SUMMARY
Ticks are remarkable vectors of a diverse and growing list of infectious agents of importance to both medical and veterinary disciplines. The tick Amblyomma americanum is one of the most frequently identified ticks in the United States with an expanding spectrum of human disease given its vast geographic range. The recently described Bourbon and Heartland viruses are likely transmitted by the Lone Star tick and are just two of the several novel tick-borne pathogens discovered in recent decades. The review will focus on these two viruses that can cause illness with similar characteristics to other diseases transmitted by the Lone Star tick. Healthcare professionals should consider these viruses in patients presenting with an ailment suggestive of a tick-born rickettsial disease that fails to improve with treatment with doxycycline. Additionally, some individuals may develop life-threatening allergic reactions triggered by the bite of the Lone Star tick.
Keywords: tickborne, Amblyoma americanum, Lone Star tick
INTRODUCTION
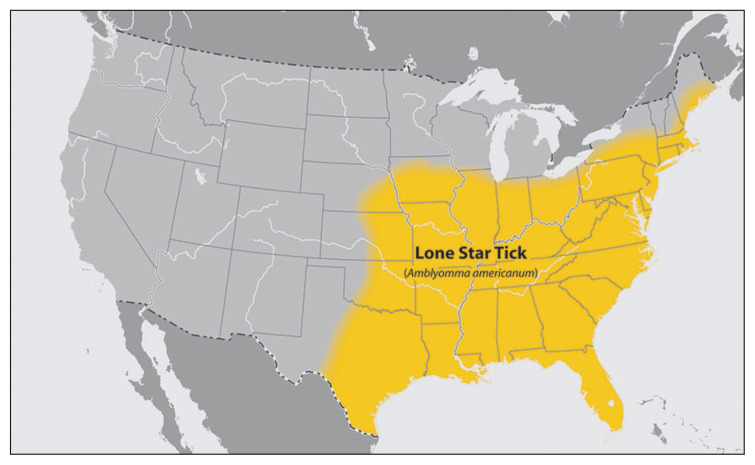
In the United States, the incidence of tick-borne diseases is increasing as these ticks expands their geographic range [1–3]. The number of reported cases of bacterial and protozoan diseases transmitted by ticks in the United Sates increased from over 22,000 cases in 2004 to over 48,000 cases in 2016 [2]. The Lone Star tick, Amblyomma americanum, has geographically expanded to the upper midwestern and northeastern regions of the United States and eastern Canada [3] (Figure 1). Imperfect surveillance and reporting systems in addition to the limitations of the currently available diagnostics underappreciate their true public health footprint [4].
Figure 1.
Geographical distribution of Amblyomma americanum in the United States. (From Centers for Disease Control and Prevention [CDC], Atlanta, GA., with permission)
Amblyomma americanum is the most common Amblyomma spp. in the United States. The female is aggressive, widely distributed, and has a characteristic white macula on the posterior region of the dorsal shield, hence the name Lone Star Tick [5] (Figure 2). Amblyomma americanum is a vector of Ehrlichia chaffeensis, E. ewingii, Franciscella tularensis, and the Southern Tick Associated Rash Illness (STARI). There is also mounting evidence of its competence as a vector of Rickettsia rickettsii [5–10]. In addition, Amblyomma americanum is likely the only vector of two newly identified viruses in the United States. Furthermore, the bite of A. americanum can trigger the alpha-gal (galactose-α-1,3-galactose) syndrome (AGS). In this review, we provide an update on these emergent viral and allergic conditions related to A. americanum.
Figure 2.
Amblyomma americanum, the Lone Star tick. Note the characteristic “lone star” mark located on the dorsum of the female at the tip of the scutum. (From Centers for Disease Control and Prevention [CDC], Atlanta, GA. Public Health Image Library, image 8683. Photo credit: James Gathany)
BOURBON VIRUS
Bourbon virus (BRBV) was first isolated in 2014 from the blood sample of a male resident of Bourbon County, Kansas [11]. The virus is a member of the genus Thogotovirus of the family Orthomyxoviridae and is the first Thogotovirus to be associated with human illness in the Western Hemisphere [11, 12].
The life cycle is poorly understood and is an area of active research. The virus has been isolated from the hard tick Amblyomma americanum in northwestern Missouri and eastern Kansas and experimental studies have confirmed the Lone Star tick as a competent vector [13–15]. Transmission of the virus by the tick is further supported by its phylogenetic relationship to Dhori and Bakten viruses within the genus Thogotovirus which have been identified in hard tick species [11]. In addition, in the report of the sentinel case an engorged tick was found attached to the shoulder of the patient. Human cases have also been reported from Payne County, Oklahoma and St. Louis, Missouri, states where this tick species is prevalent [11, 13, 16].
There is scant BRBV infection prevalence data in A. americanum ticks, animals, and humans. The first epidemiologic study was performed in Missouri in 2013. A total of 39,096 ticks collected from six sites in northwestern Missouri were retrospectively tested for BRBV. The BRBV infection rates (IR) per 1,000 adult ticks and nymphs for all sites and for the entire 2013 season were 0.32 and 0.07, respectively [13]. The second epidemiologic study was performed in Bourbon County and adjacent southern Linn County, Kansas, in 2015 [14]. In this study, the BRBV IR per 1,000 adult ticks for all sites was 0.25 [14].
Lastly, the same group of investigators, and based on the results of the 2015 study, tried to further identify virus infected ticks to better delineate the vectors and life stages involved in virus transmission to humans. A total of 14,193 ticks representing four species were collected at four sites including the sites were Heartland virus (HRTV) and BRBV were detected in 2015. All pools tested negative for BRBV [17]. The authors concluded that BRBV seems to be less frequently isolated from field-collected A. americanum ticks than HRTV. The authors further speculate that BRBV might not be as efficiently transmitted, both vertically and horizontally [17].
Antibodies to BRBV have also been detected in non-human vertebrates. In a study performed in northwest Missouri, investigators evaluated banked serum and plasma of wild and domestic animals for evidence of BRBV antibodies using a plaque-reduction neutralization assay (PRNT) [18]. Antibodies to BRBV were detected in dogs, horses, white-tail deer, raccoons, and rabbits with the deer having the highest seropositivity rate of 86% [18]. In another study performed in North Carolina, 18 out of 32 white-tailed deer had reactive antibodies to BRBV by PRNT translating to a seroprevalence of 56% [19].
The clinical manifestations of BRBV in humans are limited to two published reports [11, 16]. The first patient was a previously healthy man over the age of 50 years that presented with non-specific complaints following a tick bite. Initial symptoms included nausea, vomiting, diarrhea, anorexia, myalgias, arthralgias, headache and fever. The patient later developed decreased level of consciousness resulting in admission to a local hospital. Exam at admission was remarkable for a papular rash on his trunk. Laboratory findings showed leukopenia with lymphopenia, thrombocytopenia, mild hyponatremia and elevated levels of aspartate aminotransferase and alanine aminotransferase. The patient deteriorated despite treatment with doxycycline and required invasive mechanical ventilation and vasopressor support. The patient passed away 11 days after becoming ill. During the patient’s hospitalization a thorough evaluation for an infectious agent was unfruitful. The novel infectious agent was subsequently identified as a novel Orthomyxovirus of the genus Thogotovirus by partial-genomic high throughput sequencing and microscopy. No autopsy was performed [11].
The second case involved a 53-year-old female with history of follicular lymphoma. She had a similar flu-like illness and laboratory abnormalities as described in the case above, and approximately 1 week after several tick bites. The patient followed a similar course as the first patient, with deterioration during her hospital stay despite treatment with doxycycline and other broad-spectrum antibiotics. She also developed oral ulcers and a progressive rash with marked involvement of her palms and soles. In addition to developing respiratory failure, she also developed features suggestive of hemophagocytic lymphohistiocytosis for which she was treated with etoposide; again, without improvement. The patient died on hospital day 23 [16].
There are no routine diagnostic tests available for BRBV. Healthcare providers should contact their state health departments to coordinate testing at the Centers for Disease Control and Prevention (CDC). There is no specific treatment for BRBV other than supportive measures. Further studies to confirm the promising role of antivirals such as favipiravir seen in murine models are needed [16].
HEARTLAND VIRUS
Heartland virus was first isolated in 2009 from two men living in northwestern Missouri. The virus was identified by next-generation sequencing and based on phylogenetic analysis as a novel member of the Phlebovirus genus of the Bunyaviridae family [20]. The virus is closely related to severe fever with thrombocytopenia syndrome virus (SFTSV), a virus first identified in China in 2009 [20, 21].
Following the isolation of the virus from the sentinel human case, the virus was detected in both nymphs and adult ticks from the property of patients with HRTV [22, 23]. Further evidence to support the role of Amblyomma americanum as the vector for HRTV is its ability to transmit the virus both horizontally and transstadially [24]. Wild animals from 13 central and eastern states have been identified as harboring HRTV antibodies. The vast majority of these states are in the known geographic distribution of the Lone Star tick [25]. Nevertheless, HRTV seropositive white-tail deer have been identified in northern New England, a region where established A. americanum populations are unknown [26].
Heartland virus infected humans have been described from Oklahoma, Kansas, Missouri, Arkansas, Kentucky, Tennessee, Indiana, Illinois, Georgia, and South Carolina [27–30]. In the largest cohort of patients with HRTV, the phenotype of the disease was similar to that of other tickborne disease such as ehrlichiosis, anaplasmosis, BRBV and Colorado tick fever [30]. Most were older males that became ill between the months of April and September [27, 30]. The typical presentation is that of fever, headache, myalgias, fatigue, and nausea within a 2-week window of tick attachment or finding a tick crawling on the body. A diffuse rash is usually not seen, and some patients develop neurocognitive dysfunction characterized by gait abnormalities, dizziness and confusion. Leukopenia with lymphopenia, thrombocytopenia, hyponatremia, and elevated aminotransferases are common laboratory abnormalities. Severe and fatal complications such as hemophagocytic lymphohistiocytosis, septic shock, and multiorgan failure have also been reported [20, 29–31]. The few autopsy reports have described the identification of HRTV antigens in lymph nodes, spleen, liver, kidneys, small and large intestines, gallbladder, pancreas, lung, heart, testes and brain [32, 33].
As with BRBV, HRTV diagnostics are not readily available and testing of suspected cases should be done in coordination with state health departments. It is important to note that in acute HRTV infection, the duration of viremia is of approximately 1–2 weeks. Furthermore, the detection of both viral RNA by RT-PCR after 21 days and IgM antibodies approximately 2 weeks after symptom onset have important implications related to modes of transmission and diagnostics [30].
ALPHA-GAL SYNDROME
In the first decade of this century, reports of a higher incidence of hypersensitivity reactions to cetuximab in patients from Tennessee and North Carolina (22%) compared to other regions of the country (3%) ignited research into the mechanisms responsible for this geographic association [34, 35]. In a seminal study, pre-treatment IgE specific antibodies to the oligosaccharide, galactose-α-1,3-galactose (alpha-gal), were found in patients that experienced hypersensitivity reactions to cetuximab. The allergic reaction occurred as the oligosaccharide is present in the Fab portion of the cetuximab heavy chain. This specific oligosaccharide is not found in humans or other primates [36, 37]. Some individuals with IgE specific antibodies to alpha-gal also experienced a higher rate of allergic reactions after consumption of meat of mammalian origin. In contrast to the immediate allergic reaction seen after administration of cetuximab, sensitized individuals experienced a delayed-onset allergic reaction of several hours after consumption of animal food products [36, 38].
The link between tick bites and the alpha gal syndrome in the United States was entertained after reports of such association in Australia [39] and the geographic overlap between reported cases of allergy to mammalian meat, cetuximab, and that of the tick A. americanum [37, 40]. The sensitization to galactose-α-1,3-galactose in humans is produced by the bite of a tick that has fed on non-primate mammals containing the oligosaccharide. It is also possible that the alpha gal could be transmitted trans-stadially or be present in the tick’s saliva [41].
The alpha gal syndrome is seen in individuals with IgE specific to alpha gal who develop an allergic reaction to red meat and other animal-derived products such as milk, cheese, gelatin, certain biologics, and gelatin-containing vaccines [38, 42]. A study that characterized the phenotype of the syndrome in 248 individuals showed no difference in the nature or timing of symptomatology between adults and children. Most patients present with gastrointestinal complaints (e.g. abdominal cramping, nausea, vomiting), urticaria, angioedema, and anaphylaxis 3–6 hours after consumption of mammal-derived meat products. Symptomatology can also ensue acutely (i.e. within 2 hours), especially in women and be limited to the gastrointestinal tract. There is no association with the alpha-gal syndrome and atopy and there is a non-absolute negative association with blood group B [42]. The delayed onset of symptoms is thought to be related to the slower digestion and absorption of alpha-gal bound to lipids, and not to proteins [43].
The syndrome causes significant morbidity and is a concerning public health problem due to its increasing incidence and prevalence in the United States [44]. A retrospective study identified over 30,000 individuals with positive alpha-gal specific IgE antibodies and showed a 6-fold increase in seropositivity rates between 2011 and 2018. The locations of the identified individuals mirrored the geographic distribution of the Lone Star tick [44]. The diagnosis of the alpha-gal syndrome is challenging and based on clinical presentation, identification of alpha-gal IgE specific antibodies, and response to red meat avoidance [45, 46]. Antihistamines and elimination of consumption of mammalian animal products including dairy is the mainstay of treatment. Most can reintroduce mammalian animal products into their life with time.
DISCUSSION
In the current world order, the emergence and re-emergence of infectious diseases, some with pandemic potential, are occurring with more frequency and at a greater scale and duration [47]. Tick-borne diseases are not the exception; known tick vectors are expanding their geographic range and new tick associated pathogens continue to be discovered [48]. Warmer temperature due to climate change affect the tick’s life cycle and behavior by for example increasing the egg rate development, accelerating the tick’s developmental cycle, promoting host questing, and lengthening their active season [1, 3, 49, 50]. Amblyomma americanum has historically been established in southern, southeastern, and midwestern states but is rapidly expanding into new areas of the northwest and north including Canada. Genetic differences between the species isolated in states like New York from species located in the traditional range could facilitate pathogen transmission [3, 51, 52].
The proportion of asymptomatic infection due to both viruses is currently unknown. Adults represent the preponderance of published cases with older individuals with comorbidities, not characterized further in the reports, being at highest risk for severe disease and poor outcome. The hospitalization rate and case-fatality ratio have not been established. The largest cohort of patients with HRTV infection reported a 13% case-fatality ratio although there is likely case ascertainment bias due to physicians requesting testing for the virus in more severe cases [30]. Besides age greater than 60 years and comorbidities no further defined, no other risk factors have been consistently associated with disease severity and prognosis. The severe fever with thrombocytopenia virus, a Phlebovirus closely related to HRTV, has a case-fatality ratio of approximately 17%. Risk factors for poor outcome include older age, decreased level of consciousness and an elevated lactate dehydrogenase (>1,200 U/L) and creatine kinase levels (>800 U/L). Higher levels of serum cytokines such as interleukin 6 (IL-6), IL-10, and interferon-gamma (IFN-γ) and IL-10 were also observed in fatal cases [53].
In addition, SFTV can be transmitted from human to human through transfusion of infected blood or contact with bloody secretions [54]. Although there are no reports of transmission of HRTV by this route, the prolonged duration of viremia and its relatedness to SFTV raises the possibility of potential human-to-human transmission. Furthermore, SFTV is transmitted by the Asian Longhorn tick, Haemaphysalis longicornis, which until recently was not present in the Western Hemisphere. The tick was first found in sheep in New Jersey in 2017 and has now been reported in several other states [55, 56]. The tick is aggressive, feeding for up to 19 days from animals, and is able to reproduce through parthenogenesis resulting in massive host infestations. The tick has been found to be infected with Anaplasma, Ehrlichia, Rickettsia, and Borrelia species in other endemic regions outside the United States [56–58]. Experimental studies have shown that H. longicornis is a competent vector for R. rickettsii and unlikely to transmit Anaplasma phagocytophilum and B. burgdorferi [59–61]. Further studies are needed to define the vector’s competence and capacity for other tick-borne diseases, such as HRTV and BRBV, present in the continental United States.
Polymicrobial tick infections can increase the risk of disease severity and complicate the interpretation of available diagnostics [62]. For example, A. americanum is often infected by Rickettsia amblyommatis, a spotted-fever group rickettsia (SFGR) of undetermined pathogenicity. R. amblyommatis elicits an immune response that can cause reactivity with the antigens used commercially to diagnose other SFGR such as Rocky Mountain Spotted Fever (RMSF). Moreover, other non-rickettsial pathogens such as BRBV and HRTV could be misdiagnosed with an infection caused by a SFGR if the individual has preexistent antibodies to R. amblyommatis [63, 64]. The diagnosis of both HRTV and BRBV is restricted to a few research laboratories and CDC. There is an urgent need to refine the existent diagnostics for tick-borne diseases and to develop accurate, affordable, and more readily available test for tick-borne emergent agents.
There is no specific treatment for HRTV and BRBV and management consists of supportive care. The mechanisms by which both viruses induce severe illness remains undefined. Murine models suggest that BRBV is exquisitely sensitive to the host’s interferon (IFN) system and that the few persons with severe BRBV described in the literature might have had an inborn or acquired defect in their innate antiviral immune system [65].
As previously mentioned, SFTV is closely related to HRTV and available data suggests shared risk factors for poor outcome. In-vitro studies have shown that HRTV infection antagonizes both type I and III IFN system similar to SFTV [66]. Furthermore, SFTV leads to changes in CD4-T cell subsets such as skewing of the Th1/Th2 and Th17/Treg ratios, the later leading to increased IL-6 levels and associated with poor outcome. Blockage of IL-6 signal with tocilizumab could restore the Th17/Treg ratio [67]. In addition, a robust elevation of several cytokines such as IL-1RA, IL-6, IL-10 in SFTV is associated with poor prognosis. Identification of existent and new therapeutics targeting the virus-induced “cytokine storm” and their potential applicability and role in management should be examined further [68, 69].
It is also important to remember that HRTV and BRBV are clinically undistinguishable from treatable rickettsial diseases such as ehrlichiosis and RMSF and doxycycline should be therefore empirically prescribed when considering a tickborne disease, including children younger than 8 years of age [70].
Finally, the AGS is a relatively new entity of increasing importance given its rising incidence and associated morbidity. However, the interactions between ticks and vertebrate hosts, such as human, is not always harmful. AGS could represent the trade-off to development of immunity against organisms that produce the carbohydrate α-gal such as Plasmodium spp., Trypanosoma cruzi, and Leishmania spp. [71].
In summary, Amblyomma americanum is a prolific arachnid capable of rapidly adapting to new geographic locations. It is a competent vector of a growing list of important pathogens, and its bite is a potential allergen able to induce life-threatening allergic reactions to meat and other mammalian derived products. The health, economic, societal, and environmental impact of these three new conditions associated with the Lone Star tick and that of H. longicornis needs to be further characterized. Public health interventions aimed at prevention and control of tick-borne disease should be based on a better understanding on the effect climate change is having in the evolution of the pathogen, host, and pathogen-host interactions.
Footnotes
Conflicts of interest
None.
Funding
None.
REFERENCES
- 1.Dantas-Torres F. Climate change, biodiversity, ticks and tick-borne diseases: The butterfly effect. Int J Parasitol Parasites Wildl. 2015;4(3):452–61. doi: 10.1016/j.ijppaw.2015.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rosenberg R, Lindsey NP, Fischer M, et al. Vital signs: trends in reported vectorborne disease cases - United States and Territories, 2004–2016. MMWR Morb Mortal Wkly Rep. 2018;67(17):496–501. doi: 10.15585/mmwr.mm6717e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Molaei G, Little EAH, Williams SC, et al. Bracing for the Worst - Range Expansion of the Lone Star Tick in the Northeastern United States. N Engl J Med. 2019;381(23):2189–92. doi: 10.1056/NEJMp1911661. [DOI] [PubMed] [Google Scholar]
- 4.Paules CI, Marston HD, Bloom ME, et al. Tickborne Diseases - Confronting a growing threat. N Engl J Med. 2018;379(8):701–3. doi: 10.1056/NEJMp1807870. [DOI] [PubMed] [Google Scholar]
- 5.Mathison BA, Pritt BS. Laboratory identification of arthropod ectoparasites. Clin Microbiol Rev. 2014;27(1):48–67. doi: 10.1128/CMR.00008-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Madison-Antenucci S, Kramer LD, Gebhardt LL, et al. Emerging Tick-Borne Diseases. Clin Microbiol Rev. 2020;33(2) doi: 10.1128/CMR.00083-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Parola P, Paddock CD, Socolovschi C, et al. Update on tick-borne rickettsioses around the world: a geographic approach. Clin Microbiol Rev. 2013;26(4):657–702. doi: 10.1128/CMR.00032-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Breitschwerdt EB, Hegarty BC, Maggi RG, et al. Rickettsia rickettsii transmission by a lone star tick, North Carolina. Emerg Infect Dis. 2011;17(5):873–5. doi: 10.3201/eid1705.101530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Levin ML, Zemtsova GE, Killmaster LF, et al. Vector competence of Amblyomma americanum (Acari: Ixodidae) for Rickettsia rickettsii. Ticks Tick Borne Dis. 2017;8(4):615–22. doi: 10.1016/j.ttbdis.2017.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nicholson WL, Masters E, Wormser GP. Preliminary serologic investigation of Rickettsia amblyommii in the aetiology of Southern tick associated rash illness (STARI) Clin Microbiol Infect. 2009;15(Suppl 2):235–6. doi: 10.1111/j.1469-0691.2008.02155.x. [DOI] [PubMed] [Google Scholar]
- 11.Kosoy OI, Lambert AJ, Hawkinson DJ, et al. Novel thogotovirus associated with febrile illness and death, United States, 2014. Emerg Infect Dis. 2015;21(5):760–4. doi: 10.3201/eid2105.150150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lambert AJ, Velez JO, Brault AC, et al. Molecular, serological and in vitro culture-based characterization of Bourbon virus, a newly described human pathogen of the genus Thogotovirus. J Clin Virol. 2015;73:127–32. doi: 10.1016/j.jcv.2015.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Savage HM, Burkhalter KL, Godsey MS, Jr, et al. Bourbon Virus in Field-Collected Ticks, Missouri, USA. Emerg Infect Dis. 2017;23(12):2017–22. doi: 10.3201/eid2312.170532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Savage HM, Godsey MS, Jr, Panella NA, et al. Surveillance for Tick-Borne Viruses near the location of a fatal human case of Bourbon Virus (Family Orthomyxoviridae: Genus Thogotovirus) in Eastern Kansas, 2015. J Med Entomol. 2018;55(3):701–5. doi: 10.1093/jme/tjx251. [DOI] [PubMed] [Google Scholar]
- 15.Godsey MS, Jr, Rose D, Burkhalter KL, et al. Experimental infection of Amblyomma americanum (Acari: Ixodidae) with Bourbon Virus (Orthomyxoviridae: Thogotovirus) J Med Entomol. 2020;58(2):873–9. doi: 10.1093/jme/tjaa191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bricker TL, Shafiuddin M, Gounder AP, et al. Therapeutic efficacy of favipiravir against Bourbon virus in mice. PLoS Pathog. 2019;15(6):e1007790. doi: 10.1371/journal.ppat.1007790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Savage HM, Godsey MS, Jr, Tatman J, et al. Surveillance for Heartland and Bourbon Viruses in Eastern Kansas, June 2016. J Med Entomol. 2018;55(6):1613–6. doi: 10.1093/jme/tjy103. [DOI] [PubMed] [Google Scholar]
- 18.Jackson KC, Gidlewski T, Root JJ, et al. Bourbon Virus in wild and domestic animals, Missouri, USA, 2012–2013. Emerg Infect Dis. 2019;25(9):1752–3. doi: 10.3201/eid2509.181902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Komar N, Hamby N, Palamar MB, et al. Indirect evidence of Bourbon Virus (Thogotovirus, Orthomyxoviridae) Infection in North Carolina. N C Med J. 2020;81(3):214–5. doi: 10.18043/ncm.81.3.214. [DOI] [PubMed] [Google Scholar]
- 20.McMullan LK, Folk SM, Kelly AJ, et al. A new phlebovirus associated with severe febrile illness in Missouri. N Engl J Med. 2012;367(9):834–41. doi: 10.1056/NEJMoa1203378. [DOI] [PubMed] [Google Scholar]
- 21.Yu XJ, Liang MF, Zhang SY, et al. Fever with thrombocytopenia associated with a novel bunyavirus in China. N Engl J Med. 2011;364(16):1523–32. doi: 10.1056/NEJMoa1010095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Savage HM, Godsey MS, Jr, Panella NA, et al. Surveillance for Heartland Virus (Bunyaviridae: Phlebovirus) in Missouri during 2013: first detection of virus in adults of Amblyomma americanum (Acari: Ixodidae) J Med Entomol. 2016;53(3):607–12. doi: 10.1093/jme/tjw028. [DOI] [PubMed] [Google Scholar]
- 23.Savage HM, Godsey MS, Lambert A, et al. First detection of heartland virus (Bunyaviridae: Phlebovirus) from field collected arthropods. Am J Trop Med Hyg. 2013;89(3):445–52. doi: 10.4269/ajtmh.13-0209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Godsey MS, Savage HM, Burkhalter KL, et al. Transmission of Heartland Virus (Bunyaviridae: Phlebovirus) by experimentally infected Amblyomma americanum (Acari: Ixodidae) J Med Entomol. 2016;53(5):1226–33. doi: 10.1093/jme/tjw080. [DOI] [PubMed] [Google Scholar]
- 25.Springer YP, Eisen L, Beati L, et al. Spatial distribution of counties in the continental United States with records of occurrence of Amblyomma americanum (Ixodida: Ixodidae) J Med Entomol. 2014;51(2):342–51. doi: 10.1603/me13115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Riemersma KK, Komar N. Heartland Virus neutralizing antibodies in vertebrate wildlife, United States, 2009–2014. Emerg Infect Dis. 2015;21(10):1830–3. doi: 10.3201/eid2110.150380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Brault AC, Savage HM, Duggal NK, et al. Heartland Virus epidemiology, vector association, and disease potential. Viruses. 2018;10(9) doi: 10.3390/v10090498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tuten HC, Burkhalter KL, Noel KR, et al. Heartland Virus in humans and ticks, Illinois, USA, 2018–2019. Emerg Infect Dis. 2020;26(7):1548–52. doi: 10.3201/eid2607.200110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pastula DM, Turabelidze G, Yates KF, et al. Notes from the field: Heartland virus disease - United States, 2012–2013. MMWR Morb Mortal Wkly Rep. 2014;63(12):270–1. [PMC free article] [PubMed] [Google Scholar]
- 30.Staples JE, Pastula DM, Panella AJ, et al. Investigation of Heartland Virus Disease Throughout the United States, 2013–2017. Open Forum Infect Dis. 2020;7(5):ofaa125. doi: 10.1093/ofid/ofaa125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Carlson AL, Pastula DM, Lambert AJ, et al. Heartland Virus and Hemophagocytic Lymphohistiocytosis in immunocompromised patient, Missouri, USA. Emerg Infect Dis. 2018;24(5):893–7. doi: 10.3201/eid2405.171802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Muehlenbachs A, Fata CR, Lambert AJ, et al. Heartland virus-associated death in tennessee. Clin Infect Dis. 2014;59(6):845–50. doi: 10.1093/cid/ciu434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fill MA, Compton ML, McDonald EC, et al. Novel Clinical and Pathologic Findings in a Heartland Virus-Associated Death. Clin Infect Dis. 2017;64(4):510–2. doi: 10.1093/cid/ciw766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.O’Neil BH, Allen R, Spigel DR, et al. High incidence of cetuximab-related infusion reactions in Tennessee and North Carolina and the association with atopic history. J Clin Oncol. 2007;25(24):3644–8. doi: 10.1200/JCO.2007.11.7812. [DOI] [PubMed] [Google Scholar]
- 35.Hansen NL, Chandiramani DV, Morse MA, et al. Incidence and predictors of cetuximab hypersensitivity reactions in a North Carolina academic medical center. J Oncol Pharm Pract. 2011;17(2):125–30. doi: 10.1177/1078155209360853. [DOI] [PubMed] [Google Scholar]
- 36.Chung CH, Mirakhur B, Chan E, et al. Cetuximab-induced anaphylaxis and IgE specific for galactose-alpha-1,3-galactose. N Engl J Med. 2008;358(11):1109–17. doi: 10.1056/NEJMoa074943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Steinke JW, Platts-Mills TA, Commins SP. The alpha-gal story: lessons learned from connecting the dots. J Allergy Clin Immunol. 2015;135(3):589–96. doi: 10.1016/j.jaci.2014.12.1947. quiz 97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Commins SP, Satinover SM, Hosen J, et al. Delayed anaphylaxis, angioedema, or urticaria after consumption of red meat in patients with IgE antibodies specific for galactose-alpha-1,3-galactose. J Allergy Clin Immunol. 2009;123(2):426–33. doi: 10.1016/j.jaci.2008.10.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Van Nunen SA, O’Connor KS, Clarke LR, et al. An association between tick bite reactions and red meat allergy in humans. Med J Aust. 2009;190(9):510–1. doi: 10.5694/j.1326-5377.2009.tb02533.x. [DOI] [PubMed] [Google Scholar]
- 40.Commins SP, James HR, Kelly LA, et al. The relevance of tick bites to the production of IgE antibodies to the mammalian oligosaccharide galactose-alpha-1,3-galactose. J Allergy Clin Immunol. 2011;127(5):1286–93.e6. doi: 10.1016/j.jaci.2011.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Houchens N, Hartley S, Commins SP, et al. Hunting for a Diagnosis. N Engl J Med. 2021;384(5):462–7. doi: 10.1056/NEJMcps2017588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wilson JM, Schuyler AJ, Workman L, et al. Investigation into the alpha-Gal Syndrome: Characteristics of 261 Children and Adults Reporting Red Meat Allergy. J Allergy Clin Immunol Pract. 2019;7(7):2348–58.e4. doi: 10.1016/j.jaip.2019.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Roman-Carrasco P, Lieder B, Somoza V, et al. Only alpha-Gal bound to lipids, but not to proteins, is transported across enterocytes as an IgE-reactive molecule that can induce effector cell activation. Allergy. 2019;74(10):1956–68. doi: 10.1111/all.13873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Binder AM, Commins SP, Altrich ML, et al. Diagnostic testing for galactose-alpha-1,3-galactose, United States, 2010 to 2018. Ann Allergy Asthma Immunol. 2021 doi: 10.1016/j.anai.2020.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Commins SP. Diagnosis & management of alpha-gal syndrome: lessons from 2,500 patients. Expert Rev Clin Immunol. 2020;16(7):667–77. doi: 10.1080/1744666X.2020.1782745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Platts-Mills TAE, Li RC, Keshavarz B, et al. Diagnosis and Management of Patients with the alpha-Gal Syndrome. J Allergy Clin Immunol Pract. 2020;8(1):15–23.e1. doi: 10.1016/j.jaip.2019.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lee VJ, Aguilera X, Heymann D, et al. Preparedness for emerging epidemic threats: a Lancet Infectious Diseases Commission. Lancet Infect Dis. 2020;20(1):17–9. doi: 10.1016/S1473-3099(19)30674-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Eisen L. Stemming the Rising Tide of Human-Biting Ticks and Tickborne Diseases, United States. Emerg Infect Dis. 2020;26(4):641–7. doi: 10.3201/eid2604.191629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Suss J, Klaus C, Gerstengarbe FW, et al. What makes ticks tick? Climate change, ticks, and tick-borne diseases. J Travel Med. 2008;15(1):39–45. doi: 10.1111/j.1708-8305.2007.00176.x. [DOI] [PubMed] [Google Scholar]
- 50.Sagurova I, Ludwig A, Ogden NH, et al. Predicted northward expansion of the geographic range of the Tick Vector Amblyomma americanum in North America under future climate conditions. Environ Health Perspect. 2019;127(10):107014. doi: 10.1289/EHP5668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sonenshine DE. Range expansion of Tick Disease Vectors in North America: implications for spread of Tick-Borne Disease. Int J Environ Res Public Health. 2018;15(3) doi: 10.3390/ijerph15030478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Newman BC, Sutton WB, Moncayo AC, et al. Heartland Virus in Lone Star Ticks, Alabama, USA. Emerg Infect Dis. 2020;26(8):1954–6. doi: 10.3201/eid2608.200494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Liu W, Lu QB, Cui N, et al. Case-fatality ratio and effectiveness of ribavirin therapy among hospitalized patients in china who had severe fever with thrombocytopenia syndrome. Clin Infect Dis. 2013;57(9):1292–9. doi: 10.1093/cid/cit530. [DOI] [PubMed] [Google Scholar]
- 54.Liu Q, He B, Huang SY, et al. Severe fever with thrombocytopenia syndrome, an emerging tick-borne zoonosis. Lancet Infect Dis. 2014;14(8):763–72. doi: 10.1016/S1473-3099(14)70718-2. [DOI] [PubMed] [Google Scholar]
- 55.Rainey T, Occi JL, Robbins RG, et al. Discovery of Haemaphysalis longicornis (Ixodida: Ixodidae) parasitizing a sheep in New Jersey, United States. J Med Entomol. 2018;55(3):757–9. doi: 10.1093/jme/tjy006. [DOI] [PubMed] [Google Scholar]
- 56.Beard CB, Occi J, Bonilla DL, et al. Multistate Infestation with the Exotic Disease-Vector Tick Haemaphysalis longicornis - United States, August 2017-September 2018. MMWR Morb Mortal Wkly Rep. 2018;67(47):1310–3. doi: 10.15585/mmwr.mm6747a3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wormser GP, McKenna D, Piedmonte N, et al. First recognized human bite in the United States by the Asian Longhorned Tick, Haemaphysalis longicornis. Clin Infect Dis. 2020;70(2):314–6. doi: 10.1093/cid/ciz449. [DOI] [PubMed] [Google Scholar]
- 58.Pritt BS. Haemaphysalis longicornis is in the United States and biting humans: where do we go from here? Clin Infect Dis. 2019;70(2):317–8. doi: 10.1093/cid/ciz451. [DOI] [PubMed] [Google Scholar]
- 59.Breuner NE, Ford SL, Hojgaard A, et al. Failure of the Asian longhorned tick, Haemaphysalis longicornis, to serve as an experimental vector of the Lyme disease spirochete, Borrelia burgdorferi sensu stricto. Ticks Tick Borne Dis. 2020;11(1):101311. doi: 10.1016/j.ttbdis.2019.101311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Stanley HM, Ford SL, Snellgrove AN, et al. The ability of the invasive Asian Longhorned Tick Haemaphysalis longicornis (Acari: Ixodidae) to acquire and transmit Rickettsia rickettsii (Rickettsiales: Rickettsiaceae), the agent of Rocky Mountain Spotted Fever, under laboratory conditions. J Med Entomol. 2020;57(5):1635–9. doi: 10.1093/jme/tjaa076. [DOI] [PubMed] [Google Scholar]
- 61.Levin ML, Stanley HM, Hartzer K, et al. Incompetence of the Asian Longhorned Tick (Acari: Ixodidae) in Transmitting the Agent of Human Granulocytic Anaplasmosis in the United States. J Med Entomol. 2021;58(3):1419–23. doi: 10.1093/jme/tjab015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sanchez-Vicente S, Tagliafierro T, Coleman JL, et al. Polymicrobial nature of Tick-Borne Diseases. mBio. 2019;10(5) doi: 10.1128/mBio.02055-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Straily A, Stuck S, Singleton J, Jr, et al. Antibody Titers Reactive with Rickettsia rickettsii in blood donors and implications for surveillance of Spotted Fever Rickettsiosis in the United States. J Infect Dis. 2019;221(8):1371–8. doi: 10.1093/infdis/jiz316. [DOI] [PubMed] [Google Scholar]
- 64.McClain MT, Sexton DJ. Surveillance for Spotted Fever Group Rickettsial Infections: Problems, pitfalls, and potential solutions. J Infect Dis. 2019;221(8):1238–40. doi: 10.1093/infdis/jiz317. [DOI] [PubMed] [Google Scholar]
- 65.Fuchs J, Straub T, Seidl M, et al. Essential role of interferon response in containing Human Pathogenic Bourbon Virus. Emerg Infect Dis. 2019;25(7):1304–13. doi: 10.3201/eid2507.181062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Feng K, Deng F, Hu Z, et al. Heartland virus antagonizes type I and III interferon antiviral signaling by inhibiting phosphorylation and nuclear translocation of STAT2 and STAT1. J Biol Chem. 2019;294(24):9503–17. doi: 10.1074/jbc.RA118.006563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Li MM, Zhang WJ, Weng XF, et al. CD4 T cell loss and Th2 and Th17 bias are associated with the severity of severe fever with thrombocytopenia syndrome (SFTS) Clin Immunol. 2018;195:8–17. doi: 10.1016/j.clim.2018.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Sun Y, Jin C, Zhan F, et al. Host cytokine storm is associated with disease severity of severe fever with thrombocytopenia syndrome. J Infect Dis. 2012;206(7):1085–94. doi: 10.1093/infdis/jis452. [DOI] [PubMed] [Google Scholar]
- 69.Fajgenbaum DC, June CH. Cytokine Storm. 2020;383(23):2255–73. doi: 10.1056/NEJMra2026131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Todd SR, Dahlgren FS, Traeger MS, et al. No visible dental staining in children treated with doxycycline for suspected Rocky Mountain Spotted Fever. J Pediatr. 2015;166(5):1246–51. doi: 10.1016/j.jpeds.2015.02.015. [DOI] [PubMed] [Google Scholar]
- 71.de la Fuente J, Pacheco I, Villar M, et al. The alpha-Gal syndrome: new insights into the tick-host conflict and cooperation. Parasit Vectors. 2019;12(1):154. doi: 10.1186/s13071-019-3413-z. [DOI] [PMC free article] [PubMed] [Google Scholar]