SUMMARY
Tenofovir today exists in two pharmaceutical forms, such as Tenofovir disoproxil fumarate (TDF) and the newer Tenofovir alafenamide (TAF). The two different salts are required in order to promote intestinal absorption of the active molecule (TFV). Once absorbed the distribution of TFV into compartments is driven by the salt to which the drug is conjugated; in case of TDF, following absorption most of TFV is cleared from its link with the salt and the drug is widely distributed into different tissues, while in case of TAF the reverse is true as TFV remains mostly associated to its alafenamide salt and its distribution is restricted to cells with high carboxyesterase and catepsin A activity, such as hepatocytes and lymphocytes. This generates higher plasma levels of TFV in case of TDF while in the case of TFV much higher intracellular concentrations in target cells are achieved. The main reason for TAF development was to reduce the impact of the drug on proximal renal function and this was actually obtained by the much lower plasma concentration of TFV. Numerous clinical trials consistently demonstrated the significant lesser impact of TAF vs TDF on both renal function and structural bone integrity.
Keywords: Tenofovir disoproxil fumarate (TDF), Tenfovir alafenamide (TAF), Tenofovir (TFV), Proximal renal tubule, Low-molecular weight protein
INTRODUCTION
Tenofovir alafenamide (TAF) is the 2nd Tenofovir (TFV) prodrug released into the international market [1]. The term prodrug entails the fact that the molecule does not generate any significant therapeutic effect in its original form but requires to be metabolically transformed to became active. TFV is the molecular form undergoing intracellular phosphorylation, the final metabolic step required for the drug in order to compete with natural phosphorylated substrates of viral reverse transcriptase [2]. In the initial phase of development it was soon found that TFV as such was not absorbed at the intestinal level and that suitable pharmaceutical formulations should have been devised to allow the drug to be developed for oral intake [3]. The 1st TFV prodrug to be clinically developed was Tenofovir disoproxil fumarate (TDF), that was released into the market in 2001 (USA) for the treatment of HIV infection and in 2008 its use was also approved for the treatment of chronic HBV infection [4, 5]. The decision to develop TAF inspite of years of worldwide successful TDF use was taken with the purpose of improving several aspects of the long-term safety of the drug [6].
TENOFOVIR METABOLISM, PHARMACOKINETICS AND CLEARANCE
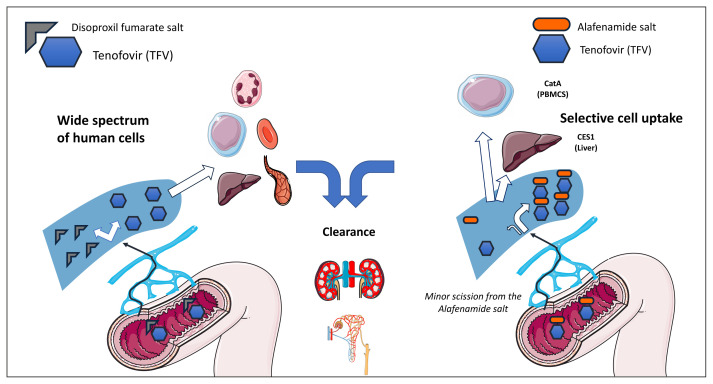
Although, by definition, the final product is the same, the clinical pharmacology of TFV is largely influenced by the prodrug considered. While both prodrugs, TDF and TAF, make the drug absorbable from the intestine, once TFV is in the circulation its distribution shows marked differences depending on which of the two oral formulations is taken. Most of TFV absorbed following TDF oral intake (25% oral bioavailability) dissolves from its link with the disoproxil fumarate salt and is evenly distributed into a wide range of different tissues [7]. The reverse is true when TFV is taken as TAF (40% oral bioavailability estimated), as the link with the alafenamide salt is stronger, and most of the drug circulates bound to it [8]. A major property of TAF is that of driving a rather selective distribution of TFV (Figure 1). Here comes the definition of “magic bullet”, as TFV when given as TAF undergoes a rather selective uptake by cells in which most of viral replication occurs. This applies both to the first-pass metabolism, where the carboxy-esterase 1(CES1)-rich hepatocytes are able to internalize the drug, and to the catepsin A (CatA) expressing PBMCs [8]. This selective distribution accounts for the much lower (25 mg) dose of TAF that is required to generate comparable clinical antiviral effects as the standard 245 mg dose of TDF [9]. Such different distribution of TFV when given as TAF or TDF was first described in a pioneer study in 2005 by Lee and coworkers, who compared the pharmacokinetics of TFV when administered by the intravenous route (IV, 1 mg/kg bw) and by the oral route as TDF (245 mg) and TAF (25 mg) [10]. While the highest plasma AUC was measured in decreasing order for IV TFV (4800 ng/h/mL), TDF (1900 ng/h/mL) and TAF (16 ng/h/mL), the PBMC/plasma ratio showed the opposite order, with TAF achieving the highest value, 150, followed by TDF, 5 and IV TFV, 1. These relevant differences in terms of intracellular distribution were mirrored by the EC50 for HIV-1 (mM), that was as low as 0.005 for TAF, 0.05 for TDF and as high as 5.0. for IV TFV [10]. The clinical relevance of these different values have been consistently confirmed in clinical studies. Plasma and intracellular pharmacokinetics of TFV was measured in 30 patients who switched from TDF- to TAF containing regimens and it was found that while TFV plasma concentrations decreased by 90% [TDF: 99.98 (2.24) ng/mL vs TAF: 10.2 (1.6) ng/mL, p<0.001] following the switch to TAF, the white cell associated TFV-diphosphate (TFV-DP) increased 2.41 fold [TAF: 834.7 (2.49) vs TDF: 346.85 (3.75) fmol/106 cells, p=0.004] [11]. The main reason why TAF was clinically developed following the extensive and successful use of TDF was not however the higher intracellular penetration of TFV achieved by TAF intake but rather its much lower plasma pK exposure. The major clinical relevance of these findings, and specifically the negligible plasma pK exposure of TFV, is thus on the toxicity side as TAF has been consistently found to be associated to a much lower impact in terms of both renal toxicity and bone structural integrity as compared to its ancestor TDF [12–15].
Figure 1.
The different tenofovir (TFV) distribution following intestinal absorption of Tenofovir disoproxil fumarate (TDF) and Tenofovir alafenamide (TAF) is represented.
The main difference in terms of clinical impact between TDF and TAF lies thus in the process of clearance of TFV, where the much lower plasma pK exposure of TFV when taken as TAF plays a key role in improving the safety profile of the drug. TFV is cleared by the renal route, with glomerular filtration accounting for approximately 2/3 and secretion by the renal proximal tubule for the rest [16]. The amount of TFV escaping glomerular filtration reaches the epithelial cells of the proximal renal tubule by the efferent arterioles. Uptake of TFV by these cells is efficient, but following internalization of the drug, the subsequent phase of apical secretion into the urine has a lower capacity, so that a variable amount of TFV tends to accumulate into proximal tubule epithelial cells [17]. Although TFV was shown to have minimal mitochondrial toxicity, once the local concentration increases alteration in mitochondrial structure and function follow, with decrease in energy production by mitochondria resulting in lower efficiency of membrane transporters [18]. A vicious circle is thus generated with chronic impairment of proximal tubule function. The latter can be measured by the capacity or reabsorbing low-molecular protein molecules, such as retinol-binding protein (RBP) and b2-microglobulin. These two markers have been extensively used in clinical trials to demonstrate the lower proximal renal tubule reabsorbing efficiency in TDF vs TAF intakers, with unambiguous results consistently showing a significantly higher preserved proximal renal function in TAF recipients [12–15]. It must be noted that these markers of proximal tubular function are rarely used in clinical practice, where creatininemia and creatinine-based calculation of glomerular filtration are more commonly measured. Creatinine renal clearance mainly occurs by glomerular filtration (85%), with a minor contribution by proximal tubular secretion [16, 19]. This explains why the increases in creatininemia and parallel decreases in the estimated value of glomerular filtration are common occurrences in TDF-treated patients, but these markers actually underestimate the impact of TDF on renal proximal tubule function. In an horizontal clinical study on 289 TDF-treated patients with steady normal creatinine values and a median exposure to TDF of 5.2 years, the measurement of the urinary RBP/creatinine ratio showed that 54% of these patients had a reduced proximal tubule function inspite of normal creatinine values. As expected, these alterations were inversely proportional to the TFV urinary concentration, thus testifying a reduced capacity of clearing the drug by tubular secretion [20].
A further difference between TDF and TAF that is also attributable to the lesser impact of the latter on renal function is the reduced impact of TAF on bone structural integrity. Lower reduction in bone mineral density (BMD) have been constantly detected in TAF vs TDF intakers, possibly reflecting a reduced phosphate loss by the proximal renal tubule [12–15]. In clinical studies evaluating the effects of switching from TDF to TAF-containing regimens an increase in patients BMD has also been regularly measured [21, 22].
All this data points on the benefit associated to the lower plasma pK exposure of TFV when administered as TAF. Before the clinical development of TAF was completed, differences in terms of BMD were already seen in TDF recipients according to the companion drugs. Depending on the companion drugs, the pK plasma exposure of TFV in patients receiving TDF may significantly differ, with both efavirenz (EFV) and raltegravir (RAL) being associated to the lowest TFV concentrations [23]. In an equivalence clinical trial comparing darunavir/ritonavir (DRV/r), atazanavir/ritonavir (ATV/r) and RAL, all associated to emtricitabine/tenofovir (FTC/TDF), the lowest impact on BMD was seen in the RAL treatment arm, and this is in full accordance to pK clinical studies measuring the TDF-associated TFV pK exposure according to companion drugs [23–25].
CONCLUSIONS
It is thus the lower concentrations of TFV in plasma that account for the significantly better safety profile of TFV, and to complete the TAF definition of “magic bullet”, the property of being less concentrated where potentially toxic (e.g. the plasma bathing the renal proximal tubule) well fits with its higher concentration into cells where HIV and HBV replicate.
Footnotes
Conflict of interest
G.Di Perri has received research grants, fees for lectures and participation to advisory boards by Abbvie, MSD, ViiV, GS, Janssen, Pfizer, Astra Zeneca, Angelini.
Funding
This is a review article with no research funding.
REFERENCES
- 1.Ray AS, Fordyce MW, Hitchcock MJM. Tenofovir alafenamide: A novel prodrug of tenofovir for the treatment of Human Immunodeficiency Virus. Antiviral Research. 206;125:63–70. doi: 10.1016/j.antiviral.2015.11.009. [DOI] [PubMed] [Google Scholar]
- 2.Birkus G, Bam RA, Willkom M, et al. Intracellular activation of Tenofovir Alafenamide and the effects of Viral and Host Protease Inhibitors. Antimicrob Agent Chemoter. 2016;60:316–322. doi: 10.1128/AAC.01834-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kearney B, Flaherty J, Shah J. Tenofovir disoproxil fumarate clinical pharmacology and pharmacokinetics. Clin Pharmacokinet. 2004;43(9):595–612. doi: 10.2165/00003088-200443090-00003. [DOI] [PubMed] [Google Scholar]
- 4.Production approval timeline. Gilead. 2018 February; https://www.gilead.com/medicines/product-approval-timeline . [Google Scholar]
- 5.Terrault N, Lok A, McMahnon B, et al. Practice guidance: update on prevention, diagnosis, and treatment of chronic hepatitis B: AASLD 2018 Hepatitis B guidance. Hepatology. 2018;67(4):1560–99. doi: 10.1002/hep.29800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Scherzer R, Estrella M, Li Y, Choi AI, et al. Association of tenofovir exposure with kidney disease risk in HIV infection. AIDS. 2012;26(7):867–75. doi: 10.1097/QAD.0b013e328351f68f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Robbins BL, Srinivas RV, Kim C, Bischofberger N, Fridland A. Anti-human immunodeficiency virus activity and cellular metabolism of a potential prodrug of the acyclic nucleoside phosphonate 9-R-(2-phosphonomethoxypropyl) adenine (PMPA), Bis(isopropyloxymethylcarbonyl) PMPA. Antimicrob Agents Chemother. 1998;42(3):612–7. doi: 10.1128/aac.42.3.612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lee W, He G, Einsenberg E, et al. Selective intracellular activation of a novel prodrug of the HIV reverse transcriptase inhibitor tenofovir leads to preferential distribution and accumulation in lymphatic tissue. Antimicrob Agents Chemother. 2005;49(5):1898–906. doi: 10.1128/AAC.49.5.1898-1906.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sax PE, Zolopa A, Brar I, et al. Tenofovir alafenamide vs tenofovir disoproxil fumarate in single tablet regimens for initial HIV-1 therapy. J Acquir Immune Defic Syndr. 2014;67(1):52–8. doi: 10.1097/QAI.0000000000000225. [DOI] [PubMed] [Google Scholar]
- 10.Lee W, He G, Einsenberg E, et al. Selective intracellular activation of a novel prodrug of the HIV reverse transcriptase inhibitor tenofovir leads to preferential distribution and accumulation in lymphatic tissue. Antimicrob Agents Chemother. 2005;49(5):1898–906. doi: 10.1128/AAC.49.5.1898-1906.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Podany AT, Bares SH, Havens J, et al. Plasma and intracellular pharmacokinetics of tenofovir in patients switched from tenofovir disoproxil fumarate to tenofovir alafenamide. AIDS. 2018;32(6):761–5. doi: 10.1097/QAD.0000000000001744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fernandez BF, Ferrer AM, Sanz AB, et al. Tenofovir nephrotoxicity: 2011 update. AIDS Res Treat. 2011;2011:354908. doi: 10.1155/2011/354908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sax PE, Zolopa A, Brar I, et al. Tenofovir alafenamide vs tenofovir disoproxil fumarate in single tablet regimens for initial HIV-1 therapy. J Acquir Immune Defic Syndr. 2014;67(1):52–8. doi: 10.1097/QAI.0000000000000225. [DOI] [PubMed] [Google Scholar]
- 14.Wohl D, Oka S, Clumeck N, et al. Brief report: a randomized, double-blind comparison of tenofovir alafenamide versus tenofovir disoproxil fumarate, each coformulated with elvitegravir, cobicistat, and emtricitabine for initial HIV-1 treatment: week 96 results. J Acquir Immune Defic Syndr. 2016;72(1):58–64. doi: 10.1097/QAI.0000000000000940. [DOI] [PubMed] [Google Scholar]
- 15.Sax PE, Wohl D, Yin MT, et al. Tenofovir alafenamide versus tenofovir disoproxil fumarate coformulated with elvitegravir, cobicistat and emtricitabine for initial treatment of HIV-1 infection: two randomized, double blind, phase 3, non-inferiority trials. Lancet. 2015;385(9987):2606–15. doi: 10.1016/S0140-6736(15)60616-X. [DOI] [PubMed] [Google Scholar]
- 16.Calcagno A, Cusato J, Marinaro L, et al. Clinical pharmacology of tenofovir clearance: a pharmacokinetic/pharmacogenetic study on plasma and urines. Pharmacogenomics J. 2016;16(6):514–8. doi: 10.1038/tpj.2015.71. [DOI] [PubMed] [Google Scholar]
- 17.Stray KM, Bam RA, Birkus G, et al. Evaluation of the Effect of Cobicistat on the In Vitro Renal Transport and Cytotoxicity Potential of Tenofovir. Antimicrob Agents Chemother. 2013;57:4982–9. doi: 10.1128/AAC.00712-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Samuels R, Bayerri CR, Sayer JA, Price DA, Payne BAI. Tenofovir disoproxil fumarate-associated renal tubular dysfunction: noninvasive assessment of mitochondrial injury. AIDS. 2017;31(9):1297–301. doi: 10.1097/QAD.0000000000001466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lin Y, Bansal N, Vittinghoff E, et al. Determinants of the creatinine clearance to glomerular filtration rate ratio in patients with chronic kidney disease: a cross-sectional study. BMC Nephrol. 2013;14:268. doi: 10.1186/1471-2369-14-268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Calcagno A, Cusato J, Marinaro L, et al. Tenofovir clearance is reduced in HIV-positive patients with sub-clinical tubular impairment. AIDS. 2016;27:915–20. doi: 10.1097/QAD.0000000000000995. [DOI] [PubMed] [Google Scholar]
- 21.Mills A, Arribas JR, Andrade-Villanueva J, et al. GS-US-292-0109 team. Switching from tenofovir disoproxil fumarate to tenofovir alafenamide in antiretroviral regimens for virologically suppressed adults with HIV-1 infection: a randomised, active-controlled, multicentre, open-label, phase 3, non-inferiority study. Lancet Infect Dis. 2016;16:43–52. doi: 10.1016/S1473-3099(15)00348-5. [DOI] [PubMed] [Google Scholar]
- 22.Pozniak A, Arribas JR, Gathe J, et al. Switching to Tenofovir Alafenamide, Coformulated With Elvitegravir, Cobicistat, and Emtricitabine, in HIV-Infected Patients With Renal Impairment: 48-Week Results From a Single-Arm, Multicenter, Open-Label Phase 3 Study. J Acquir Immune Defic Syndr. 2016;71(5):530–7. doi: 10.1097/QAI.0000000000000908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Calcagno A, Gonzalez de Requena D, Simiele M, et al. Tenofovir plasma concentrations according to companion drugs: a cross-sectional study of HIV-positive patients with normal renal function. Antimicrob Agents Chemother. 2013;57(4):1840–3. doi: 10.1128/AAC.02434-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Brown TT, Moser C, Currier JS, et al. Changes in Bone Mineral Density After Initiation of Antiretroviral Treatment With Tenofovir Disoproxil Fumarate/Emtricitabine Plus Atazanavir/Ritonavir, Darunavir/Ritonavir, or Raltegravir. J Infect Dis. 2015;212(8):1241–9. doi: 10.1093/infdis/jiv194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ray AS, Fordyce MW, Hitchcock MJM. Tenofovir alafenamide: A novel prodrug of tenofovir for the treatment of Human Immunodeficiency Virus. Antivir Res. 2016;125:63–70. doi: 10.1016/j.antiviral.2015.11.009. [DOI] [PubMed] [Google Scholar]