Abstract
Background
This is an update of a Cochrane Review first published in Issue 3, 2004 of The Cochrane Library and previously updated in 2007.
The use of botulinum toxin for the treatment of spasmodic dysphonia is currently the treatment of choice for management of this neurological voice disorder. Over the past 20 years, botulinum toxin has been used to treat both adductor and abductor forms of the disorder, with vocal improvement noted after treatment for both. A large number of studies have attempted to document the efficacy of botulinum toxin for improvement of vocal symptoms in individuals with spasmodic dysphonia.
Objectives
To determine the effectiveness of botulinum toxin for treating spasmodic dysphonia.
Search methods
We searched the Cochrane Ear, Nose and Throat Disorders Group Trials Register; the Cochrane Central Register of Controlled Trials (CENTRAL); PubMed; EMBASE; CINAHL; Web of Science; BIOSIS Previews; Cambridge Scientific Abstracts; mRCT and additional sources for published and unpublished trials. The date of the most recent search was 22 July 2009, following a previous search update in 2007.
Selection criteria
All studies in which the participants were randomly allocated prior to intervention and in which botulinum toxin was compared to either an alternative treatment, placebo or non‐treated control group.
Data collection and analysis
Two authors independently evaluated all potential studies meeting the selection criteria noted above for inclusion. One study met the inclusion criteria and was included in the final analysis.
Main results
Only one study in the literature met the inclusion criteria. This was the only study identified which reported a treatment/no treatment comparison. It reported significant beneficial effects for fundamental frequency (Fo), Fo range, spectrographic analysis, independent ratings of voice severity and patient ratings of voice improvement.
Authors' conclusions
The evidence from randomized controlled trials does not allow firm conclusions to be drawn about the effectiveness of botulinum toxin for all types of spasmodic dysphonia, or for patients with different behavioral or clinical characteristics.
Plain language summary
Botulinum toxin injections for the treatment of spasmodic dysphonia
Botulinum toxin is currently the gold standard of treatment for patients with spasmodic dysphonia. It has been used over the past two decades to treat both adductor and abductor forms of the disorder. The results of this review of randomized controlled trials indicate that botulinum toxin is effective for some aspects of voice production, including perceptual measures of improvement post‐injection, variability of fundamental frequency, vocal intensity and subglottal air pressure. These benefits may be dependent on certain subject variables, such as the amount of voice use immediately post‐injection and treatment variables such as dosage and location of injection. These results should currently be interpreted with caution, however, as studies have used small sample sizes and have methodological differences which prevent between‐study comparisons.
Background
This is an update of a Cochrane Review first published in Issue 3, 2004 of The Cochrane Library and previously updated in 2007.
Spasmodic dysphonia is a voice disorder resulting from disrupted laryngeal motor control which causes involuntary movements of the laryngeal musculature during phonation. These involuntary movements may cause the vocal folds to inappropriately hyper‐adduct (close) (adductor spasmodic dysphonia) or abduct (open) (abductor spasmodic dysphonia), or in some cases do both (Aronson 1990; Cannito 1981). Adductor spasmodic dysphonia is the more common form of the disorder and is characterized by laryngeal muscle strain, a strained‐strangled and harsh voice quality, pitch breaks, and abnormally low fundamental frequency (glottal fry). Abductor spasmodic dysphonia is characterized by intermittent glottal widening and a transient breathy voice quality (Aronson 1990; Blitzer 1991; Cannito 1981). The severity of symptoms and disabling nature of the disorder can vary from patient to patient.
Understanding of the etiology of spasmodic dysphonia has evolved over time from theories of underlying psychological causes to current opinion which emphasises a primary neurological cause (Brin 1998a; Cannito 2001; Whurr 1993). As a type of dystonia, spasmodic dysphonia has been characterized as a chronic neurological disorder of central motor processing causing action‐induced muscular spasms (Blitzer 2001). On average the first signs of spasmodic dysphonia are seen in individuals of around 40 years (Brin 1998a). The disorder appears to occur more often in females, with familial involvement in approximately 12% of all cases (Brin 1998a; Cannito 2001).
Primary behavioral treatment for spasmodic dysphonia is relatively ineffective (Boone 2000; Cannito 2001). When combined with pharmacological therapy, however, behavioral treatment may assist in improving voice quality and prolonging the benefit of pharmacological effects (Murry 1995; Stemple 2000). In this regard, a number of complementary behavioral management strategies for spasmodic dysphonia have been suggested, including laryngeal tension‐reducing exercises, breath flow regulation, decreasing effort during phonation and co‐ordination of the speech subsystems (Cannito 2001; Murry 1995; Stemple 2000).
Botulinum toxin is generally regarded as the primary pharmacological treatment for adductor spasmodic dysphonia, and may also be beneficial in cases of abductor spasmodic dysphonia or mixed spasmodic dysphonia (Bielamowicz 2001; Cannito 2001). Botulinum toxin inhibits the release of acetylcholine at the motor end plates, resulting in a temporary paresis or paralysis of the injected muscle (Blitzer 2001; Langeveld 1998). Botulinum toxin is administered either unilaterally (injected into the left or right vocal fold muscle) or bilaterally (injected into the vocal fold muscles on both sides), with smaller dosage levels reported for effective bilateral injections (Adams 1995; Bielamowicz 2000; Bielamowicz 2001). There are also different injection techniques that may be utilized, including an electromyographic guided percutaneous technique, and a nasolaryngoscopic guided technique (Bielamowicz 2001; Rhew 1994).
Over the past 20 years, a large number of articles have been published which have described the use of botulinum toxin for the treatment of spasmodic dysphonia. A recent search of the literature revealed over 100 published articles that investigated this topic, characterized as either clinical studies, review articles, animal studies or non‐clinical studies. The majority of these manuscripts were clinical studies that attempted to document the effectiveness of botulinum toxin for the treatment of vocal symptoms secondary to spasmodic dysphonia. The methodology used in these studies is extremely variable. As an example, the independent (manipulated) variables selected for examination in the published literature have included injection type (unilateral versus bilateral, in both separate groups and in series), injection procedure, disorder type (adductor‐type versus abductor‐type), injection location, patient characteristics and treatment combinations (botulinum toxin with voice therapy or acupuncture) among others (Adams 1993; Bielamowicz 2002; Blitzer 1998; Drost 1998; Ford 1990; Ford 1992; Green 1992; Ludlow 1992; Lundy 1998; Schonweiler 1998). Dependent (measured) variables studied have included perceptual, electroglottographic, electromyographic, acoustic, endoscopic, stroboscopic, duration of benefit, aerodynamic and subjective rating measures (Fisher 1999; Mehta 2001; Lundy 1998; Papathanasiou 1997; Rodriguez 1994; Sapienza 2002; Whurr 1998; Wong 1995a; Zwirner 1991). The exact method in which independent and dependent variables have been studied is rarely consistent between any two investigations. However, the collective literature has provided ample evidence of the positive effectiveness of botulinum toxin for treating spasmodic dysphonia.
To date there have been two systematic reviews (Duffy 2003; Whurr 1997) and two meta‐analyses in the literature that have considered the effectiveness of botulinum toxin for spasmodic dysphonia (Boutsen 2002; Whurr 1998). However, the ability to draw conclusions from these studies is limited as data from randomized control studies were combined with data from a variety of research designs, or data from individual studies were reported separately and not combined for analysis. Numerous questions still need to be answered by randomized clinical trials:
Is botulinum toxin treatment of spasmodic dysphonia more effective in providing temporary symptomatic relief of vocal spasms (e.g. improving voice quality) than no treatment?
Are bilateral injections more effective for improving voice quality than unilateral injections?
Is botulinum toxin more effective for improving voice quality in abductor spasmodic dysphonia, adductor spasmodic dysphonia, or mixed spasmodic dysphonia?
Are bilateral injections associated with more frequent adverse events compared to unilateral injections?
Are different dosage levels associated with differences in the frequency of reported adverse events?
Is botulinum toxin alone more effective for improving voice quality compared to behavioral voice therapy alone, or in conjunction with post‐injection voice therapy?
Is one type of botulinum toxin product more effective for improving voice quality than another?
There have been no systematic reviews evaluating the effectiveness of botulinum toxin treatment for spasmodic dysphonia using only randomized trials. Thus the purpose of this review is to assess the effectiveness of the use of botulinum toxin for the treatment of spasmodic dysphonia using only randomized controlled trials as the standard for summary and analysis.
Objectives
To assess the effectiveness of botulinum toxin in the treatment of spasmodic dysphonia using data from randomized controlled trials.
Methods
Criteria for considering studies for this review
Types of studies
All randomized controlled trials that compared the use of botulinum toxin with a placebo, no treatment, or alternative treatments.
Types of participants
Participants in these trials were adults whose primary diagnosis was adductor, abductor or mixed spasmodic dysphonia.
Types of interventions
Experimental interventions included any unilateral or bilateral injection of botulinum toxin into a muscle or muscles of the laryngeal mechanism. We set no restriction on dosage, number of treatments or time to outcome measure.
Types of outcome measures
The major outcome measures studied in the randomized controlled trials included the following.
Acoustic function, or instrumental measurement of the voice spectrum via computerized analysis (e.g. fundamental frequency, frequency and amplitude perturbation, signal‐to‐noise ratio);
Perceptual ratings, or subjective ratings of voice quality or ability (e.g. subjective voice improvement, spectrographic ratings of normalcy);
Aero‐dynamic function, or instrumental measurement of the air flow and air pressure that is needed to vibrate the vocal folds (e.g. mean airflow, coefficient of airflow variation, air pressure).
Search methods for identification of studies
We conducted systematic searches for randomized controlled trials. There were no language, publication year or publication status restrictions. The date of the last search was 22 July 2009, following a previous search update in 2007.
Electronic searches
For the update of this review we searched: the Cochrane Ear, Nose and Throat Disorders Group Trials Register; the Cochrane Central Register of Controlled Trials (CENTRAL, The Cochrane Library Issue 2, 2009); PubMed; EMBASE; CINAHL; AMED; LILACS; KoreaMed; IndMed; PakMediNet; CAB Abstracts; Web of Science; BIOSIS Previews; CNKI; mRCT (Current Controlled Trials); ClinicalTrials.gov; ICTRP (International Clinical Trials Registry Platform) and Google.
We modelled subject strategies for databases on the search strategy designed for CENTRAL. Where appropriate, we combined subject strategies with adaptations of the highly sensitive search strategy designed by the Cochrane Collaboration for identifying randomized controlled trials and controlled clinical trials (as described in The Cochrane Handbook for Systematic Reviews of Interventions Version 5.0.1, Box 6.4.b. (Handbook 2008)). Search strategies for the major databases including CENTRAL are provided in Appendix 1.
Searching other resources
For the review update, we scanned reference lists of identified studies for further trials. We searched PubMed, TRIPdatabase, NHS Evidence ‐ ENT and Audiology and Google to retrieve existing systematic reviews possibly relevant to this systematic review, in order to search their reference lists for additional trials. We sought abstracts from conference proceedings via the Cochrane Ear, Nose and Throat Disorders Group Trials Register. In 2007, we also performed the following searches.
We handsearched journals relevant to the topic (e.g. Medical Journal of Speech Language Pathology, Journal of Voice, Journal of Speech‐Language‐Hearing Research, Annals of Otology, Rhinology and Laryngology, Laryngoscope, Otolaryngology ‐ Head and Neck Surgery, International Journal of Human Communication Disorders, Journal of Neurology, Neurosurgery, Psychiatry).
We contacted universities/hospitals/centres where there were individuals who engaged in spasmodic dysphonia treatment using botulinum toxin for additional citations and details of ongoing research.
We reviewed conference proceedings and we attempted to retrieve relevant citations from presenters.
We contacted authors who have researched in this area and asked them to provide any unpublished data or studies.
Data collection and analysis
Selection of studies
We obtained abstracts for all studies which were potentially appropriate for inclusion in the review. Two authors assessed whether or not the complete study should be retrieved. For all studies clearly appropriate to the review we obtained a complete copy of the study. For those studies in which the abstract was not clear regarding the appropriateness, we obtained a copy of the full study. We collected complete articles and two authors read and evaluated each study independently for inclusion criteria (i.e. random allocation). Authors were not blind to author or source, and any differences in study selection were resolved by discussion between the authors.
Assessment of risk of bias in included studies
Each author independently assessed the methodological quality of the studies identified for possible inclusion. Any discrepancy was resolved by consensus discussion.
The categorization of the methodological quality included an assessment of each study according to the following categories:
A ‐ adequate concealment;
B ‐ unclear concealment;
C ‐ inadequate concealment.
Adequate concealment, category A, included any form of random assignment in which the individual participant's group assignment was unknown prior to the actual assignment and an acceptable randomization procedure, such as computer‐generated allocation, was used for group assignment. If the author(s) of the study indicated that participant assignment to the experimental and control conditions was accomplished using a randomized process but gave no specific information regarding the details of the randomizing process, that study would at best be classified in category B, as using an unclear concealment procedure. Only studies reporting adequate or unclear concealment were assigned to the included studies group. Studies reporting no randomizing procedure were automatically categorized as reflecting an inadequate concealment procedure, category C, and were not included for the review or analysis. No study reporting more than 20% attrition was assigned to the included studies group.
Data synthesis
We collated the data from the studies using RevMan 5.0 (RevMan 2008). Studies reporting binary outcomes were to be summarized using odds ratios (OR) with 95% confidence intervals (CI). Studies reporting continuous outcomes were summarized using weighted or standardized mean differences (WMD/SMD) with a 95% confidence interval. Where the outcome had been measured using the same measure, a weighted mean difference was to be used. Where different measures were used to measure the same outcome the standardized mean difference was to be calculated to allow comparison.
Sensitivity analyses were to be performed to investigate heterogeneity of results based on design quality.
It was planned that, where possible, subgroup analyses would be performed to look at the comparative efficacy. Areas of subgroup analysis were to include (if applicable):
different types of intervention compared with untreated controls;
injection site;
dosage and dilution;
type of toxin used;
side effects and adverse events; and
first treatment versus subsequent treatment(s).
Results
Description of studies
Results of the search
From the 2009 update searches a total of 150 abstracts were retrieved; 10 of the references were duplicates and the remaining 140 abstracts were ineligible for inclusion due to subject scope, methodology (e.g. review article, non‐clinical study) and lack of non‐randomized control design. From the searches conducted in 2007, a total of 77 abstracts were identified. Of these, 70 were found to be ineligible for inclusion due to methodology. Of the seven randomized controlled trials, four articles did not report appropriate data for analysis and authors were either unable to provide the appropriate data or did not respond to contact attempts. Of the three remaining studies, two did not meet inclusion requirements due to the nature of the treatment protocol (Finnegan 1999; Wong 1995b). The remaining study (Troung 1991) met the inclusion criteria.
Included studies
Troung 1991 was the only study which could be included in the review. Troung utilized a double‐blind, placebo‐controlled study to examine the effects of botulinum toxin (BotoxTM) on voice quality (via spectrographic analysis), perceived voice improvement, and acoustic measures in subjects with adductor spasmodic dysphonia who received either drug or saline injection into the thyroarytenoid muscles. Thirteen subjects were randomly assigned to either the botulinum toxin or saline treatment groups. Perceptual and acoustic analyses were applied to recordings of each subject producing sustained vowel productions. Perceptual variables included a speech rating scale completed by the investigators that judged the normalcy of spectrograms generated from the vowel productions, related to periodicity, the presence of high frequency energy and voice breaks. In addition, experimental subjects completed a self‐rating scale related to degree of improvement post‐injection and an independent judge rated the degree of voice severity pre‐injection and post‐injection. Acoustic variables included fundamental frequency, phonation time, fundamental frequency range and perturbation measures.
Outcome measures revealed that subjects injected with botulinum toxin exhibited significantly decreased perturbation and fundamental frequency range compared to subjects who received saline. In addition, the botulinum toxin group exhibited a significant improvement in ratings of speech quality (spectrographic analysis) and perceived improvements compared to the subjects who received saline (for both self and independent ratings). The authors proposed that results demonstrated an effective outcome for botulinum toxin, both for perceptual ratings and objective measures of voice production, at least in the treatment of adductor spasmodic dysphonia.
Excluded studies
Finnegan 1999 utilized a randomized, double‐blinded cross‐over design to study airflow measures as a function of injection site (intrinsic laryngeal muscle only versus intrinsic laryngeal muscle plus laryngeal strap muscles). Participants were randomly assigned to one of two treatment protocols. However, while both groups of participants received the 2.5 unit bilateral injection into the intrinsic muscle (thyroarytenoid), both groups received differing bilateral injections during the alternative intervention. One group received 2.5 units of saline in the thyroarytenoid and sternothyroid while the second group received 7.5 units of botulinum toxin. Thus, the effects of the botulinum toxin cannot be assessed.
Wong 1995b examined acoustic and aerodynamic measurements subsequent to botulinum toxin injection in two experimental groups of speakers with spasmodic dysphonia: a non‐vocalization group who remained silent for 30 minutes after botulinum toxin injection, and a vocalization group who read aloud at normal conversational loudness for 30 minutes after the injection. Twenty subjects in total were randomly assigned to one of the two groups. Each subject received 2.5 units of botulinum toxin to the thyroarytenoid muscles, bilaterally. Acoustic measures were obtained from audio recordings of the subjects producing sustained vowel prolongation of /a/. Aerodynamic measures were obtained in the context of having each subject produce three trials of uttering the syllable /pi/ repetitively. Both acoustic and aerodynamic measures were obtained prior to injection (baseline), and at two and ten weeks post‐injection.
Wong 1995b could not be included in the analysis because, although participants were randomly assigned to different groups, both groups received botulinum toxin injections as part of the treatment protocol. Thus, although each group received a different secondary intervention as part of the treatment protocol (i.e. vocalization or non‐vocalization), it is not possible to compare directly the effects of the botulinum toxin for treatment of spasmodic dysphonia.
Risk of bias in included studies
Only one study (Troung 1991) was identified as meeting the inclusion criteria based on methodological quality and availability of appropriate data for analysis. Troung 1991 used a double‐blind, randomly assigned placebo‐controlled design. The precise method used to assign subjects randomly to the experimental and control groups was not described, and the study was therefore given a level B 'unclear concealment' rating for methodological quality.
Effects of interventions
Troung 1991 was the only study comparing treated and non‐treated groups. A total of five measures were taken at four days post‐injection for both groups. Data were analyzed using the nonparametric Mann‐Whitney U test for ranked data which yielded significant between group differences for spectrographic analysis, fundamental frequency range, self rating of improvement and professional spectrographic rating. Non‐significant group differences were reported for phonation time.
Effect sizes were calculated using the individual group means and standard deviations reported for the seven dependent variables. Results of these analyses yielded significant group differences for:
fundamental frequency (standardized mean difference (SMD) ‐1.60; 95% confidence interval (CI) = ‐2.92 to ‐0.29);
fundamental frequency range (SMD ‐4.39; 95% CI = ‐6.68 to ‐2.09);
perturbation (SMD ‐2.36; 95% CI = ‐3.90 to ‐0.82)
spectrographic analysis (SMD ‐4.28; 95% (CI) = ‐6.53 to ‐2.03);
professional rating of improvement (SMD ‐4.42; 95% CI = ‐6.72 to ‐2.11).
Non‐significant differences emerged for phonation time (SMD 0.40; 95% CI = ‐0.70 to 1.51). Data provided for the self‐rating of improvement did not allow for a calculation of effect. A meta‐analysis was not possible since data from only one study were available for each outcome measured.
Discussion
Although three studies reported randomized participant assignment, only one study representing a total of 13 participants with spasmodic dysphonia could ultimately be included in this review. The study reported a positive effect of botulinum toxin on both physiological functioning and listener perception.
The outcomes measured included basic physiological performance and perceived vocal quality resulting from improvement in these parameters of vocal production. Troung 1991 found that botulinum toxin improved the basic efficiency of the physiological functioning of the vocal mechanism in four of the five physiologically related dimensions measured (fundamental frequency, fundamental frequency range, perturbation, spectrographic analysis). In addition, Troung showed that patient rating of their speech production and independent ratings of speech severity improved significantly as a result of treatment.
Also noticeably absent from this study is long‐term follow up of effects and side effects. Others have reported that the effects of the botulinum toxin treatment typically last between 3 and 12 months. Troung 1991 assessed post‐treatment measures after four days, and reported an average improvement lasting three months. Breathiness was reported as a side effect in two participants. No information was provided with regard to the measurement of vocal production beyond the four days. This is in contrast to some studies which have reported that positive effects of botulinum toxin may not be observed for up to two weeks post‐treatment. Certainly it would be important to provide quantitative support for the both the longer term effects and the severity of the side effects.
A major problem with any interpretation of the effects of botulinum toxin in the treatment of spasmodic dysphonia, based on this one included study, is the small sample size. While significant differences were observed, any attempt make a population generalization would be suspect, with only a single study representing the effects of intervention.
This does not imply that the use of botulinum toxin for the treatment of spasmodic dysphonia should be rejected. The paucity of outcome data should be considered in the context of certain methodological shortcomings and differences that should be addressed in future randomized controlled trials. However, although small sample sizes were utilized in included and excluded studies and the observed effects were relatively inconsistent for the measurements at the post‐treatment times reported, the fact remains that the overwhelming clinical evidence suggests that botulinum toxin is very effective in treating spasmodic dysphonia. Of the 77 citations identified, none of the clinical reports, case studies, single subject design studies, or excluded group designs studies indicated a negative effect for botulinum toxin treatment. In fact, these excluded citations are noteworthy for the similarity in their report of positive effects related to length of treatment effect, degree of improvement, patient satisfaction and observed side effects. This is not to suggest that better evidence is not warranted but only to point out that the gold standard data reported in this review may not represent the current practice regarding effective use of botulinum toxin for treatment of spasmodic dysphonia.
Future studies should utilize larger sample sizes to increase power for detecting the presence of significant outcomes. In addition, the lack of uniformity in methodological design, not only in the randomized controlled trials cited in the literature, but also the entire corpus of clinical literature related to the topic of botulinum toxin for spasmodic dysphonia, points to the need for replication studies and research designs that will allow for data comparisons with previously published research.
Authors' conclusions
Implications for practice.
In the randomized controlled trials published in the literature, botulinum toxin has been shown to demonstrate a benefit with regard to subjective measures of voice production and select acoustic and aerodynamic variables. However, due to the small number of studies available for this review, and the methodological differences inherent in the studies, generalizations regarding the degree of effectiveness of botulinum toxin for all forms of spasmodic dysphonia or patients with different behavioral or clinical characteristics must be withheld at this point.
Implications for research.
Of 77 articles published in the area of botulinum toxin treatment for spasmodic dysphonia, only seven articles reported randomized controlled clinical trials. Of these seven, only three published adequate data that could be further analyzed via a systematic review. However, on further inspection it was found that only one study presented an appropriate and analyzable randomized trial thus limiting any conclusions that might be drawn. There is a large body of data from non‐randomized clinical studies that, taken together, suggest a very positive clinical outcome from the use of botulinum toxin for spasmodic dysphonia. However, well constructed randomized controlled trials are insufficient in this area. In addition, methodological variation across all studies in the clinical botulinum toxin literature make it difficult to compare one study with another. In order to facilitate more valid, reliable and specific decisions regarding treatment benefit, more randomized controlled trials are needed that control for or investigate the following variables: dosage, injection location, spasmodic dysphonia type, subject characteristics and time post‐injection that dependent variables are measured. These factors should be incorporated into methodologies that investigate a number of variables that are of clinical relevance. These include subjective and objective measures of the degree and duration of effectiveness for post‐injection vocal improvement and neuromuscular functioning, as well as acoustic, aerodynamic and endoscopic measures of the effects of botulinum toxin on laryngeal function in patients with spasmodic dysphonia.
What's new
| Date | Event | Description |
|---|---|---|
| 22 July 2009 | New search has been performed | New search 22 July 2009. No new studies identified. |
| 20 October 2008 | Amended | Converted to new review format. |
| 20 April 2007 | Amended | New search 20 April 2007. No new studies identified. |
History
Protocol first published: Issue 3, 2003 Review first published: Issue 3, 2004
| Date | Event | Description |
|---|---|---|
| 26 May 2004 | New citation required and conclusions have changed | Substantive amendment |
Appendices
Appendix 1. Search strategies
| CENTRAL | PubMed | EMBASE (Ovid) |
| #1 VOICE DISORDERS single term (MeSH) #2 LARYNGISMUS single term (MeSH) #3 spasm* NEAR dysphoni* or spastic NEAR dysphoni* OR flaccid NEAR dysphoni* OR hyperkinetic NEAR dysphoni* OR respiratory NEAR dysphoni* OR laryngospasm* NEAR dysphoni* #4 phonation NEXT disorder* OR laryngeal NEXT dystonia* OR neurologic* NEXT voice NEXT disorder* #5 ABSD OR ADSD #6 #1 OR #2 OR #3 OR #4 OR #5 #7 BOTULINUM TOXINS explode all trees (MeSH) #8 botulin* OR botox* OR dysport* OR oculinum* OR myobloc* OR neurobloc* OR botb* OR cs NEXT bot* OR vistabel* #9 #7 OR #8 #10 #6 AND #9 | (("VOICE DISORDERS"[MeSH]) OR ("LARYNGISMUS"[Mesh]) OR ((SPASM*[tiab] OR SPASTIC[tiab] OR FLACCID[tiab] OR HYPERKINETIC[tiab] OR RESPIRATORY[tiab] OR LARYNGOSPASM[tiab]) AND DYSPHONI*[tiab]) OR ((PHONATION[tiab] OR DISORDER*[tiab]) OR (LARYNGEAL[tiab] AND DYSTONIA[tiab]) OR NEUROLOGIC[tiab] AND VOICE[tiab] AND DISORDER*[tiab) OR ((PHONATION[tiab] OR DISORDER*[tiab]) OR (LARYNGEAL[tiab] AND DYSTONIA[tiab]) OR (NEUROLOGIC[tiab] AND VOICE[tiab] AND DISORDER*[tiab])) OR (ABSD[tiab])) AND ((BOTULIN*[tiab] OR BOTOX*[tiab] OR DYSPORT*[tiab] OR OCULINUM*[tiab] OR MYOBLOC*[tiab] OR NEUROBLOC*[tiab] OR BOTB*[tiab] OR (CS[tiab] AND BOT*[tiab]) OR VISTABEL*[tiab]) OR ("Botulinum Toxins"[MeSH])) | 1 LARYNX DISORDER/ or LARYNX SPASM/ or DYSPHONIA/ 2 LARYNX/ and DYSTONIA/ 3 ((SPASM* or SPASTIC or FLACCID or HYPERKINETIC or RESPIRATORY or LARYNGOSPASM*) and DYSPHONI*).tw. 4 ((PHONATION adj DISORDER*) or (LARYNGEAL adj DYSTONIA) or (NEUROLOGIC adj VOICE adj DISORDER*)).tw. 5 ABSD.tw. 6 4 or 1 or 3 or 2 or 5 7 BOTULINUM TOXIN/ or BOTULINUM TOXIN A/ or BOTULINUM TOXIN B/ 8 (BOTULIN* or BOTOX* or DYSPORT* or OCULINUM* or MYOBLOC* or NEUROBLOC* or BOTB* or (CS adj BOT*) or VISTABEL*).tw. 9 8 or 7 10 6 and 9 |
| CINAHL | Web of Science | mRCT |
| S1 (MH "Voice Disorders") S2 TX ( SPASM* OR SPASTIC OR FLACCID OR HYPERKINETIC OR RESPIRATORY OR LARYNGOSPASM* ) and TX dysphoni* S3 TX ABSD S4 (MH "Botulinum Toxins") S5 TX ( BOTULIN* OR BOTOX* OR DYSPORT* OR OCULINUM* OR MYOBLOC* OR NEUROBLOC* OR BOTB* OR VISTABEL* ) or ( CS AND BOT* ) S6 S4 or S5 S7 S1 or S2 or S3 S8 S6 and S7 | #1 TS=((SPASM* or SPASTIC or FLACCID or HYPERKINETIC or RESPIRATORY or LARYNGOSPASM*) and DYSPHONI*) #2 TS=((PHONATION adj DISORDER*) or (LARYNGEAL adj DYSTONIA) or (NEUROLOGIC adj VOICE adj DISORDER*)) #3 TS=(BOTULIN* or BOTOX* or DYSPORT* or OCULINUM* or MYOBLOC* or NEUROBLOC* or BOTB* or (CS adj BOT*) or VISTABEL*) #4 TS=ABSD #5 #4 OR #2 OR #1 #6 #5 AND #3 | (dysphon% OR dyston% OR voice OR phonation OR ABSD) AND (botulin% OR botox OR DYSPORT% OR OCULINUM% OR MYOBLOC% OR NEUROBLOC% OR BOT% OR VISTABEL%) |
Data and analyses
Comparison 1. Fundamental frequency (Hz).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Treatment vs control at 4 days post | 1 | 13 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐1.60 [‐2.92, ‐0.29] |
1.1. Analysis.
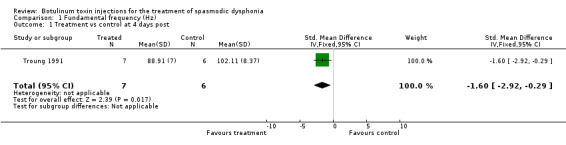
Comparison 1 Fundamental frequency (Hz), Outcome 1 Treatment vs control at 4 days post.
Comparison 2. Mean improvement post‐injection.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Botox versus placebo at 4 days | 1 | 13 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
2.1. Analysis.
Comparison 2 Mean improvement post‐injection, Outcome 1 Botox versus placebo at 4 days.
Comparison 3. Fundamental frequency range.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Fundamental frequency range at 4 days post | 1 | 13 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐4.39 [‐6.68, ‐2.09] |
3.1. Analysis.
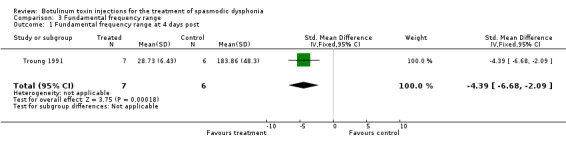
Comparison 3 Fundamental frequency range, Outcome 1 Fundamental frequency range at 4 days post.
Comparison 4. Perturbation.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Perturbation at 4 days | 1 | 13 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐2.36 [‐3.90, ‐0.82] |
4.1. Analysis.
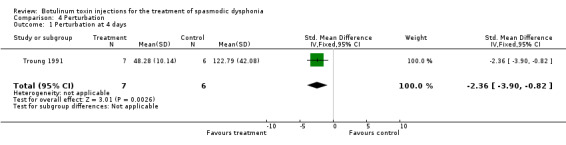
Comparison 4 Perturbation, Outcome 1 Perturbation at 4 days.
Comparison 5. Spectrographic ratings (degree of normalcy).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Botox versus placebo at 4 days | 1 | 13 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐4.28 [‐6.53, ‐2.03] |
5.1. Analysis.
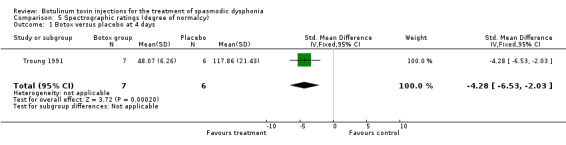
Comparison 5 Spectrographic ratings (degree of normalcy), Outcome 1 Botox versus placebo at 4 days.
Comparison 6. Phonation time.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Phonation time treatment versus control | 1 | 13 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.40 [‐0.70, 1.51] |
6.1. Analysis.
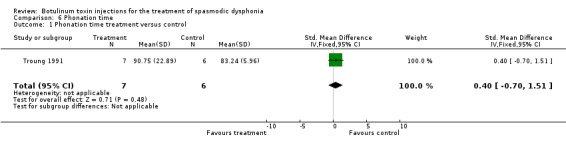
Comparison 6 Phonation time, Outcome 1 Phonation time treatment versus control.
Comparison 7. Audio ratings (degree of severity).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Perceptual audio ratings treatment versus control | 1 | 13 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐4.42 [‐6.72, ‐2.11] |
7.1. Analysis.
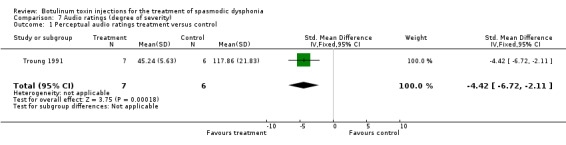
Comparison 7 Audio ratings (degree of severity), Outcome 1 Perceptual audio ratings treatment versus control.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Troung 1991.
| Methods | Double‐blinded, cross‐over, placebo‐controlled, random assignment | |
| Participants | 13 adductor spasmodic dysphonia | |
| Interventions | Bilateral thyroarytenoid muscle injections of 2.5 U Botox or 2.5 U saline | |
| Outcomes | Fo MPT Fo range Pertubation Spectrographic analysis Perceptual ratings | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| AANTTAS 1990 | Allocation: Not randomized |
| Adams 1993 | Allocation: Not randomized |
| Adams 1995 | Allocation: Random Participants: Adults with spasmodic dysphonia Interventions: Unilateral versus bilateral botulinum toxin injections |
| Adams 1996 | Allocation: Not randomized |
| Aronson 1993 | Allocation: Not randomized |
| Benninger 2001 | Allocation: Not randomized |
| Bhattacharyya 2001 | Allocation: Not randomized |
| Bielamowicz 2000 | Allocation: Not randomized |
| Bielamowicz 2001 | Allocation: Random Participants: Adults with abductor spasmodic dysphonia Interventions: Percutaneous versus transnasal injection of botulinum toxin |
| Bielamowicz 2002 | Allocation: Not randomized |
| Blitzer 1985 | Allocation: Not randomized |
| Blitzer 1988 | Allocation: Not randomized |
| Blitzer 1991 | Allocation: Not randomized |
| Blitzer 1992a | Allocation: Not randomized |
| Blitzer 1992b | Allocation: Not randomized |
| Blitzer 1998 | Allocation: Not randomized |
| Brin 1988 | Allocation: Not randomized |
| Brin 1989 | Allocation: Not randomized |
| Brin 1998b | Allocation: Not randomized |
| Brin 2001 | Allocation: Random Participants: Adults with spasmodic dysphonia Interventions: Unilateral versus bilateral botulinum toxin injections |
| Cannito 1994 | Allocation: Not randomized |
| Ceballos 1992 | Allocation: Not randomized |
| Courey 2000 | Allocation: Not randomized |
| Crevier‐Buchman 1997 | Allocation: Not randomized |
| Cyrus 2001 | Allocation: Not randomized |
| Davidson 1996 | Allocation: Not randomized |
| Devriese 1994 | Allocation: Not randomized |
| Finnegan 1999 | Allocation: Random Participants: Adults with spasmodic dysphonia Interventions: Botulinum toxin injections into both intrinsic and extrinsic laryngeal muscles versus botulinum toxin injections into intrinsic plus saline into extrinsic muscles |
| Fisher 1996 | Allocation: Not randomized |
| Fisher 1999 | Allocation: Not randomized |
| Ford 1990 | Allocation: Not randomized |
| Ford 1992 | Allocation: Not randomized |
| Galardi 2001 | Allocation: Not randomized |
| George 1992 | Allocation: Not randomized |
| Green 1992 | Allocation: Not randomized |
| Inagi 1996 | Allocation: Not randomized |
| Jankovic 1990 | Allocation: Not randomized |
| Klap 1991 | Allocation: Not randomized |
| Klap 1993 | Allocation: Not randomized |
| Kobayashi 1993 | Allocation: Not randomized |
| Koriwchak 1996 | Allocation: Not randomized |
| Langeveld 1998 | Allocation: Not randomized |
| Langeveld 2001 | Allocation: Not randomized |
| Lees 1992 | Allocation: Not randomized |
| Liu 1996 | Allocation: Not randomized |
| Loven 1993 | Allocation: Not randomized |
| Loven 1994 | Allocation: Not randomized |
| Ludlow 1988 | Allocation: Not randomized |
| Ludlow 1990 | Allocation: Not randomized |
| Ludlow 1991 | Allocation: Not randomized |
| Ludlow 1992 | Allocation: Not randomized |
| Lundy 1998 | Allocation: Not randomized |
| Maloney 1994 | Allocation: Not randomized |
| Marion 1992 | Allocation: Not randomized |
| Maurri 1992 | Allocation: Not randomized |
| Mehta 2001 | Allocation: Not randomized |
| Meleca 1997 | Allocation: Not randomized |
| Miller 1987 | Allocation: Not randomized |
| Murry 1995 | Allocation: Not randomized |
| Papathanasiou 1997 | Allocation: Not randomized |
| Poungvarin 1995 | Allocation: Not randomized |
| Rhew 1994 | Allocation: Not randomized |
| Rodriguez 1994 | Allocation: Not randomized |
| Rontal 1991 | Allocation: Not randomized |
| Rosen 1999 | Allocation: Not randomized |
| Ruiz 1998 | Allocation: Not randomized |
| Sapienza 2002 | Allocation: Not randomized |
| Schonweiler 1998 | Allocation: Not randomized |
| Smith 2000 | Allocation: Not randomized |
| Teive 2001 | Allocation: Not randomized |
| Thomas 2006 | Allocation: Randomized Participants: Adult patients with adductor spasmodic dysphonia Interventions: Frozen versus fresh reconstituted botulinum toxin type A |
| Tish 2003 | Allocation: Not randomized |
| Whurr 1993 | Allocation: Not randomized |
| Whurr 1997 | Allocation: Not randomized |
| Whurr 1998 | Allocation: Not randomized |
| Wong 1995a | Allocation: Randomized Participants: Adults with spasmodic dysphonia Interventions: Botulinum toxin with vocalization versus botulinum toxin without vocalization |
| Wong 1995b | Allocation: Randomized Participants: Adults with spasmodic dysphonia Interventions: Botulinum toxin with vocalization versus botulinum toxin without vocalization |
| Zwirner 1991 | Allocation: Not randomized |
| Zwirner 1992 | Allocation: Not randomized |
| Zwirner 1993a | Allocation: Not randomized |
| Zwirner 1993b | Allocation: Not randomized |
| Zwirner 1997 | Allocation: Not randomized |
Contributions of authors
All authors contributed to the collection and evaluation of included/excluded studies, and preparing the text. Watts and Nye were responsible for the data input and analysis.
Declarations of interest
None known.
New search for studies and content updated (no change to conclusions)
References
References to studies included in this review
Troung 1991 {published data only}
- Troung D, Rontal M, Rolnick M, Aronson A, Mistura K. Double‐blind controlled study of botulinum toxin in adductor spasmodic dysphonia. Laryngoscope 1991;101:630‐4. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
AANTTAS 1990 {published data only}
- American Academy of Neurology Therapeutics and Technology Assessment Subcommittee. Assessment: the clinical usefulness of botulinum toxin‐A in treating neurologic disorders. Neurology 1990;40:1332‐6. [DOI] [PubMed] [Google Scholar]
Adams 1993 {published data only}
- Adams SG, Hunt EJ, Charles DA, Lang AE. Unilateral versus bilateral botulinum toxin injections in spasmodic dysphonia: acoustic and perceptual results. The Journal of Otolaryngology 1993;22(3):171‐5. [PubMed] [Google Scholar]
Adams 1995 {published data only}
- Adams SG, Hunt EJ, Irish JC, Charles DA, Lang AE, Durkin LC, Wong DLH. Comparison of botulinum toxin injection procedures in adductor spasmodic dysphonia. Journal of Otolaryngology 1995;24(6):345‐51. [PubMed] [Google Scholar]
Adams 1996 {published data only}
- Adams SG, Durkin LC, Irish JC, Wong DL, Hunt EJ. Effects of botulinum toxin type A injections on aerodynamic measures of spasmodic dysphonia. Laryngoscope 1996;106:296‐300. [DOI] [PubMed] [Google Scholar]
Aronson 1993 {published data only}
- Aronson AE, McCaffrey TV, Litchey WJ, Lipton RJ. Botulinum toxin injection for adductor spasmodic dysphonia: patient self‐ratings of voice and phonatory effort after three successive injections. Laryngoscope 1993;103:683‐92. [DOI] [PubMed] [Google Scholar]
Benninger 2001 {published data only}
- Benninger MS, Gardner G, Grywalski C. Outcomes of botulinum toxin treatment for patients with spasmodic dysphonia. Archives of Otolaryngology ‐ Head & Neck Surgery 2001;127:1083‐5. [DOI] [PubMed] [Google Scholar]
Bhattacharyya 2001 {published data only}
- Bhattacharyya N, Tarsy D. Impact on quality of life of botulinum toxin treatments for spasmodic dysphonia and oromandibular dystonia. Archives of Otolaryngology ‐ Head and Neck Surgery 2001;127:389‐92. [DOI] [PubMed] [Google Scholar]
Bielamowicz 2000 {published data only}
- Bielamowicz S, Ludlow CL. Effects of botulinum toxin on pathophysiology in spasmodic dysphonia. Annals of Otology, Rhinology, Laryngology 2000;109:194‐203. [DOI] [PubMed] [Google Scholar]
Bielamowicz 2001 {published data only}
- Bielamowicz S, Bidus K, Squire S, Ludlow CL. Assessment of posterior cricoarytenoid botulinum toxin injections in patients with abductor spasmodic dysphonia. The Annals of Otology, Rhinology and Laryngology 2001;110:406‐12. [DOI] [PubMed] [Google Scholar]
Bielamowicz 2002 {published data only}
- Bielamowicz S, Sheila SV, Badillo A, Godlewski A. Unilateral versus bilateral injections of botulinum toxin in patients with adductor spasmodic dysphonia. Journal of Voice 2002;16(1):117‐23. [DOI] [PubMed] [Google Scholar]
Blitzer 1985 {published data only}
- Blitzer A, Lovelace RE, Brin MF, Fahn S, Fink ME. Electromyographic findings in focal laryngeal dystonia (spasmodic dysphonia). Annals of Otology, Rhinology, Laryngology 1985;94(6):591‐4. [DOI] [PubMed] [Google Scholar]
Blitzer 1988 {published data only}
- Blitzer A, Brin MF, Fahn S, Lovelace RE. Localized injections of botulinum toxin for the treatment of focal laryngeal dystonia (spastic dysphonia). Laryngoscope 1988;98(2):193‐7. [DOI] [PubMed] [Google Scholar]
Blitzer 1991 {published data only}
- Blitzer A, Brin MF. Laryngeal dystonia: a series with botulinum toxin therapy. Annals of Otology, Rhinology, & Laryngology 1991;100:85‐9. [DOI] [PubMed] [Google Scholar]
Blitzer 1992a {published data only}
- Blitzer A, Brin MF, Stewart C, Aviv JE, Fahn S. Abductor laryngeal dystonia: a series treated with botulinum toxin. Laryngoscope 1992;102:163‐7. [DOI] [PubMed] [Google Scholar]
Blitzer 1992b {published data only}
- Blitzer A, Brin MF. Treatment of spasmodic dysphonia (laryngeal dystonia) with local injections of botulinum toxin. Journal of Voice 1992;6(4):365‐9. [Google Scholar]
Blitzer 1998 {published data only}
- Blitzer A, Brin MF, Stewart CF. Botulinum toxin management of spasmodic dysphonia (laryngeal dystonia): a 12‐year experience in more than 900 patients. Laryngoscope 1998;108:1435‐41. [DOI] [PubMed] [Google Scholar]
Brin 1988 {published data only}
- Brin MF, Fahn S, Moskowitz C, Friedman A, Shale HM, Greene PE, Blitzer A, List T, Lange D, Lovelace RE. Localized injections of botulinum toxin for the treatment of focal dystonia and hemifacial spasm. Advances in Neurology 1988;50:599‐608. [PubMed] [Google Scholar]
Brin 1989 {published data only}
- Brin MF, Blitzer A, Fahn S, Gould W, Lovelace RE. Adductor laryngeal dystonia: treatment with local injections of botulinum toxin. Movement Disorders 1989;4(4):287‐96. [DOI] [PubMed] [Google Scholar]
Brin 1998b {published data only}
- Brin MF, Blitzer A, Stewart C. Laryngeal dystonia (spasmodic dysphonia): observations of 901 patients and treatment with botulinum toxin. Advances in Neurology 1998;78:237‐52. [PubMed] [Google Scholar]
Brin 2001 {published data only}
- Brin MF, Blitzer A, Stewart CF, Diamond B, Pogoda JM. Botulinum toxin type A for adductor spasmodic dysphonia (laryngeal dystonia): double blind placebo controlled assessment of dose and technique. Neurology 2001;56(8 (Suppl 3)):A346. [Google Scholar]
Cannito 1994 {published data only}
- Cannito MP, Murry T, Woodson GE. Attitudes toward communication in adductor spasmodic dysphonia before and after botulinum toxin injection. Journal of Medical Speech‐Language Pathology 1994;2(2):125‐33. [Google Scholar]
Ceballos 1992 {published data only}
- Ceballos Bauman AG. Spasmodic dysphonia: clinical features and treatment with laryngeal botulinum toxin. Otolaryngology, Rhinology, Laryngology 1992;2(1):33‐8. [Google Scholar]
Courey 2000 {published data only}
- Courey MS, Garrett CG, Portell MS, Billante, CR, Smith TL, Stone RE, Netterville JL. Outcomes assessment following treatment of spasmodic dysphonia with botulinum toxin. Annals of Otology, Rhinology and Laryngology 2000;109:819‐22. [DOI] [PubMed] [Google Scholar]
Crevier‐Buchman 1997 {published data only}
- Crevier‐Buchman L, Laccourreye O, Papon JF, Nurit D, Brasnu D. Adductor spasmodic dysphonia: case reports with acoustic analysis following botulinum toxin injection and acupuncture. Journal of Voice 1997;11(2):232‐7. [DOI] [PubMed] [Google Scholar]
Cyrus 2001 {published data only}
- Cyrus CB, Bielamowicz S, Evans FJ, Ludlow CL. Adductor muscle activity abnormalities in abductor spasmodic dysphonia. Otolaryngology ‐ Head and Neck Surgery 2001;124(1):23‐30. [DOI] [PubMed] [Google Scholar]
Davidson 1996 {published data only}
- Davidson BJ, Ludlow CL. Long‐term effects of botulinum toxin injections in spasmodic dysphonia. Annals of Otology, Rhinology and Laryngology 1996;105:33‐42. [DOI] [PubMed] [Google Scholar]
Devriese 1994 {published data only}
- Devriese PP, Speelman JD. The treatment of vocal‐cord dyskinesia with botulinum toxin. Nederlands Tijdschrift voor Geneeskunde 1994;138(19):944‐7. [PubMed] [Google Scholar]
Finnegan 1999 {published data only}
- Finnegan E, Luschei E, Gordon J, Barkmeier J, Hoffman H. Increased stability of airflow following botulinum toxin injection. Laryngoscope 1999;109:1300‐6. [DOI] [PubMed] [Google Scholar]
Fisher 1996 {published data only}
- Fisher KV, Scherer RC, Guo CG, Owen AS. Longitudinal phonatory characteristics after botulinum toxin injection. Journal of Speech and Hearing Research 1996;39:968‐80. [DOI] [PubMed] [Google Scholar]
Fisher 1999 {published data only}
- Fisher KV, Scherer RC, Swank PR, Giddens CG, Patten D. Electroglottographic tracking of phonatory response to Botox. Journal of Voice 1999;13(2):203‐9. [DOI] [PubMed] [Google Scholar]
Ford 1990 {published data only}
- Ford CN, Bless DM, Lowery JD. Indirect laryngoscopic approach for injection of botulinum toxin in spasmodic dysphonia. Otolaryngology ‐ Head Neck Surgery 1990;103:752‐8. [DOI] [PubMed] [Google Scholar]
Ford 1992 {published data only}
- Ford CN, Bless DM, Patel NY. Botulinum toxin treatment of spasmodic dysphonia: techniques, indications, efficacy. Journal of Voice 1992;6(4):370‐6. [Google Scholar]
Galardi 2001 {published data only}
- Galardi G, Guerriero R, Amadio S, Leocani L, Teggi R, Mellani G, Comi G. Sporadic failure of botulinum toxin treatment in usually responsive patients with adductor spasmodic dysphonia. Neurological Sciences 2001;22(4):303‐6. [DOI] [PubMed] [Google Scholar]
George 1992 {published data only}
- George EF, Zimbler M, Wu BL, Biller HF, Sanders I. Quantitative mapping of the effect of botulinum toxin injections in the thyroarytenoid muscle. Annals of Otology, Rhinology, Laryngology 1992;101(11):888‐92. [DOI] [PubMed] [Google Scholar]
Green 1992 {published data only}
- Green DC, Ward PH, Berke GS, Gerratt BR. Point‐touch technique of botulinum toxin injection for the treatment of spasmodic dysphonia. Annals of Otology, Rhinology and Laryngology 1992;101:883‐7. [DOI] [PubMed] [Google Scholar]
Inagi 1996 {published data only}
- Inagi K, Ford CN, Bless DM, Heisey D. Analysis of factors affecting botulinum toxin results in spasmodic dysphonia. Journal of Voice 1996;10(3):306‐13. [DOI] [PubMed] [Google Scholar]
Jankovic 1990 {published data only}
- Jankovic J, Schwartz K, Donovan D. Botulinum toxin treatment of cranial‐cervical dystonia, spasmodic dysphonia, and other focal dystonias and hemifacial spasm. Journal of Neurology, Neurosurgery and Psychiatry 1990;53:633‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Klap 1991 {published data only}
- Klap P, Marion MH, Perrin A, Fresnel‐Elbaz E. Treatment of spasmodic dysphonia with botulinum toxin [Traitement de la dysphonie spasmodicque par la toxine botulique]. Annales d'Oto‐laryngologie et de Chirurgie Cervico Faciale 1991;108:477‐83. [PubMed] [Google Scholar]
Klap 1993 {published data only}
- Klap P, Marion MH, Perrin A, Fresnel‐Elbaz E, Cohen M. Indications of botulinus toxin in laryngology. Revue de Laryngologie 1993;114(4):281‐7. [PubMed] [Google Scholar]
Kobayashi 1993 {published data only}
- Kobayashi T, Miimi S, Kumada M, Kosaki H, Hirose H. Botulinum toxin treatment for spasmodic dysphonia. Acta Otolaryngologica 1993;504 (supp):155‐7. [DOI] [PubMed] [Google Scholar]
Koriwchak 1996 {published data only}
- Koriwchak MJ, Netterville JL, Snowden T, Courey M, Ossoff RH. Alternating unilateral botulinum toxin type A (BOTOX) injections for spasmodic dysphonia. Laryngoscope 1996;106:1476‐81. [DOI] [PubMed] [Google Scholar]
Langeveld 1998 {published data only}
- Langeveld TP, Drost HA, Baatenburg de Jong RJ. Unilateral versus bilateral botulinum toxin injections in adductor spasmodic dysphonia. Annals of Otology, Rhinology and Laryngology 1998;107:280‐4. [DOI] [PubMed] [Google Scholar]
Langeveld 2001 {published data only}
- Langeveld TP, Edgar HH, Jeroen JB, Rossum M, Zwinderman AH, Baatenburg RJ. Evaluation of voice quality in adductor spasmodic dysphonia before and after botulinum toxin treatment. Annals of Otology, Rhinology and Laryngology 2001;110:627‐34. [DOI] [PubMed] [Google Scholar]
Lees 1992 {published data only}
- Lees AJ, Turjanski N, Rivest J, Whurr R, Lorch M, Brookes G. Treatment of cervical dystonia hand spasms and laryngeal dystonia with botulinum toxin. Journal of Neurology 1992;239:1‐4. [DOI] [PubMed] [Google Scholar]
Liu 1996 {published data only}
- Liu TC, Irish JC, Adams SG, Durkin LC, Hunt EJ. Prospective study of patients' subjective responses to botulinum toxin injection for spasmodic dysphonia. The Journal of Otolaryngology 1996;25(2):66‐74. [PubMed] [Google Scholar]
Loven 1993 {published data only}
- Loven J, Brondbo K, Ganes T. Botulinum toxin. A new therapeutic alternative in spastic dysphonia (laryngeal abductor dystonia) [Et nytt behandlingsalternativ ved spastisk dysfoni (laryngeal adduktordystoni)]. Tidsskr for den Norske laegeforening: tidsskrift for praktisk 1993;113(7):841‐3. [PubMed] [Google Scholar]
Loven 1994 {published data only}
- Loven JO, Brondbo K, Ganes T, Tveteras G, Frydenbo J. Botulinum toxin treatment for spasmodic dysphonia (adductor laryngeal dystonia): Experience with 31 patients. Scandinavia Journal of Logopedics & Phoniatrics 1994;19:107‐11. [Google Scholar]
Ludlow 1988 {published data only}
- Ludlow CL, Naunton RF, Sedory SE, Schulz GM, Hallett M. Effects of botulinum toxin injections on speech in adductor spasmodic dysphonia. Neurology 1988;38:1220‐5. [DOI] [PubMed] [Google Scholar]
Ludlow 1990 {published data only}
- Ludlow CL, Naunton RF, Fujita M, Sedory SE. Spasmodic dysphonia: botulinum toxin injection after recurrent nerve surgery. Otolaryngology ‐ Head and Neck Surgery 1990;102:122‐31. [DOI] [PubMed] [Google Scholar]
Ludlow 1991 {published data only}
- Ludlow CL, Naunton RF, Terada S, Anderson BJ. Successful treatment of selected cases of abductor spasmodic dysphonia using botulinum toxin injection. Otolaryngology ‐ Head and Neck Surgery 1991;104:849‐55. [DOI] [PubMed] [Google Scholar]
Ludlow 1992 {published data only}
- Ludlow CL, Bagley J, Yin SG, Koda J. A comparison of injection techniques using botulinum toxin injection for treatment of the spasmodic dysphonias. Journal of Voice 1992;6(4):380‐6. [Google Scholar]
Lundy 1998 {published data only}
- Lundy DS, Ling Lu F, Casiano RR, Xue JW. The effect of patient factors on response outcomes to botox treatment for spasmodic dysphonia. Journal of Voice 1998;4:460‐6. [DOI] [PubMed] [Google Scholar]
Maloney 1994 {published data only}
- Maloney AP, Morrison MD. A comparison of the efficacy of unilateral versus bilateral botulinum toxin injections in the treatment of adductor spasmodic dysphonia. The Journal of Otolaryngology 1994;23(3):160‐4. [PubMed] [Google Scholar]
Marion 1992 {published data only}
- Marion MH, Perrin KA, Elbaz E. Les dysphonies spasmodiques: methodes d'investigation et de traitement. Rev. Neurol. (Paris) 1992;148(3):180‐3. [PubMed] [Google Scholar]
Maurri 1992 {published data only}
- Maurri S Barontini F. Botulinum toxin. A new therapeutic alternative in spastic dysphonia (laryngeal abductor dystonia) [Breve aggiornamento sulla tereapia della disfonia spasmodica. Efficacia della tossina botulinnica purificata di tipo]. Nuova Rivista di Neurologia 1992;3(1):35‐8. [Google Scholar]
Mehta 2001 {published data only}
- Mehta RP, Goldman SN, Orloff LA. Long‐term therapy for spasmodic‐dysphonia: acoustic and aerodynamic outcomes. Archives of Otolaryngology ‐ Head and Neck Surgery 2001;127:393‐9. [DOI] [PubMed] [Google Scholar]
Meleca 1997 {published data only}
- Meleca RJ, Hogikyan ND, Bastian RW. A comparison of methods of botulinum toxin injection for abductory spasmodic dysphonia. Otolaryngology ‐ Head and Neck Surgery 1997;117:487‐92. [DOI] [PubMed] [Google Scholar]
Miller 1987 {published data only}
- Miller RH, Woodson GE, Jankovic J. Botulinum toxin injection of the vocal fold for spasmodic dysphonia. Archives of Otolaryngology ‐ Head and Neck Surgery 1987;113:603‐5. [DOI] [PubMed] [Google Scholar]
Murry 1995 {published data only}
- Murry T, Woodson GE. Combined‐modality treatment of adductor spasmodic dysphonia with botulinum toxin and voice therapy. Journal of Voice 1995;9(4):460‐5. [DOI] [PubMed] [Google Scholar]
Papathanasiou 1997 {published data only}
- Papathanasiou I, MacDonald L, Whurr R, Brookes G, Jahanshahi M. Perceived stigma among patients with spasmodic dysphonia. Journal of Medical Speech‐Language Pathology 1997;5(4):251‐61. [Google Scholar]
Poungvarin 1995 {published data only}
- Poungvarin N, Devahastin V, Viriyavejakul A. Treatment of various movement disorders with botulinum toxin A injection: an experience of 900 patients. Journal of the Medical Association of Thailand 1995;78(6):281‐7. [PubMed] [Google Scholar]
Rhew 1994 {published data only}
- Rhew K, Fiedler DA, Ludlow CL. Technique for injection of botulinum toxin through the flexible nasolaryngoscope. Otolaryngology ‐ Head and Neck Surgery 1994;111:787‐94. [DOI] [PubMed] [Google Scholar]
Rodriguez 1994 {published data only}
- Rodriguez AA, Ford CN, Bless DM, Harmon RL. Electromyographic assessment of spasmodic dysphonia patients prior to botulinum toxin injection. Electromyography & Clinical Neurophysiology 1994;34:403‐7. [PubMed] [Google Scholar]
Rontal 1991 {published data only}
- Rontal M, Rontal E, Rolnick M, Merson R, Silverman B, Truong D. A method for the treatment of abductor spasmodic dysphonia with botulinum toxin injections: a preliminary report. Laryngoscope 1991;101:911‐4. [DOI] [PubMed] [Google Scholar]
Rosen 1999 {published data only}
- Rosen CA, Murry T. Botox for hyperadduction of the false vocal folds: a case report. Journal of Voice 1999;13:234‐9. [DOI] [PubMed] [Google Scholar]
Ruiz 1998 {published data only}
- Ruiz PJG, Bernardos VS, Astarloa R, Sanabria J, Yebenes JG. Botulinum toxin treatment for spasmodic dysphonia: percutaneous versus transoral apporach. Clinical Neuropharmacology 1998;21(3):196‐8. [PubMed] [Google Scholar]
Sapienza 2002 {published data only}
- Sapienza CM, Cannito MP, Murry T, Branski R, Woodson G. Acoustic variations in reading produced by speakers with spasmodic dysphonia pre‐botox injection and within early stages of post‐botox injection. Journal of Speech, Language and Hearing Research 2002;45:830‐43. [DOI] [PubMed] [Google Scholar]
Schonweiler 1998 {published data only}
- Schonweiler R, Wohlfarth K, Dengler R, Ptok M. Supraglottal injection of botulinum toxin type A in adductor spasmodic dysphonia with both intrinsic and extrinsic hyperfunction. Laryngoscope 1998;108:55‐63. [DOI] [PubMed] [Google Scholar]
Smith 2000 {published data only}
- Smith ME, Ford CN. Resistance to botulinum toxin injections for spasmodic dysphonia. Archives of Otolaryngology ‐ Head and Neck Surgery 2000;126(4):533‐5. [DOI] [PubMed] [Google Scholar]
Teive 2001 {published data only}
- Teive HAG, Scola HS, Werneck LC, Quadros A, Gasparetto EL, Sa DS, Brandi IV, Filho EDM. Use of botulinum toxin in the treatment of laryngeal dystonia (spasmodic dysphonia): preliminary study of twelve patients. Arquivos de Neuro‐psiquiatria 2001;59(1):97‐100. [DOI] [PubMed] [Google Scholar]
Thomas 2006 {published data only}
- Thomas JP, Siupsinskiene N. Frozen versus fresh reconstituted botox for laryngeal dystonia. Otolaryngology ‐ Head and Neck Surgery 2006;135(2):204‐8. [DOI] [PubMed] [Google Scholar]
Tish 2003 {published data only}
- Tish SH, Brake HM, Law M, Cole IE, Darveniza P. Spasmodic dysphonia: clinical features and effects of botulinum toxin therapy in 169 patients ‐ an Australian experience. Journal of Clinical Neuroscience 2003;10(4):434‐8. [DOI] [PubMed] [Google Scholar]
Whurr 1993 {published data only}
- Whurr R, Lorch M, Fontana H, Brookes G, Lees A, Marsden CD. The use of botulinum toxin in the treatment of adductor spasmodic dysphonia. Journal of Neurology, Neurosurgery and Psychiatry 1993;56:526‐30. [DOI] [PMC free article] [PubMed] [Google Scholar]
Whurr 1997 {published data only}
- Whurr R, Lorch M, Nye C. The treatment of spasmodic dysphonia with botulinum toxin. Neurology Reviews International 1997;2:11‐15. [Google Scholar]
Whurr 1998 {published data only}
- Whurr R, Lorch M, Lindsay M, Brookes G, Marsden C. Psychological function in spasmodic dysphonia before and after treatment with botulinum toxin. Journal of Medical Speech‐Language Pathology 1998;2:81‐91. [Google Scholar]
Wong 1995a {published data only}
- Wong DLH, Irish JC, Adams SG, Durkin LC, Hunt EJ. Laryngeal image analysis following botulinum toxin injections in spasmodic dysphonia. Journal of Otolaryngology 1995;24(1):64‐8. [PubMed] [Google Scholar]
Wong 1995b {published data only}
- Wong DL, Adams SG, Irish JC, Durkin LC, Hunt EJ, Charlton MP. Effect of neuromuscular activity on the response to botulinum toxin injections in spasmodic dysphonia. The Journal of Otolaryngology 1995;24(4):209‐16. [PubMed] [Google Scholar]
Zwirner 1991 {published data only}
- Zwirner P, Murry T, Swenson M, Woodson GE. Acoustic changes in spasmodic dysphonia after botulinum toxin injection. Journal of Voice 1991;5(1):78‐84. [Google Scholar]
Zwirner 1992 {published data only}
- Zwirner P, Murry T, Swenson M, Woodson GE. Effects of botulinum toxin therapy in patients with adductor spasmodic dysphonia: acoustic, aerodynamic and videoendoscopic findings. Laryngoscope 1992;102:400‐6. [DOI] [PubMed] [Google Scholar]
Zwirner 1993a {published data only}
- Zwirner P, Murry T, Woodson GE. A comparison of bilateral and unilateral botulinum toxin treatments for spasmodic dysphonia. European Archives of Otorhinolaryngology 1993;250:271‐6. [DOI] [PubMed] [Google Scholar]
Zwirner 1993b {published data only}
- Zwirner P, Murry T, Woodson GE. Perceptual‐acoustic relationships in spasmodic dysphonia. Journal of Voice 1993;7(2):165‐71. [DOI] [PubMed] [Google Scholar]
Zwirner 1997 {published data only}
- Zwirner P, Murry T, Woodson GE. Effects of botulinum toxin on vocal tract steadiness in patients with spasmodic dysphonia. European Archives of Otorhinolaryngology 1997;254:391‐5. [DOI] [PubMed] [Google Scholar]
Additional references
Aronson 1990
- Aronson A. Clinical Voice Disorders: An Interdisciplinary Approach. New York: Thieme, 1990. [Google Scholar]
Blitzer 2001
- Blitzer A, Sulica L. Botulinum toxin: basic science and clinical uses in otolaryngology. The Laryngoscope 2001;111:218‐26. [DOI] [PubMed] [Google Scholar]
Boone 2000
- Boone D, McFarlane S. The Voice and Voice Therapy. Boston: Allyn & Bacon, 2000. [Google Scholar]
Boutsen 2002
- Boutsen F, Cannito M, Taylor M, Bender B. Botox treatment for adductor spasmodic dysphonia: a meta‐analysis. Journal of Speech, Language and Hearing Research 2002;45:469‐81. [DOI] [PubMed] [Google Scholar]
Brin 1998a
- Brin M, Blitzer A, Stewart C. Botulinum toxin management of spasmodic dysphonia (laryngeal dystonia): a 12 year experience in more than 900 patients. The Laryngoscope 1998;98:193‐7. [DOI] [PubMed] [Google Scholar]
Cannito 1981
- Cannito M, Johson P. Spastic dysphonia: a continuum disorder. Journal of Communication Disorders 1981;14:215‐23. [DOI] [PubMed] [Google Scholar]
Cannito 2001
- Cannito M. Neurological aspects of spasmodic dysphonia. In: Vogel D, Cannito M editor(s). Treating Disordered Speech Motor Control. Austin: Pro‐Ed, 2001. [Google Scholar]
Drost 1998
- Drost HA, Baatenburg de Jong RJ. Unilateral versus bilateral botulinum toxin injections in adductor spasmodic dysphonia. Annalos of Otology, Rhinology and Laryngology 1998;107:280‐4. [DOI] [PubMed] [Google Scholar]
Duffy 2003
- Duffy JR, Yorkston K. Medical interventions for spasmodic dysphonia and some related conditions: a systematic review. Journal of Medical Speech Language Pathology 2003;11(4):ix‐lviii. [Google Scholar]
Handbook 2008
- Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions 5.0.0 [updated February 2008]. The Cochrane Collaboration, 2008. Available from www.cochrane‐handbook.org.
RevMan 2008 [Computer program]
- The Nordic Cochrane Centre. The Cochrane Collaboration. Review Manager (RevMan). Version 5.0. Copenhagen: The Nordic Cochrane Centre. The Cochrane Collaboration, 2008.
Stemple 2000
- Stemple J. Voice Therapy: Clinical Studies. San Diego: Singular, 2000. [Google Scholar]


