Introduction
Decreased bone mass has been reported in both cross-sectional and longitudinal studies among children living with HIV (CLWH) on antiretroviral therapy (ART) in comparison to children without HIV, even after adjustment for pubertal stage, weight, and height.[1-6] Most prior studies demonstrating low bone mass among CLWH used dual x-ray absorptiometry (DXA) to measure bone mineral content (BMC, grams [g]) and bone mineral density (BMD, g/centimeters[cm]2). DXA estimates a two-dimensional areal BMD (aBMD) rather than a three-dimensional volumetric BMD (vBMD), by taking a ratio of the amount of bone and the area scanned. It is therefore affected by bone size. In children with impaired growth, delayed pubertal development, and smaller bone size, aBMD by DXA may be inaccurate.[7] DXA is further limited as it is unable to distinguish between cortical and trabecular bone compartments, or provide a specific estimate of bone strength.
Peripheral quantitative computed tomography (pQCT) is a bone imaging modality that may overcome some of the limitations of DXA, and be better suited for studies of CLWH who commonly experience poor somatic growth and delays in pubertal development.[8-12] pQCT measures both cortical and trabecular bone compartments, provides information on bone geometry and trabecular structure, and can quantify vBMD. It also involves little exposure to ionizing radiation and has good accuracy and precision. There are few studies of pQCT imaging in CLWH,[13] and none involving CLWH in sub-Saharan Africa, the region most affected by the pediatric HIV epidemic. The aim of this study is to compare bone architecture and strength by pQCT in school-aged CLWH and uninfected children as controls in South Africa, as well as between CLWH on a lopinavir/ritonavir (LPV/r)-based regimen vs. an efavirenz-based regimen.
Methods
Study participants
This study included a subgroup of CLWH and HIV-uninfected controls enrolled in the CHANGES Bone Study at the Empilweni Services and Research Unit (ESRU) at Rahima Moosa Mother and Child Hospital in Johannesburg, South Africa who underwent a pQCT scan during a single study visit.[5] All children were of black race. Controls without HIV were recruited from among eligible siblings or household members of CLWH or those attending the study site for routine outpatient health services. Excluded were controls with known chronic medical conditions, including known bone, renal, or liver disease, malabsorption syndrome, or inflammatory bowel disease and children taking antiepileptic medication. The Institutional Review Boards of Columbia University, New York, NY, USA, and the University of the Witwatersrand, Johannesburg, South Africa approved the study. Children’s guardians provided informed consent and children over 7 years of age provided assent.
Measurements
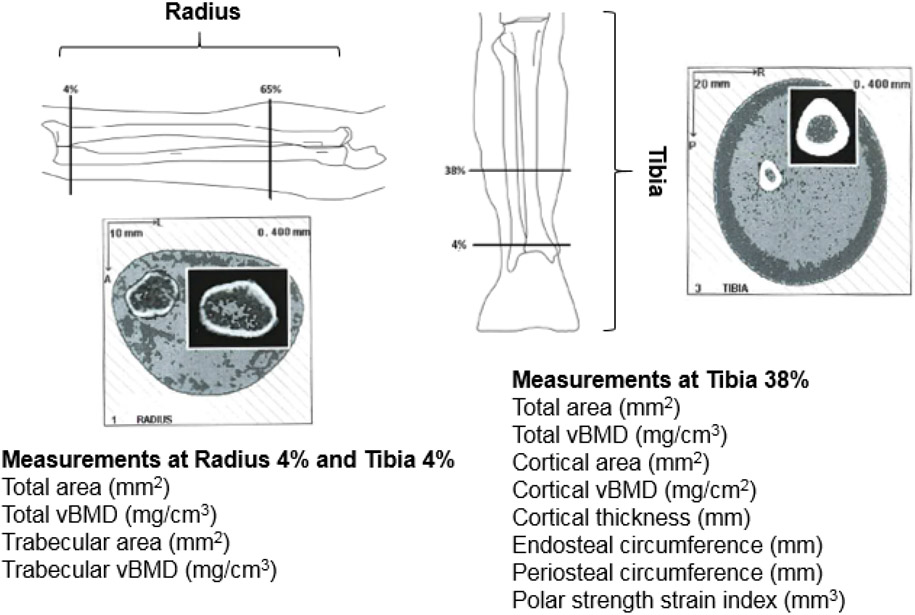
Image slices (2.3 mm) at the 4% radius and tibia and 38% tibia of the non-dominant forearm and lower leg were conducted using pQCT (Stratec XCT-2000 bone scanner, Stratec Medical, Pforzheim, Germany) to measure parameters that describe bone strength at the MRC/WITS Developmental Pathways for Health Research Unit (Figure 1). Forearm length was measured from the tip of the olecranon process to the most distal end of the ulna styloid process, and the tibial length was measured from the distal end of the medial malleolus to the superior aspect of the medial tibial condyle. Scanner positioning and image processing was completed as previously described.[14] In brief, a scout scan identified the distal epiphysial plate and allowed placement of a reference line at its proximal edge. The technician then identified the 4% site using this reference line in relation to the measured limb length. No scans were removed from the analysis due to positioning issues. A voxel size of 0.4 mm was used and the scan speed was set at 25 mm/s. Image processing and calculation of numerical values were conducted using the software package provided by the manufacturer (version 6; Stratec Medical, Pforzheim, Germany). All measurements and analyses were performed by a single trained operator.
Figure 1.
Peripheral quantitative computed tomography (pQCT) images from an 8-year-old child living with HIV at the radius and tibia and measurement
The 4% (distal) radial and tibial sites were selected to assess cross sections of primarily trabecular bone. They were analyzed using the CALCBD analysis algorithm using contour mode 1, peel mode 1, and a threshold of 180 mg/cm3. At these sites the following parameters were obtained: total area, total vBMD, trabecular area, and trabecular vBMD. The 38% tibia site was selected to assess cross-sections of primarily cortical bone. It was analyzed using Cort mode 1 at a threshold of 710 mg/cm3. At this site the following parameters were obtained: total area, cortical area, cortical vBMD, cortical thickness, endosteal circumference, and periosteal circumference. Bone strength was estimated by the polar strength strain index (SSI), an estimate of bending and torsional bone strength for cortical bone, and a validated measure of fracture risk.
In addition, demographic data were collected and participants underwent physical examination to obtain anthropometric measures and to assess pubertal development. Weight was measured to the nearest 0.1 kg using a digital scale, and standing height was measured to the nearest 0.1 cm using a wall-mounted stadiometer. BMI was calculated as weight (kg) divided by height squared (m2). Weight-for-age Z-scores (WAZ), height-for-age Z-scores (HAZ), and BMI-for-age Z-scores (BAZ) were calculated using WHO standards.[15] Underweight was defined as WAZ less than −2 and stunted was defined as HAZ less than −2. Pubertal status was assessed and graded by trained study physicians (RS, FP, MB) according to Tanner’s Sexual Maturation Scale.[16, 17] Girls were staged using the highest score of either breast or pubic hair development. For CLWH, blood was drawn at the visit and plasma HIV-RNA levels (lower limit of detection 40 copies/mL) were measured by the Abbott RealTime HIV-1 Assay (Abbott Park, Illinois, USA). CD4 counts and percentage were measured by the TruCount Method (BD Biosciences, Germany).
Statistical analysis
We compared characteristics and pQCT outcomes between CLWH and controls, and CLWH on a LPV/r-based regimen compared to an efavirenz-based regimen. All analyses were stratified by sex. Where applicable, a chi-squared or Fisher’s exact test was used to compare proportions and t-test was used to compare means. Linear regression was used to adjust comparisons between the CLWH and controls by sex, age, Tanner stage, and radial/tibial length. A P-value <0.05 was considered statistically significant. All statistical analyses were performed using SAS (version 9.4; SAS Institute, Cary, North Carolina, USA).
Results
Characteristics of the 172 CLWH on ART and 98 controls with pQCT scans are shown in Table 1. At the time of scan, CLWH (50% male) and controls (62% male) were an average of 10.2 and 10.8 years of age, respectively (range 7.8 years to 14.2 years). Among boys, 94.2% of CLWH and 88.5% of controls were in Tanner Stage 1 or 2 (p=0.22). Among girls, 87.2% of CLWH and 73.0% of controls were in Tanner Stage 1 or 2 (p=0.054). Among CLWH, 122 (70.9%) were on an efavirenz-based regimen, 49 (28.5%) on a LPV/r-based regimen, and 1 on an atazanavir/ritonavir-based regimen. For nucleoside backbones, most children (95%) were on lamivudine/abacavir, 7 (4.1%) were on lamivudine/zidovudine, and 2 (1.2%) on lamivudine/stavudine. No children received a tenofovir-containing regimen. CLWH had been on ART for an average of 9.5 years (range 6.3 to 12.8). At the time of evaluation, 93% of CLWH had a plasma HIV-1 RNA concentration <400 copies/mL and a mean CD4 percentage of 37.3.
Table 1.
Characteristics of children living with HIV and controls in Johannesburg, South Africa
| Characteristics1 | CLWH (N=172) |
Controls (N=98) |
P | LPV/r (N=49) |
EFV (N=122) |
P |
|---|---|---|---|---|---|---|
| Male, N (%) | 86 (50.0) | 61 (62.2) | 0.05 | 26 (53.1) | 60 (49.2) | 0.65 |
| Age (years), Mean (SD) | 10.2 (1.4) | 10.8 (1.8) | 0.002 | 10.0 (1.4) | 10.2 (1.4) | 0.43 |
| Weight (kg), Mean (SD) | 28.6 (6.7) | 34.7 (11.1) | <0.001 | 28.5 (6.9) | 28.5 (6.7) | 0.98 |
| Height (cm), Mean (SD) | 131.3 (8.8) | 139.8 (10.6) | <0.001 | 130.9 (8.8) | 131.3 (8.7) | 0.80 |
| HAZ, Mean (SD) | −1.15 (0.97) | −0.42 (1.0) | <0.001 | −1.10 (0.88) | −1.17 (1.02) | 0.69 |
| BAZ, Mean (SD) | −0.31 (0.97) | −0.09 (1.2) | 0.10 | −0.22 (0.92) | −0.35 (0.99) | 0.44 |
| Tanner Stage, N (%) | ||||||
| 1 | 111 (64.9) | 54 (55.1) | 0.23 | 33 (67.4) | 78 (63.9) | 0.46 |
| 2 | 45 (26.3) | 27 (27.6) | 13 (26.5) | 32 (26.2) | ||
| 3 | 10 (5.8) | 11 (11.2) | 3 (6.1) | 6 (4.9) | ||
| 4 | 6 (3.5) | 6 (6.1) | 0 (0.0) | 6 (4.9) | ||
| ART regimen, N (%) | ||||||
| LPV/r-based | 49 (28.5) | |||||
| EFV-based | 122 (70.9) | |||||
| Other | 1 (0.6) | |||||
| Plasma HIV RNA <400 (copies/mL), N (%) | 155 (93.9) | 43 (89.6) | 112 (95.7) | 0.13 | ||
| CD4 percentage (%), Mean (SD) | 37.3 (7.3) | 35.8 (6.3) | 38.1 (7.2) | 0.06 | ||
| CD4 count (cells/mm3), Mean (SD) | 987 (317) | 997 (283) | 990 (324) | 0.89 | ||
| Age at ART start (months), Mean (SD) | 8.5 (6.7) | 8.4 (6.1) | 8.5 (6.8) | 0.94 | ||
| Age at ART start (months), N (%) | ||||||
| <6 | 87 (50.6) | 23 (46.9) | 64 (52.5) | 0.43 | ||
| 6-12 | 42 (24.4) | 15 (30.6) | 26 (21.3) | |||
| >12 | 43 (25.0) | 11 (22.5) | 32 (26.2) |
Abbreviations: HAZ – height-for-age Z-score; BAZ – BMI-for-age Z-score; ART – antiretroviral therapy
Characteristics at the time of pQCT scan, unless specified
pQCT scans were obtained for all participants except 4 CLWH at the radius and 2 CLWH at the tibia. CLWH had a lower radial length (boys: 208 vs. 216 mm, p=0.018; girls: 207.1 vs. 218.9 mm, p=0.002) and tibial length (boys: 299 vs. 327 mm, p<0.001; girls: 300 vs. 330 mm, p<0.001). Trabecular area of the radius and tibia, as well as cortical area of the tibia were smaller in male and female CLWH compared to controls (Table 2).
Table 2.
Unadjusted and adjusted differences in peripheral quantitative computed tomography (pQCT) measures between children living with HIV (CLWH) and controls, stratified by sex
| Boys | Girls | |||||||
|---|---|---|---|---|---|---|---|---|
| Measurement, Mean (SD) | Unadjusted Estimate (SE) |
P |
*Adjusted Estimate (SE) |
P | Unadjusted Estimate (SE) |
P |
*Adjusted Estimate (SE) |
P |
| Radius 4% | ||||||||
| Total area (mm2) | −36.1 (9.2) | <0.001 | −25.1 (8.1) | 0.002 | −37.6 (10.6) | <0.001 | −18.8 (8.7) | 0.034 |
| Total vBMD (mg/cm3) | 4.9 (6.2) | 0.43 | 2.5 (6.3) | 0.69 | 23.0 (6.8) | 0.001 | 19.7 (7.1) | 0.007 |
| Trabecular area (mm2) | −16.2 (4.1) | 0.0001 | −11.3 (3.6) | 0.002 | −16.9 (4.7) | 0.0005 | −8.5 (3.9) | 0.0336 |
| Trabecular vBMD (mg/cm3) | −14.3 (7.1) | 0.045 | −17.3 (7.2) | 0.018 | 9.2 (6.8) | 0.18 | 7.2 (7.1) | 0.32 |
| Tibia 4% | ||||||||
| Total area (mm2) | −114.7 (24.2) | <0.001 | −71.5 (17.4) | <0.001 | −107.8 (23.7) | <0.001 | −54.9 (17.9) | 0.003 |
| Total vBMD (mg/cm3) | −2.01 (6.3) | 0.75 | −3.15 (6.5) | 0.63 | 18.3 (7.3) | 0.014 | 14.6 (7.5) | 0.055 |
| Trabecular area (mm2) | −51.6 (10.9) | <0.001 | −32.2 (7.9) | <0.001 | −48.1 (10.7) | <0.001 | −24.7 (8.1) | 0.003 |
| Trabecular vBMD (mg/cm3) | −6.01 (8.2) | 0.47 | −7.2 (8.5) | 0.40 | 16.3 (9.5) | 0.09 | 10.1 (9.8) | 0.31 |
| Tibia 38% | ||||||||
| Total area (mm2) | −48.5 (10.1) | <0.001 | −30.1 (6.4) | <0.001 | −34.8 (10.0) | <0.001 | −11.7 (6.9) | 0.09 |
| Cortical area (mm2) | −30.5 (6.5) | <0.001 | −18.2 (3.9) | <0.001 | −23.2 (6.2) | <0.001 | −8.4 (3.9) | 0.033 |
| Cortical vBMD (mg/cm3) | 5.8 (6.3) | 0.36 | 6.5 (6.6) | 0.33 | 14.2 (8.1) | 0.08 | 17.1 (8.2) | 0.038 |
| Cortical thickness (mm) | −0.29 (0.10) | 0.003 | −0.15 (0.08) | 0.056 | −0.29 (0.10) | 0.004 | −0.11 (0.08) | 0.13 |
| Endosteal circumference (mm) | −2.94 (0.90) | 0.001 | −1.92 (0.82) | 0.02 | −1.74 (0.93) | 0.06 | −0.40 (0.85) | 0.64 |
| Periosteal circumference (mm) | −4.97 (1.03) | <0.001 | −3.06 (0.67) | <0.001 | −3.54 (1.05) | 0.001 | −1.11 (0.72) | 0.13 |
| Polar strength strain index (mm3)** | −153.9 (30.2) | <0.001 | −71.8 (36.2) | 0.0496 | ||||
Adjusted for age, radial/tibial length, and Tanner stage
Adjusted for age and Tanner stage
As shown in Table 2, male CLWH had a lower trabecular vBMD of the radius than controls (221 vs. 235 mg/cm3, p=0.045). This finding remained after adjustment for age, radial length, and Tanner stage (β=−17.3, SE=7.2, p=0.018). Trabecular vBMD of the tibia was also lower in male CLWH (β=−7.2, SE=8.5, p=0.40), but not statistically significant. Trabecular vBMD of both the radius and tibia did not differ between female CLWH and controls.
A trend towards higher cortical vBMD of the tibia was seen in female CLWH compared with controls (1094 vs. 1080 mg/cm3, p=0.08), including after adjustment for age, tibial length, and Tanner stage (p=0.038). In boys, cortical vBMD of the tibia did not differ between groups.
Cortical thickness was lower in both male CLWH (3.38 vs. 3.67 mm, p=0.003) and female CLWH (3.27 vs. 3.56 mm, p=0.004) compared with controls. In adjusted analyses, cortical thickness remained lower in males (β=−0.15, SE=0.08, p=0.056) and females (β=−0.11, SE=0.08, p=0.13), although not statistically significant. Endosteal and periosteal circumferences were significantly lower for male CLWH compared with controls after adjustment for age, tibial length, and Tanner Stage and lower for female CLWH although not significantly lower (Table 2).
Bone strength by polar SSI was lower in CLWH than controls (778 vs. 972 mm3, p<0.01); this finding was consistent in boys and girls, even after adjustment after age and Tanner Stage (Table 2).
pQCT results for CLWH by treatment group are presented in Table 3, stratified by sex. Radial length, tibial length, trabecular area of the radius and tibia, and cortical area of the tibia did not differ between male and female CLWH on a LPV/r-based regimen and those on an efavirenz-based regimen. Cortical thickness was also similar between groups. CLWH on a LPV/r-based regimen had lower trabecular vBMD (199 vs. 222 mg/cm3, p<0.001) than those on an efavirenz-based regimen; this finding was consistent in boys (206 vs. 228 mg/cm3, p=0.014) and girls (191 vs. 216 mg/cm3, p=0.005). Similarly, CLWH on a LPV/r-based regimen had lower cortical vBMD (1074 vs. 1093 mg/cm3, p=0.004) than those on an efavirenz-based regimen; this finding was consistent in boys (1069 vs. 1086 mg/cm3, p=0.056) and girls (1079 vs. 1100 mg/cm3, p=0.040). No difference in bone strength by polar SSI was observed between treatment groups.
Table 3.
Peripheral quantitative computed tomography (pQCT) measures in children living with HIV (CLWH) by treatment regimen, stratified by sex
| Boys | Girls | |||||||
|---|---|---|---|---|---|---|---|---|
| Measurement, Mean (SD) | Controls (N=61) |
LPV/r (N=26) |
EFV (N=60) |
P (LPV/r vs. EFV) |
Controls (N=37) |
LPV/r (N=23) |
EFV (N=62) |
P (LPV/r vs. EFV) |
| RADIUS | ||||||||
| Missing | 0 | 0 | 2 | 0 | 0 | 2 | ||
| Radial length (mm) | 216.1 (20.8) | 207.6 (20.6) | 208.5 (18.3) | 0.83 | 218.9 (22.2) | 208.3 (17.4) | 206.3 (16.8) | 0.63 |
| Radius 4% | ||||||||
| Total area (mm2) | 253.7 (55.3) | 212.4 (59.6) | 220.0 (51.6) | 0.55 | 243.8 (64.1) | 205.3 (42.2) | 205.3 950.0) | 1.0 |
| Total vBMD (mg/cm3) | 307.1 (36.0) | 297.8 (39.5) | 318.4 (35.1) | 0.019 | 269.9 (30.6) | 279.8 (37.6) | 297.3 (34.9) | 0.048 |
| Trabecular area (mm2) | 114.1 (24.9) | 95.5 (26.8) | 98.9 (23.2) | 0.56 | 109.6 (28.8) | 92.3 (19.0) | 92.3 (22.5) | 1.0 |
| Trabecular vBMD (mg/cm3) | 235.4 (46.6) | 205.8 (41.6) | 227.9 (35.3) | 0.014 | 200.6 (29.9) | 191.3 (27.1) | 215.8 (36.6) | 0.005 |
| TIBIA | ||||||||
| Missing | 0 | 1 | 1 | 0 | 0 | 0 | ||
| Tibial length (mm) | 325.4 (36.5) | 296.6 (33.4) | 299.6 (30.0) | 0.69 | 330.4 (35.1) | 303.1 (24.9) | 298.1 (24.5) | 0.41 |
| Tibia 4% | ||||||||
| Total area (mm2) | 881.1 (155.5) | 750.9 (149) | 773.0 (129) | 0.50 | 812.9 (138.9) | 723.4 (126) | 695.8 (102) | 0.30 |
| Total vBMD (mg/cm3) | 323.2 (38.4) | 311.4 (32.8) | 325.4 (37.5) | 0.11 | 282.2 (30.6) | 287.8 (38.8) | 303.9 (38.2) | 0.09 |
| Trabecular area (mm2) | 396.4 (70.0) | 337.8 (67.2) | 347.8 (58.1) | 0.50 | 365.7 (62.5) | 325.4 (56.6) | 313.0 (46.0) | 0.30 |
| Trabecular vBMD (mg/cm3) | 282.5 (45.8) | 260.9 (43.8) | 283.1 (52.8) | 0.07 | 242.1 (33.3) | 251.1 (52.7) | 259.6 (53.0) | 0.51 |
| Tibia 38% | ||||||||
| Total area (mm2) | 316.0 (67.1) | 270.7 (62.1) | 266.1 (49.8) | 0.72 | 296.5 (65.8) | 266.2 (39.9) | 258.7 (43.0) | 0.47 |
| Cortical area (mm2) | 190.0 (46.0) | 160.4 (34.3) | 159.1 (30.9) | 0.87 | 177.1 (39.0) | 151.7 (31.1) | 153.8 (26.5) | 0.76 |
| Cortical vBMD (mg/cm3) | 1075 (36.9) | 1069 (34.7) | 1086 (38.5) | 0.056 | 1080 (41.5) | 1079 (48.0) | 1100 (37.4) | 0.040 |
| Cortical thickness (mm) | 3.67 (0.69) | 3.37 (0.45) | 3.38 (0.48) | 0.95 | 3.56 (0.55) | 3.18 (0.57) | 3.30 (0.41) | 0.27 |
| Endosteal circumference (mm) | 39.4 (5.5) | 36.8 (5.9) | 36.4 (4.8) | 0.73 | 38.3 (5.8) | 37.7 (4.1) | 36.1 (4.1) | 0.11 |
| Periosteal circumference (mm) | 62.7 (6.6) | 58.0 (6.5) | 57.6 (5.3) | 0.78 | 60.7 (6.6) | 57.7 (4.3) | 56.8 (4.7) | 0.45 |
| Polar strength strain index (mm3) | 1015 (321) | 803 (285) | 787 (232) | 0.78 | 901 (309) | 744 (180) | 765 (190) | 0.65 |
Abbreviations: vBMD – volumetric bone mineral density
Discussion
To our knowledge, this is the first study to examine bone microarchitecture by pQCT in a cohort of CLWH living in sub-Saharan Africa who initiated ART early in life with well-controlled HIV. Sex-stratified differences were noted in various pQCT parameters. Male CLWH had reduced trabecular vBMD, cortical thickness, and bone strength than controls, but no difference in cortical vBMD. Female CLWH had decreased cortical thickness and bone strength, but higher cortical vBMD and no difference in trabecular vBMD compared with controls.
We previously reported mean whole body BMC z-score by DXA was 0.17 lower for CLWH than controls after adjustment for physical activity, dietary calcium and serum calcium (p=0.03).[5] The deficits in structural bone parameters in CLWH detected by pQCT in the current study provide additional insight into the reduced DXA-derived BMD observed in this cohort. A study in Canada found higher cortical bone BMD z-scores by pQCT at the tibia in CLWH (71% on ART) compared with a cohort of healthy controls, and pQCT findings did not reflect deficits in bone outcomes in CLWH found with imaging by DXA.[13] In addition to a mix of ethnicities, participants in this study were considerably older and a greater proportion had undergone puberty than those in our study, possibly explaining the different findings.
The reduced cortical thickness, lower trabecular vBMD, and bone strength among CLWH, especially amongst the boys, in these largely pre-pubertal children are of concern with respect to future bone health. Although limited, studies of young adults with HIV acquired early in life suggest that the deficits in bone mineral accrual and bone architecture persist and impair skeletal development, resulting in reduced peak adult bone mass and bone strength. In a study by Yin and colleagues in New York City of 30 young African-American and Hispanic men with HIV on ART and 15 controls without HIV aged 20-25 years (the age when peak bone mass is typically attained), deficits in bone by high-resolution pQCT in cortical and trabecular compartments were detected, as well as decreased bone stiffness, which is a measure of bone strength.[18] These deficits place individuals at increased long term risk for osteopenia and fractures, which is reported among older people living with HIV.[19, 20] Of note, for male CLWH, decreased trabecular vBMD was observed at the radius but not the tibia. It is possible that the weight-bearing site (tibia) may be stronger and less affected by HIV than the non-weight bearing site (radius). We did not observe similar patterns of reduced cortical thickness or lower trabecular vBMD in girls; sex differences have been reported in other HIV studies, including pre-pubertal CLWH.[21, 22]
We found lower bone strength as measured by polar SSI in CLWH compared with controls. While polar SSI is a parameter that has been shown to be an accurate and precise indicator of long-bone bending strength and fracture risk in studies of adults,[23] its significance is not as certain in children, since there are no data on whether SSI in children at this age predicts fracture risk. One issue is that bone size, represented by area, thickness, and circumference, is a major determinant of polar SSI. Given that we observed delayed maturation in female CLWH, with only 10% of CLWH girls in Tanner stage 3-4 as compared with 27% of controls, accounting for Tanner stage was necessary. While adjustment for age and Tanner stage mitigated the between-group differences in polar SSI in girls, polar SSI was still significantly lower in female CLWH (p<0.05) even after this adjustment. Other estimates of bone strength, such as finite element analysis or individual trabecular segmentation that are not dependent upon bone size can be obtained with high resolution pQCT,[24-26] but polar SSI was the only bone strength variable available for pQCT.
In addition, lower total vBMD by pQCT was found in CLWH on a LPV/r-based regimen compared with those on an efavirenz-based regimen. These differences were reflected in both lower trabecular vBMD at the radius and lower cortical vBMD at the tibia in the LPV/r group, and are consistent with our previously reported findings of lower aBMD by DXA in the LPV/r group.[5] Of interest, bone strength SSI did not differ between the treatment groups. Bone size did not differ between treatment groups, so it is possible that the relative weight placed upon bone size by the polar SSI formula is greater than that of vBMD. Without histomorphometric studies which require more invasive bone biopsy, insight into the clinical significance of the pQCT differences observed between treatment regimen is limited.
A strength of our study is the use of pQCT to obtain separate measures of bone strength and architecture of the trabecular and cortical bone in CLWH in sub-Saharan Africa. In addition, our study is strengthened by a control group of the same age with the same racial/ethnic background and similar social conditions from the same community as the CLWH. Measures of pubertal development were also taken into account. A longitudinal study will provide a more thorough understanding of changes in bone architecture and strength during skeletal maturation and whether deficits persist after puberty. Furthermore, to confirm that deficits in bone strength occur in CLWH, fracture outcome data is essential.
In summary, we demonstrate differences in bone architecture and strength by pQCT in a cohort of children living with HIV compared with controls in Johannesburg, South Africa. Follow up of these children is critical to determine whether these differences will be clinically significant as these children age. In addition, studies of interventions to normalize bone growth and homeostasis and mitigate fracture risk, during childhood and adolescence, which are critical periods for bone mineralization and skeletal development, are warranted.
Supplementary Material
Acknowledgements
We would like to acknowledge the children and families who participated in the study, as well as the dedicated study teams at the Empilweni Services and Research Unit and the SAMRC Developmental Pathways for Health Research Unit.
Conflicts of Interest and Source of Funding:
The authors have no conflicts of interest to declare. This study was supported by grants from the Eunice Kennedy Shriver National Institute of Child Health & Human Development (HD 073977, HD 073952).
References
- 1.Jacobson DL, Lindsey JC, Gordon CM, Moye J, Hardin DS, Mulligan K, et al. Total body and spinal bone mineral density across Tanner stage in perinatally HIV-infected and uninfected children and youth in PACTG 1045. AIDS 2010; 24(5):687–696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arpadi SM, Horlick M, Thornton J, Cuff PA, Wang J, Kotler DP. Bone mineral content is lower in prepubertal HIV-infected children. J Acquir Immune Defic Syndr 2002; 29(5):450–454. [DOI] [PubMed] [Google Scholar]
- 3.DiMeglio LA, Wang J, Siberry GK, Miller TL, Geffner ME, Hazra R, et al. Bone mineral density in children and adolescents with perinatal HIV infection. Aids 2013; 27(2):211–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sudjaritruk T, Bunupuradah T, Aurpibul L, Kosalaraksa P, Kurniati N, Prasitsuebsai W, et al. Adverse bone health and abnormal bone turnover among perinatally HIV-infected Asian adolescents with virological suppression. HIV Med 2016. [DOI] [PubMed] [Google Scholar]
- 5.Arpadi SM, Shiau S, Strehlau R, Patel F, Mbete N, McMahon DJ, et al. Efavirenz is associated with higher bone mass in South African children with HIV. AIDS 2016; 30(16):2459–2467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lima LR, Silva RC, Giuliano Ide C, Sakuno T, Brincas SM, Carvalho AP. Bone mass in children and adolescents infected with human immunodeficiency virus. J Pediatr (Rio J) 2013; 89(1):91–99. [DOI] [PubMed] [Google Scholar]
- 7.Crabtree NJ, Arabi A, Bachrach LK, Fewtrell M, El-Hajj Fuleihan G, Kecskemethy HH, et al. Dual-energy X-ray absorptiometry interpretation and reporting in children and adolescents: the revised 2013 ISCD Pediatric Official Positions. J Clin Densitom 2014; 17(2):225–242. [DOI] [PubMed] [Google Scholar]
- 8.Stagi S, Cavalli L, Cavalli T, de Martino M, Brandi ML. Peripheral quantitative computed tomography (pQCT) for the assessment of bone strength in most of bone affecting conditions in developmental age: a review. Ital J Pediatr 2016; 42(1):88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Adams JE, Engelke K, Zemel BS, Ward KA. Quantitative computer tomography in children and adolescents: the 2013 ISCD Pediatric Official Positions. J Clin Densitom 2014; 17(2):258–274. [DOI] [PubMed] [Google Scholar]
- 10.Williams PL, Abzug MJ, Jacobson DL, Wang J, Van Dyke RB, Hazra R, et al. Pubertal onset in children with perinatal HIV infection in the era of combination antiretroviral treatment. Aids 2013; 27(12):1959–1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ramteke SM, Shiau S, Foca M, Strehlau R, Pinillos F, Patel F, et al. Patterns of Growth, Body Composition, and Lipid Profiles in a South African Cohort of Human Immunodeficiency Virus-Infected and Uninfected Children: A Cross-Sectional Study. J Pediatric Infect Dis Soc 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bellavia A, Williams PL, DiMeglio LA, Hazra R, Abzug MJ, Patel K, et al. Delay in sexual maturation in perinatally HIV-infected youths is mediated by poor growth. Aids 2017; 31(9):1333–1341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Macdonald HM, Chu J, Nettlefold L, Maan EJ, Forbes JC, Cote H, et al. Bone geometry and strength are adapted to muscle force in children and adolescents perinatally infected with HIV. J Musculoskelet Neuronal Interact 2013; 13(1):53–65. [PubMed] [Google Scholar]
- 14.Neu CM, Manz F, Rauch F, Merkel A, Schoenau E. Bone densities and bone size at the distal radius in healthy children and adolescents: a study using peripheral quantitative computed tomography. Bone 2001; 28(2):227–232. [DOI] [PubMed] [Google Scholar]
- 15.Organization WH. Child Growth Standards. Available at: http://www.who.int/childgrowth/en. 2007.
- 16.Marshall WA, Tanner JM. Variations in pattern of pubertal changes in girls. Arch Dis Child 1969; 44(235):291–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Marshall WA, Tanner JM. Variations in the pattern of pubertal changes in boys. Arch Dis Child 1970; 45(239):13–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yin MT, Lund E, Shah J, Zhang CA, Foca M, Neu N, et al. Lower peak bone mass and abnormal trabecular and cortical microarchitecture in young men infected with HIV early in life. AIDS 2014; 28(3):345–353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Heaney RP, Abrams S, Dawson-Hughes B, Looker A, Marcus R, Matkovic V, et al. Peak bone mass. Osteoporos Int 2000; 11(12):985–1009. [DOI] [PubMed] [Google Scholar]
- 20.Shiau S, Broun EC, Arpadi SM, Yin MT. Incident fractures in HIV-infected individuals: a systematic review and meta-analysis. AIDS 2013; 27(12):1949–1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shiau S, Kuhn L, Strehlau R, Martens L, McIlleron H, Meredith S, et al. Sex differences in responses to antiretroviral treatment in South African HIV-infected children on ritonavir-boosted lopinavir- and nevirapine-based treatment. BMC pediatrics 2014; 14:39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cornell M, Schomaker M, Garone DB, Giddy J, Hoffmann CJ, Lessells R, et al. Gender differences in survival among adult patients starting antiretroviral therapy in South Africa: a multicentre cohort study. PLoS medicine 2012; 9(9):e1001304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Varghese B, Short D, Hangartner T. Development of quantitative computed-tomography-based strength indicators for the identification of low bone-strength individuals in a clinical environment. Bone 2012; 50(1):357–363. [DOI] [PubMed] [Google Scholar]
- 24.Wang J, Zhou B, Hu Y, Zhang Z, Yu YE, Nawathe S, et al. Accurate and Efficient Plate and Rod Micro Finite Element Whole Bone Models Based on High-Resolution Peripheral Computed Tomography. J Biomech Eng 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Stein EM, Kepley A, Walker M, Nickolas TL, Nishiyama K, Zhou B, et al. Skeletal structure in postmenopausal women with osteopenia and fractures is characterized by abnormal trabecular plates and cortical thinning. J Bone Miner Res 2014; 29(5):1101–1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Boutroy S, Khosla S, Sornay-Rendu E, Zanchetta MB, McMahon DJ, Zhang CA, et al. Microarchitecture and Peripheral BMD are Impaired in Postmenopausal White Women With Fracture Independently of Total Hip T-Score: An International Multicenter Study. J Bone Miner Res 2016; 31(6):1158–1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.