Abstract
A sensitive and specific PCR method to detect Treponema pallidum in clinical specimens was developed. PCR primers were designed based on two unique features of the DNA polymerase I gene (polA). The first distinctive characteristic is that the region codes for a high cysteine content and has low homology with similar regions of DNA polymerase I gene from known microorganisms. The second unique feature is the presence of four insertions in the gene. PCR tests using primers designed on the basis these regions reacted with various pathogenic T. pallidum subspecies but did not react with nonpathogenic treponemal species or other spirochetes. An additional 59 species of bacteria and viruses, including those that cause genital ulcers, tested negative. This PCR method is extremely robust and sensitive. The detection limit is about 10 to 25 organisms when analyzed on gel. However, the analytic sensitivity can be increased by at least 1 log, to a detection limit of a single organism, when the ABI 310 Prism Genetic Analyzer is used to detect fluorescence-labeled amplicons. We further used this test in a clinical setting and compared the results with results from a previously reported multiplex-PCR test (for T. pallidum, Haemophilus ducreyi, and herpes simplex virus). We tested 112 genital ulcer specimens by the polA PCR, obtaining a sensitivity of 95.8% and a specificity of 95.7%. These results suggest that the polA PCR is applicable as a routine clinical diagnostic test for syphilis.
Current efforts toward the elimination of syphilis in the United States depend heavily on early identification of infected persons and prompt treatment to prevent the transmission of the infection (19). The diagnosis of syphilis is based on clinical features, observation of the organisms by dark-field microscopy, and serologic tests (12, 13). Detecting syphilis through clinical presentation is highly subjective and depends on factors such as the completeness of the case history, the presence of lesions, and the experience of the physician. Serologic tests for syphilis are relatively insensitive in the early stage of infection. Neither nontreponemal tests nor treponemal tests can detect antibodies until the infection has progressed 1 to 3 weeks after the development of the chancre (12, 13). Thus, direct detection of treponemes from clinical specimens has become an important method for the early diagnosis of Treponema pallidum infection. Among methods used for direct detection, dark-field microscopy of the ulcers is easy to perform; however, this method is relatively insensitive (sensitivity is approximately 105 organisms/ml) and requires special equipment (dark-field microscope) and trained and experienced laboratorians. Immunostaining of ulcer specimens with fluorescent antibodies (DFA-TP) is more sensitive than dark-field microscopy and is used for confirmation. However, interpretation of DFA-TP results can be subjective and also requires a good deal of experience. The rabbit infectivity test (RIT), which is still considered the “gold standard” test, has a detection limit of a single organism and can be used to confirm T. pallidum infection (12, 20). Performing this test, however, requires access to an animal facility and is extremely time-consuming and expensive.
The newest test for the direct detection of T. pallidum involves PCR. Several PCR methods have been reported. Each test uses a different target gene (2, 3, 7, 15, 18). The sensitivity of these assays varies from the equivalent of 10−3 organisms, obtained by reverse transcriptase PCR (RT-PCR) (3), to 10 to 50 organisms when the gene encoding the 47-kDa protein is used as the target. Although RT-PCR is extremely sensitive, isolating RNA can be time-consuming, because great care is required to prevent contamination of the specimen by unrelated organisms. A Southern blot step may be needed in addition to confirm the specificity. A multiplex-PCR method has been developed for the simultaneous detection of T. pallidum, Haemophilus ducreyi, and herpes simplex virus (15). The T. pallidum target is the 47-kDa gene, and enzyme-linked immunosorbent assay is used to detect the amplicon. Although some have hypothesized that the 47-kDa protein is involved in cell wall synthesis and would be expected to be conserved in related spirochetes, the exact function of the 47-kDa antigen is unclear (21). The lack of clarity has rendered the optimization of primers difficult, because homologous sequences are not available for comparison.
Here we report on the development of a highly sensitive and specific test in which primers are rationally designed based on two unique characteristics of the DNA polymerase I gene of T. pallidum (17).
(Part of the data in this work was presented at the International Conference on Emerging Infectious Diseases in Atlanta, Ga., July 2000).
MATERIALS AND METHODS
Microorganism strains and genital ulcer specimens.
Organisms used for specificity tests are listed in Table 1. T. pallidum subsp. pallidum (Nichols strain), T. pallidum subsp. pertenue (Gauthier strain), T. pallidum subsp. endemicum (Bosnia strain), Treponema denticola, Treponema phagedenis (Reiter strain), Treponema refringens (Noguchi strain), Borrelia burgdorferi, Neisseria gonorrhoeae, Neisseria sicca, Neisseria flavescens, Chlamydia trachomatis, and herpes simplex virus type 1 (HSV-1) and HSV-2 were maintained at the Centers for Disease Control and Prevention (CDC), Atlanta, Ga. Genomic DNA from Brachyspira hyodysenteriae (B78), Brachyspira innocens (B256), Brachyspira pilosicoli (P43), Brachyspira intermedia (PwS/A), Brachyspira murdochii (56/150), and Brachyspira alvinipulli (C1) were provided by Thad Stanton of the Animal Disease Research Center (Ames, Iowa). Leptospira subspecies were provided by the Special Pathogens Branch, CDC; Mycoplasma fermentans, Mycoplasma salivarium, Mycoplasma penetrans, Mycoplasma hominis, Mycoplasma genitalium, Mycoplasma pirum, Mycoplasma faucium, Mycoplasma pneumoniae, Clostridium tertium, Haemophilus influenzae, Bordetella pertussis, Chlamydia pneumoniae, Streptococcus pyogenes, Streptococcus mitis, Streptococcus oralis, Streptococcus sanguis, Streptococcus crista, Streptococcus parasanguis, Streptococcus gordonii, Streptococcus vestibularius, and Eikenella corrodens were provided by the Respiratory Disease Branch, CDC. Trichomonas vaginalis was provided by Allan Pillay, CDC. Genital ulcer specimens were obtained from routine surveillance (5).
TABLE 1.
Organisms used for specificity tests
| Organism |
|---|
| Spirochetes |
| Treponema pallidum subsp. pallidum (Nichols) |
| Treponema pallidum subsp. pertenue (Gauthier) |
| Treponema pallidum subsp. endemicum (Bosnia) |
| Treponema denticola |
| Treponema phagedenis Reiter |
| Treponema refringens Noguchi |
| Borrelia burgdorferi |
| Leptospira interrogans serogroups |
| Australis |
| Autumnalis |
| Ballum |
| Bataviae |
| Canicola |
| Celledoni |
| Cynopteri |
| Djasiman |
| Grippotyphosa |
| Hebdomadis |
| Icterohaemorrhagiae |
| Javanica |
| Pomona |
| Pyrogenes |
| Tarassovi |
| Brachyspira hyodysenteriae (B78) |
| Brachyspira innocens (B256) |
| Brachyspira pilosicoli (P43) |
| Brachyspira intermedia (PwS/A) |
| Brachyspira murdochii (56/150) |
| Brachyspira alvinipulli (C1) |
| Nonspirochetes |
| Trichomonas vaginalis |
| Haemophilus ducreyi |
| HSV-1 |
| HSV-2 |
| Neisseria gonorrheae |
| Chlamydia trachomatis |
| Mycoplasma fermentans |
| Mycoplasma salivarium |
| Mycoplasma penetrans |
| Mycoplasma hominis |
| Mycoplasma genitalium |
| Mycoplasma fermentans incognitas |
| Mycoplasma pirum |
| Mycoplasma faucium |
| Mycoplasma pneumoniae |
| Haemophilus influenzae (serotypes B and F) |
| Clostridium tertium |
| Eikenella corrodens |
| Neisseria sicca |
| Neisseria flavescens |
| Chlamydia pneumoniae |
| Bordetella pertussis |
| Streptococcus pneumoniae |
| Streptococcus pyogenes |
| Streptococcus oralis |
| Streptococcus mitis |
| Streptococcus sanguis |
| Streptococcus crista |
| Streptococcus parasanguis |
| Streptococcus gordonii |
| Streptococcus vestibularis |
PCR primers.
The two primer sets (F1-R1 and F2-R2) chosen for evaluation with clinical specimens give rise to amplicons of 377 or 395 bp, respectively, from the polA gene of T. pallidum. The sequences of the two primers and their positions in the published sequence (GenBank accession number TPU57757) are as follows: for primer set 1: F1 (forward primer), TGCGCGTGTGCGAATGGTGTGGTC (nucleotides 1759 to 1783, corresponding to amino acids CACANGVV) (in polA 1390 to 1413); R1 (reverse primer), CACAGTGCTCAAAAACGCCTGCACG (nucleotides 2111 to 2135, corresponding to amino acids RAGVFEHCA); for primer set 2: F2 (forward primer), CGTCTGGTCGATGTGCAAATGAGTG (nucleotides 1539 to 1563, corresponding to amino acids TSGRCANEC); R2 (reverse primer), TGCACATGTACACTGAGTTGACTCGG (nucleotides 1908 to 1933, corresponding to amino acids TESTQCTCA). The primers were synthesized either by the Biotechnology Core Facility, CDC, or by Integrated DNA Technologies, Inc. (Coralville, Iowa). The primers F1-F2 were labeled at the 5′ end with fluorescein phosphoramidite. The primers R1-R2 were labeled with tetrachloro-fluorescein phosphoramidite.
DNA preparation and amplification conditions.
DNA was extracted from 200 μl of the bacterial or viral cultures or ulcer specimen using a Blood-Tissue kit or DNA Mini Kit (Qiagen, Valencia, Calif.) following the manufacturer's instructions. All PCR assays were performed in 50-μl PCR vials. The reaction mixture contained 5 μl of 10× PCR buffer (50 mM KCl, 10 mM Tris hydrochloride, 3 mM MgCl2 [pH 8.3], 12% glycerol), 1 μl (50 mM final concentration) of each of the primers, 1 μl of each deoxynucleoside triphosphate (10 mM concentrations [each] of dATP, dGTP, and dCTP; 30 mM dUTP) 1 U of uracil-N-glycosylase (AmpErase; Roche Molecular Biochemicals, Indianapolis, Ind.), 5 μl of template DNA, and 1.75 U of Expand high-fidelity Taq polymerase (Roche Molecular Biochemicals). Amplification was performed in a model 9700 thermal cycler (Applied Biosystems, Foster City, Calif.). The following conditions were used for the first cycle: 50°C for 5 min to allow uracil-N-glycosylase inactivation of any carryover product and then 94°C for 5 min, 65°C for 1 min, and 72°C for 1 min. The following conditions were used for the subsequent 45 cycles: 94°C for 1 min, 65°C for 1 min, and 72°C for 1 min. Final extension consisted of one cycle at 72°C for 7 min, and then tubes were stored at 4°C until analyzed. Precautions were taken to avoid contamination. Briefly, all instruments and pipettes were cleaned regularly by wiping them with Windex or another glass cleaner, soaking them in a 1:100 dilution of bleach for 1 to 4 h, rinsing them with large quantities of double-distilled H2O, air drying them, and then exposing them to UV light (120 J) by using a Stratalinker 2400 device (Stratagene, La Jolla, Calif.). In addition, aerosol-resistant tips were used in all the experiments. All DNA extractions were performed in a room designated solely for this purpose, and all PCRs were performed in a hood dedicated to PCR use. Multiplex PCR (Roche Molecular Systems, Alameda, Calif.) was performed as described previously (5, 15).
Detection of amplicons using agarose gel electrophoresis and ABI 310 Genetic Analyzer.
Amplicons were detected by using a 1.5% agarose gel or the ABI 310 Prism Genetic Analyzer (Applied Biosystems). The gels were run with a 100-bp ladder (Life Technologies, Grand Island, N.Y.) at 100 V for 1 h. Bands were visualized on a UV transilluminator after staining with ethidium bromide (0.5 mg/ml; Sigma Chemical Co., St. Louis, Mo.). For ABI 310 analyses, each vial contained 1 μl of TAMRA standards varying in size from 100 to 500 bp, 14 μl of formamide, and 1.5 μl of amplicon. Next, the prepared vials were heated at 94°C for 4 min to denature the DNA; the contents were then injected into a 47-cm-long capillary filled with gel mixture (POP 4; Applied Biosystems). The capillary run was performed at 15 kV, 9 mA, and 60°C for 30 min for each sample. The specific band was analyzed with GenScan software (Applied Biosystems).
Computer methods and GenBank accession number.
The sequence for the polA gene and surrounding DNA has been assigned GenBank accession number TPU57757. Comparisons to known gene sequences were made by using the Genetics Computer Group package from the University of Wisconsin and the MacVector software package (version 5.0) of Eastman Kodak. Sequence alignments were made with the Gap and Bestfit programs in the Genetics Computer Group package.
RESULTS
Rational design of PCR primers.
Two pairs of PCR primers were designed to take advantage of the unique high cysteine content and the additional inserts in the DNA polymerase I gene of T. pallidum (17). In the first primer set, F1 and R1 are from amino acids 404 to 411 (CACANGVV) and 529 to 521 (RAGVFEHCA), respectively, and cover a total of three cysteines (two on the forward primer and one on the reverse primer). In the second primer set, F2 and R2 are from amino acids 430 to 438 (TSGRCANEC) and 454 to 461 (ESTOCTCA), respectively. They are located in the two additional insert regions in protein and DNA sequences compared with E. coli (17) and cover a total of four cysteines (two on forward and two on reverse) (Fig. 1). Data presented here utilized both sets of primers. The predicted amplicon was 377 bp for the first set of primers and 395 bp for the second set of primers. Both primer sets demonstrated similar sensitivity and specificity.
FIG. 1.
Primer selection and design. Primers were chosen from DNA sequences corresponding to the amino acid region that has additional insertions and cysteine residues.
Specificity of polA PCR.
To determine the specificity of the polA PCR method, we tested a panel of microorganisms, including pathogens involved in sexually transmitted diseases (such as N. gonorrheae, C. trachomatis, HSV-1 and -2, and T. vaginalis) and a variety of spirochetes (Table 1). The two sets of primers amplified DNA from only the three subspecies of T. pallidum (subsp. pallidum, pertenue, and endemicum). In addition, dot blot analyses of related spirochetes were performed using a probe corresponding to the polymerase domain of polA. The results indicate that this probe was specific for pathogenic T. pallidum but not for nonpathogenic spirochetes such as T. denticola, T. phagedenis, T. refringens, and B. burgdorferi (data not shown).
Sensitivity of polA PCR.
The analytic sensitivity of polA PCR was tested using known concentrations of organisms. T. pallidum harvested from rabbits was centrifuged at 1,000 × g to remove debris, and the organisms were enumerated with a dark-field microscope and suspended in double-distilled H2O. The enumerations were done by using a bacterium counting chamber, according to the method described by Turner et al. (20). The extracted DNA from these known concentrations of microorganism was then tested by using polA PCR. The polA PCR could consistently detect 10 organisms in the samples (Fig. 2). To improve the detection limit, we labeled the primers with the fluorescent dyes fluorescein phosphoramidite and tetrachloro-fluorescein phosphoramidite. The resulting amplicon was further analyzed with the ABI 310 Prism Genetic Analyzer. This fluorescence-detecting method can increase the sensitivity of the assay to one organism per sample (Fig. 3).
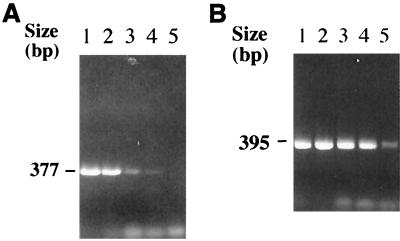
FIG. 2.
Results of agarose gel electrophoresis of polA PCR from serial dilutions of known concentrations of T. pallidum DNA extract. (A) Primer set I. The arrow indicates a 377-bp product. (B) Primer set II. The arrow indicates a 395-bp product. Lanes 1, 2 × 104 T. pallidum organisms per reaction; lanes 2, 2 × 103 organisms per reaction; lanes 3, 2 × 102 organisms per reaction; lanes 4, 2 × 101 organisms per reaction; lanes 5, 2 × 100 organisms per reaction.
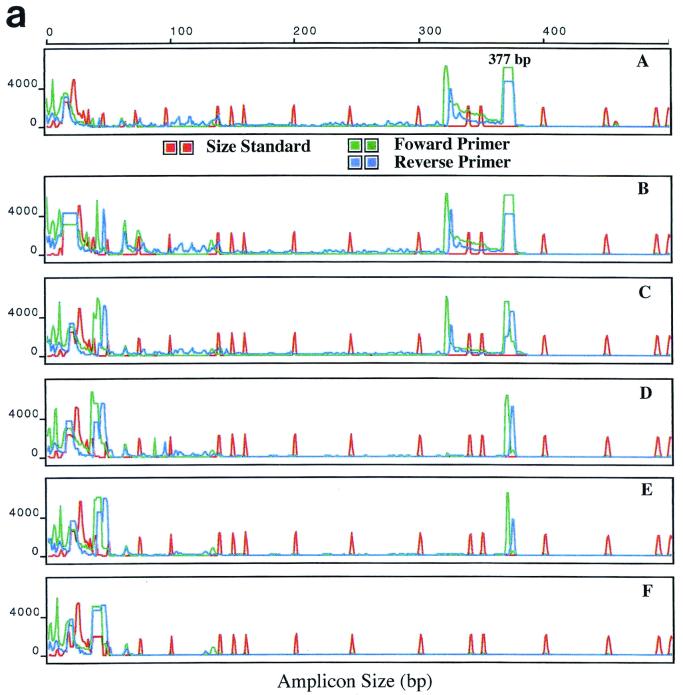
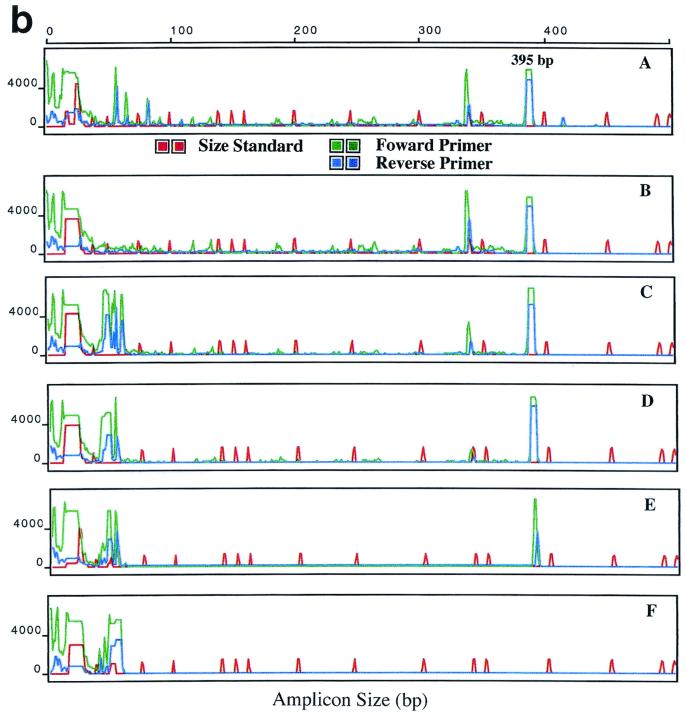
FIG. 3.
ABI 310 analyses of the polA PCR amplicon. PCR conditions were similar to those shown in Fig. 2. Amplicons from PCR of serial dilutions of T. pallidum DNA by using primer set I (a) and primer set II (b). Panels A to E show results obtained with 2 × 104, 2 × 103, 2 × 102 2 × 101, and 2 × 100 T. pallidum organisms per reaction; panel F, negative control. The x axis shows amplicon size in base pairs; the y axis shows relative fluorescence.
Detection of T. pallidum in clinical specimens.
To validate the method, we compared the results from polA PCR with results from a previously reported multiplex PCR (Roche Molecular System). The polA PCR amplicons were detected by using the agarose gel electrophoresis method in this experiment. A total of 112 genitourinary ulcer specimens were examined by the two methods. Twenty-two specimens tested positive by both methods; 85 specimens tested negative by both methods. One specimen tested negative by the polA PCR but positive by the multiplex PCR; four specimens tested positive by the polA PCR but negative by the multiplex PCR. Using multiplex PCR as the reference method, the polA PCR showed a sensitivity of 95.8% and specificity of 95.7%.
DISCUSSION
During the past 10 years, several target genes of T. pallidum have been used for detection by PCR. These genes include tpf-1 (14), the BMP gene (10, 14, 22), the tmpA and tmpB genes (7, 8), and the 47-kDa protein gene (2, 6, 11, 15, 18, 20); most recently RT-PCR has been performed by using the 16S rRNA (3). Most functions of the target genes are unknown or poorly characterized (e.g., those of tpf-1, the BMP gene, tmp, and the 47-kDa protein gene). The selection of primers based on these sequences requires testing a large repertoire of organisms to verify the specificity of the assay. The 47-kDa protein gene is the most commonly reported target for PCR, which may then require a DNA hybridization step to confirm the specificity. Nonspecific reactions have been reported from utilizing the 47-kDa protein gene PCR (3). The RT-PCR with the 16S rRNA also requires a Southern blot step to guarantee specificity because of the close relatedness of the 16S rRNA sequences among species. Thus, the specificity of current PCR methods is a major concern. Genetic variability within species may also affect the specificity of the PCR results (4, 16). Despite the fact that the T. pallidum genome is relatively conserved among strains, genes of unknown functions may contain insertions or deletions, as indicated by the recent reports of heterogeneities among T. pallidum species (arp, tpr, etc.) (4, 16). Unlike these other PCR targets, however, DNA polymerase I is a housekeeping enzyme important in DNA replication and repair. The sequence of polA is well described and is conserved between organisms, especially in the functional domains. The sequence comparison clearly illustrates the uniqueness of the regions that are rich in cysteine and the four insertions (17).
We designed two pairs of primers from regions that have insertions and are high in cysteine. In most organisms, DNA polymerase I contains only one to two cysteines. The DNA polymerase I of T. pallidum has 24 cysteines, which comprise 2.4% of its total amino acids. The first set of primers was selected from regions that have two cysteines on the forward strand primer and one cysteine on the reverse primer. The second set of primers has two cysteines on the forward strand primer and two cysteines on the reverse primer. These primers have been utilized in routine diagnostic PCR in this laboratory and have proven to be specific as well as sensitive. In addition, these primer sets can tolerate extreme variations of PCR conditions; variations in the PCR buffer concentration as great as twofold did not affect the results (data not shown).
In this study, we compared the polA PCR with a previously reported multiplex-PCR assay which has a sensitivity of 10 organisms (15). The multiplex PCR utilized a colorimetric step to amplify the signal and also to increase the specificity. The results of the two PCR methods are comparable. However, the multiplex-PCR method is not commercially available at this time.
The use of the ABI 310 Genetic Analyzer has advantages over agarose gel electrophoresis for the detection of the amplicons. The presence of nonspecific bands is seen in many PCRs. This could be due to variations in melting temperature during the priming phase, a delayed start of annealing between primers and templates, mutations of the templates, or mistakes in primer synthesis. Using fluorescence detection coupled with the use of an internal size standard, we were able to identify the specific band size of the DNA amplicon within five nucleotides of the predicted amplicon size. All nonspecific bands which have a molecular weight outside the predicted range can be excluded from data analyses, thus eliminating the subjective determination of banding patterns. To further reduce the possibility of a false-positive reaction, e.g., a false-positive band located in the correct size range, duplicate samples and duplicate PCRs were routinely used for specimens that potentially contained extremely low copy numbers of DNA.
The PCR detection limit of amplicons on agarose gel is usually 10 to 50 organisms. However, by using the fluorescence-labeled primers and the ABI 310 Genetic Analyzer, we can increase the sensitivity by approximately 1 log without affecting specificity (Fig. 3). Thus, in most of the clinical isolates, we were able to detect fewer than five organisms per PCR. The use of the ABI 310 device is especially advantageous for clinical specimens in which the number of organisms is low, such as whole blood and cerebrospinal fluid.
The theoretical detection limit of the polA PCR, when the ABI 310 device is used for analysis, is 200 organisms per ml or two organisms per PCR. This limit was calculated based on results from a 200-μl sample processed for DNA extraction, which resulted in 100 μl of eluted DNA. The current PCR method uses 5 μl of extracted DNA in a PCR. This improved sensitivity, to nearly one organism per PCR, has made the sensitivity comparable with that of the RIT. It is important to note, however, that the sensitivity of detection is limited by the sampling method. When the concentration of organisms is lower than one per 5 μl, sampling errors could affect detection. In addition, another limiting factor is the amount of extracted DNA that can be added to a PCR. It has been reported that inhibitors exist in the PCR template fraction (1, 9); this excludes the possibility of adding a large amount of DNA template to the reaction mixture. The current Qiagen extraction kit seems to have removed most of the inhibitors; however, we have not tested this factor directly. By contrast, in the RIT, relatively large quantities of sample (e.g., 0.5 ml) can be injected into the rabbit, increasing the test's sensitivity dramatically.
The PCR method we have reported here is not completely satisfactory, because of the time required for the entire procedure. The current method requires a DNA extraction step which usually takes about half an hour to 1 h, depending on the number of samples to be processed. The PCR takes 2 to 3 h, and the gel analyses take 1 h. ABI 310 analysis takes 30 min per sample. In the future, the sample processing procedure could be automated, real-time detection of the PCR could be used, and the procedure could be reduced to less than 1 h. The drawback, however, is that real-time PCR utilizes a fluorescence-based 5′ exonuclease assay (Taqman) detection and has a sensitivity level of 10 copies, which is about 1 log less sensitive than fluorescence detection with the ABI 310 (H. Liu, K. McCaustland, and B. Holloway, Int. Soc. Sex. Transm. Dis. Res. Meet., abstr. 19, 2001).
This PCR method has been successfully used to detect T. pallidum in a variety of specimens, such as whole blood, blood fractions, cerebrospinal fluid amniotic fluid, and genitourinary ulcers. These rationally designed primer pairs and detection with the ABI 310 device can be a useful addition to the methods available for diagnosing syphilis.
ACKNOWLEDGMENTS
We thank Brian Holloway for assistance in ABI 310 analyses; David Cox for assisting in enumerating T. pallidum organisms; Robert George, Mindy Zhang, Thad Stanton, Allan Pillay, and Robert Weyant for providing organisms; and Stephen Morse for helpful discussions.
REFERENCES
- 1.Abu Al-Soud W, Jonsson L J, Radstrom P. Identification and characterization of immunoglobulin G in blood as a major inhibitor of diagnostic PCR. J Clin Microbiol. 2000;38:345–350. doi: 10.1128/jcm.38.1.345-350.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Burstain J M, Grimprel E, Lukehart S A, Norgard M V, Radolf J D. Sensitive detection of Treponema pallidum by using the polymerase chain reaction. J Clin Microbiol. 1991;29:62–69. doi: 10.1128/jcm.29.1.62-69.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Centurion-Lara A, Castro C, Shaffer J M, Van Voorhis W C, Marra C M, Lukehart S A. Detection of Treponema pallidum by a sensitive reverse transcriptase PCR. J Clin Microbiol. 1997;35:1348–1352. doi: 10.1128/jcm.35.6.1348-1352.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Centurion-Lara A, Sun E S, Barrett L K, Castro C, Lukehart S A, Van Voorhis W C. Multiple alleles of Treponema pallidum repeat gene D in Treponema pallidum isolates. J Bacteriol. 2000;182:2332–2335. doi: 10.1128/jb.182.8.2332-2335.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen C Y, Ballard R C, Beck-Sague C M, Dangor Y, Radebe F, Schhmid S, Weiss J B, Tshabalala V, Fehler G, Htun Y, Morse S A. Human immunodeficiency virus infection and genital ulcer disease in South Africa. The herpetic connection. Sex Transm Dis. 2000;27:21–29. doi: 10.1097/00007435-200001000-00005. [DOI] [PubMed] [Google Scholar]
- 6.Grimprel E, Sanchez P J, Wendel G D, Burstain J M, McCracken G H, Radolf J D, Norgard M V. Use of polymerase chain reaction and rabbit infectivity testing to detect Treponema pallidum in amniotic fluid. J Clin Microbiol. 1991;29:1711–1718. doi: 10.1128/jcm.29.8.1711-1718.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hay P E, Clarke J R, Strugnell R A, Taylor-Robinson D, Goldmeier D. Use of polymerase chain reaction to detect DNA sequences specific to pathogenic treponemes in cerebrospinal fluid. FEMS Microbiol Lett. 1990;68:428–432. doi: 10.1111/j.1574-6968.1990.tb13943.x. [DOI] [PubMed] [Google Scholar]
- 8.Hay P E, Taylor-Robinson D, Goldmeier D. Detection of treponemal DNA in the CSF of patients with syphilis and HIV infection using polymerase chain reaction. Genitourin Med. 1990;66:428–432. doi: 10.1136/sti.66.6.428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Holodniy M, Kim S, Katzenstein D, Konrad M, Groves E, Merigan T C. Inhibition of human immunodeficiency virus gene amplification by heparin. J Clin Microbiol. 1991;29:676–679. doi: 10.1128/jcm.29.4.676-679.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Horowitz H W, Valsamis M P, Wicher V, Abbruscato F, Larsen S A, Wormser G P, Wicher K. Brief report: cerebral syphilitic gumma confirmed by the polymerase chain reaction in a man with human immunodeficiency virus infection. N Engl J Med. 1994;331:1488–1491. doi: 10.1056/NEJM199412013312204. [DOI] [PubMed] [Google Scholar]
- 11.Jethwa H S, Schmitz J L, Dallabetta G, Behets F, Hoffman I, Hamilton H, Lule G, Cohen M, Folds J D. Comparison of molecular and microscopic techniques for detection of Treponema pallidum in genital ulcers. J Clin Microbiol. 1995;33:180–183. doi: 10.1128/jcm.33.1.180-183.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Larsen S A, Steiner B M, Rudolph A H. Laboratory diagnosis and interpretation of tests for syphilis. Clin Microbiol Rev. 1995;8:1–21. doi: 10.1128/cmr.8.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Larsen S A, Norris S J, Pope V. Treponema and other host-associated spirochetes. In: Murray P R, Baron E J, Pfaller M A, Tenover F C, Yolken R H, editors. Manual of clinical microbiology. 7th ed. Washington, D.C.: ASM Press; 1999. pp. 759–776. [Google Scholar]
- 14.Noordhoek G T, Woltes T E C, De Jonge M E J, van Embden J D A. Detection by polymerase chain reaction of Treponema pallidum DNA in cerebrospinal fluid from neurosyphilis patients before and after antibiotic treatment. J Clin Microbiol. 1991;29:1976–1984. doi: 10.1128/jcm.29.9.1976-1984.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Orle K A, Gates C A, Martin D H, Body B A, Weiss J B. Simultaneous PCR detection of Haemophilus ducreyi, Treponema pallidum, and herpes simplex virus types 1 and 2 from genital ulcers. J Clin Microbiol. 1996;34:49–54. doi: 10.1128/jcm.34.1.49-54.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pillay A, Liu H, Chen C-Y, Holloway B, Sturm A, Steiner B, Morse S A. Molecular subtyping of Treponema pallidum subspecies pallidum. Sex Transm Dis. 1998;25:408–414. doi: 10.1097/00007435-199809000-00004. [DOI] [PubMed] [Google Scholar]
- 17.Rodes B, Liu H, Johnson S, George R, Steiner B. Cloning and characterization of a gene (polA) coding for an unusual DNA polymerase I from the obligate parasite Treponema pallidum. J Med Microbiol. 2000;49:657–667. doi: 10.1099/0022-1317-49-7-657. [DOI] [PubMed] [Google Scholar]
- 18.Sanchez P J, Wendel G D, Grimprel E, Goldberg M, Hall M, Arencibia-Mireles O, Radolf J D, Norgard M V. Evaluation of molecular methodologies and rabbit infectivity testing for the diagnosis of congenital syphilis and neonatal central nervous system invasion by Treponema pallidum. J Infect Dis. 1993;167:148–157. doi: 10.1093/infdis/167.1.148. [DOI] [PubMed] [Google Scholar]
- 19.St. Louis M. Strategies for syphilis prevention in the 1990s. Sex Transm Dis. 1996;23:58–67. doi: 10.1097/00007435-199601000-00012. [DOI] [PubMed] [Google Scholar]
- 20.Turner T B, Hardy P H, Newman B. Infectivity tests in syphilis. Br J Vener Dis. 1969;45:183. doi: 10.1136/sti.45.3.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Weigel L M, Radolf J D, Norgard M V. The 47-kDa major lipoprotein immunogen of Treponema pallidum is a penicillin-binding protein with carboxypeptidase activity. Proc Natl Acad Sci USA. 1994;91:11611–11615. doi: 10.1073/pnas.91.24.11611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wicher K, Noordhoek G T, Abbruscato F, Wicher V. Detection of Treponema pallidum in early syphilis by DNA amplification. J Clin Microbiol. 1992;30:497–500. doi: 10.1128/jcm.30.2.497-500.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]