Abstract
The intestinal immune system samples the luminal contents to induce adaptive immune responses characterized by tolerance in the steady state and protective immunity during infection. How luminal substances are acquired by the immune system for this task is of significant interest and investigation. Goblet cells perform a vital by delivering luminal substances to the immune system through the formation of goblet cell associated antigen passages (GAPs). Soluble antigens in the intestinal lumen are transported across the epithelium transcellularly through GAPs and acquired by dendritic cells for presentation to T cells for the induction of immune responses. GAPs can be identified and quantified using fluorescently labeled dextran which is taken up goblet cells forming GAPs. Here we describe a method to visualize GAPs, and other cells that have the capacity to take up luminal substances, by intraluminal injection of fluorescent dextran with the animal under anesthesia, tissue preservation and sectioning for slide preparation, followed by fluorescent microscopy. In contrast to in vivo two photon imaging to identify GAPs. this technique is not limited by anatomy and can be used to assess GAP formation throughout the length of the intestine. In addition, it can be combined with common immunohistochemistry protocols to visualize other cell types. This approach can be used to compare GAP formation following treatments or changes to the luminal environment to obtain insight into how sampling of luminal substance is altered during pathophysiologic conditions.
Keywords: Goblet Cells, antigen delivery, intestinal epithelium, intravital imaging, immunofluorescence, mucosal immunology
SUMMARY:
This protocol describes how to identify and quantify intestinal epithelial cells that have the capacity to take up luminal substances, using fluorescent microscopy of fixed tissue sections. Goblet cell associated antigen passages (GAPs) can deliver luminal substances to antigen presenting cells underlying the epithelium to induce antigen specific T cell responses. Accordingly, this technique can be used to assess the capacity of the intestine to deliver luminal antigens from the diet or microbiota across the epithelium to the intestinal immune system to induce adaptive immune responses.
INTRODUCTION:
The introduction of substances from the lumen to the immune cells within the lamina propria of the intestine is important for the maintenance of tolerance to antigens originating from the diet and commensal microbiota, and for the monitoring of potential pathogen threats. A principal mechanism for antigens crossing the epithelium and being loaded onto dendritic cells to induce antigen specific T cell responses is through the formation of goblet cell-associated antigen passages (GAPs) 1. Goblet cells are secretory epithelial cells found along the length of the intestine. Goblet cells form GAPs upon acetylcholine stimulation of muscarinic acetylcholine receptors expressed by goblet cells2. Luminal antigens are taken up by the goblet cell, cross the goblet cell transcellularly, and are passed to dendritic cells below, that form contacts with the GAPs1,3. GAP formation is a dynamic process. Currently there are no identified markers to denote which goblet cell is forming a GAP at any given time other than the capacity of goblet cells to take up luminal substances. Therefore, to monitor GAP formation, luminal fluorescent model antigens are used to visualize and quantify GAP formation by immunofluorescence microscopy.
GAP formation in the small intestine occurs spontaneously in the steady-state, and this protocol represents a method to measure GAP formation at the steady-state and in response to various luminal and environmental influences1,2. Indeed, GAP formation is highly adaptable to different luminal conditions; GAP formation is limited during enteric infection as a means to limit pathogen dissemination and prevent inflammatory responses against dietary antigens 4. Additionally, GAP formation in the colon is regulated throughout early life, as GAPs form prior to weaning allowing for the introduction of commensal antigens and induction of tolerogenic responses toward the microbiota and are inhibited post-weaning as the microbiota becomes more complex5. As the process of GAP formation is dynamic through the intestinal regions, during different phases of life, and in response to lumen environmental changes, this protocol for quantifying GAP formation represents a robust assay for assessing the capacity of antigen delivery to the lamina propria immune system.
PROTOCOL DEVELOPMENT:
The ability of goblet cells to endocytose and transcytose luminal proteins has long been appreciated6–9. This protocol is adapted from the intravital imaging technique preformed with two-photon microscopy 1, and uses methodology based on the previously described techniques for assessing transport of proteins across the intestinal epithelium. As two-photon microscopy systems are specialized equipment not available to all researchers, this protocol has been revised into a bench-top procedure using commonly available fluorescent microscopy to visualize GAP formation in vivo 2,4,5,10. Additionally, the use of fluorescence-based antigens makes this technique suitable to be coupled with fluorescent-labeled antibodies to identify a desired cell type. Furthermore, this procedure is suitable to perform on multiple animals simultaneously for efficient experimentation. When designing experiments, the number of mice chosen should reflect inclusion of control groups and experimental groups with at least three mice per group. The maximum number of mice one individual can feasibly perform this procedure on is approximately eight mice. Experimental groups could utilize intraluminal or systemic substances to alter the rate of luminal sampling by GAPs. While the procedure presented here is described as labeling of GAPs, this procedure identifies other epithelial cell types than can take up luminal substances, which has also been shown to occur in enteroendocrine cells using a similar protocol 11. With extended rests at step 2F, this procedure could be adapted to identify which cells in the lamina propria obtain luminal antigens.
MATERIALS:
Heating Pad: NC0590272 SnuggleSafe, www.snugglesafe.co.uk
Bench top pads: VWR Deluxe Absorbent Underpads, 56616–018, VWR International Corp, us.vwr.com
Wound closure autoclipper and 9mm staples: 205016 MikRon Precision, Inc
Flash freezing compound: Super Friendly Freeze’It, 23–022524, Fisherbrand, www.fishersci.com
Cryomold: Tissue-Tek Cryomold Standard 4557 Sakura www.sakuraus.com
OCT: Tissue Plus O.C.T. Compound Clear, 23–730-571 Fisher HealthCare, www.fishersci.com
Slides: ColorFrost Plus Microscope Slides Precleaned, 12–550-17, Fisher Scientific, www.fishersci.com
1.5 ml conical polypropylene microcentrifuge tubes, Eppendorf snap-cap safe-lock tubes, 05–402-23 Fisher Scientific, colored to prevent photobleaching of dextran
Sterile surgical drapes, NC0493742, Fisher Scientific, www.fishersci.com
Insulin syringe: 28G ½” needle with 500ul syringe, 329461, Fisher Scientific, www.fishersci.com
Iris curved surgical scissors: 10 cm, 14394, World Precision Instruments, Fisher Scientific, www.fishersci.com
Iris curved serrated non-toothed forceps: 10cm, 50–822-332, World Precision Instruments, Fisher Scientific, www.fishersci.com
Straight surgical scissors: 10cm, 14393, World Precision Instruments, www.fishersci.com
Covered Slide Folder: 48443–850, VWR, us.vwr.com
Lipid Pen, ImmEdge Hydrophobic Barrier PAP Pen: H-4000, Vector Laboratories, Inc. www.vectorlabs.com
REAGENTS:
Phosphate buffered saline (PBS), No added calcium chloride or magnesium chloride, #14190–136, Gibco
- Dextran, tetramethylrhodamine, 10,000 MW, lysine fixable (fluoro-Ruby): D1817, Thermofisher
- 25mg reconstituted with 1ml PBS to 25mg/ml can be stored at 4° C for 6 months as a stock solution
- Make a working solution of 2mg/ml dextran in PBS, approximately 300 μl per mouse will be required.
- DAPI: D1306, Thermofisher
- 10mg, reconstituted with 1ml DMSO for a stock solution that can be stored at −20° C for 6 months
- Make a working solution of 1 ug/ml in PBS for counter staining tissue sections
UEA I: FL1061–2, Vector Laboratories (Optional)
WGA: W11261, Thermofisher (Optional)
- Ketamine/ xylazine anesthesia : ketamine (100 mg/kg) xylazine (10 mg/kg)
- CAUTION, use of anesthesia should be performed in accordance with relevant guidelines and regulations.
- Mice: C57Bl/6 mice, aged 6–8 weeks.
- CAUTION, all experiments should be performed in accordance with relevant guidelines and regulations.
- Note: this protocol has been performed on mice as young as 5 days old and as old as 18 months old.
- 10% Buffered formalin: SF100–4, Fisher chemical, www.fishersci.com
- CAUTION: combustible liquid, may cause serious eye damage, skin irritation, respiratory irritation, and cancer. Use proper PPE when working with formalin fixative.
- 30% sucrose solution in sterile deionized water:
- Sucrose, S3–500, Fisher Chemical, www.fishersci.com, dissolved in deionized water
70% ethanol
EQUIPTMENT:
Cytostat (Leica, CM1860)
Immunofluorescence microscope: fluorescent wide field microscopy was performed with an axioskop 2 microscope and xenon lamp, using Axiovision software (Carl Zeiss, Thornwood, NY)
PROCEDURE:
1. Anesthesia and mouse preparation
NOTE: The following procedure, including animal preparation, surgery, and tissue extraction will take approximately one hour. Prior to the surgery, warm up a SnuggleSafe heating pad in microwave (1000 watts, 5 minutes). Drape absorbent underpad over heating pad and slice an incision in the pad.
Experiments are performed on 8–10 weeks old C57BL/6 mice (Jackson Laboratory). According to IACUC-approved procedure, anesthetize the mouse by intraperitoneal injection of ketamine (100 mg/kg) and xylazine (10 mg/kg) based on the weight of the animal (150–200 μL approximate volume). The level of anesthesia will be evaluated by respiration rate, and toe pinch reflex.
Once animals are properly anesthetized, place mouse on the heating pad with tail tucked in the incision to keep tail directly on the heating pad to maintain body temperature. If the anesthesia begins to wear off, administer ketamine/xylazine in 50 μL doses until the mouse is fully sedated.
Prepare the working solution of fluorescent dextran (2 mg/mL) in sterile PBS. Prepare petri dish with cold PBS. Prepare microcentrifuge tubes with 500 μl of 10% buffered formalin solution, labeled, one for every segment of intestine per mouse.
2. Nonsurgery and dextran administration
NOTE: During surgery care should be given to avoid puncturing the intestine or disrupting the mesenteric vasculature. To reduce damage, use non-toothed forceps, and minimal pressure. Also, avoid grabbing or pinching the intestines between the forceps and instead manipulate tissue with forceps by gentling “pushing” tissue around body cavity. Pinching the intestines could pierce or damage the intestines, and introduce more leak or excessive acetyl choline release.
Wet mouse fur with 70% alcohol. Cover animal with sterile surgical drapes. Using a 4cm curved scissor, make a 2cm vertical incision through the skin and peritoneum along the midline of the lower abdomen to expose the intestines.
Locate the cecum and carefully manipulate the intestine with curved forceps within the peritoneal cavity to identify the area of small intestine or colon to be analyzed.
Locate a region of interest within intestine or 2–3 cm in length. If analyzing the small intestine: segment should be free of or contain minimal detritus content and should avoid Peyer’s patches. Select similar regions (ie, proximal ileum, distal ileum) of the small intestine if analyzing multiple mice by comparing the distance from the region to the cecum. If analyzing the colon: segment should be free of or contain minimal fecal content. Preference should be given for the proximal colon, directly below the cecal junction above the formation of any fecal pellets. Care should be given to manipulate the intestinal segment to allow for injection of dextran yet attempt to keep the intestines within the peritoneum cavity to avoid drying out the tissue.
-
With a 28-gauge insulin syringe, inject 2 mg/ml dextran into the desired segment. Injection volume should be 100 μl for each intestinal area.
Note: this protocol does not utilize tying off intestinal loops, to avoid introducing damage to the mesenteric tissue or to the epithelium. Take care to note where injection of dextran was made based on distance from cecum or other anatomical markers.
Manipulate the intestines back fully into the body cavity and with two pairs of forceps, pull the skin and peritoneum up around the body cavity. Using an animal wound closure autoclipper, staple the incision closed with 9mm autoclips, taking care not to staple through intestines.
Wrap the mouse with a sterile drape and place animal on its side on the heating pad for 30 minutes while monitoring. An incubation step of 30 min allows for the dextran to fill GAPs; this incubation time is also suitable for mice of younger or older ages.
Note: surgery is presented in supplemental movie 1.
3. Tissue Preparation for immunohistochemistry
Note: if performing these steps on the same day, prepare 30% sucrose, and a cooler with dry ice surrounding a 500ml beaker. Locate Freezing compound (Freezeit).
After 30 minutes, sacrifice mouse using carbon dioxide or approved euthanasia method. Wet mouse fur with 70% alcohol, using curved scissors, cut the incision open removing the staples, taking care not to pierce the intestines.
-
Identify the region of intestines where dextran was injected.
Note: The dextran may have moved from original injection site due to peristalsis. This protocol recommends taking region where dextran was initially injected for optimal loading of GAPs or other epithelial cells.
-
Using 4cm straight scissors cut 2–3 cm segment around original dextran injection. Place intestinal segment directly in petri dish containing cold PBS. Remove mesenteric fat from intestine. Using straight scissors and forceps, open intestinal segment and briefly rinse with PBS to remove residual dextran and luminal detritus. Carefully removed any luminal matter that remains on the tissue, taking care not to scrape and damage the epithelium
Note: these steps are presented in supplemental movie 1.
-
Place opened segment of intestine into microcentrifuge with 10% formalin. Fix for 30 minutes at room temperature (or overnight at 4°C if necessary). Following fixation, remove 10% formalin with pipet, being careful not to damage the intestinal segment, and replace with 500 μl 30% sucrose. Incubate at room temperature for 6–12 hours, or overnight.
Note: The tissue will initially float in sucrose. As sucrose replaces the water in the tissue, the tissue will begin to sink to the bottom of the tube, depending on size of tissue and volume of sucrose. Once tissue sinks to the bottom of the tube it is ready to be blocked in a cryomold with OCT. This step helps prevent tissue from shrinking during the flash freezing process.
Add Freezeit to 500ml beaker in dry ice. Freezing process should require 1 cm of liquid covering the bottom of beaker. More freezeit may need to be added to beaker as experiment continues if freezeit evaporates off.
-
Using forceps remove tissue from sucrose, gently place on kimwipe to allow kimwipe to absorb extra liquid remaining on tissue.
Note: Liquid droplets on the tissue can prevent thorough freezing and introduce crystals in between the tissue and the OCT interface, which can result in torn tissue sections during sectioning.
Place dried tissue in a cryomold and add OCT to fill mold surrounding the tissue. Care should be given to avoid any bubbling of the OCT. Using a 26gauge syringe, pop any bubbles that may form. Label cryomold with permanent marker.
Using forceps, flash freeze the cryomold with the tissue by placing directly into beaker with Freezeit. Take care to keep cryomold level to avoid moving the tissue, or introducing bubbles into the cryomold. As tissue freezes, OCT will turn white. After approximately 1 minute, remove cryomold from Freezeit and place either on dry ice while other blocks are frozen, or directly into −80°C storage. Let freeze solid overnight for best results.
Note: these steps are presented in supplemental video 2.
4. Prepare slides and preform immunohistochemistry
-
Move OCT blocks from −80°C storage to cryostat set between −22 to −20°C. Let blocks sit in the cryostat for 30 minutes to allow blocks to warm to −20°C from −80°C.
Note: optimal temperature for sectioning frozen sections is between −22 and −20°C. Temperatures warmer that −20°C may result in melting of the block and smeared tissue. Temperatures colder than −22 may result in shattering of the block and tissue.
Place a drop of OCT, approximately 1cm in size, on chuck. Take blocks from cryomold tray and place on drop of OCT. Place chuck back in cryostat to allow block to freeze to the chuck for 10 minutes.
-
Section tissue to obtain 7 μm thick sections onto colorfrost plus microscope slides.
Note: for more instruction on sectioning tissue using a cryostat, process is presented in supplemental movie 3.
Allow slides to dry for 30 minutes in a covered slide folder to avoid photobleaching of slides.
Using a lipid pen draw a circle around the tissue on slide.
Add 1ml PBS to slide to rinse residual OCT from slide. Let sit 1 minute in covered slide folder.
-
Tap slide onto a paper towel to allow PBS to run off slide. Remove extra PBS from slide with pipet or vacuum with care not to scrap or touch tissue with pipet tip.
Note: this protocol can be performed with other immunohistochemistry protocols to stain intestinal tissue with other markers. This should be performed between steps 7 and 8 if desired.
Optional: Fluorescein labeled lectins, such as wheat germ agglutinin (WGA) or ulex europeaus agglutinin (UEA-I) can be used to identify goblet cells. Add WGA or UEA-I in PBS (1μg/ml) in 100 μl of volume onto slide to counterstain, incubate room temperature for 15 minutes in covered slide folder. Rinse slides with PBS. Dry slides by removing PBS with pipet or vacuum with care not to scrap or touch tissue with pipet tip
Add DAPI (1μg/ml) in 100 μl of volume onto slide to counterstain, incubate room temperature for 5 minutes in covered slide folder.
Rinse slides with PBS. Dry slides by removing PBS with pipet or vacuum with care not to scrap or touch tissue with pipet tip.
Add 10 ul 50% glycerol in PBS on top of each tissue section, with care not to scrap or touch tissue with pipet tip. Place coverslip on tissue.
5. Imaging of sections and GAP quantification
Image sections using standard fluorescent microscope with Xenon lamp. Tetramethylrhodamine labeled dextran will excite/emit at 555 nm/580 nm, DAPI will excite/emit at 358 nm/461 nm, fluorescein labeled lectins will excite/emit at 490 nm/525 nm.
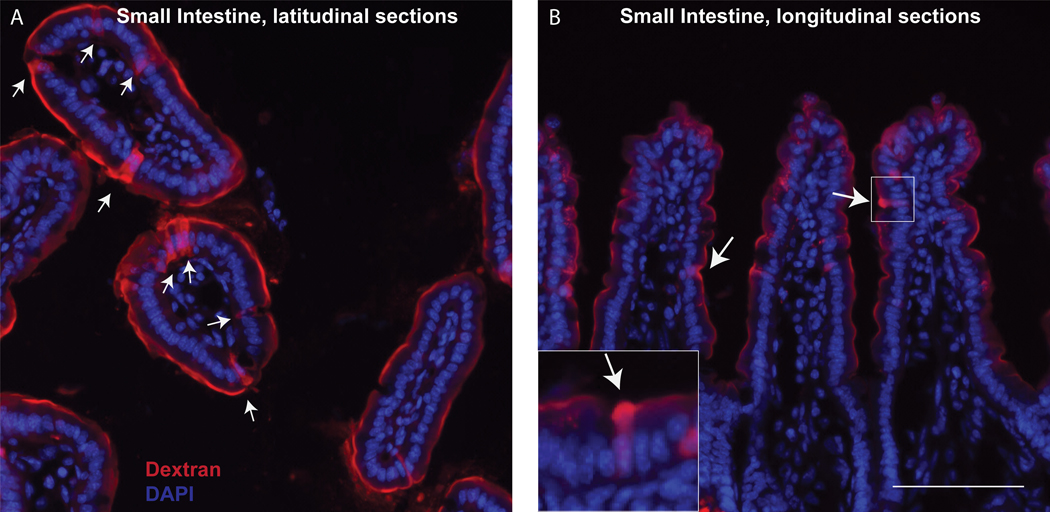
GAPs and epithelium will be visible at 20x and 40x dry lenses. GAPs can be identified as columnar epithelial cells containing a DAPI nucleus and containing dextran (Figure 1).
Count GAPs per villi (if small intestine) or GAPs per crypt (if colon), (Figure 1).
Figure 1:
Sections of small intestine in latitudinal (left) or longitudinal (right) following GAP labeling. White labels denote dextran-filled GAP. Scale bar = 100 μm
Note: This protocol uses 10kD rhodamine labeled lysine fixable dextran, resulting in labeling of GAPs brightly visible at using standard filters for Cy-3, PE, or Texas-Red channel of a fluorescent microscope, excitation 540nm, emission 565nm. Other sizes of dextrans, or other fluorescently labeled dextrans, can be used as desired. Lysine fixable dextran is required to fix the dextran in place for imaging on fixed tissue sections.
REPRESENTATIVE RESULTS:
DISCUSSION:
Increased intestinal permeability, or ‘leak’, thought to occur paracellularly through tight junctions 12 is associated with a variety of conditions13 including inflammatory bowel disease 14–16, Celiac disease17,18, obesity19,20, and cirrhosis21,22. In addition, it has become appreciated that in the healthy state the immune system surveys the luminal contents to initiate tolerogenic adaptive immune responses23. How substances traverse the epithelial barrier is a topic of significant interest and importance to health and disease. Assessing the intestinal epithelial barrier is often accomplished by the administration of undigestible sugars and their measurement in the urine or measuring 4kD fluorescent dextran in the serum following gavage24,25. While these approaches give insight into the permeability of the epithelial barrier, they do not identify how substances are crossing this barrier, which may also occur transcellularly26. In addition, the substances used in these approaches are generally too small for the induction of antigen specific T cell responses, as 4kD is on the lower end of the spectrum for peptides associated with MHC class II presentation 27,28. Indeed, several groups have shown targeting goblet cells with nanoparticles can improve effective delivery of orally administered proteins 29–35, supporting a role for goblet cells and GAPs in transporting larger proteins across the intestinal epithelium.
The protocol outlined above uses model substances of larger molecular weight and fixes these substances in place to evaluate how they cross the epithelial barrier. Translocation of larger substances to induce antigen specific T cell responses has been shown to utilize GAPs 1. While this protocol uses 10 kD dextran, other size dextrans can be utilized to assess the translocation of larger weight markers via GAPs. Soluble antigens of a variety of molecular weights such as ovalbumin (43kDa), bovine serum antigen (66 kDa), peanut agglutinin (110kDa), and lipopolysaccride (50–100 kDa) are also able to pass through GAPs 3. Passage of macromolecules, such as horse-radish peroxidase (40 kDa) occurs via goblet cells9, and is not associated with paracellular leak through tight junctions36. Using this protocol, we have visualized GAPs with fluorescent dextrans of 70 kDa to 1000 kDa (data not shown), consistent with work showing larger proteins, such as cationized ferritin (450 kDa) can be endocytosed by goblet cells6,7, though it was noted larger molecular weight markers required increased time to pass through the mucous barrier and gain access to the GAPs. Cursory experiments assessing GAP formation with fluorescent inert beads 200 nm in diameter were inconclusive, however live bacteria, including pathogens and gut commensal bacteria, have been observed to traverse the epithelium when GAPs are present 4,10.
This protocol does not use intestinal “loops” where sections of intestine are secured with sutures. Blockage of blood flow and pressure from the secured suture can introduce epithelial damage. Dextran may become introduced into apoptotic cells with disrupted cell membranes or through disrupted tight junctions, resulting in sub-optimal images. Additionally increased damage to the intestine may release acetylcholine, an activator of GAP formation2, or inflammatory cytokines, inhibitors of GAP formation4, which may falsely disrupt results of GAP formation.
The intestinal epithelium is, perhaps, the most common mucosal surface analyzed for translocation of antigens. However, other groups using similar dextran-based protocols have explored how antigens may cross the conjunctiva epithelium37, and the lung epithelium38, both epithelial surfaces that contain goblet cells. Indeed, goblet cells within the conjunctiva epithelium were found to contain dextran and deliver antigens to antigen-processing cells in a mechanism similar to intestinal GAP formation39. Thus, adapted protocols based off the procedure outlined here may be useful to study other mucosal surfaces and understand how antigens may cross the epithelium in the steady state, or breach the epithelium in times of inflammation.
When selecting a fluorescent marker care should be given to select a marker that can be fixed in place such as a lysine fixable dextran. If this is not available then alternative approaches such as the in vivo two photon imaging approach would be necessary to visualize epithelial cells taking up luminal substances. The presence of lysine ensures the marker will be crosslinked inside the tissue during fixation and tissue processing, necessary to prevent dextran from being washed out of the tissue during tissue processing, sectioning, and staining of slides. This fixation step may be incompatible with immunohistochemistry with some antibodies without using antigen retrieval methods. Antigen retrieval processes, such as citrate buffer antigen retrieval, can be used following sectioning to make the sections compatible with IHC for monoclonal antibodies for cell surface markers. Here we show a simple staining procedure using DAPI, a nuclear marker that is compatible with formalin fixed tissue, as a counterstain to the dextran labeled GAPs. The optional step to identify all goblet cells based on the lectins WGA+ or UEA-I+ binding mucus within the goblet cells allows for the assessment of the percentage of goblet cells to form GAPs within desired parameters. The presence of GAPs was found to directly correlate with the ability of lamina propria dendritic cells to initiate antigen specific T cell responses to luminal substances 5 and therefore this approach can be used as a surrogate to evaluate a pathway for sampling the luminal contents for the induction of adaptive immune responses to luminal substance.
Supplementary Material
Supplemental movie 1: Mouse surgery and intraluminal dextran administration
Supplemental movie 2: Embedding and freezing of tissue
Supplemental movie 3: Sectioning of tissue for slide preparation
Figure 2:
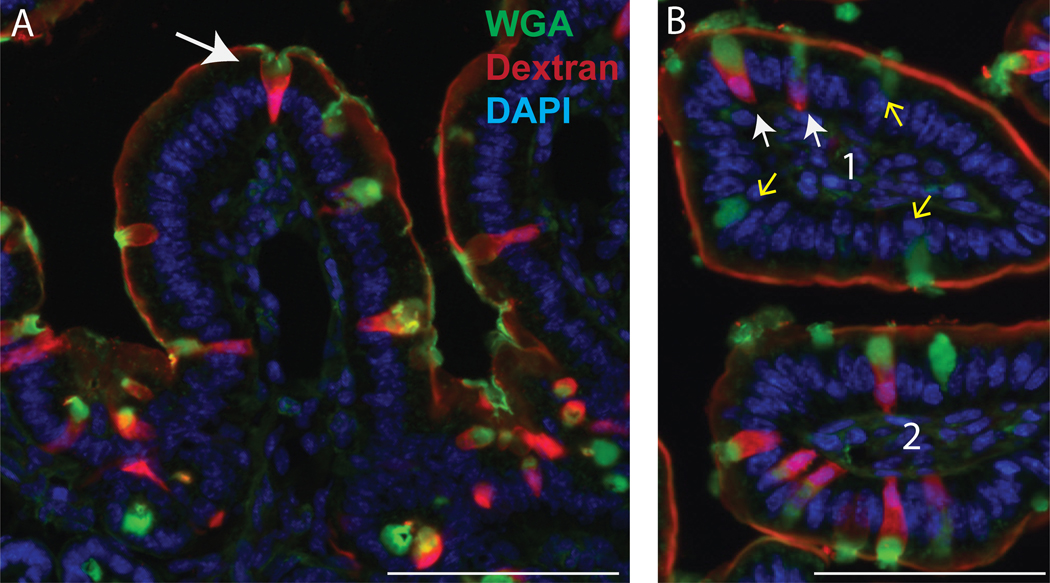
sections of small intestine in longitudinal sections (left) and latitudinal sections (right) following GAP labeling (dextran, red) with optional WGA staining (WGA, green), as described in section 4 step H. White arrows denote GAP: goblet cell containing dextran and WGA, green arrow denotes a WGA+ Goblet cell that is not forming a GAP and does not contain dextran. Scale bar = 100 μm
Figure 3:
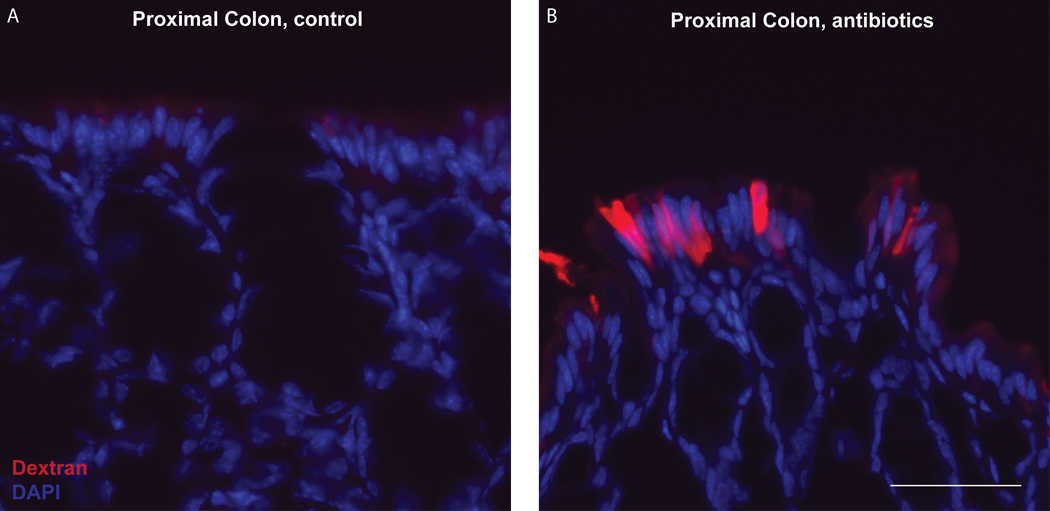
Sections of proximal colon in untreated mouse (control, left) or in a mouse 3 days following a single oral gavage of antibiotics (0.4mg/ml ampicillin in 200 ml drinking water). Dextran filled GAPs can be found following antibiotic disruption of microbial signals that inhibit colonic GAPs during the steady state. Scale bar = 50 μm
Figure 4:
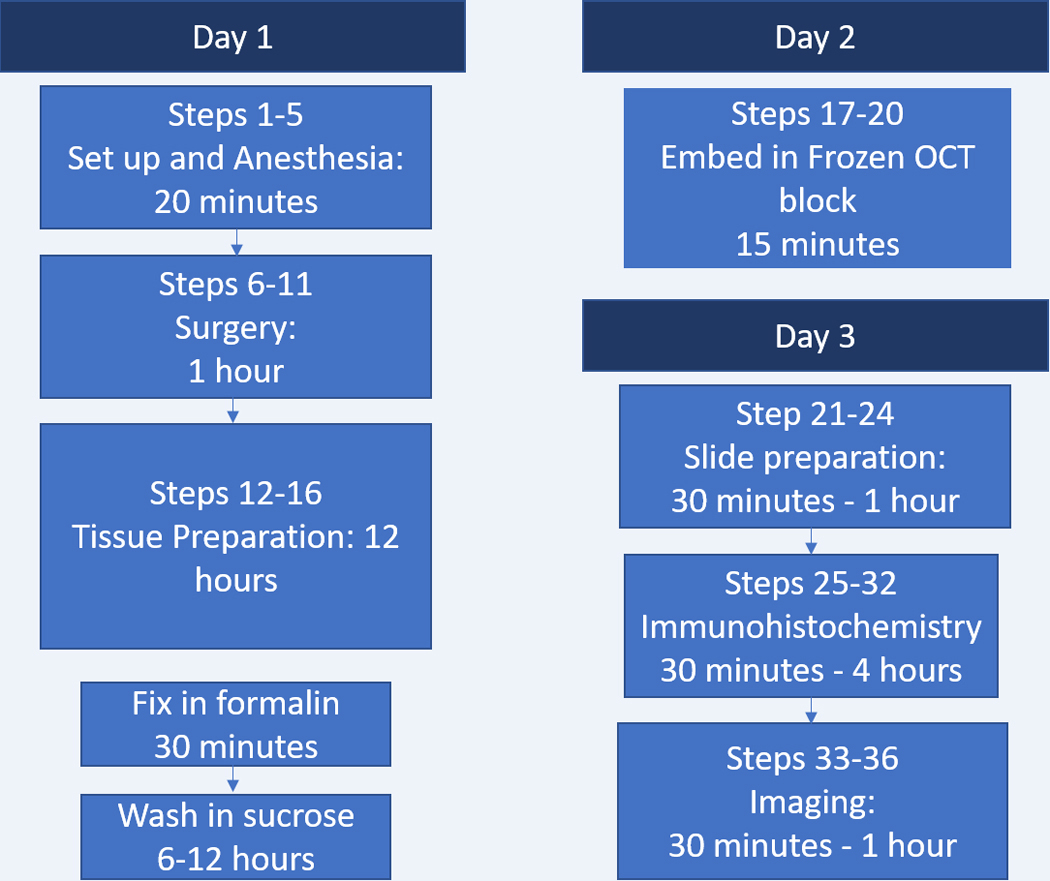
Timeline of Protocol
ACKNOWLEDGMENTS:
Supported by grants: DK64798-RDN, AI009550-RDN, DK097317-RDN, Children’s Discovery Institute Grant MD-II-2015–481–RDN, AGA Providence Food Intolerance Award–RDN, DK052574-KAK, DK09789-KAK, AI095542-KAK, Swedish Research Council International Postdoc Award 2014–00366 -JKG, Crohn’s and Colitis Foundation 610605-DHK
Footnotes
DISCLOSURES: RDN, KAK, and KMG are inventors on U.S. Nonprovisional Application Serial No. 15/880,658 Compositions And Methods For Modulation Of Dietary And Microbial Exposure.
REFERENCES:
- 1.McDole JR et al. Goblet cells deliver luminal antigen to CD103+ dendritic cells in the small intestine. Nature 483, 345–349, doi: 10.1038/nature10863 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Knoop KA, McDonald KG, McCrate S, McDole JR & Newberry RD Microbial sensing by goblet cells controls immune surveillance of luminal antigens in the colon. Mucosal Immunol 8, 198–210, doi: 10.1038/mi.2014.58 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Knoop KA et al. Antibiotics promote the sampling of luminal antigens and bacteria via colonic goblet cell associated antigen passages. Gut microbes 8, 400–411, doi: 10.1080/19490976.2017.1299846 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kulkarni DH et al. Goblet cell associated antigen passages are inhibited during Salmonella typhimurium infection to prevent pathogen dissemination and limit responses to dietary antigens. Mucosal Immunology 11, 1103–1113, doi: 10.1038/s41385-018-0007-6 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Knoop KA et al. Microbial antigen encounter during a preweaning interval is critical for tolerance to gut bacteria. Sci Immunol 2, doi: 10.1126/sciimmunol.aao1314 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Barbour WM & Hopwood D. Uptake of cationized ferritin by colonic epithelium. J Pathol 139, 167–178, doi: 10.1002/path.1711390208 (1983). [DOI] [PubMed] [Google Scholar]
- 7.Colony PC & Specian RD Endocytosis and vesicular traffic in fetal and adult colonic goblet cells. Anat Rec 218, 365–372, doi: 10.1002/ar.1092180403 (1987). [DOI] [PubMed] [Google Scholar]
- 8.Weiner ML Intestinal transport of some macromolecules in food. Food Chem Toxicol 26, 867–880 (1988). [DOI] [PubMed] [Google Scholar]
- 9.Kiliaan AJ et al. Stress stimulates transepithelial macromolecular uptake in rat jejunum. Am J Physiol 275, G1037–1044, doi: 10.1152/ajpgi.1998.275.5.G1037 (1998). [DOI] [PubMed] [Google Scholar]
- 10.Knoop KA, McDonald KG, Kulkarni DH & Newberry RD Antibiotics promote inflammation through the translocation of native commensal colonic bacteria. Gut 65, 1100–1109, doi: 10.1136/gutjnl-2014-309059 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nagatake T, Fujita H, Minato N. & Hamazaki Y. Enteroendocrine Cells Are Specifically Marked by Cell Surface Expression of Claudin-4 in Mouse Small Intestine. PLOS ONE 9, e90638, doi: 10.1371/journal.pone.0090638 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yu QH & Yang Q. Diversity of tight junctions (TJs) between gastrointestinal epithelial cells and their function in maintaining the mucosal barrier. Cell Biol Int 33, 78–82, doi: 10.1016/j.cellbi.2008.09.007 (2009). [DOI] [PubMed] [Google Scholar]
- 13.Turner JR Intestinal mucosal barrier function in health and disease. Nat Rev Immunol 9, 799–809, doi: 10.1038/nri2653 (2009). [DOI] [PubMed] [Google Scholar]
- 14.Heller F. et al. Interleukin-13 is the key effector Th2 cytokine in ulcerative colitis that affects epithelial tight junctions, apoptosis, and cell restitution. Gastroenterology 129, 550–564, doi: 10.1016/j.gastro.2005.05.002 (2005). [DOI] [PubMed] [Google Scholar]
- 15.Chang J. et al. Impaired Intestinal Permeability Contributes to Ongoing Bowel Symptoms in Patients With Inflammatory Bowel Disease and Mucosal Healing. Gastroenterology 153, 723–731.e721, doi: 10.1053/j.gastro.2017.05.056 (2017). [DOI] [PubMed] [Google Scholar]
- 16.Michielan A. & D’Incà R. Intestinal Permeability in Inflammatory Bowel Disease: Pathogenesis, Clinical Evaluation, and Therapy of Leaky Gut. Mediators of inflammation 2015, 628157–628157, doi: 10.1155/2015/628157 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cukrowska B. et al. Intestinal epithelium, intraepithelial lymphocytes and the gut microbiota - Key players in the pathogenesis of celiac disease. World journal of gastroenterology 23, 7505–7518, doi: 10.3748/wjg.v23.i42.7505 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Obrenovich ME Leaky Gut M, Leaky Brain? Microorganisms 6, 107, doi: 10.3390/microorganisms6040107 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hamilton MK & Raybould HE Bugs, guts and brains, and the regulation of food intake and body weight. International journal of obesity supplements 6, S8–S14, doi: 10.1038/ijosup.2016.3 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Araújo JR, Tomas J, Brenner C. & Sansonetti PJ Impact of high-fat diet on the intestinal microbiota and small intestinal physiology before and after the onset of obesity. Biochimie 141, 97–106, doi: 10.1016/j.biochi.2017.05.019 (2017). [DOI] [PubMed] [Google Scholar]
- 21.Shen L, Weber CR, Raleigh DR, Yu D. & Turner JR Tight Junction Pore and Leak Pathways: A Dynamic Duo. Annual Review of Physiology 73, 283–309, doi: 10.1146/annurev-physiol-012110-142150 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Utzeri E. & Usai P. Role of non-steroidal anti-inflammatory drugs on intestinal permeability and nonalcoholic fatty liver disease. World journal of gastroenterology 23, 3954–3963, doi: 10.3748/wjg.v23.i22.3954 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Knoop KA, Miller MJ & Newberry RD Transepithelial antigen delivery in the small intestine: different paths, different outcomes. Curr Opin Gastroenterol 29, 112–118, doi: 10.1097/MOG.0b013e32835cf1cd (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Volynets V. et al. Assessment of the Intestinal Barrier with Five Different Permeability Tests in Healthy C57BL/6J and BALB/cJ Mice. Digestive Diseases and Sciences 61, 737–746, doi: 10.1007/s10620-015-3935-y (2016). [DOI] [PubMed] [Google Scholar]
- 25.Wang L. et al. Methods to determine intestinal permeability and bacterial translocation during liver disease. Journal of immunological methods 421, 44–53, doi: 10.1016/j.jim.2014.12.015 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wu L-L et al. Commensal Bacterial Endocytosis in Epithelial Cells Is Dependent on Myosin Light Chain Kinase–Activated Brush Border Fanning by Interferon-γ. The American Journal of Pathology 184, 2260–2274, doi: 10.1016/j.ajpath.2014.05.003 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rock KL, Reits E. & Neefjes J. Present Yourself! By MHC Class I and MHC Class II Molecules. Trends in Immunology 37, 724–737, doi: 10.1016/j.it.2016.08.010 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Blum JS, Wearsch PA & Cresswell P. Pathways of Antigen Processing. Annual Review of Immunology 31, 443–473, doi: 10.1146/annurev-immunol-032712-095910 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kang SK et al. Identification of a peptide sequence that improves transport of macromolecules across the intestinal mucosal barrier targeting goblet cells. J Biotechnol 135, 210–216, doi: 10.1016/j.jbiotec.2008.01.021 (2008). [DOI] [PubMed] [Google Scholar]
- 30.Jin Y. et al. Goblet cell-targeting nanoparticles for oral insulin delivery and the influence of mucus on insulin transport. Biomaterials 33, 1573–1582, doi: 10.1016/j.biomaterials.2011.10.075 (2012). [DOI] [PubMed] [Google Scholar]
- 31.Fan T. et al. Design and evaluation of solid lipid nanoparticles modified with peptide ligand for oral delivery of protein drugs. Eur J Pharm Biopharm 88, 518–528, doi: 10.1016/j.ejpb.2014.06.011 (2014). [DOI] [PubMed] [Google Scholar]
- 32.Lee JY et al. Production of recombinant human growth hormone conjugated with a transcytotic peptide in Pichia pastoris for effective oral protein delivery. Molecular biotechnology 57, 430–438, doi: 10.1007/s12033-014-9835-0 (2015). [DOI] [PubMed] [Google Scholar]
- 33.Xia D. et al. Wheat germ agglutinin nanocage stabilized drug nanocrystals cross intestinal epithelium barrier via goblet cells. J Control Release 213, e25–26, doi: 10.1016/j.jconrel.2015.05.039 (2015). [DOI] [PubMed] [Google Scholar]
- 34.Xia D. et al. Enhanced transport of nanocage stabilized pure nanodrug across intestinal epithelial barrier mimicking Listeria monocytogenes. Biomaterials 37, 320–332, doi: 10.1016/j.biomaterials.2014.10.038 (2015). [DOI] [PubMed] [Google Scholar]
- 35.Kenngott EE et al. Identification of Targeting Peptides for Mucosal Delivery in Sheep and Mice. Mol Pharm 13, 202–210, doi: 10.1021/acs.molpharmaceut.5b00635 (2016). [DOI] [PubMed] [Google Scholar]
- 36.Madara JL & Trier JS Structure and permeability of goblet cell tight junctions in rat small intestine. J Membr Biol 66, 145–157 (1982). [DOI] [PubMed] [Google Scholar]
- 37.Barbosa FL et al. Goblet Cells Contribute to Ocular Surface Immune Tolerance-Implications for Dry Eye Disease. Int J Mol Sci 18, doi: 10.3390/ijms18050978 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mammoto A. et al. Control of lung vascular permeability and endotoxin-induced pulmonary oedema by changes in extracellular matrix mechanics. Nature Communications 4, 1759, doi:10.1038/ncomms2774 https://www.nature.com/articles/ncomms2774#supplementary-information (2013). [DOI] [PubMed] [Google Scholar]
- 39.Ko BY, Xiao Y, Barbosa FL, de Paiva CS & Pflugfelder SC Goblet cell loss abrogates ocular surface immune tolerance. JCI Insight 3, doi: 10.1172/jci.insight.98222 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental movie 1: Mouse surgery and intraluminal dextran administration
Supplemental movie 2: Embedding and freezing of tissue
Supplemental movie 3: Sectioning of tissue for slide preparation