Abstract
Background
The aims of this study were to (1) calculate the correlation between different tensile force levels and corresponding muscle stiffness both in vitro and in vivo; (2) determine whether muscle stiffness assessed using a MyotonPRO myotonometer can be used to accurately estimate muscle activity level; and (3) evaluate the inter-operator reliability of MyotonPRO-based measurement in assessing biceps brachii muscle (BBM) stiffness.
Material/Methods
In Experiment I, muscle stiffness, as measured using the MyotonPRO, was obtained at 0 N, 2 N, 4 N, 6 N, 8 N, and 10 N of applied force on 6 fresh medial gastrocnemius muscle specimens. In Experiment II, 11 healthy subjects were recruited. BBM stiffness, assessed by the same device, was obtained at different tensile force levels, from 0 to 50% of maximal voluntary contraction (MVC). For the reliability test, the score for each subject was quantified by 2 operators (I and II), thrice, at 30-minute intervals on the same day.
Results
A strong correlation was found between the different tensile force levels, which corresponded to muscle stiffness in vitro (r=0.71–0.95, all P<0.05). In vivo, muscle stiffness increased linearly with an increase of the tensile force levels from 0 to 50% of MVC (r=0.99, P=0.00) and there was a significant difference in BBM stiffness among the incremental isometric tasks (F [1.76, 17.60]=91.52, P=0.00). The inter-operator reliability for the measurement of BBM stiffness was good (ICC=0.86).
Conclusions
Our findings indicate that muscle stiffness measured using the MyotonPRO is strongly related to muscle activity level and that the MyotonPRO is a feasible tool for quantifying BBM stiffness as well as for quantifying changes in MVC levels.
Keywords: Elasticity; Muscle Strength; Muscle, Skeletal
Background
It is well established that skeletal muscle is made up of contractile components and noncontractile components. Clinically, it is difficult to accurately assess muscle stiffness due to the capacity of the spatial structure of muscle fibers and muscle stiffness to vary with muscle length and activation level. In addition, abnormal muscle stiffness has been demonstrated as an indicator of various disease states, such as in patients with Parkinson’s disease [1], chronic stroke [2], and low back pain [3]. In a study by Kalkhoven et al [4], higher levels of stiffness appeared to be good for athletic performance for football players. However, muscle functions are more than likely to be compromised if muscle tissue is too hard and is therefore unable to absorb adequate energy during functional activities [5]. Therefore, the objective measurement of muscle stiffness is essential to identify individuals who are at risk of disease and to prevent sports injuries.
Many technologies have been applied to evaluate musculoskeletal stiffness, such as magnetic resonance elastography (MR elastography) and shear wave elastography (SWE) [6,7]. However, each instrument has its own inherent limitations. For example, one published study proposed that the propagation of shear waves in nonhomogeneous media is complex, and that external interference may lead to the failure of accurate tissue stiffness evaluation by MR elastography [8]. SWE also has this limitation, similar to MR elastography. In addition, there is evidence that the pressure of the ultrasound probe on tissue may cause errors in the measurement of tissue stiffness via SWE [9]. The MyotonPRO is a new technology for assessing musculoskeletal stiffness; it is used widely in scientific fields [10–12] and can remedy the portability limitations of MRI and the control pressure limitations of SWE. However, the MyotonPRO device also has some limitations. One such limitation is that the device cannot be used for the measurement of nonpalpable muscles. Another limitation is thin muscles (< 3 mm) and muscles with small mass (<20 g). Our studies were conducted using the MyotonPRO to assess the upper trapezius, gastrocnemius muscle, and Achilles tendon, all of which showed high reliabilities [13–15].
Unfortunately, human muscle stiffness is commonly concentrated on the entire joint, including on the skeletal muscle and tendon. This limits the improvement of our clinical and scientific understanding of it. A reliable and valid method to assess the stiffness of skeletal muscle is important not only to gain insight into changes in skeletal muscle stiffness but also to improve diagnosis and intervention after an injury.
The present studies were untaken (1) to investigate the associations between tensile force levels and corresponding muscle stiffness in vitro and in vivo, (2) to determine whether muscle stiffness measured using the MyotonPRO can be used to accurately estimate muscle activity level, and (3) to evaluate inter-operator reliability of the MyotonPRO in measuring biceps brachii muscle (BBM) stiffness.
Material and Methods
Experiment I: Specimen Preparation
Four freshly slaughtered chickens (60 days old) were bought from our local food market (body mass 1.9±0.1 kg). The medial gastrocnemius muscles of the chickens were dissected at the Animal Laboratory in the Luoyang Orthopedic Hospital of Henan Province. A total of 8 fresh medial gastrocnemius muscle specimens were carefully harvested from the calcaneus and femur, and all soft tissue was cut off around the knee joints, such that all that remained was the small calcaneal tuberosity and distal femur. Then, each specimen was labeled. The length of the specimens was measured by a plastic meter that was used to adapt to the distance between the 2 clamps of a material testing machine (AGX-10KN, Shimadzu, Japan). The indoor temperature was controlled at 25 °C.
Experiment I: Experimental Procedure
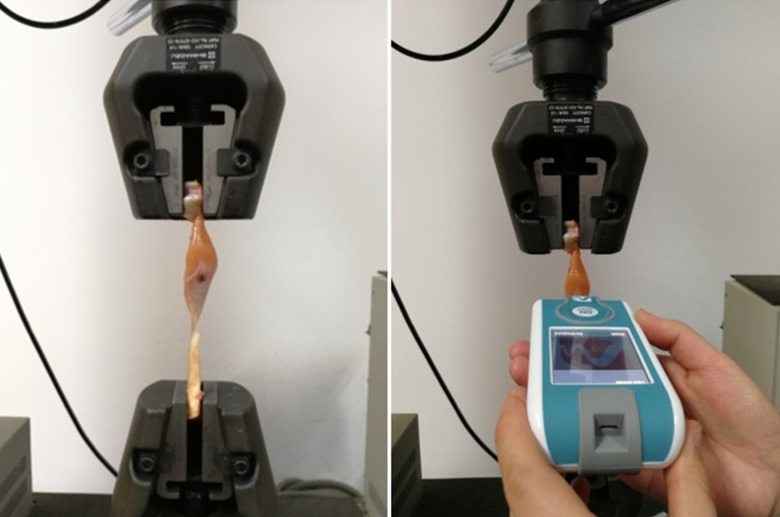
The location for stiffness assessment was at the junction of the upper third and middle third of the lines joining both tendon insertion sites and was marked with a permanent marker. For specimen insertion, a custom jig was used to firmly clamp the calcaneal tuberosity and distal femur of the harvested medial gastrocnemius muscles. A coupling device that fit around the head of the specimens was used to pull out each muscle at a loading rate of 2 mm/min until the force extended to 10 N. A maximum force of 10 N was chosen based on our own pilot study (using 2 specimens). The specimens were easily broken when the exerted force exceeded 10N. The strength was recorded by the load cells, and the displacement of the custom jig was metered by an extensometer. Both values were displayed online by the computer attached to the material testing machine. Muscle stiffness (unit: N/m) was obtained at 0 N, 2 N, 4 N, 6 N, 8 N, and 10 N of the applied force using the MyotonPRO (Figure 1). In order to minimize the effect of muscle creep, muscle stiffness was measured only once for each tensile force level.
Figure 1.
Experimental setup. The stiffness measurements of the medial gastrocnemius muscle specimens were obtained using the MyotonPRO.
Experiment II: Ethics Statement
The study was conducted at the Rehabilitation Therapy Center, Luoyang Orthopedic Hospital of Henan Province. This study was approved by the Ethics Committee (KY2019-001-01) of Henan Provincial Luoyang Orthopedic Hospital. The trial was registered in the International Clinical Trials Registry Platform (ChiCTR2000029282). The subjects were informed in detail of the purpose of the study and methods used and informed written consent was obtained before testing. The experimental procedures were conducted according to the Declaration of Helsinki.
Experiment II: Subjects
This study was conducted in March 2019. Eleven healthy subjects (6 men, 5 women; aged 25.4±3.9 years, height 169.9±9.1 cm, weight 61.5±15.0 kg, BMI 21.0±3.3) were recruited from a convenience sample at the rehabilitation therapy center of Luoyang Orthopedic Hospital of Henan Province. Exclusion criteria consisted of histories of neck pain, shoulder pain, lower back pain, joint instability, or upper limb surgery.
Experiment II: Experimental Procedure
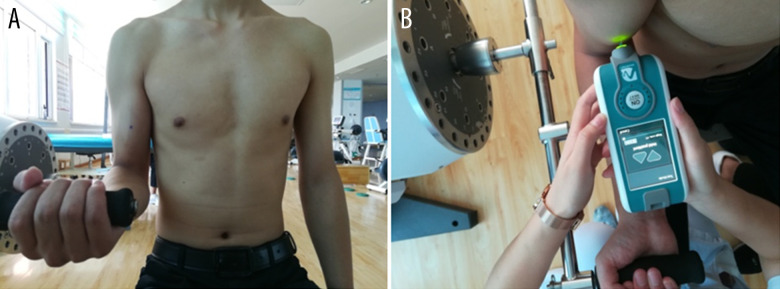
The dominant BBM of each subject was tested in this study. The dominant side was defined as the side of the subject’s writing hand [16]. Muscle activation was measured by the BTE Primus work stimulation training system (Baltimore Therapeutic Equipment, MD, USA) while the subject was seated in an upright position: shoulder flexion 0°, elbow flexion 90°. The right arm was placed on a platform that was adjustable in height. The point for stiffness measurement was determined centrally on BBM at about one-third of the total muscle length [17] (Figure 2A). Subjects were asked to lean their back against the back of the chair. First, each subject was asked to perform 3 maximal isometric voluntary elbow flexions (3 s each, with 1-min intervals between flexions) to determine maximal voluntary contraction (MVC). Second, each subject performed incremental isometric tasks (with 1-min rests between tasks) from 0 to 50% of MVC using direct visual feedback of the torque signal [18]. BBM stiffness was obtained at different tensile force levels using the MyotonPRO (Figure 2B). Three measurements (with 1-min rests between measurements) on each level of MVC were conducted, and the mean was computed for further analysis.
Figure 2.
(A) Subject was sitting with his elbow in 90° flexion and the point for stiffness measurement was marked on the BBM at about one-third of the total muscle length. (B) The stiffness measurements for the BBM were acquired using the MyotonPRO.
Reliability
For the inter-operator reliability test, each subject was assessed by 2 operators (ZJZ & YPL) with shoulder flexion 0° and elbow flexion 90° at 0 of MVC, with 30-min intervals between measurements. Both operators were blinded to the results during testing.
Data Analysis
SPSS (Version 23.0, SPSS Inc, Chicago, IL) was used for statistical analysis. All demographic data were calculated using descriptive statistics and are shown as mean±standard error of the mean (SEM). Pearson’s product-moment correlation coefficients were computed to explore the relationship between different tensile force levels and corresponding muscle stiffness. Significant effects were determined among incremental isometric tasks from 0 to 50% of MVC using a one-way repeated ANOVA, and the Bonferroni adjustment was used for multiple comparisons. The inter-operator reliability was examined using the intraclass correlation coefficient (ICC) based on a 2-way random-effects model. Reliability coefficients were categorized as follows: below 0.49 as poor, 0.50 to 0.69 as moderate, 0.70 to 0.89 as good, and 0.90 to 1.00 as excellent [15]. Minimum detectable change (MDC) and SEM were determined using the formula below: MDC=1.96×SEM×√2 and SEM=standard deviation×√1-ICC. Differences were considered significant at an alpha level of P<0.05.
Results
Experiment I: Relationship Between the Different Tensile Force Levels Corresponding to Muscle Stiffness in Vitro
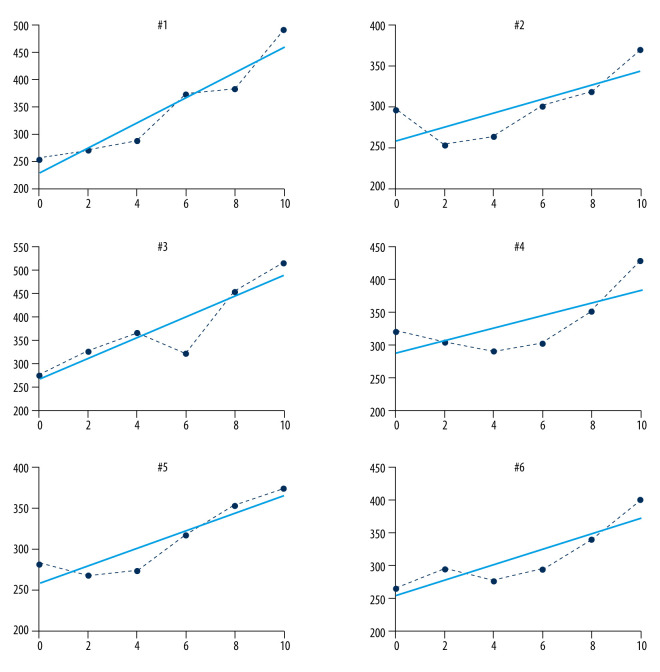
The mean in vitro stiffness values are shown inTable 1. Muscle stiffness changed slowly as the tensile force increased from 0 N to 2 N (from 284.17 to 287.17 N/m) and muscle stiffness increased sharply when the tensile force increased from 4 N to 10 N (from 293.67 to 431.33 N/m). The relationship between tensile force and stiffness is shown in Table 2. A significant, exponential correlation was revealed between the tensile force and stiffness values acquired from the material testing machine and the MyotonPRO in all tested specimens, with the correlation coefficient ranging from 0.71 to 0.95 (all P<0.05, Figure 3).
Table 1.
The difference in tensile force levels and corresponding muscle stiffness in vitro and in vivo (N/m).
| Descriptive statistics – stiffness for the different tensile force levels (mean±SD) | ||||||
|---|---|---|---|---|---|---|
| Experiment I (in vitro) | 0 N | 2 N | 4 N | 6 N | 8 N | 10 N |
| 284.17±25.25 | 287.17±27.67 | 293.67±37.73 | 319.17±28.36 | 367.83±47.46 | 431.33±61.73 | |
| Experiment II (in vivo) | Rest | 10% of MVC | 20% of MVC | 30% of MVC | 40% of MVC | 50% of MVC |
| 186.21±17.09 | 282.97±42.64 | 324.03±43.27 | 381.42±70.35 | 435.48±84.56 | 472.55±80.23 | |
SD – standard deviation; MVC – maximal voluntary contraction.
Table 2.
Pearson correlation coefficient between the tensile force and stiffness obtained from the material testing machine and the MyotonPRO, respectively.
| Chicken | r | P |
|---|---|---|
| #1 | 0.95 | 0.00 |
| #2 | 0.76 | 0.08 |
| #3 | 0.91 | 0.01 |
| #4 | 0.71 | 0.12 |
| #5 | 0.91 | 0.01 |
| #6 | 0.88 | 0.02 |
Figure 3.
Correlations between the stiffness of the medial gastrocnemius muscle specimens as measured by the MyotonPRO, and tensile force obtained using the material testing machine.
Experiment II: Relationship Between the Different Levels of MVC Corresponding to Muscle Stiffness in Vivo
The mean in vivo BBM stiffness values are shown in Table 1. Muscle stiffness increased linearly, with an increase in the tensile force levels (from 186.21 to 472.55 N/m). Similar to Experiment I, there was a strong correlation between the different tensile force levels that corresponded to muscle stiffness (r=0.99, P=0.00). Muscle stiffness also increased with increases in the incremental isometric tasks (Figure 4, dotted line).
Figure 4.
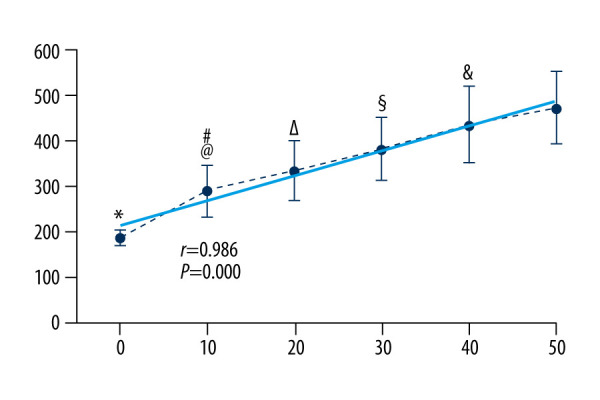
BBM stiffness obtained by the MyotonPRO at all measurement conditions; significant difference for conditions: * rest vs 10%, 20%, 30%, 40%, 50% of MVC; # 10% vs 30%, 40%, 50% of MVC; @ 10% vs 20% of MVC; Δ 20% vs 30%, 40%, 50% of MVC; § 30% vs 40%, 50% of MVC; & 40% vs 50% of MVC. Data are presented as group means±standard deviation.
Stiffness Differences Among Incremental Isometric Tasks, from 0 to 50% of MVC
A one-way repeated ANOVA for stiffness data indicated a significant difference in BBM stiffness among incremental isometric tasks from 0 to 50% of MVC (F [1.76, 17.60]=91.52, P=0.00). The 10% versus 20% of MVC and 40% versus 50% of MVC were P=0.03 and P=0.02, respectively (Figure 4).
Reliability
The inter-operator reliabilities for the assessments of BBM in the 11 healthy subjects were good (2-way random-effects model; ICC=0.86). The value for MDC was 13.33 N/m, corresponding to an SEM of 4.81 N/m as well as to a 95% CI; 0.49–0.96 (Table 3).
Table 3.
The inter-operator reliability of the MyotonPRO in measurement of BBM stiffness.
| Operator 1 (N/m) | Operator 2 (N/m) | SEM (N/m) | ICC | 95% CI | MDC (N/m) | |
|---|---|---|---|---|---|---|
| Dominant arm | 186.21±17.09 | 182.39±15.94 | 4.81 | 0.86 | 0.49–0.96 | 13.33 |
SEM – standard error of the mean; ICC – intraclass correlation coefficient; MDC – minimum detectable change, 95% CI – 95% confidence interval.
Discussion
The present study was designed to clarify the associations between differences in tensile force levels and corresponding muscle stiffness, in vitro and in vivo. Furthermore, this study aimed to assess whether muscle stiffness measured using the MyotonPRO can be used to accurately estimate muscle activity level, and likewise aimed to evaluate the inter-operator reliability of the MyotonPRO in measuring BBM stiffness. Our results indicate a significant correlation between the different tensile force levels and corresponding muscle stiffness in vitro and vivo. There was a significant difference in BBM stiffness between incremental isometric tasks from 0 to 50% of MVC. The results also demonstrate good inter-operator reliability in measuring BBM stiffness using the MyotonPRO.
Relationship Between Different Tensile Force Levels and Corresponding Muscle Stiffness in Vitro
In Experiment I, we demonstrated a significant exponential correlation between the different tensile force levels and corresponding levels of muscle stiffness. This correlation may be due to the fact that muscles are elastic tissue. In the initial stages of traction, muscle stiffness increased slowly in the elastic zone of muscle tissue. Furthermore, an increase in the length of muscle in the plastic zone was accompanied by a rapid increase in muscle stiffness. To the best of our knowledge, the present investigation is the first to show the relationship between different tensile force levels, and corresponding levels of muscle stiffness, as determined by the MyotonPRO in vitro. The findings were similar to those from earlier work by Eby et al [19], which evaluated deformation throughout tensile loading of porcine brachialis whole-muscle tissue specimens, while simultaneously making SWE measurements of those same specimens. The results of their study demonstrated that parallel SWE and the materials testing system showed increased stiffness measures with an increasing tensile load. Coincidentally, similar results were reported by Hatta et al [20]. Their study applied similar technologies to human supraspinatus muscle and evaluated the correlations between the SWE modulus and the extensibility of the muscle under 30 and 60 N loads. The results of their study showed that SWE measurements for the supraspinatus muscle were highly correlated with experimental extensibility. Haen et al [21] investigated the correlation between the shear moduli of human cadaveric Achilles tendon, obtained by SWE, and the elastic moduli of those tendons, acquired by tensile tests. The results of both measurements manifested that there was a statistical correlation (P<0.01) between shear moduli and apparent elastic moduli with a correlation coefficient R2=0.95±0.05. In our previous study, Zhang et al [22] revealed that the shear elastic modulus of the patellar tendon, as assessed by SWE, was related to the tangent traction modulus, quantified by the material testing machine (r=0.82–1.00, all P<0.05).
Relationship Between the Different Levels of MVC and Corresponding Muscle Stiffness in Vivo
In Experiment II, muscle stiffness increased with increases in incremental isometric tasks from 0 to 50% of MVC. Leonard et al [23] studied the relationship between BBM stiffness and sEMG during various levels of voluntary isometric contractions. They observed the strongest correlations between myotonometer and sEMG measurement (from −0.70 to −0.90). Jarocka et al [24] achieved similar results, reporting a high correlation between muscle stiffness using a Myoton-3 device and % MVC (<80% of MVC). A pilot study conducted by Nordez et al [18] was designed to explore whether the shear elastic modulus assessed using SWE can be used to accurately estimate muscle activity level (from 0 to 40% of MVC). The results showed there was a strong linear regression between shear elastic modulus and EMG activity level (P<0.00). Moreover, Ateş et al [25] determined a correlation between muscle shear elastic modulus and muscle force across the full range of contraction intensity (from 0 to 100% of MVC). Moreover, there was a linear regression between shear elastic modulus and joint torque over the entire range of contraction intensity (0 to 100% of MVC).
Stiffness Differences Among Incremental Isometric Tasks from 0 to 50% of MVC
The mean stiffness values differed significantly among the 6 groups of records. A recent study published by Marusiak et al [26] measured BBM stiffness at rest and at 10% of MVC in healthy elderly subjects and in subjects with Parkinson’s disease using a Myoton-3 device. They found that BBM stiffness was greater at 10% of MVC than at rest in each subpopulation, with an increase of 48.44% in elderly subjects and an increase of 44.83% in Parkinson’s disease patients. In our study, muscle stiffness was 51.61% higher at 10% of MVC than at rest. The reason our results differ from Marusiak’s may be due to our study focusing on healthy young adults. Interestingly, significant differences were reported by Ikezoe et al [27] in muscle stiffness between rest and contraction conditions among young, but not elderly, women using the myotonometer. Skeletal muscle consists of contractile components and noncontractile components. During contraction, increased muscle stiffness may be due to the activation of the motor units that cause changes in the spatial structure of the muscle. The subsequent results may be related to changes in the alignment of muscle structural proteins and the increase in intramuscular pressure.
Reliability
The present findings demonstrated good inter-operator reliability in measuring BBM stiffness, which was likewise in accordance with the results of previous studies. Van Deun et al [17] reported that inter-rater reliability was good to excellent in healthy subpopulations (ICC=0.82–0.90). These findings are also in accordance with the results of the present studies. In individuals with paratonia, inter-rater reliability ranged from low to high (ICC=0.65–0.73). In a study by Lo et al [28], which assessed BBM stiffness in the acute stroke population, the within-session inter-rater reliability of the MyotonPRO covered a broad range, with ICCs between 0.63 and 0.97. In our study, MDC and SEM values were 13.33 N/m and 4.81 N/m, respectively. Of note, the MDC percentage values (using the formula MDC%=(MDC/mean)×100%) were less than 10%, a value which mirrors the SEM percentage values (SEM%=(SEM/mean)×100%), which were also less than 10%. Chuang et al [29] suggested that an MDC% smaller than 10% can be considered excellent, while Flansbjer et al [30] showed that an SEM percentage below 10% was arbitrarily considered to be small and should be considered to be acceptable.
Limitations
This study has several limitations. In Experiment I, it should be noted that the stiffness and the extensibility outcomes obtained from the isolated muscle might differ from those observed in vivo. This difference warrants further investigation. Moreover, due to the creep of the mechanical properties of biological tissue, the results of muscle stiffness will be affected during the pulling process. In Experiment II, one limitation is that only healthy young adults were investigated. Therefore, our results cannot be considered to be representative of the entire population. Further studies, including studies with the elderly or different age groups, are required to clarify the association between age-related changes in muscle characteristics, such as muscle stiffness and muscle function, and to confirm the findings reported in the present study.
Conclusions
In conclusion, muscle stiffness measured using the MyotonPRO is highly related to muscle activity level. Our findings indicate that the MyotonPRO device is a feasible tool to quantify BBM stiffness as well as changes at the different levels of MVC.
Footnotes
Conflict of interest: The authors declare no commercial conflict of interest relevant to this article
Declaration of Figures’ Authenticity
All figures submitted were created by the authors, who confirm that the images are original, with no duplication, and have not been previously published in whole or in part.
Financial support: None declared
References
- 1.Du LJ, He W, Cheng LG, et al. Ultrasound shear wave elastography in assessment of muscle stiffness in patients with Parkinson’s disease: A primary observation. Clin Imaging. 2016;40:1075–80. doi: 10.1016/j.clinimag.2016.05.008. [DOI] [PubMed] [Google Scholar]
- 2.Eby S, Zhao H, Song P, et al. Quantitative evaluation of passive muscle stiffness in chronic stroke. Am J Phys Med Rehabil. 2016;95:899–910. doi: 10.1097/PHM.0000000000000516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Masaki M, Aoyama T, Murakami T, et al. Association of low back pain with muscle stiffness and muscle mass of the lumbar back muscles, and sagittal spinal alignment in young and middle-aged medical workers. Clin Biomech (Bristol, Avon) 2017;49:128–33. doi: 10.1016/j.clinbiomech.2017.09.008. [DOI] [PubMed] [Google Scholar]
- 4.Kalkhoven JT, Watsford ML. The relationship between mechanical stiffness and athletic performance markers in sub-elite footballers. J Sports Sci. 2018;36:1022–29. doi: 10.1080/02640414.2017.1349921. [DOI] [PubMed] [Google Scholar]
- 5.Chiu TC, Ngo HC, Lau LW, et al. An investigation of the immediate effect of static stretching on the morphology and stiffness of Achilles tendon in dominant and non-dominant legs. PLoS One. 2016;11:e0154443. doi: 10.1371/journal.pone.0154443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hong SH, Hong SJ, Yoon JS, et al. Magnetic resonance elastography (MRE) for measurement of muscle stiffness of the shoulder: Feasibility with a 3 T MRI system. Acta Radiol. 2016;57:1099–106. doi: 10.1177/0284185115571987. [DOI] [PubMed] [Google Scholar]
- 7.Sarabon N, Kozinc Z, Podrekar N. Using shear-wave elastography in skeletal muscle: A repeatability and reproducibility study on biceps femoris muscle. PLoS One. 2019;14:e0222008. doi: 10.1371/journal.pone.0222008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Souchon R, Salomir R, Beuf O, et al. Transient MR elastography (t-MRE) using ultrasound radiation force: Theory, safety, and initial experiments in vitro. Magn Reson Med. 2008;60:871–81. doi: 10.1002/mrm.21718. [DOI] [PubMed] [Google Scholar]
- 9.Mikołajowski G, Pałac M, Wolny T, et al. Lateral abdominal muscles shear modulus and thickness measurements under controlled ultrasound probe compression by external force sensor: A comparison and reliability study. Sensors (Basel) 2021;21:4036. doi: 10.3390/s21124036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kong PW, Chua YH, Kawabata M, et al. Effect of post-exercise massage on passive muscle stiffness measured using myotonometry – a double-blind study. J Sports Sci Med. 2018;17:599–606. [PMC free article] [PubMed] [Google Scholar]
- 11.Schneider S, Peipsi A, Stokes M, et al. Feasibility of monitoring muscle health in microgravity environments using Myoton technology. Med Biol Eng Comput. 2015;53:57–66. doi: 10.1007/s11517-014-1211-5. [DOI] [PubMed] [Google Scholar]
- 12.Chuang LL, Wu CY, Lin KC, Lur SY. Quantitative mechanical properties of the relaxed biceps and triceps brachii muscles in patients with subacute stroke: A reliability study of the myoton-3 myometer. Stroke Res Treat. 2012;2012:617694. doi: 10.1155/2012/617694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liu CL, Feng YN, Zhang HQ, et al. Assessing the viscoelastic properties of upper trapezius muscle: Intra- and inter-tester reliability and the effect of shoulder elevation. J Electromyogr Kinesiol. 2018;43:226–29. doi: 10.1016/j.jelekin.2017.09.007. [DOI] [PubMed] [Google Scholar]
- 14.Huang J, Qin K, Tang C, et al. Assessment of passive stiffness of medial and lateral heads of gastrocnemius muscle, achilles tendon, and plantar fascia at different ankle and knee positions using the MyotonPRO. Med Sci Monit. 2018;24:7570–76. doi: 10.12659/MSM.909550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Feng YN, Li YP, Liu CL, Zhang ZJ. Assessing the elastic properties of skeletal muscle and tendon using shearwave ultrasound elastography and MyotonPRO. Sci Rep. 2018;8:17064. doi: 10.1038/s41598-018-34719-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Leong HT, Ng GY, Leung VY, Fu SN. Quantitative estimation of muscle shear elastic modulus of the upper trapezius with supersonic shear imaging during arm positioning. PLoS One. 2013;8:e67199. doi: 10.1371/journal.pone.0067199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Van Deun B, Hobbelen JSM, Cagnie B, et al. Reproducible measurements of muscle characteristics using the MyotonPRO dfevice: Comparison between individuals with and without paratonia. J Geriatr Phys Ther. 2018;41:194–203. doi: 10.1519/JPT.0000000000000119. [DOI] [PubMed] [Google Scholar]
- 18.Nordez A, Hug F. Muscle shear elastic modulus measured using supersonic shear imaging is highly related to muscle activity level. J Appl Physiol (1985) 2010;108(5):1389–94. doi: 10.1152/japplphysiol.01323.2009. [DOI] [PubMed] [Google Scholar]
- 19.Eby SF, Song P, Chen S, et al. Validation of shear wave elastography in skeletal muscle. J Biomech. 2013;46:2381–87. doi: 10.1016/j.jbiomech.2013.07.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hatta T, Giambini H, Itoigawa Y, et al. Quantifying extensibility of rotator cuff muscle with tendon rupture using shear wave elastography: A cadaveric study. J Biomech. 2017;61:131–36. doi: 10.1016/j.jbiomech.2017.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Haen TX, Roux A, Soubeyrand M, Laporte S. Shear waves elastography for assessment of human Achilles tendon’s biomechanical properties: An experimental study. J Mech Behav Biomed Mater. 2017;69:178–84. doi: 10.1016/j.jmbbm.2017.01.007. [DOI] [PubMed] [Google Scholar]
- 22.Zhang ZJ, Fu SN. Shear elastic modulus on patellar tendon captured from supersonic shear imaging: Correlation with tangent traction modulus computed from material testing system and test-retest reliability. PLoS One. 2013;8:e68216. doi: 10.1371/journal.pone.0068216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Leonard CT, Brown JS, Price TR, et al. Comparison of surface electromyography and myotonometric measurements during voluntary isometric contractions. J Electromyogr Kinesiol. 2004;14:709–14. doi: 10.1016/j.jelekin.2004.06.001. [DOI] [PubMed] [Google Scholar]
- 24.Jarocka E, Marusiak J, Kumorek M, et al. Muscle stiffness at different force levels measured with two myotonometric devices. Physiol Meas. 2012;33:65–78. doi: 10.1088/0967-3334/33/1/65. [DOI] [PubMed] [Google Scholar]
- 25.Ates F, Hug F, Bouillard K, et al. Muscle shear elastic modulus is linearly related to muscle torque over the entire range of isometric contraction intensity. J Electromyogr Kinesiol. 2015;25:703–8. doi: 10.1016/j.jelekin.2015.02.005. [DOI] [PubMed] [Google Scholar]
- 26.Marusiak J, Jarocka E, Jaskólska A, Jaskólski A. Influence of number of records on reliability of myotonometric measurements of muscle stiffness at rest and contraction. Acta Bioeng Biomech. 2018;20(4):123–31. [PubMed] [Google Scholar]
- 27.Ikezoe T, Asakawa Y, Fukumoto Y, et al. Associations of muscle stiffness and thickness with muscle strength and muscle power in elderly women. Geriatr Gerontol Int. 2012;12(1):86–92. doi: 10.1111/j.1447-0594.2011.00735.x. [DOI] [PubMed] [Google Scholar]
- 28.Lo WLA, Zhao JL, Li L. Relative and absolute interrater reliabilities of a hand-held myotonometer to quantify mechanical muscle properties in patients with acute stroke in an inpatient ward. 2017;2017:4294028. doi: 10.1155/2017/4294028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chuang LL, Lin KC, Wu CY, et al. Relative and absolute reliabilities of the myotonometric measurements of hemiparetic arms in patients with stroke. Arch Phys Med Rehabil. 2013;94:459–66. doi: 10.1016/j.apmr.2012.08.212. [DOI] [PubMed] [Google Scholar]
- 30.Flansbjer UB, Holmback AM, Downham D, et al. Reliability of gait performance tests in men and women with hemiparesis after stroke. J Rehabil Med. 2005;37:75–82. doi: 10.1080/16501970410017215. [DOI] [PubMed] [Google Scholar]