence of gastric atrophy. Helicobacter. (2002) 7:337–41. 10.1046/j.1523-5378.2002.00106.x [DOI] [PubMed] [Google Scholar]
- 61.Ozer B, Serin E, Gumurdulu Y, Kayaselcuk F, Anarat R, Gur G, et al. Helicobacter pylori eradication lowers serum homocysteine level in patients without gastric atrophy. World J Gastroenterol. (2005) 11:2764–67. 10.3748/wjg.v11.i18.2764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Chuang CH, Sheu BS, Huang AH, Yang HB, Wu JJ. Vitamin C and E supplements to lansoprazole-amoxicillin-metronidazole triple therapy may reduce the eradication rate of metronidazole-susceptible Helicobacter pylori infection. Helicobacter. (2002) 7:310–16. 10.1046/j.1523-5378.2002.00095.x [DOI] [PubMed] [Google Scholar]
- 63.Everett SM, Drake IM, White KLM, Mapstone NP, Chalmers DM, Schorah CJ, et al. Antioxidant vitamin supplements do not reduce reactive oxygen species activity in Helicobacter pylori gastritis in the short term. Br J Nutr. (2002) 87:3–11. 10.1079/BJN2001477 [DOI] [PubMed] [Google Scholar]
- 64.Sezikli M, Cetinkaya ZA, Güzelbulut F, Sezikli H, Özkara S, Coşgun S, et al. Efficacy of vitamins supplementation to therapy on Helicobacter pylori eradication in patients with low antioxidant capacity. Clin Res Hepatol Gastroenterol. (2011) 35:745–49. 10.1016/j.clinre.2011.07.001 [DOI] [PubMed] [Google Scholar]
- 65.Sezikli M, Çetinkaya ZA, Güzelbulut F, Yeşil A, Coşgun S, Kurdaş OÖ. Supplementing vitamins C and E to standard triple therapy for the eradication of Helicobacter pylori. J Clin Pharm Ther. (2012) 37:282–85. 10.1111/j.1365-2710.2011.01286.x [DOI] [PubMed] [Google Scholar]
- 66.Demirci H, Uygun Ilikhan S, Öztürk K, Üstündag Y, Kurt Ö, Bilici M, et al. Influence of vitamin C and E supplementation on the eradication rates of triple and quadruple eradication regimens for Helicobacter pylori infection. Turk J Gastroenterol. (2015) 26:456–60. 10.5152/tjg.2015.0233 [DOI] [PubMed] [Google Scholar]
- 67.Koçkar C, Oztürk M, Bavbek N. Helicobacter pylori eradication with beta carotene, ascorbic acid and allicin. Acta Medica (Hradec Kralove). (2001) 44:97–100. 10.14712/18059694.2019.92 [DOI] [PubMed] [Google Scholar]
- 68.Chuang CH, Sheu BS, Kao AW, Cheng HC, Huang AH, Yang HB, et al. Adjuvant effect of vitamin C on omeprazole-amoxicillin-clarithromycin triple therapy for Helicobacter pylori eradication. Hepatogastroenterology. (2007) 54:320–24. [PubMed] [Google Scholar]
- 69.Kaboli SA, Zojaji H, Mirsattari D, Talaie R, Derakhshan F, Zali MR, et al. Effect of addition of vitamin C to clarithromycin-amoxicillin-omeprazol triple regimen on Helicobacter pylori eradication. Acta Gastroenterol Belg. (2009) 72:222–24. [PubMed] [Google Scholar]
- 70.Lahner E, Persechino S, Annibale B. Micronutrients (Other than iron) and Helicobacter pylori infection: a systematic review. Helicobacter. (2012) 17:1–15. 10.1111/j.1523-5378.2011.00892.x [DOI] [PubMed] [Google Scholar]
- 71.Reynolds E. Vitamin B12, folic acid, and the nervous system. Lancet Neurol. (2006) 5:949–60. 10.1016/S1474-4422(06)70598-1 [DOI] [PubMed] [Google Scholar]
- 72.Tamura T, Picciano MF. Folate and human reproduction. Am J Clin Nutr. (2006) 83:993–1016. 10.1093/ajcn/83.5.993 [DOI] [PubMed] [Google Scholar]
- 73.Sun LP, Yan LB, Liu ZZ, Zhao WJ, Zhang CX, Chen YM, et al. Dietary factors and risk of mortality among patients with esophageal cancer: a systematic review. BMC Cancer. (2020) 20:287. 10.1186/s12885-020-06767-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ashor AW, Siervo M, Lara J, Oggioni C, Afshar S, Mathers JC. Effect of vitamin C and vitamin E supplementation on endothelial function: a systematic review and meta-analysis of randomised controlled trials. Br J Nutr. (2015) 113:1182–94. 10.1017/S0007114515000227 [DOI] [PubMed] [Google Scholar]
- 75.Gunton JE, Girgis CM, Lau T, Vicaretti M, Begg L, Flood V. Vitamin C improves healing of foot ulcers: a randomised, double-blind, placebo-controlled trial. Br J Nutr. (2020) 126:1–8. 10.1017/S0007114520003815 [DOI] [PubMed] [Google Scholar]
- 76.Eshak ES, Okada C, Baba S, Kimura T, Ikehara S, Sato T, et al. Maternal total energy, macronutrient and vitamin intakes during pregnancy associated with the offspring's birth size in the Japan Environment and Children's Study. Br J Nutr. (2020) 124:558–66. 10.1017/S0007114520001397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Wanibuchi K, Hosoda K, Ihara M, Tajiri K, Sakai Y, Masui H, et al. Indene compounds synthetically derived from Vitamin D have selective antibacterial action on helicobacter pylori. Lipids. (2018) 53:393–401. 10.1002/lipd.12043 [DOI] [PubMed] [Google Scholar]
- 78.Pero R, Coretti L, Nigro E, Lembo F, Laneri S, Lombardo B, et al. β-Defensins in the Fight against Helicobacter pylori. Molecules. (2017) 22:424. 10.3390/molecules22030424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Caicedo Ochoa EY, Quintero Moreno CO, Méndez Fandiño YR, Sánchez Fonseca SC, Cortes Motta HF, Guio Guerra SA. Assessment of the use of vitamin C and E supplements concomitantly to antibiotic treatment against Helicobacter pylori: a systematic review and meta-analysis. Med Clin (Barc). (2018) 151:45–52. 10.1016/j.medcle.2018.05.016 [DOI] [PubMed] [Google Scholar]
- 80.Yang-Ou YB, Hu Y, Zhu Y, Lu NH. The effect of antioxidants on Helicobacter pylori eradication: a systematic review with meta-analysis. Helicobacter. (2018) 23:e12535. 10.1111/hel.12535 [DOI] [PubMed] [Google Scholar]
- 81.Li WQ, Zhang JY, Ma JL, Li ZX, Zhang L, Zhang Y, et al. Effects of treatment and vitamin and garlic supplementation on gastric cancer incidence and mortality: follow-up of a randomized intervention trial. BMJ. (2019) 366:l5016. 10.1136/bmj.l5016 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding authors.